Centennial Fertilization-Induced Soil Processes Control Trace Metal Dynamics. Lessons from a Long-Term Bare Fallow Experiment
Abstract
1. Introduction
2. Materials and Methods
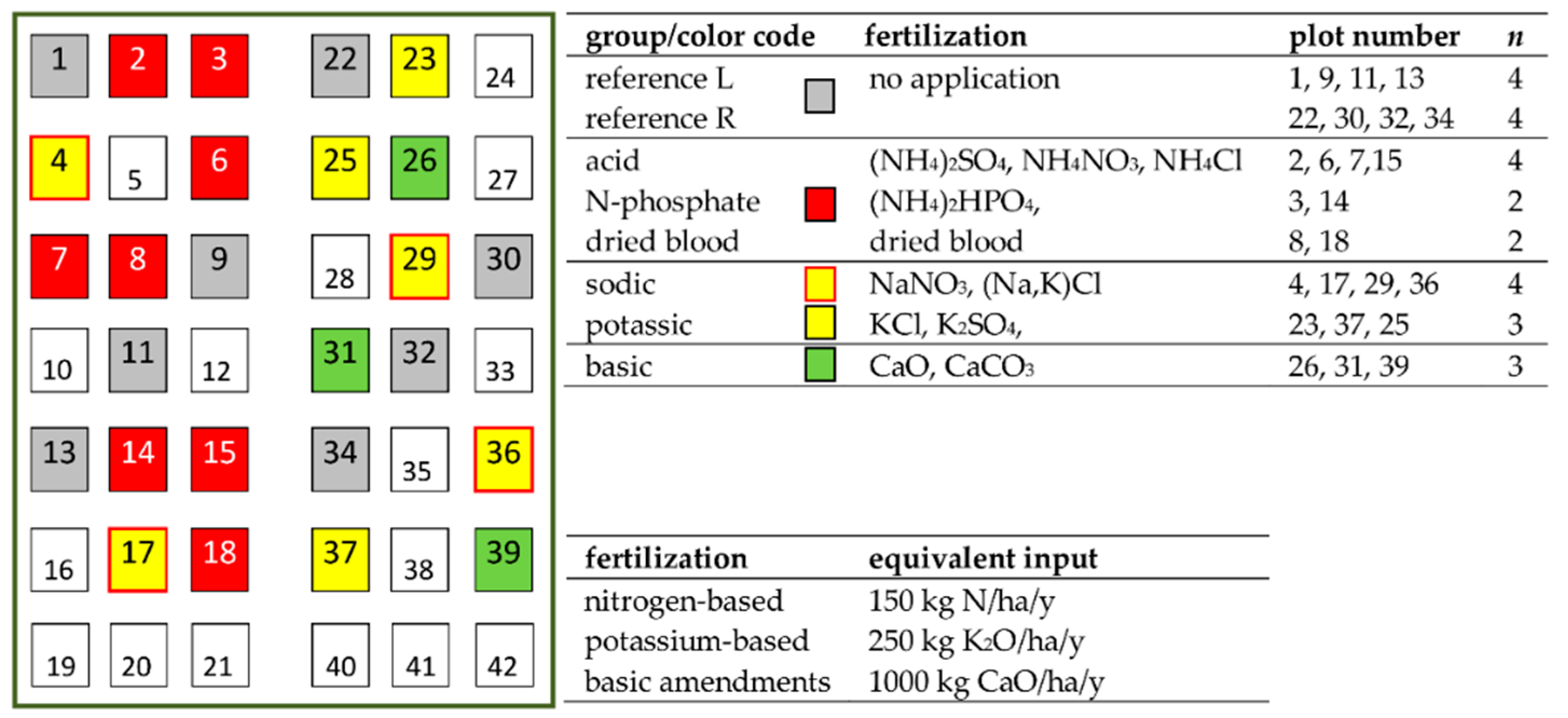
2.1. Long-Term Bare Fallow Experiment of INRA Versailles
2.2. Analysis of Soil Characteristics and Total Element Concentrations
2.3. Computation of Element Stocks and Budgets
2.4. Validity of Calculations
2.5. Data Presentation and Statistical Treatment
3. Results
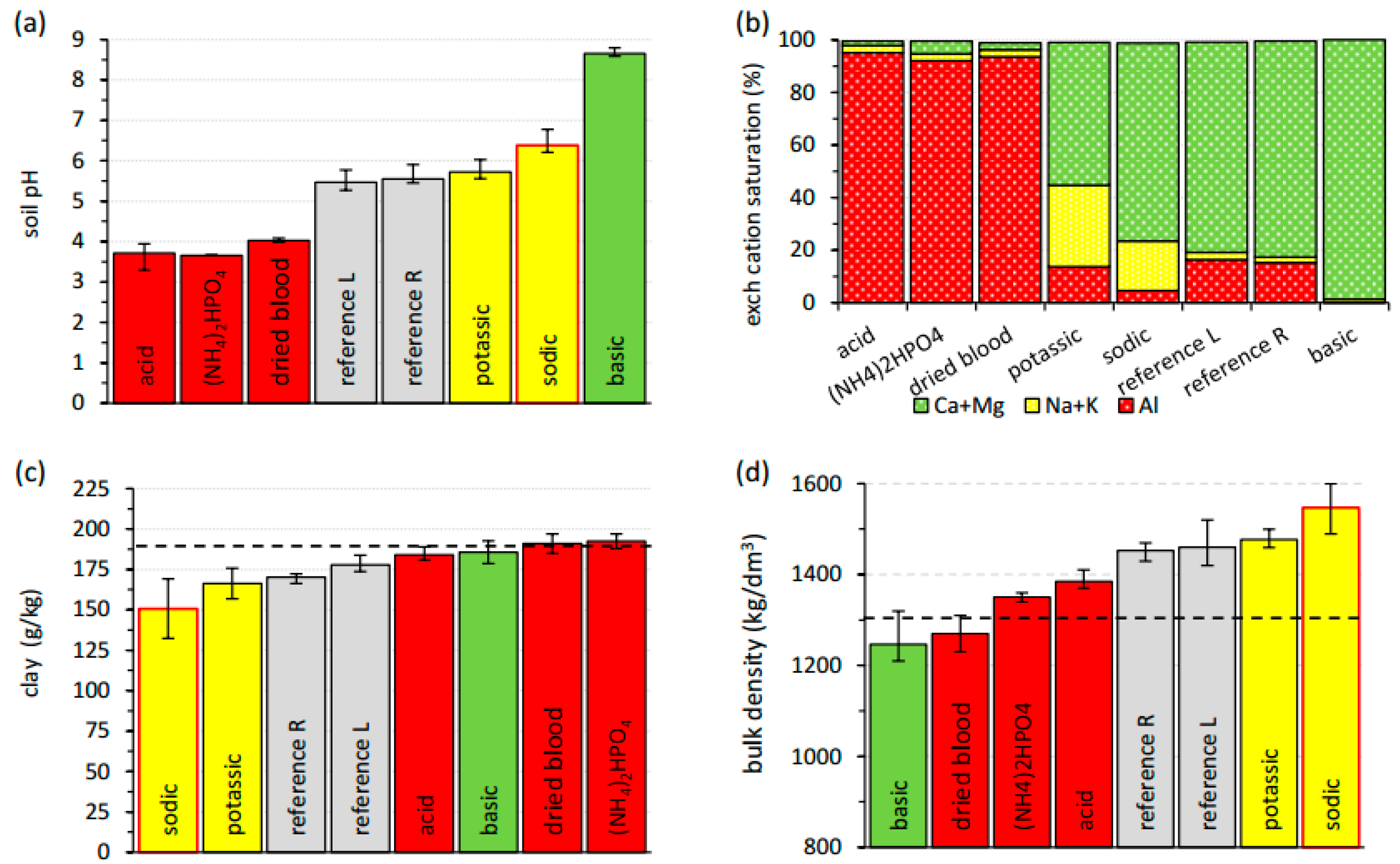
3.1. Present Physicochemical Soil Characteristics
3.2. Geochemical Budgets of Major and Trace Elements between 1929 and 2014
3.2.1. Element Budgets in Nonamended Reference Plots
3.2.2. Element Budgets in Fertilized Plots
3.3. Selected Exploratory Analytical Data from Subsurface Horizons in 2014
3.3.1. Chemistry
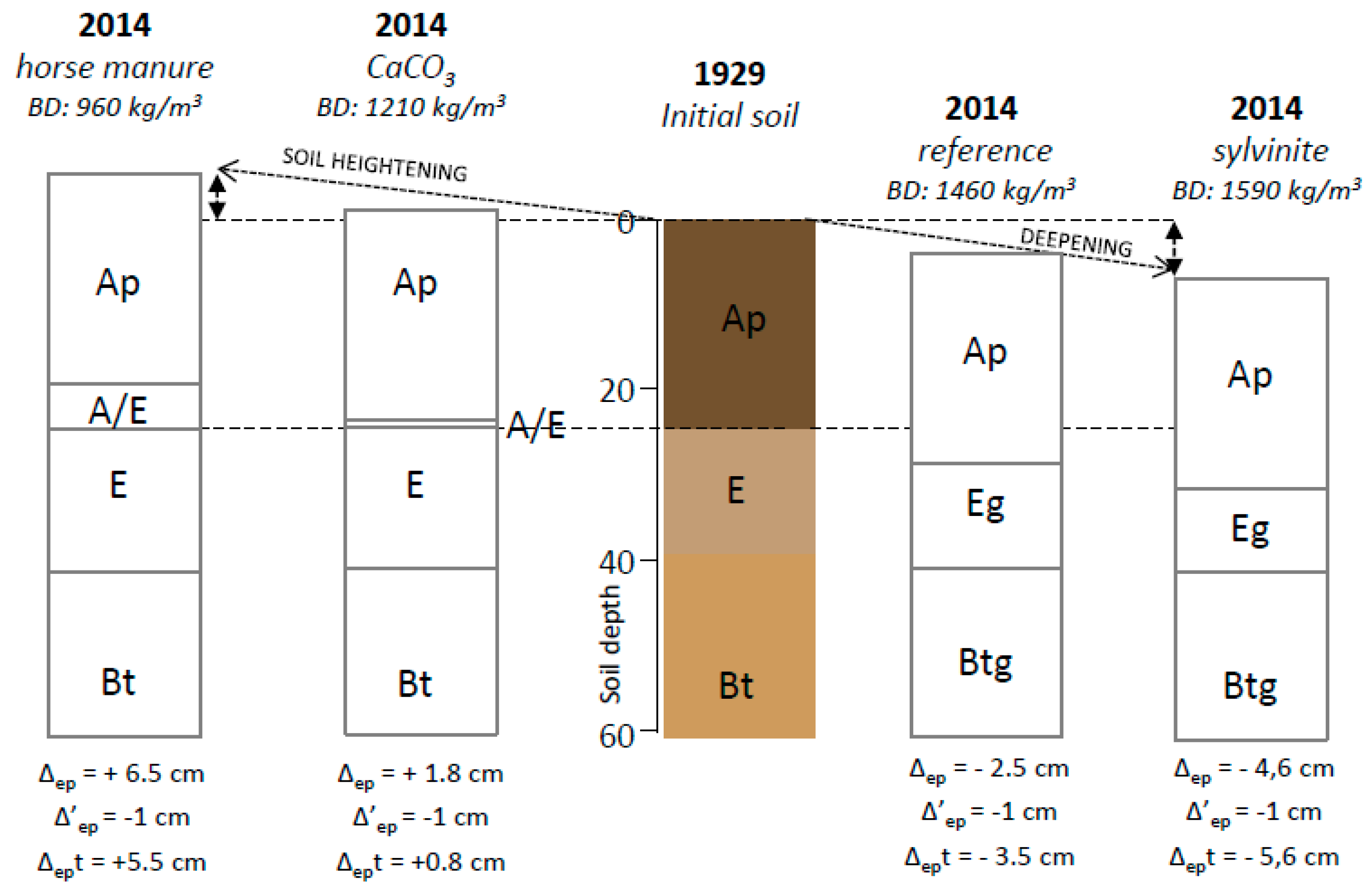
3.3.2. Micromorphology
4. Discussion
4.1. Dynamics of Major and Trace Element in Soils: Lessons from the 42-Plots LTBF Experiment
4.1.1. Sources of Element Inputs
4.1.2. Soil Processes
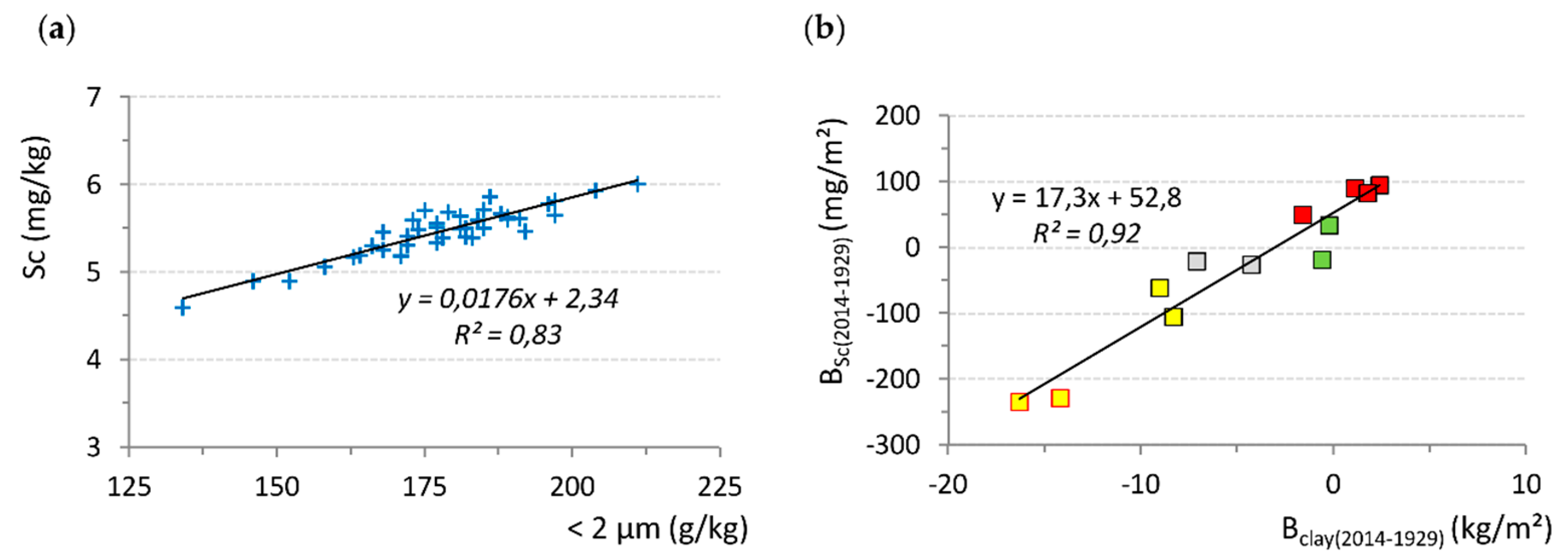
Clay Leaching
Acidification, Lixiviation, and Enhanced Chemical Weathering
Alkalinization
4.2. Significance of Agronomic Experiments to Long-Term Soil Evolution Studies
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Paradelo, R.; van Oort, F.; Chenu, C. Water-dispersible clay in bare fallow soils after 80 years of continuous fertilizers addition. Geoderma 2013, 200–201, 40–44. [Google Scholar] [CrossRef]
- Manlay, R.; Feller, C.; Swift, M. Historical evolution of soil organic matter concepts and their relationships with the fertility and sustainability of cropping systems. Agric. Ecosyst. Environ. 2007, 119, 217–233. [Google Scholar] [CrossRef]
- Rajan, K.; Natarajan, A.; Kumar, K.; Badrinath, M.; Gowda, R. Soil organic carbon—The most reliable indicator for monitoring land degradation by soil erosion. Curr. Sci. 2010, 99, 823–827. [Google Scholar]
- Reeves, D.W. The role of soil organic matter in maintaining soil quality in continuous cropping systems. Soil Tillage Res. 1997, 43, 131–167. [Google Scholar] [CrossRef]
