Cu, Pb, and Zn Sorption to Biogenic Iron (Oxyhydr)Oxides Formed in Circumneutral Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biogenic Iron (Oxyhydr)Oxide (BIOS) Sampling and Sorbent Preparation
2.3. Characterization of BIOS and Two-Line Ferrihydrite (2LFh)
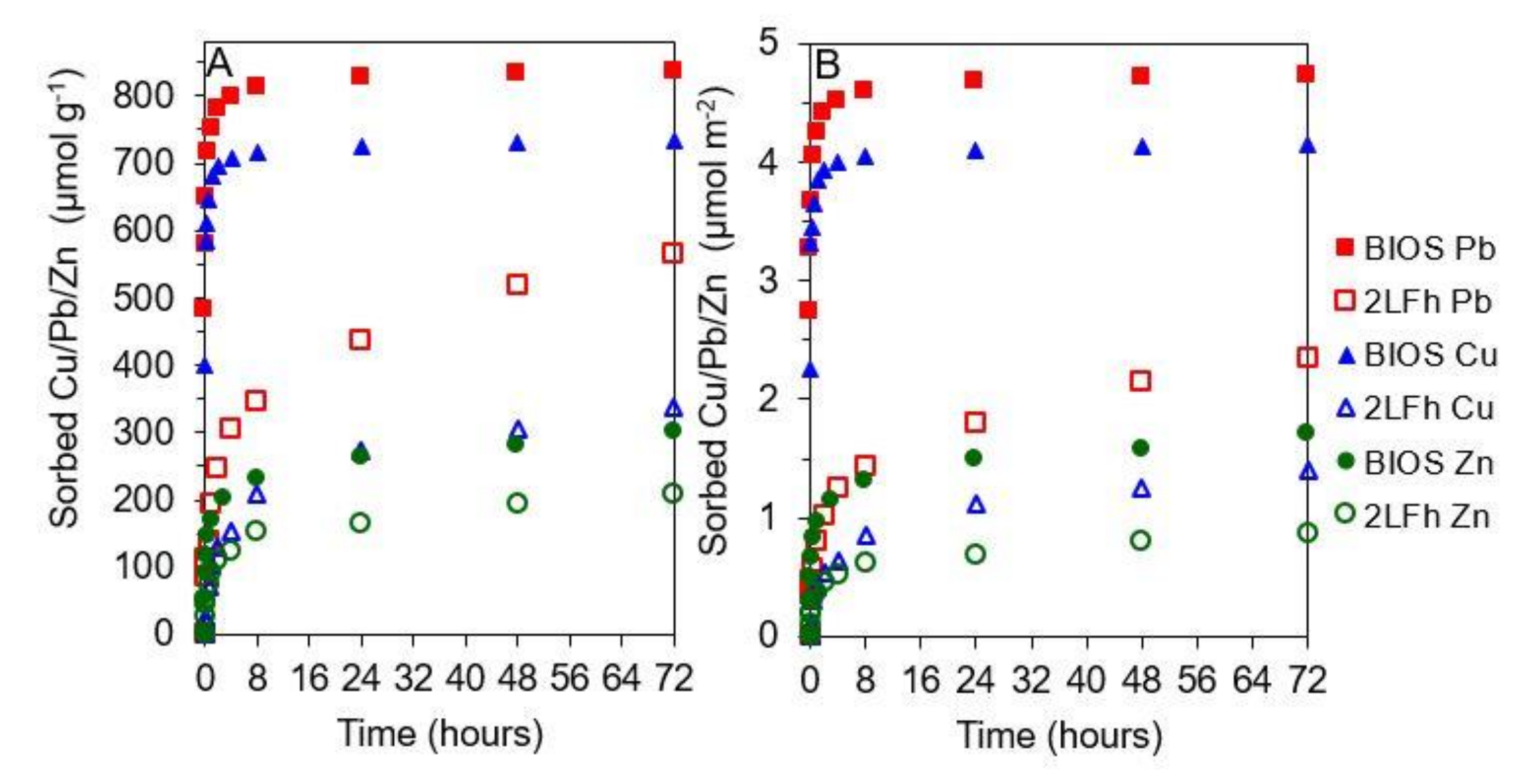
2.4. Rates of Copper, Lead, and Zinc Sorption to Iron Minerals
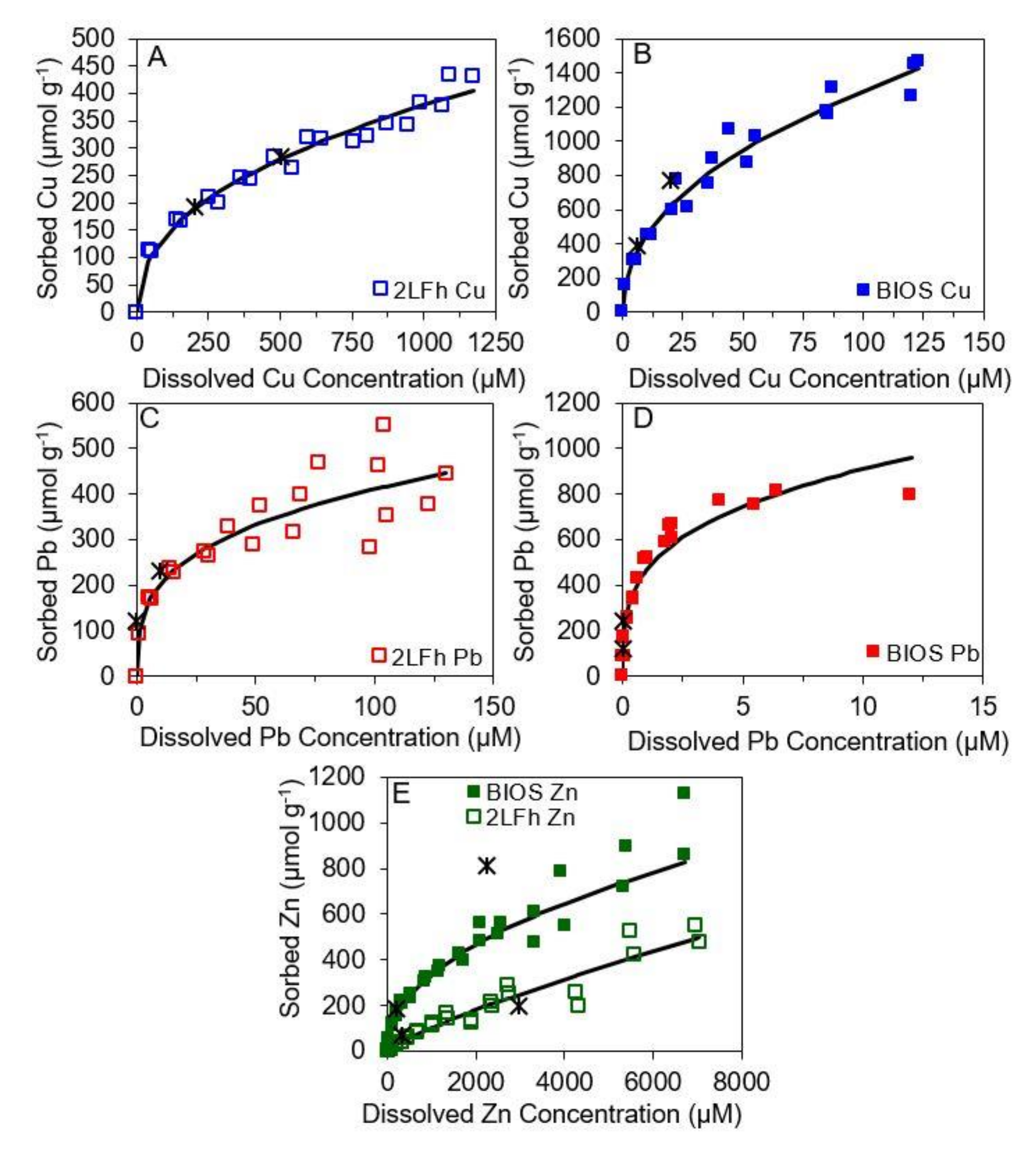
2.5. Adsorption Isotherms
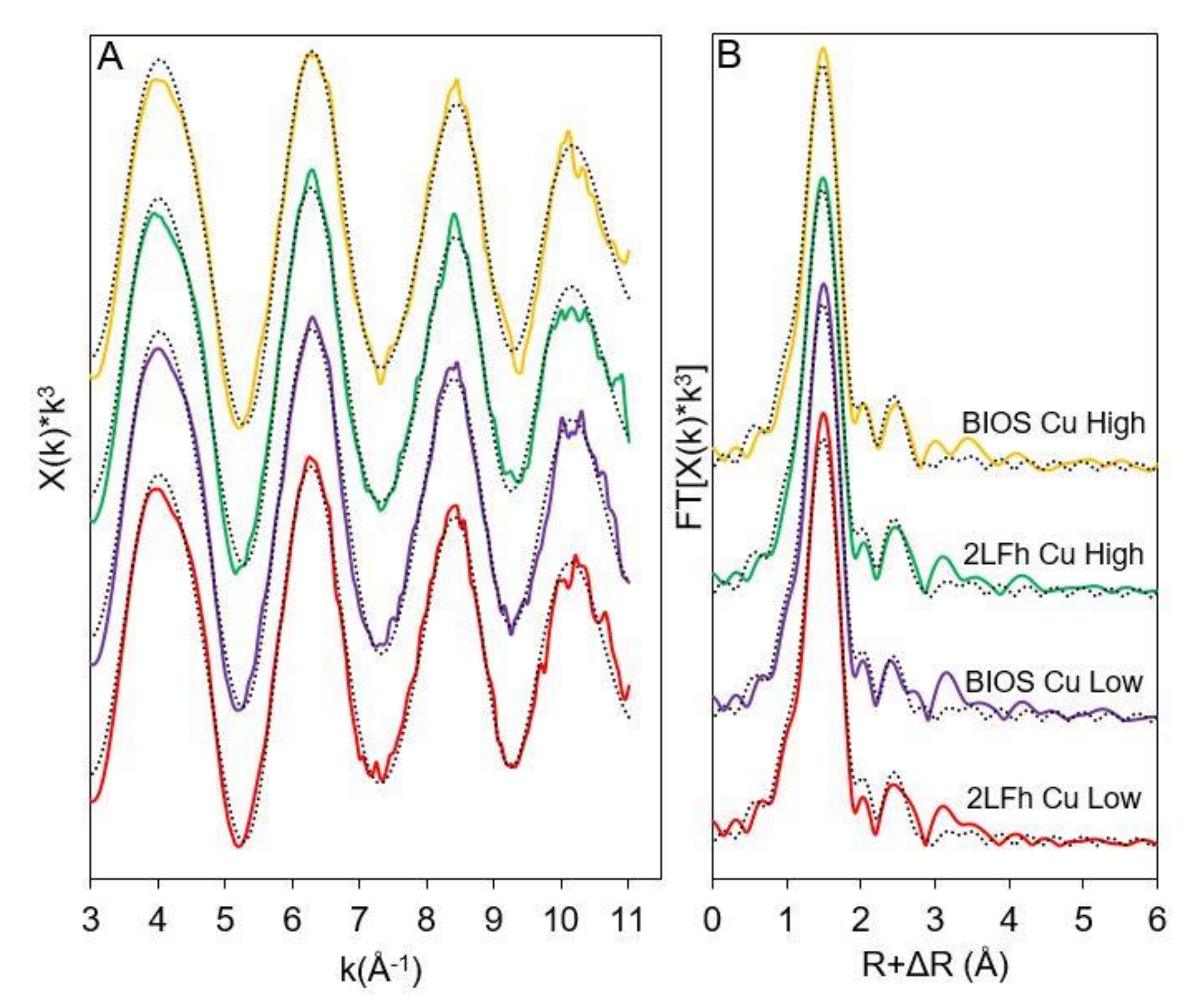
2.6. X-ray Absorption Spectroscopy Sample Preparation and Analysis
2.7. X-ray Total Scattering
3. Results and Discussion
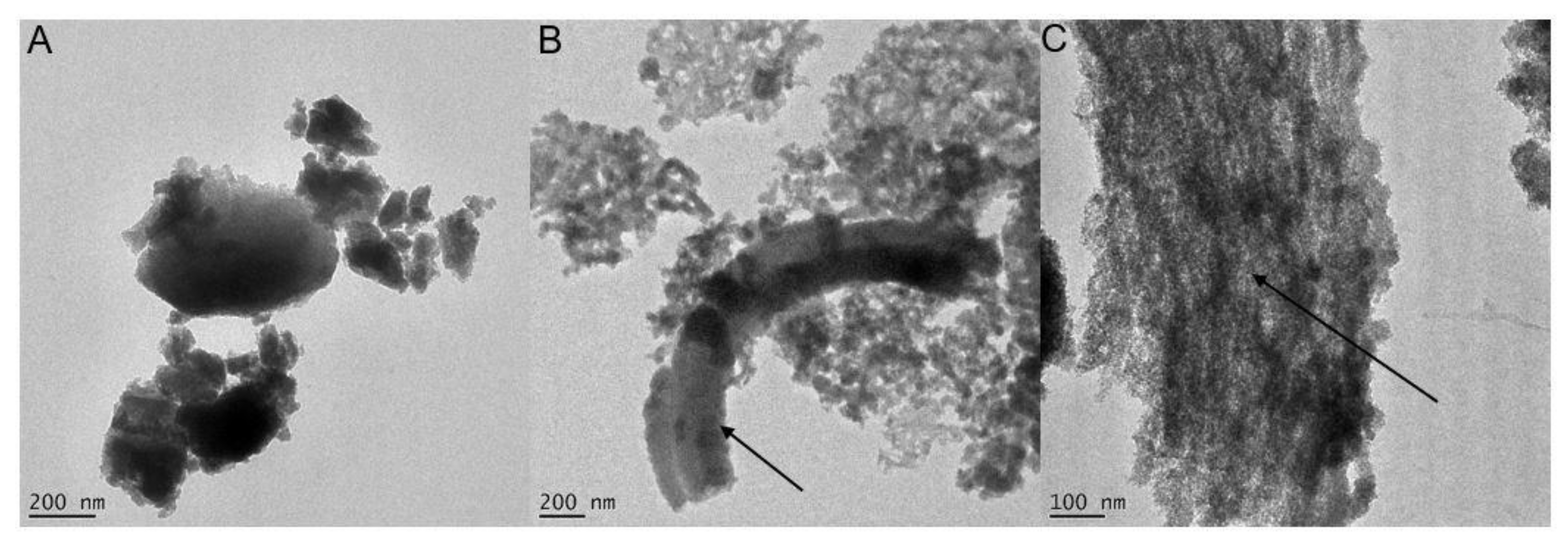
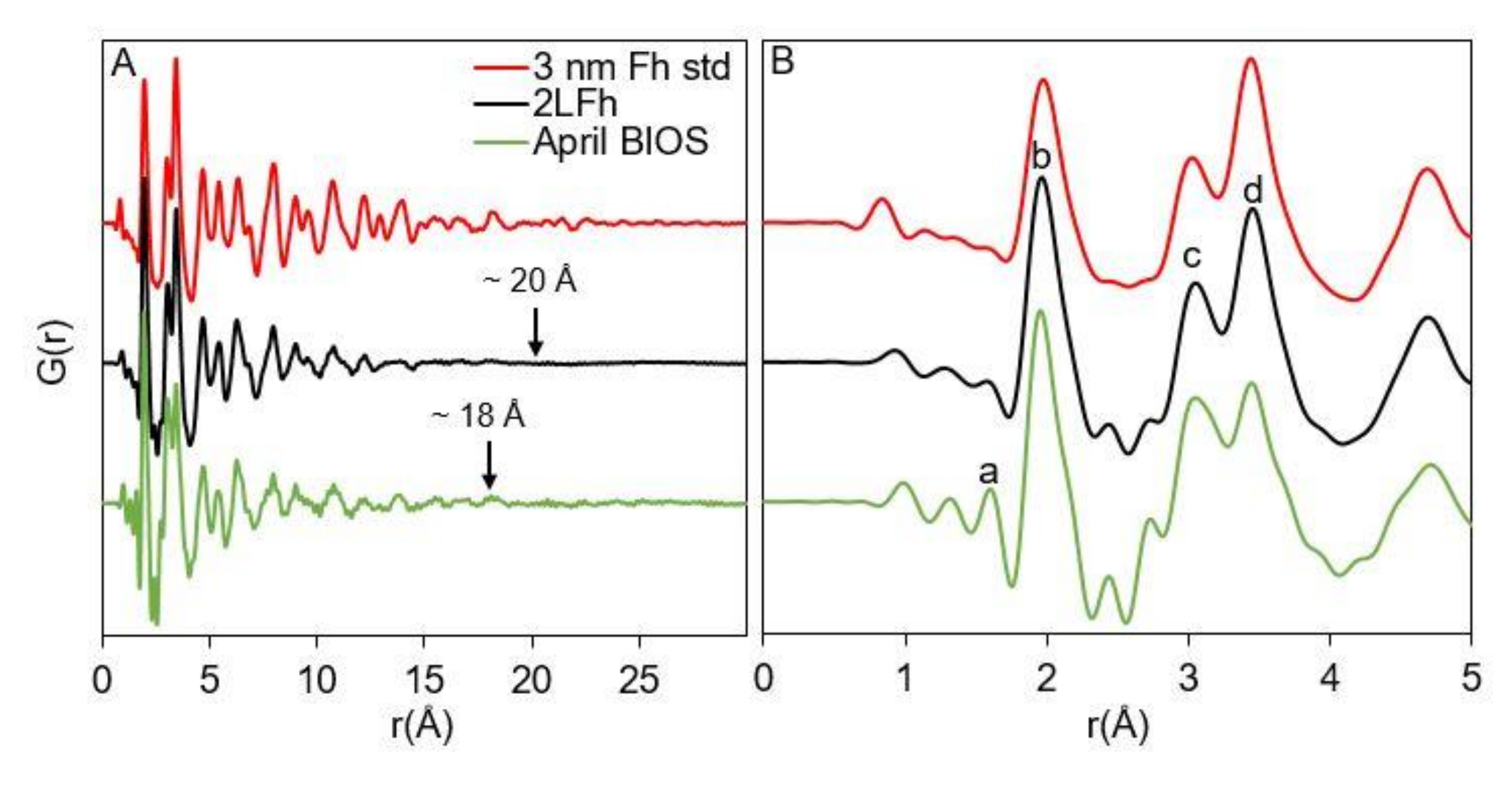
3.1. 2LFh and BIOS Mineral Characterization
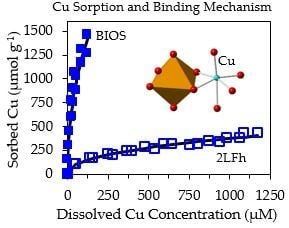
3.2. Cu, Pb, and Zn sorption to 2LFh and BIOS
3.3. Partitioning to Mineral Surfaces and Biomass
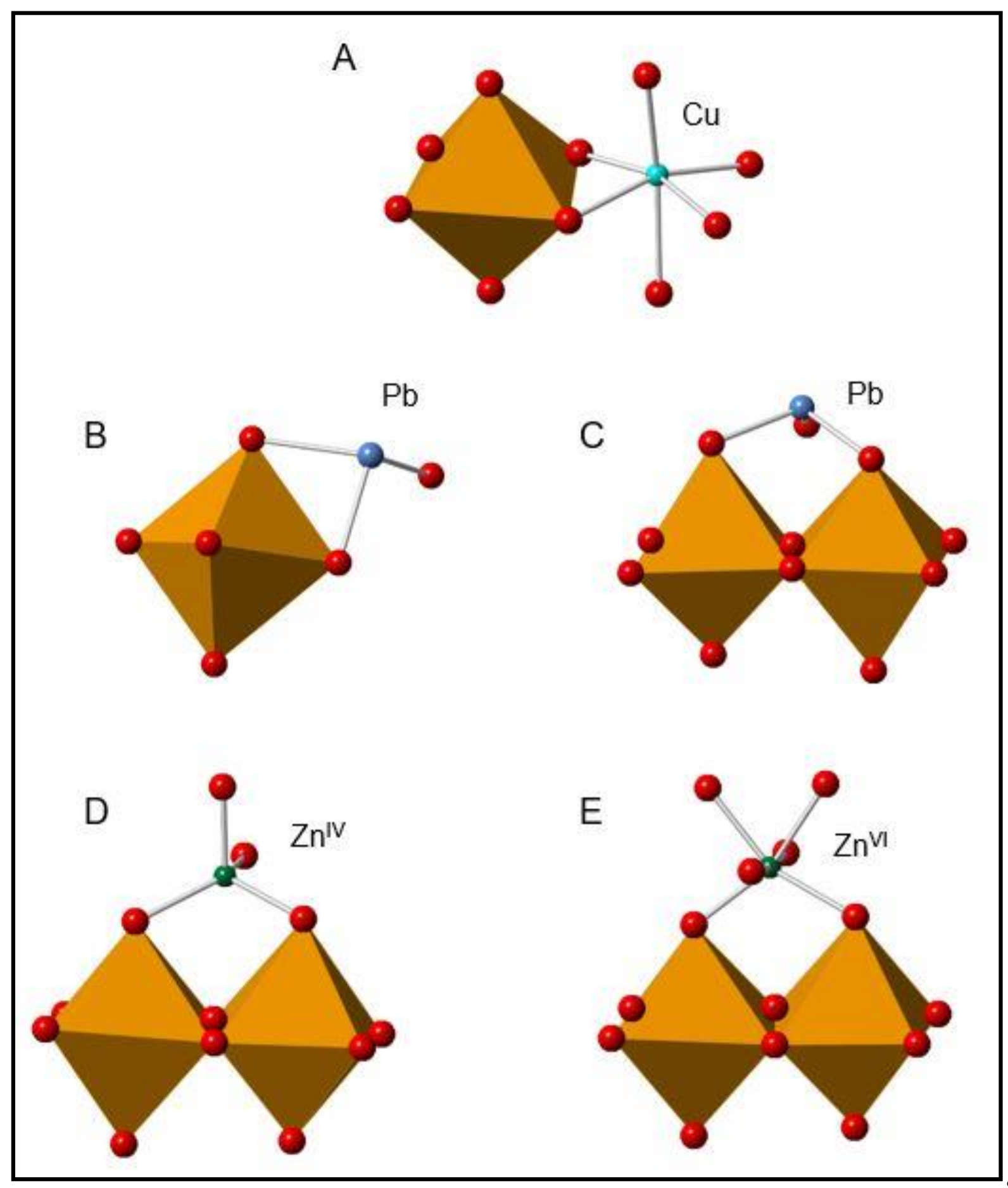
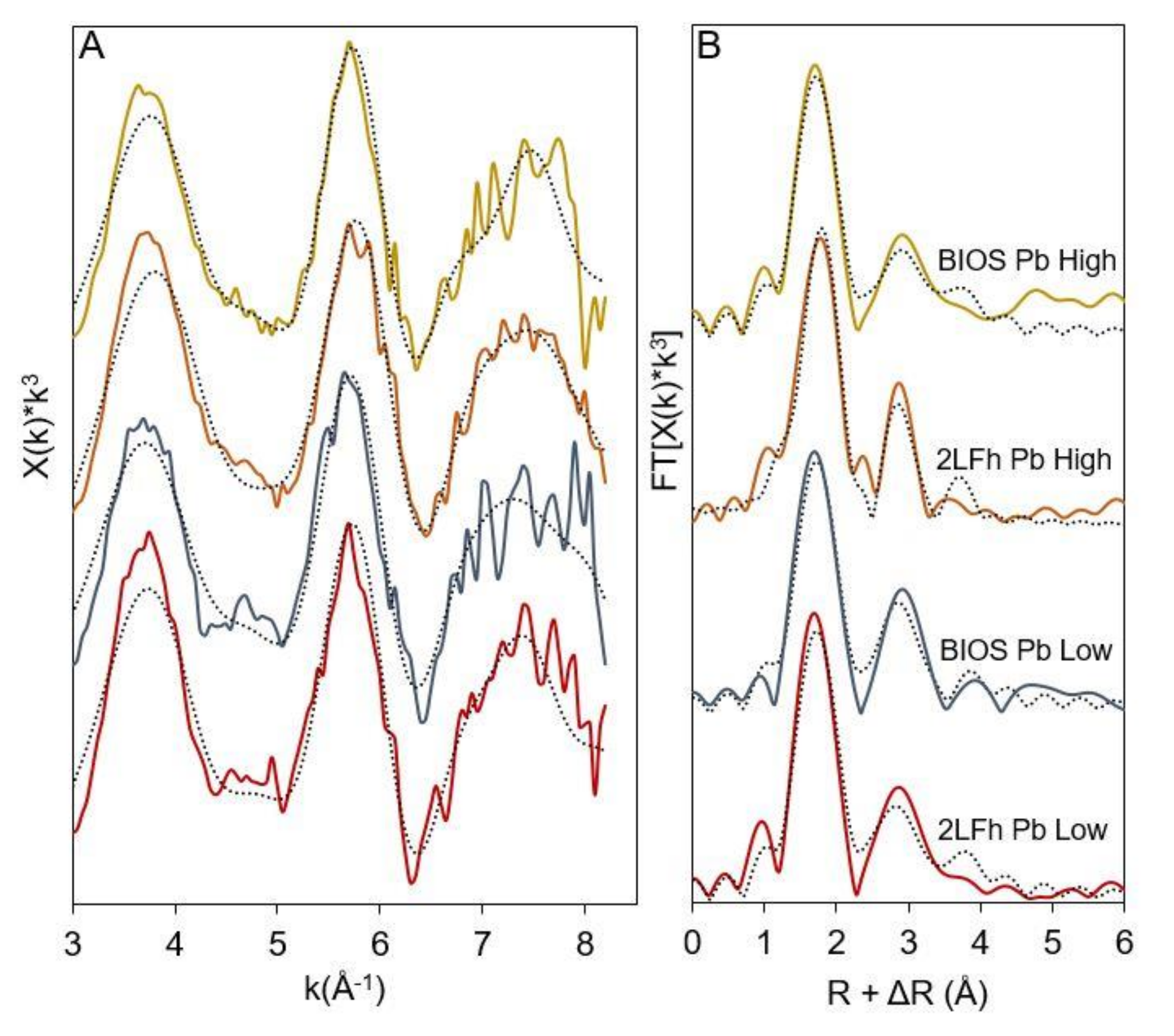
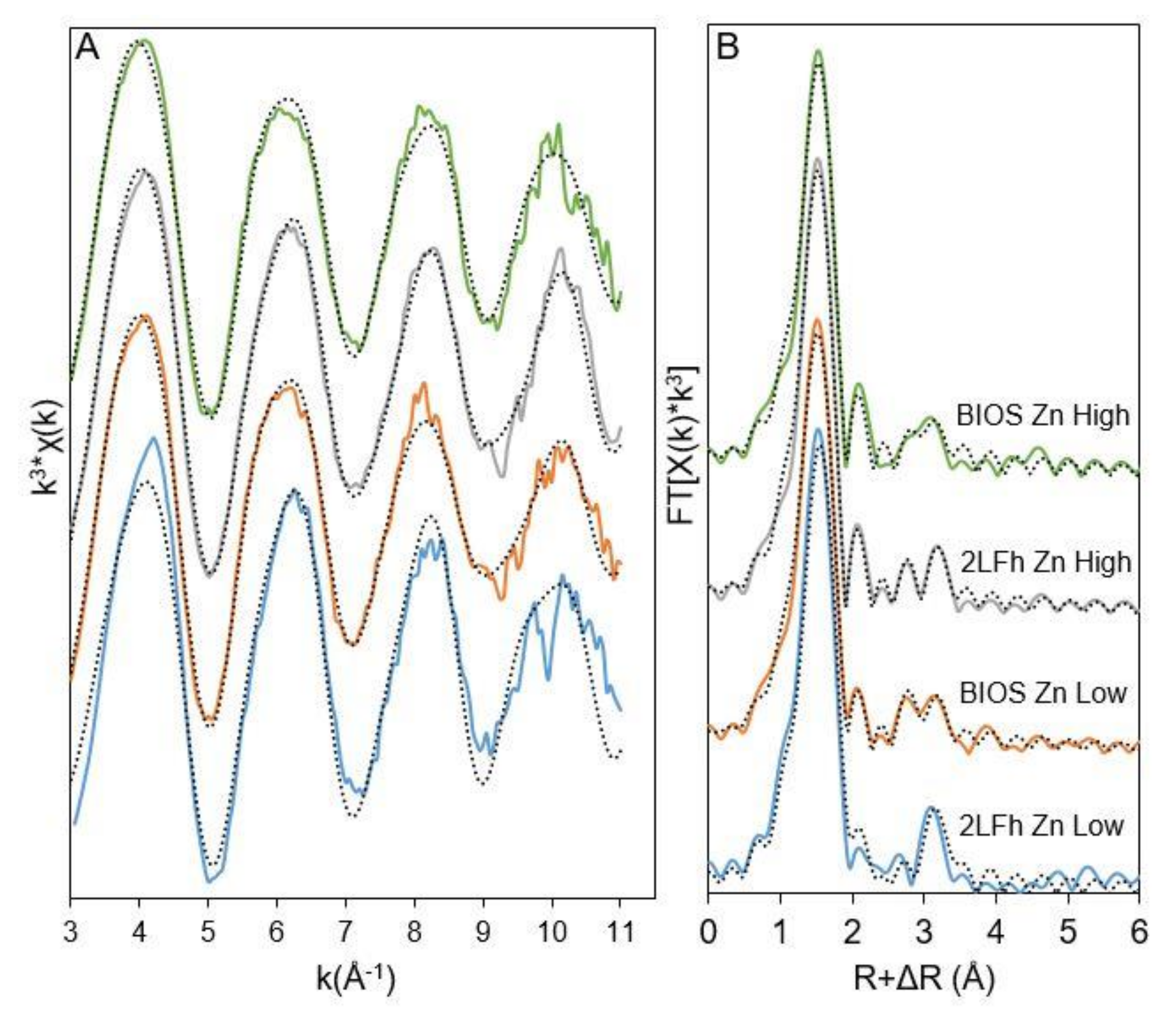