- Balesdent, J.; Wagner, G.H.; Mariotti, A. Soil organic matter turnover in long-term field experiments as revealed by C-13 natural abundance. Soil Sci. Soc. Am. J. 1988, 52, 118–124. [Google Scholar] [CrossRef]
- Dixon, J.B.; Weed, S.B. Minerals in Soil Environments, 2nd ed.; SSSA Book Series 1; Soil Scientific Society American: Madison, WI, USA, 1989; ISBN 0-89118-787-1. [Google Scholar]
- Tessier, D. Behaviour and microstructure of clay minerals. Soil Colloids and Their Associations in Aggregates; De Boodt, M.F., Hayes, M.H.B., Herbillon, A., Eds.; Nato ASI Series B: Physics; Plenum Press: New York, NY, USA, 1990; Volume 215, pp. 387–415. ISBN 0-306-43419-9. [Google Scholar]
- Van Oort, F.; Thiry, M.; Foy, E.; Fujisaki, K.; Delarue, G.; Dairon, R.; Jongmans, A.G. Impacts of one century of wastewater discharge on soil transformation through ferrolysis and related metal pollutant distributions. Sci. Total Environ. 2017, 590–591, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Burgevin, H.; Hénin, S. Dix années d’expériences sur l’action des engrais sur la composition et les propriétés d’un sol de limon. Ann. Agron. 1939, 9, 771–799. [Google Scholar]
- Barré, P.; Eglin, T.; Christensen, B.T.; Ciais, P.; Houot, S.; Kätterer, T.; van Oort, F.; Peylin, P.; Poulton, P.R.; Romanenkov, V.; et al. Quantifying and isolating stable carbon using long-term bare fallow experiments. Biogeosciences 2010, 7, 3839–3850. [Google Scholar] [CrossRef]
- Semlali, R.; Dessogne, J.; Monna, F.; Bolte, J.; Azimi, S.; Denaix, L.; Loubet, M.; van Oort, F. Modeling lead input and output in soils by using lead isotopic geochemistry. Environ. Sci. Technol. 2004, 38, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Monna, F.; van Oort, F.; Hubert, P.; Dominik, J.; Bolte, J.; Loizeau, J.L.; Labanowski, J.; Lamri, J.; Petit, C.; Le Roux, G.; et al. Modelling of 137Cs migration in soils using an 80-years soil archive. Role of fertilizers and agricultural amendments. J. Environ. Radioact. 2009, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Barré, P.; Plante, A.F.; Cécillon, L.; Lutfalla, S.; Baudin, F.; Bernard, S.; Christensen, B.T.; Fernandez, J.M.; Houot, S.; Kätterer, T.; et al. The energetic and chemical signatures of persistent soil organic matter. Biogeochemistry 2016, 1–2, 1–12. [Google Scholar] [CrossRef]
- Paradelo, R.; van Oort, F.; Barré, P.; Billiou, D.; Chenu, C. Soil organic matter stabilization at the pluri-decadal scale: Insight from bare fallow soils with contrasting physicochemical properties and macrostructures. Geoderma 2016, 275, 48–54. [Google Scholar] [CrossRef]
- Haney, R.J. Are Fertilizers Punishing Our Soils? Yale School of Forestry & Environmental Studies: New Haven, CT, USA, 2017; Available online: http://e360.yale.edu/features/why-its-time-to-stop-punishing-our-soils-with-fertilizers-and-chemicals/ (accessed on 5 December 2017).
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014; ISBN 978-92-5-108369-7. [Google Scholar]
- Van Oort, F.; Proix, N.; Paradelo, R.; Delarue, G.; Breuil, S.; Baize, D.; Richard, A. Dernières nouvelles de 42 vieilles parcelles. Indicateurs d’évolutions pédologiques infra-centenaires en Néoluvisol de lœss nu, sous contrainte d’applications continues de matières fertilisantes. Étude et Gestion des Sols 2016, 23, 143–162. [Google Scholar]
- Van Oort, F.; Paradelo, R.; Proix, N.; Breuil, S.; Delarue, G.; Trouvé, A.; Baize, D.; Monna, F.; Richard, A. Arsenic et Vieilles Parcelles. États et bilans géochimiques dans un Néoluvisol de lœss nu, avec ou sans apports de matières fertilisantes depuis 1928. Étude et Gestion des Sols 2017, 24, 99–126. [Google Scholar]
- AFNOR. Méthodes D’analyse Chimique. In Evaluation de la Qualité des Sols; Recueil Normes & Réglementation; AFNOR: Paris, France, 2004; Volume 1, pp. 401–409. ISBN 2.12.213151-9. [Google Scholar]
- Baize, D. Teneurs Totales en Eléments Traces Métalliques Dans les Sols (France); INRA: Paris, France, 1997; ISBN 2-7380-0747-3. [Google Scholar]
- Bresson, L.M.; Boiffin, J. Morphological characterization of soil crust development stages on an experimental field. Geoderma 1990, 47, 301–325. [Google Scholar] [CrossRef]