3.4. Surface Complexes of Cu, Pb, and Zn on 2LFh and BIOS
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cornell, R.; Schwertmann, U. Structure, properties, reactions, occurrence and uses. In The Iron Oxides; VCH: Weinheim, Germany, 1996; pp. 375–395. [Google Scholar]
- Jambor, J.L.; Dutrizac, J.E. Occurrence and constitution of natural and synthetic ferrihydrite, a widespread iron oxyhydroxide. Chem. Rev. 1998, 98, 2549–2586. [Google Scholar] [CrossRef] [PubMed]
- Bargar, J.; Brown, G.; Parks, G. Surface complexation of Pb(II) at oxide-water interfaces: II. XAFS and bond-valence determination of mononuclear Pb(II) sorption products and surface functional groups on iron oxides. Geochim. Cosmochim. Acta 1997, 61, 2639–2652. [Google Scholar] [CrossRef]
- Bargar, J.; Brown, G.; Parks, G. Surface complexation of Pb(II) at oxide-water interfaces: III. XAFS determination of Pb(II) and Pb(II)-chloro adsorption complexes on goethite and alumina. Geochim. Cosmochim. Acta 1998, 62, 193–207. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Leckie, J.O. Multiple-site adsorption of Cd, Cu, Zn, and Pb on amorphous iron oxyhydroxide. J. Colloid Interface Sci. 1981, 79, 209–221. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.; Persson, P.; Skyllberg, U. Complexation of copper (II) in organic soils and in dissolved organic matter—EXAFS evidence for chelate ring structures. Environ. Sci. Technol. 2006, 40, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.; Martinez, R.; Scott, S.D.; Ferris, F. Surface chemistry and reactivity of bacteriogenic iron oxides from Axial Volcano, Juan de Fuca Ridge, north-east Pacific Ocean. Geobiology 2003, 1, 59–69. [Google Scholar] [CrossRef]
- Lee, S.; Anderson, P.R. EXAFS study of Zn sorption mechanisms on hydrous ferric oxide over extended reaction time. J. Colloid Interface Sci. 2005, 286, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.E.; McBride, M.B. Dissolved and Labile Concentrations of Cd, Cu, Pb, and Zn in Aged Ferrihydrite-Organic Matter Systems. Environ. Sci. Technol. 1999, 33, 745–750. [Google Scholar] [CrossRef]
- Moon, E.M.; Peacock, C.L. Adsorption of Cu(II) to ferrihydrite and ferrihydrite-bacteria composites: Importance of the carboxyl group for Cu mobility in natural environments. Geochim. Cosmochim. Acta 2012, 92, 203–219. [Google Scholar] [CrossRef]
- Sauvé, S.; Martínez, C.E.; McBride, M.; Hendershot, W. Adsorption of Free Lead (Pb2+) by Pedogenic Oxides, Ferrihydrite, and Leaf Compost. Soil Sci. Soc. Am. J. 2000, 64, 595–599. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Abend, S.; Pandya, K.I.; Sparks, D.L. Kinetic Controls on Cu and Pb Sorption by Ferrihydrite. Environ. Sci. Technol. 2001, 35, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Axe, L. Modeling Cd and Zn sorption to hydrous metal oxides. Environ. Sci. Technol. 2000, 34, 2215–2223. [Google Scholar] [CrossRef]
- Trivedi, P.; Dyer, J.A.; Sparks, D.L. Lead sorption onto ferrihydrite. 1. A macroscopic and spectroscopic assessment. Environ. Sci. Technol. 2003, 37, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Waychunas, G.; Fuller, C.; Davis, J. Surface complexation and precipitate geometry for aqueous Zn(II) sorption on ferrihydrite I: X-ray absorption extended fine structure spectroscopy analysis. Geochim. Cosmochim. Acta 2002, 66, 1119–1137. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 126. [Google Scholar]
- Davison, W.; Seed, G. The kinetics of the oxidation of ferrous iron in synthetic and natural waters. Geochim. Cosmochim. Acta 1983, 47, 67–79. [Google Scholar] [CrossRef]
- Martin, S.T. Precipitation and Dissolution of Iron and Manganese Oxides. Available online: https://www.researchgate.net/file.PostFileLoader.html?id=5774bc85615e27a79348dd21&assetKey=AS%3A378564546449408%401467268229098 (accessed on 28 January 2018).