- Pernes-Debuyser, A.; Tessier, D. Soil physical properties affected by long-term fertilization. Eur. J. Soil Sci. 2004, 55, 505–512. [Google Scholar] [CrossRef]
- Doran, J.W.; Sarrantonio, M.; Liebig, M.A. Soil health and sustainability. Adv. Agron. 1996, 56, 1–54. [Google Scholar] [CrossRef]
- Ellert, B.H.; Bettany, J.R. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef]
- Balabane, M.; Faivre, D.; van Oort, F.; Dahmani-Muller, H. Mutual effects of soil organic matter dynamics end heavy metals fate in a metallophyte grassland. Environ. Pollut. 1999, 105, 45–54. [Google Scholar] [CrossRef]
- Isambert, M. Etudes des Sols des Parcelles Expérimentales du CNRA Versailles, Parcelles des Closeaux; Internal Report; SESCPF: Orléans, France, 1979. [Google Scholar]
- Monni, C. Stabilisation Physique et Physico-Chimique de la Matière Organique dans les Horizons Profonds du Sol. Ph.D. Thesis, Université Pierre et Marie Curie, Paris, France, 2008. [Google Scholar]
- Horovitz, C.T.; Gschneidner, K.A., Jr.; Melson, G.A.; Youngblood, D.H.; Schock, H.H. Scandium. Its Occurrence, Chemistry, Physics, Metallurgy, Biology and Technology; Academic Press: London, UK, 1975; ISBN 978-0124125353. [Google Scholar]
- Mitchel, R.L. Trace Elements in Soils. Chemistry of the Soil, 2nd ed.; Bear, F.E., Ed.; Rheinholt: New York, NY, USA, 1964. [Google Scholar]
- Shotyk, W.; Weiss, D.; Kramers, J.; Frei, R.; Cherbukin, A.K.; Gloor, M.; Reese, S. Geochemistry of the peat bog at Étang de la Gruère, Jura Mountains, Switzerland, and its record of atmospheric Pb and lithogenic trace metals (Sc, Ti, Y, Zr, and REE) since 12 370 14C yr BP. Geochem. Cosmochim. Acta 2001, 65, 2337–2360. [Google Scholar] [CrossRef]
- Kabata Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4200-9368-1. [Google Scholar]
- Pernes-Debuyser, A.; Tessier, D. Influence de matières fertilisantes sur les propriétés des sols : Cas des 42 parcelles de L’INRA à Versailles. Étude et Gestion des Sols 2002, 9, 177–186. [Google Scholar]
- Stoops, G.; Marcellino, V.; Mees, F. Interpretation of Micromorphological Features of Soils and Regoliths; Elsevier: Amsterdam, The Netherlands, 2010; 720p, ISBN 978-0-444-53156-8. [Google Scholar]
- Yunta, F.; Di Foggia, M.; Bellido-Díaz, V.; Morales-Calderón, M.; Tessarin, P.; López-Rayo, S.; Tinti, A.; Kovács, K.; Klencsár, Z.; Fodor, F.; et al. Blood meal-based compound. Good choice as iron fertilizer for organic farming. J. Agric. Food Chem. 2013, 61, 3995–4003. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Heavy Metals in Soils, 3rd ed.; Springer: Dordrecht, The Netherlands, 2013; ISBN 9789400744691. [Google Scholar]
- Juste, C.; Tauzin, J. Evolution du contenu en métaux lourds d’un sol de limon maintenu en jachère nue après 56 années d’application continue de divers engrais et amendements. CR Acad. Agric. 1986, 72, 739–746. [Google Scholar]
- Ma, Y.; Hooda, P.S. Chromium, Nickel and Cobalt. In Trace Elements in Soils; Hooda, P.S., Ed.; Whiley: Chichester, UK, 2010; pp. 461–479. ISBN 978-1-405-16037-7. [Google Scholar]
- Van Leeuwen, E.P.; Draaijer, G.P.J.; Erisman, J.W. Mapping wet deposition of acidifying components and base cations over Europe using measurements. Atmos. Environ. 1996, 30, 2495–2511. [Google Scholar] [CrossRef]
- Belon, E.; Boisson, M.; Deportes, I.Z.; Eglin, T.K.; Feix, I.; Bispo, A.O.; Galsomies, L.; Leblond, S.; Guellier, C.R. An inventory of trace elements inputs to French agricultural soils. Sci. Total Environ. 2012, 439, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Saby, N.; Arrouays, D.; Boulonne, L.; Jolivet, C.; Pochot, A. Geostatistical assessment of Pb in soil around Paris, France. Sci. Total Environ. 2006, 367, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Rampazzo, G.; Innocente, E.; pecorari, E.; Squizzato, S.; Valotto, G. Potential harmful elements in the atmosphere. In PHE’s, Environment and Human Health; Bini, C., Bech, J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–36. ISBN 978-94-017-8964-6. [Google Scholar]
- Granier, L.; Chevreuil, M.; Carru, A.M.; Chesterikoff, A. Atmospheric fallout of organochlorines and heavy metals on the Paris area (France). Sci. Total Environ. 1992, 126, 165–172. [Google Scholar] [CrossRef]