- Druschel, G.K.; Emerson, D.; Sutka, R.; Suchecki, P.; Luther, G.W. Low-oxygen and chemical kinetic constraints on the geochemical niche of neutrophilic iron (II) oxidizing microorganisms. Geochim. Cosmochim. Acta 2008, 72, 3358–3370. [Google Scholar] [CrossRef]
- James, R.; Ferris, F. Evidence for microbial-mediated iron oxidation at a neutrophilic groundwater spring. Chem. Geol. 2004, 212, 301–311. [Google Scholar] [CrossRef]
- Fleming, E.J.; Cetinić, I.; Chan, C.S.; King, D.W.; Emerson, D. Ecological succession among iron-oxidizing bacteria. ISME J. 2014, 8, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Bruun, A.-M.; Finster, K.; Gunnlaugsson, H.P.; Nornberg, P.; Friedrich, M.W. A comprehensive investigation on iron cycling in a freshwater seep including microscopy, cultivation and molecular community analysis. Geomicrobiol. J. 2010, 27, 15–34. [Google Scholar] [CrossRef]
- Duckworth, O.W.; Holmström, S.J.; Peña, J.; Sposito, G. Biogeochemistry of iron oxidation in a circumneutral freshwater habitat. Chem. Geol. 2009, 260, 149–158. [Google Scholar] [CrossRef]
- Haaijer, S.C.; Harhangi, H.R.; Meijerink, B.B.; Strous, M.; Pol, A.; Smolders, A.J.; Verwegen, K.; Jetten, M.S.; Den Camp, H.J.O. Bacteria associated with iron seeps in a sulfur-rich, neutral pH, freshwater ecosystem. ISME J. 2008, 2, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.; Weiss, J.V. Bacterial iron oxidation in circumneutral freshwater habitats: Findings from the field and the laboratory. Geomicrobiol. J. 2004, 21, 405–414. [Google Scholar] [CrossRef]
- Weiss, J.V.; Emerson, D.; Megonigal, J.P. Geochemical control of microbial Fe(III) reduction potential in wetlands: Comparison of the rhizosphere to non-rhizosphere soil. FEMS Microbiol. Ecol. 2004, 48, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.V.; Emerson, D.; Megonigal, J.P. Rhizosphere Iron (III) Deposition and Reduction in a L.-Dominated Wetland. Soil Sci. Soc. Am. J. 2005, 69, 1861–1870. [Google Scholar] [CrossRef]
- Neubauer, S.C.; Toledo-Durán, G.E.; Emerson, D.; Megonigal, J.P. Returning to their roots: Iron-oxidizing bacteria enhance short-term plaque formation in the wetland-plant rhizosphere. Geomicrobiol. J. 2007, 24, 65–73. [Google Scholar] [CrossRef]
- Weiss, J.V.; Emerson, D.; Backer, S.M.; Megonigal, J.P. Enumeration of Fe(II)-oxidizing and Fe(III)-reducing bacteria in the root zone of wetland plants: Implications for a rhizosphere iron cycle. Biogeochemistry 2003, 64, 77–96. [Google Scholar] [CrossRef]
- Weiss, J.V.; Rentz, J.A.; Plaia, T.; Neubauer, S.C.; Merrill-Floyd, M.; Lilburn, T.; Bradburne, C.; Megonigal, J.P.; Emerson, D. Characterization of neutrophilic Fe(II)-oxidizing bacteria isolated from the rhizosphere of wetland plants and description of Ferritrophicum radicicola gen. nov. sp. nov., and Sideroxydans paludicola sp. nov. Geomicrobiol. J. 2007, 24, 559–570. [Google Scholar] [CrossRef]
- Ferris, F. Biogeochemical properties of bacteriogenic iron oxides. Geomicrobiol. J. 2005, 22, 79–85. [Google Scholar] [CrossRef]
- Ferris, F.; Hallberg, R.; Lyven, B.; Pedersen, K. Retention of strontium, cesium, lead and uranium by bacterial iron oxides from a subterranean environment. Appl. Geochem. 2000, 15, 1035–1042. [Google Scholar] [CrossRef]
- Sowers, T.D.; Harrington, J.M.; Polizzotto, M.L.; Duckworth, O.W. Sorption of arsenic to biogenic iron (oxyhydr) oxides produced in circumneutral environments. Geochim. Cosmochim. Acta 2017, 198, 194–207. [Google Scholar] [CrossRef]
- Cismasu, A.C.; Michel, F.M.; Tcaciuc, A.P.; Tyliszczak, T.; Brown, G.E., Jr. Composition and structural aspects of naturally occurring ferrihydrite. Comptes Rendus Geosci. 2011, 343, 210–218. [Google Scholar] [CrossRef]
- Ferris, F.; Konhauser, K.; Lyven, B.; Pedersen, K. Accumulation of metals by bacteriogenic iron oxides in a subterranean environment. Geomicrobiol. J. 1999, 16, 181–192. [Google Scholar]
- Martinez, R.E.; Pedersen, K.; Ferris, F.G. Cadmium complexation by bacteriogenic iron oxides from a subterranean environment. J. Colloid Interface Sci. 2004, 275, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Langley, S.; Gault, A.G.; Ibrahim, A.; Takahashi, Y.; Renaud, R.; Fortin, D.; Clark, I.D.; Ferris, F.G. Sorption of strontium onto bacteriogenic iron oxides. Environ. Sci. Technol. 2009, 43, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Rentz, J.A.; Ullman, J.L. Copper and Zinc Removal Using Biogenic Iron Oxides. In Proceedings of the World Environmental and Water Resources Congress 2012: Crossing Boundaries, Albuquerque, NM, USA, 20–24 May 2012; pp. 687–698. [Google Scholar]
- Manceau, A.; Charlet, L.; Boisset, M.; Didier, B.; Spadini, L. Sorption and speciation of heavy metals on hydrous Fe and Mn oxides. From microscopic to macroscopic. Appl. Clay Sci. 1992, 7, 201–223. [Google Scholar] [CrossRef]
- Rout, K.; Mohapatra, M.; Anand, S. 2-Line ferrihydrite: Synthesis, characterization and its adsorption behaviour for removal of Pb(II), Cd(II), Cu(II) and Zn(II) from aqueous solutions. Dalton Trans. 2012, 41, 3302–3312. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Dyer, J.A.; Sparks, D.L.; Pandya, K. Mechanistic and thermodynamic interpretations of zinc sorption onto ferrihydrite. J. Colloid Interface Sci. 2004, 270, 77–85. [Google Scholar] [CrossRef]