- Azimi, S.; Ludwig, A.; Thévenot, D.R.; Colin, J.L. Trace metal determination in total atmospheric deposition in rural and urban areas. Sci. Total Environ. 2003, 308, 247–256. [Google Scholar] [CrossRef]
- Evans, L.J.; Barabash, S.J. Molybdenum, Silver, Thallium and Vanadium. In Trace Elements in Soils; Hooda, P.S., Ed.; Whiley: Chichester, UK, 2010; pp. 515–549. ISBN 978-1-405-16037-7. [Google Scholar]
- Tremel, A.; Masson, P.; Sterckeman, T.; Baize, D.; Mench, M. Thallium in French agrosystems. I. Thallium contents in arable soils. Environ. Pollut. 1997, 95, 293–302. [Google Scholar] [CrossRef]
- Punshon, T.; Jackson, B.P.; Meharg, A.A.; Warczak, T.; Scheckel, K.; Guerinot, M.L. Understanding arsenic dynamics in agronomic systems to predict and prevent uptake by crop plants. Sci. Total Environ. 2017, 581–582, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.A.; Goldberg, S. Adsorption and stability of arsenic(III) at the clay mineral-water interface. Environ. Sci. Technol. 1997, 31, 2005–2011. [Google Scholar] [CrossRef]
- Party, J.P.; Probst, A.; Dambrine, E.; Thomas, A.L. Critical loads of acidity to France: Sensitivity areas in the north-eastern France. Water Soil Air Pollut. 1995, 85, 2407–2412. [Google Scholar] [CrossRef]
- UNEP. Global Environment Outlook; GEO4; United Nations Environment Programme: Valetta, Malta, 2007; ISBN 978-92-807-2836-1. [Google Scholar]
- Van Breemen, N.; Buurman, P. Soil Formation; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-1-4020-0718-7. [Google Scholar]
- Hardy, M.; Jamagne, M.; Elsass, F.; Robert, M.; Chesneau, D. Mineralogical development of the silt fractions of a Podzoluvisol on lœss in the ParisBasin (France). Eur. J. Soil Sci. 1999, 50, 443–456. [Google Scholar] [CrossRef]
- Bohn, H.L.; McNeal, B.L.; O’Connor, G.A. Soil Chemistry, 4th ed.; John Wiley & Sons: New York, NY, USA, 2015; ISBN 978-1118629253. [Google Scholar]
- Bolt, G.H.; Bruggenwert, M.G.M. Soil Chemistry. A. Basic Elements; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Eeckman, J.P.; Laudelout, H. Chemical stability of hydrogen-montmorillonite suspensions. Colloid Polym. Sci. 1961, 178, 99–107. [Google Scholar] [CrossRef]