- Trivedi, P.; Axe, L.; Tyson, T.A. An analysis of zinc sorption to amorphous versus crystalline iron oxides using XAS. J. Colloid Interface Sci. 2001, 244, 230–238. [Google Scholar] [CrossRef]
- Almaraz, N.; Whitaker, A.H.; Andrews, M.Y.; Duckworth, O.W. Assessing Biomineral Formation by Iron-oxidizing Bacteria in a Circumneutral Creek. J. Contemp. Water Res. Educ. 2017, 160, 60–71. [Google Scholar] [CrossRef]
- Andrews, M.Y.; Duckworth, O. A universal assay for the detection of siderophore activity in natural waters. BioMetals 2016, 29, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory: Preparation and Characterization; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Whitaker, A.H.; Peña, J.; Amor, M.; Duckworth, O.W. Cr(VI) Uptake and Reduction in Biogenic Iron Oxides Assemblages. 2017. Submitted. [Google Scholar]
- Gustafsson, J. Visual MINTEQ ver. 3.0; Department of Land and Water Resources Engineering, Royal Institute of Technology: Stokholm, Sweden, 2011. [Google Scholar]
- Bolster, C.H.; Hornberger, G.M. On the use of linearized Langmuir equations. Soil Sci. Soc. Am. J. 2007, 71, 1796–1806. [Google Scholar] [CrossRef]
- Kelly, S.; Hesterberg, D.; Ravel, B. Analysis of soils and minerals using X-ray absorption spectroscopy. Methods Soil Anal. 2008, 5, 387–463. [Google Scholar]
- Webb, S. SIXpack: A graphical user interface for XAS analysis using IFEFFIT. Phys. Scr. 2005, 2005, 1011. [Google Scholar] [CrossRef]
- Newville, M. IFEFFIT: Interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 2001, 8, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.M.; Parker, D.L.; Bargar, J.R.; Jarzecki, A.A.; Tebo, B.M.; Sposito, G.; Duckworth, O.W. Structural dependence of Mn complexation by siderophores: Donor group dependence on complex stability and reactivity. Geochim. Cosmochim. Acta 2012, 88, 106–119. [Google Scholar] [CrossRef]
- O’day, P.A.; Rivera, N.; Root, R.; Carroll, S.A. X-ray absorption spectroscopic study of Fe reference compounds for the analysis of natural sediments. Am. Mineral. 2004, 89, 572–585. [Google Scholar] [CrossRef]
- Pena, J. Contaminant Metal Immobilization by Biogenic Manganese Oxide Nanoparticles: Implications for Natural Attenuation and Bioremediation; University of California: Berkeley, CA, USA, 2009. [Google Scholar]
- Toner, B.; Manceau, A.; Marcus, M.A.; Millet, D.B.; Sposito, G. Zinc sorption by a bacterial biofilm. Environ. Sci. Technol. 2005, 39, 8288–8294. [Google Scholar] [CrossRef] [PubMed]
- Rehr, J.J.; Kas, J.J.; Vila, F.D.; Prange, M.P.; Jorissen, K. Parameter-free calculations of X-ray spectra with FEFF9. Phys. Chem. Chem. Phys. 2010, 12, 5503–5513. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, O.W.; Bargar, J.R.; Sposito, G. Quantitative structure-activity relationships for aqueous metal-siderophore complexes. Environ. Sci. Technol. 2008, 43, 343–349. [Google Scholar] [CrossRef]
- Villalobos, M.; Bargar, J.; Sposito, G. Mechanisms of Pb(II) sorption on a biogenic manganese oxide. Environ. Sci. Technol. 2005, 39, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Toner, B.; Manceau, A.; Webb, S.M.; Sposito, G. Zinc sorption to biogenic hexagonal-birnessite particles within a hydrated bacterial biofilm. Geochim. Cosmochim. Acta 2006, 70, 27–43. [Google Scholar] [CrossRef]
- Alcacio, T.E.; Hesterberg, D.; Chou, J.W.; Martin, J.D.; Beauchemin, S.; Sayers, D.E. Molecular scale characteristics of Cu(II) bonding in goethite-humate complexes. Geochim. Cosmochim. Acta 2001, 65, 1355–1366. [Google Scholar] [CrossRef]
- Cismasu, A.C.; Levard, C.; Michel, F.M.; Brown, G.E. Properties of impurity-bearing ferrihydrite II: Insights into the surface structure and composition of pure, Al-and Si-bearing ferrihydrite from Zn(II) sorption experiments and Zn K-edge X-ray absorption spectroscopy. Geochim. Cosmochim. Acta 2013, 119, 46–60. [Google Scholar] [CrossRef]
- Downward, L.; Booth, C.; Lukens, W.; Bridges, F. A Variation of the F-Test for Determining Statistical Relevance of Particular Parameters in EXAFS Fits. AIP Conf. Proc. 2007, 882, 129–131. [Google Scholar]
- Hamilton, W.C. Significance tests on the crystallographic R factor. Acta Crystallogr. 1965, 18, 502–510. [Google Scholar] [CrossRef]
- Webb, S.M.; Tebo, B.; Bargar, J. Structural characterization of biogenic Mn oxides produced in seawater by the marine Bacillus sp. strain SG-1. Am. Mineral. 2005, 90, 1342–1357. [Google Scholar] [CrossRef]
- Hammersley, A. FIT2D: An introduction and overview. Eur. Synchrotron Radiat. Facil. Intern. Rep. 1997, 68, 58. [Google Scholar]
- Juhás, P.; Davis, T.; Farrow, C.L.; Billinge, S.J. PDFgetX3: A rapid and highly automatable program for processing powder diffraction data into total scattering pair distribution functions. J. Appl. Crystallogr. 2013, 46, 560–566. [Google Scholar] [CrossRef]