- Robert, M.; Razzaghe, M.H.; Ranger, J. Rôle du facteur biochimique dans la podzolisation. In Podzols et Podzolisation; Righi, D., Chauvel, A., Eds.; AFES-INRA: Paris, France, 1987; pp. 207–223. [Google Scholar]
- Robert, M.; Razzaghe, M.H.; Vicente, M.A.; Veneau, G. Rôle du facteur biochimique dans l’altération des minéraux silicatés. Science du Sol 1979, 2–3, 153–174. [Google Scholar]
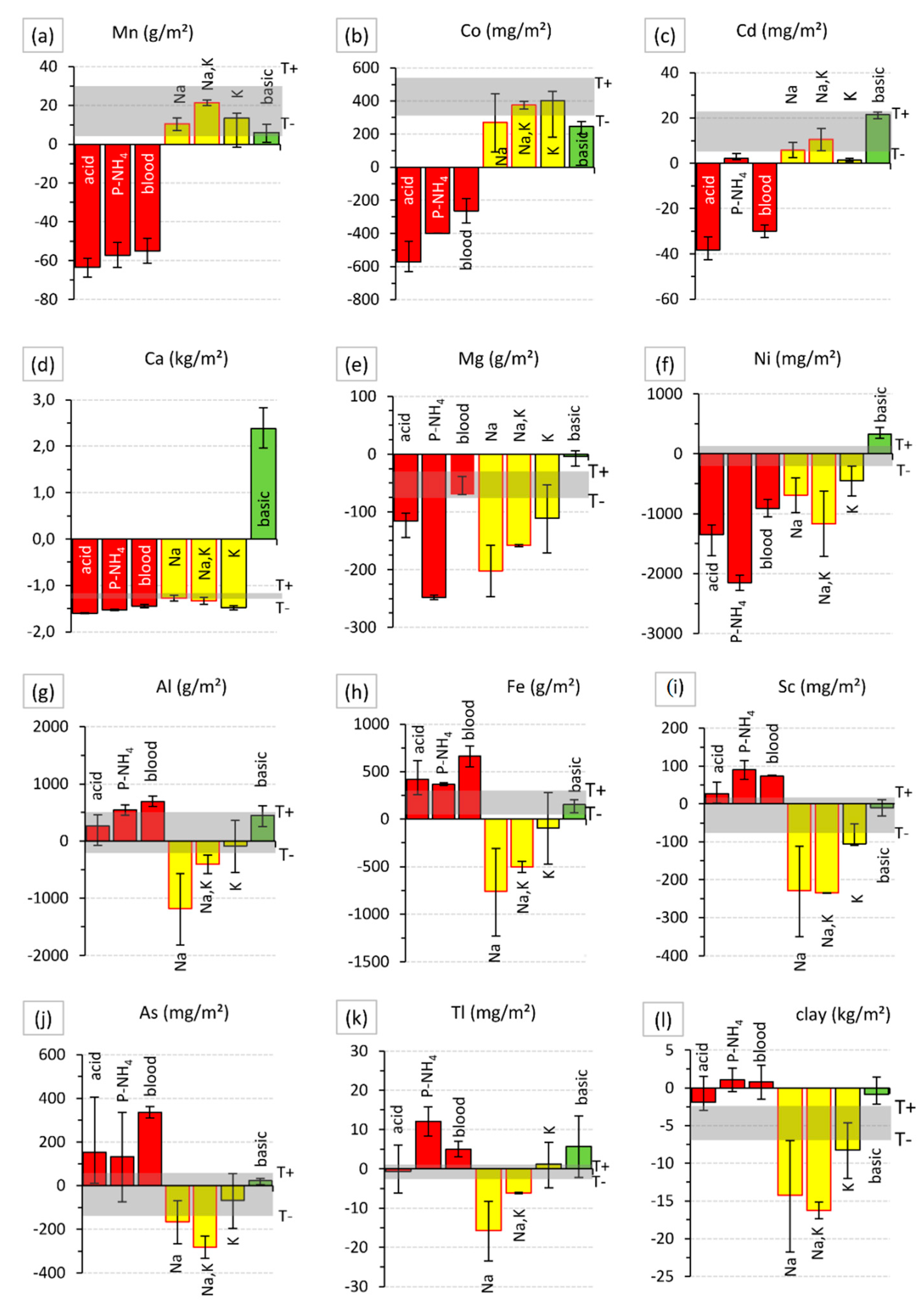
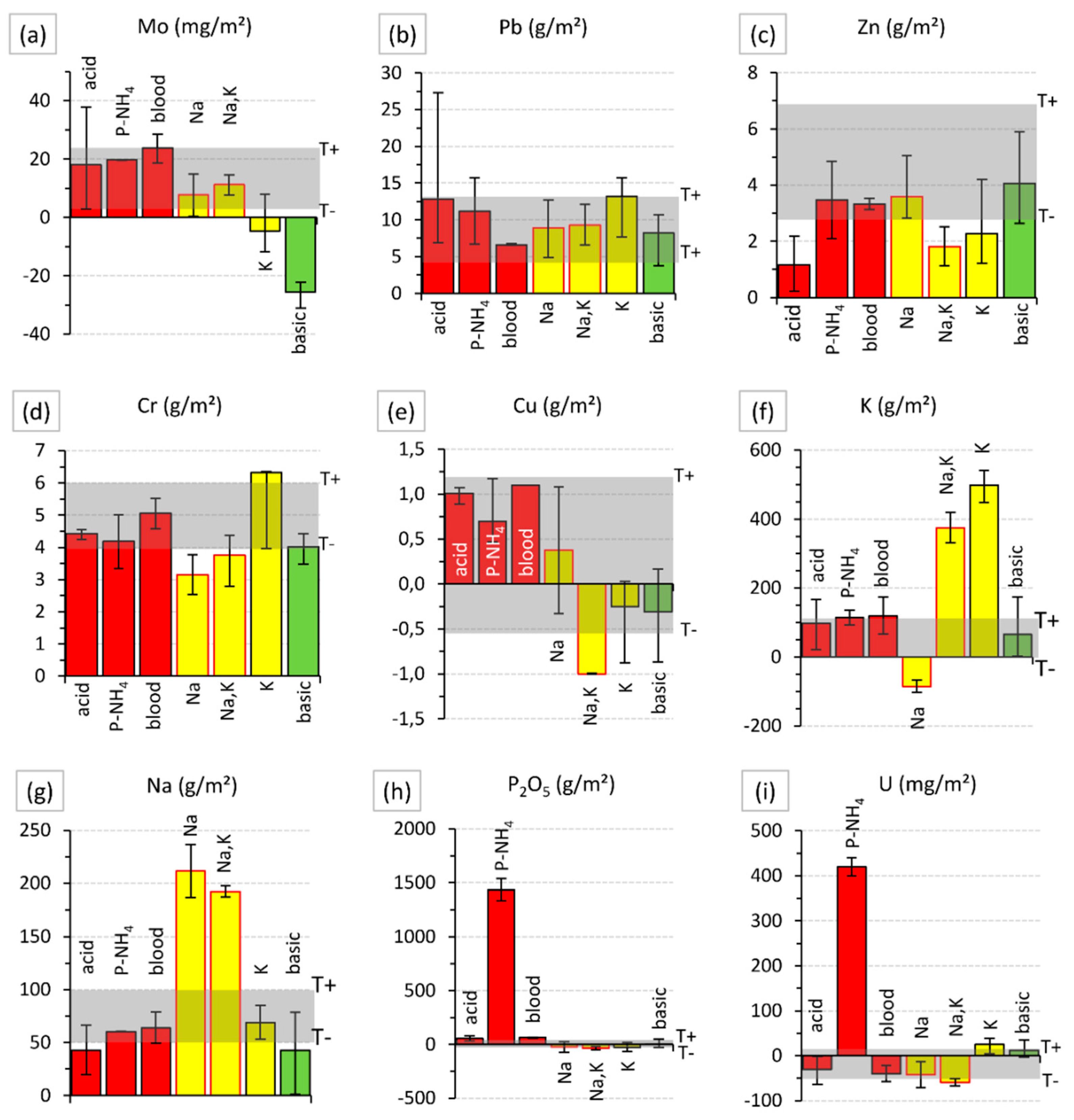


| Element | P | K | Ca | Mg | Na | Al | Fe | Mn |
|---|---|---|---|---|---|---|---|---|
| S1929 (g/m2) | 156 | 4250 | 2069 | 918 | 1766 | 11,823 | 5913 | 114 |
| S2014 (g/m2) | 158 | 4248 | 876 | 862 | 1877 | 12,006 | 6091 | 129 |
| p-value | 0.64 | 0.15 | <0.01 ** | <0.01 ** | <0.01 ** | 0.25 | 0.055 | 0.31 |
| B2014–1929 (kg/m2) | – | – | −1.2 | −0.05 | +0.1 | – | – |
| Element | Cu | Zn | Co | Cr | Ni | Mo | As | Cd | Pb | U | Tl | Sc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1929 (mg/m2) | 6530 | 15,380 | 2062 | 13,312 | 5328 | 161 | 2583 | 39.5 | 16,753 | 615 | 129 | 1781 |
| S2014 (mg/m2) | 6895 | 20,437 | 2492 | 18,374 | 5285 | 178 | 2539 | 52.0 | 25,856 | 593 | 128 | 1743 |
| p-value | 0.31 | <0.01 ** | <0.01 ** | <0.01 ** | 0.55 | <0.01 ** | 0.31 | <0.01 ** | <0.01 ** | 0.31 | 0.55 | 0.11 |
| B2014−1929 (mg/m2) | – | +5057 | +430 | +5026 | – | +17 | – | +12.5 | +9103 | – | – | – |
| Treatment | n | P | K | Ca | Mg | Na | Al | Fe | Mn | Cu | Zn |
| g/m2 | mg/m2 | ||||||||||
| acid | 4 | +30 | – | −1600 | −110 | – | - | +420 | −65 | – | −2000 |
| N–PH4 | 2 | +630 | – | −1500 | −250 | – | +550 | +370 | −60 | – | – |
| dried blood | 2 | +25 | – | −1400 | −70 | – | +700 | +660 | −55 | – | – |
| Na | 2 | – | −80 | −1300 | −200 | +110 | −1200 | −760 | – | – | – |
| Na + K | 2 | – | +380 | −1300 | −160 | +90 | −400 | −500 | – | −1000 | −1000 |
| K | 3 | – | +500 | −1500 | −110 | – | - | −100 | – | – | – |
| basic | 3 | – | – | +2500 | – | – | - | - | – | – | – |
| Co | Cr | Ni | Mo | As | Cd | Pb | U | Tl | Sc | ||
| mg/m2 | |||||||||||
| acid | 4 | −800 | – | −1350 | – | – | −45 | – | – | – | +30 |
| N–PH4 | 2 | −700 | – | −2150 | – | – | +3.5 | – | +4200 | +12 | +90 |
| dried blood | 2 | −600 | – | −900 | – | +330 | −35 | – | – | +5 | +70 |
| Na | 2 | – | −1000 | −700 | – | – | – | – | – | −16 | −230 |
| Na + K | 2 | – | −500 | −1200 | – | −280 | – | – | – | −6 | −230 |
| K | 3 | – | – | −500 | −15 | – | −5 | – | – | −100 | |
| basic | 3 | −70 | – | +330 | −35 | – | – | – | – | +7 | – |
| Element Input Sources | Major Elements | Trace Elements |
|---|---|---|
| ammonium phosphate | P, N | U, Cd, Tl, As |
| dried blood | N, Fe | Tl, As |
| monovalent | K, Na | |
| basic amendments | Ca | Tl, Ni |
| atmospheric deposition (reference plots) | Na | Pb, Zn, Cr, Co, Mo, Cd |
| Soil Processes Controlling Element Dynamics | ||
| clay leaching | Fe, Al, Ca, Mg, K | Sc, As, Tl, Ni, Cr, As |
| acid lixiviation | Ca, Mg | Mn, Cd, Co, Zn, Ni |
| alkalinization | Mo | |
| mineral weathering (relative accumulation) | Al, Fe | Sc, Tl, As |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Oort, F.; Paradelo, R.; Proix, N.; Delarue, G.; Baize, D.; Monna, F. Centennial Fertilization-Induced Soil Processes Control Trace Metal Dynamics. Lessons from a Long-Term Bare Fallow Experiment. Soil Syst. 2018, 2, 23. https://doi.org/10.3390/soilsystems2020023
Van Oort F, Paradelo R, Proix N, Delarue G, Baize D, Monna F. Centennial Fertilization-Induced Soil Processes Control Trace Metal Dynamics. Lessons from a Long-Term Bare Fallow Experiment. Soil Systems. 2018; 2(2):23. https://doi.org/10.3390/soilsystems2020023
Chicago/Turabian StyleVan Oort, Folkert, Remigio Paradelo, Nicolas Proix, Ghislaine Delarue, Denis Baize, and Fabrice Monna. 2018. "Centennial Fertilization-Induced Soil Processes Control Trace Metal Dynamics. Lessons from a Long-Term Bare Fallow Experiment" Soil Systems 2, no. 2: 23. https://doi.org/10.3390/soilsystems2020023
APA StyleVan Oort, F., Paradelo, R., Proix, N., Delarue, G., Baize, D., & Monna, F. (2018). Centennial Fertilization-Induced Soil Processes Control Trace Metal Dynamics. Lessons from a Long-Term Bare Fallow Experiment. Soil Systems, 2(2), 23. https://doi.org/10.3390/soilsystems2020023