- Chupas, P.J.; Qiu, X.; Hanson, J.C.; Lee, P.L.; Grey, C.P.; Billinge, S.J. Rapid-acquisition pair distribution function (RA-PDF) analysis. J. Appl. Crystallogr. 2003, 36, 1342–1347. [Google Scholar] [CrossRef]
- Michel, F.; Ehm, L.; Liu, G.; Han, W.; Antao, S.; Chupas, P.; Lee, P.; Knorr, K.; Eulert, H.; Kim, J.; et al. Similarities in 2-and 6-line ferrihydrite based on pair distribution function analysis of X-ray total scattering. Chem. Mater. 2007, 19, 1489–1496. [Google Scholar] [CrossRef]
- Toner, B.M.; Berquó, T.S.; Michel, F.M.; Sorensen, J.V.; Templeton, A.S.; Edwards, K.J. Mineralogy of iron microbial mats from Loihi Seamount. Front. Microbiol. 2012, 3, 118. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.; Zanchet, D.; Ugarte, D. Estimating nanoparticle size from diffraction measurements. J. Appl. Crystallogr. 2000, 33, 1335–1341. [Google Scholar] [CrossRef]
- Kennedy, C.B.; Scott, S.D.; Ferris, F.G. Characterization of Bacteriogenic Iron Oxide Deposits from Axial Volcano, Juan de Fuca Ridge, Northeast Pacific Ocean. Geomicrobiol. J. 2003, 20, 199–214. [Google Scholar] [CrossRef]
- Michel, F.M.; Barrón, V.; Torrent, J.; Morales, M.P.; Serna, C.J.; Boily, J.-F.; Liu, Q.; Ambrosini, A.; Cismasu, A.C.; Brown, G.E. Ordered ferrimagnetic form of ferrihydrite reveals links among structure, composition, and magnetism. Proc. Natl. Acad. Sci. USA 2010, 107, 2787–2792. [Google Scholar] [CrossRef] [PubMed]
- Stanjek, H.; Weidler, P. The effect of dry heating on the chemistry, surface area, and oxalate solubility of synthetic 2-line and 6-line ferrihydrites. Clay Miner. 1992, 27, 397. [Google Scholar] [CrossRef]
- Toner, B.M.; Santelli, C.M.; Marcus, M.A.; Wirth, R.; Chan, C.S.; McCollom, T.; Bach, W.; Edwards, K.J. Biogenic iron oxyhydroxide formation at mid-ocean ridge hydrothermal vents: Juan de Fuca Ridge. Geochim. Cosmochim. Acta 2009, 73, 388–403. [Google Scholar] [CrossRef]
- Van Genuchten, C.M.; Gadgil, A.J.; Peña, J. Fe(III) nucleation in the presence of bivalent cations and oxyanions leads to subnanoscale 7 Å polymers. Environ. Sci. Technol. 2014, 48, 11828–11836. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.M.; Ehm, L.; Antao, S.M.; Lee, P.L.; Chupas, P.J.; Liu, G.; Strongin, D.R.; Schoonen, M.A.; Phillips, B.L.; Parise, J.B. The structure of ferrihydrite, a nanocrystalline material. Science 2007, 316, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, M.; Koopal, L.K.; Li, W.; Xu, W.; Liu, F.; Zhang, J.; Liu, Q.; Feng, X.; Sparks, D.L. Effects of crystallite size on the structure and magnetism of ferrihydrite. Environ. Sci. Nano 2016, 3, 190–202. [Google Scholar] [CrossRef]
- Cismasu, A.C.; Michel, F.M.; Tcaciuc, A.P.; Brown, G.E. Properties of impurity-bearing ferrihydrite III. Effects of Si on the structure of 2-line ferrihydrite. Geochim. Cosmochim. Acta 2014, 133 (Suppl. C), 168–185. [Google Scholar] [CrossRef]
- Waychunas, G.; Rea, B.; Fuller, C.; Davis, J. Surface chemistry of ferrihydrite: Part 1. EXAFS studies of the geometry of coprecipitated and adsorbed arsenate. Geochim. Cosmochim. Acta 1993, 57, 2251–2269. [Google Scholar] [CrossRef]
- Mikutta, C. X-ray absorption spectroscopy study on the effect of hydroxybenzoic acids on the formation and structure of ferrihydrite. Geochim. Cosmochim. Acta 2011, 75, 5122–5139. [Google Scholar] [CrossRef]
- Charlet, L.; Manceau, A.A. X-ray absorption spectroscopic study of the sorption of Cr(III) at the oxide-water interface: II. Adsorption, coprecipitation, and surface precipitation on hydrous ferric oxide. J. Colloid Interface Sci. 1992, 148, 443–458. [Google Scholar] [CrossRef]
- Zhu, J.; Pigna, M.; Cozzolino, V.; Caporale, A.G.; Violante, A. Competitive sorption of copper (II), chromium (III) and lead (II) on ferrihydrite and two organomineral complexes. Geoderma 2010, 159, 409–416. [Google Scholar] [CrossRef]
- Kennedy, C.; Gault, A.; Fortin, D.; Clark, I.; Ferris, F. Retention of iodide by bacteriogenic iron oxides. Geomicrobiol. J. 2011, 28, 387–395. [Google Scholar] [CrossRef]
- Martinez, R.E.; Smith, D.S.; Kulczycki, E.; Ferris, F.G. Determination of Intrinsic Bacterial Surface Acidity Constants using a Donnan Shell Model and a Continuous pKa Distribution Method. J. Colloid Interface Sci. 2002, 253, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Childs, C.; Downes, C.; Wells, N. Hydrous iron oxide minerals with short range order deposited in a spring/stream system, Tongariro National Park, New Zealand. Soil Res. 1982, 20, 119–129. [Google Scholar] [CrossRef]
- Langley, S.; Igric, P.; Takahashi, Y.; Sakai, Y.; Fortin, D.; Hannington, M.; Schwarz-Schampera, U. Preliminary characterization and biological reduction of putative biogenic iron oxides (BIOS) from the Tonga-Kermadec Arc, southwest Pacific Ocean. Geobiology 2009, 7, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Eusterhues, K.; Wagner, F.E.; Häusler, W.; Hanzlik, M.; Knicker, H.; Totsche, K.U.; Kögel-Knabner, I.; Schwertmann, U. Characterization of ferrihydrite-soil organic matter coprecipitates by X-ray diffraction and Mossbauer spectroscopy. Environ. Sci. Technol. 2008, 42, 7891–7897. [Google Scholar] [CrossRef] [PubMed]
- Axe, L.; Anderson, P.R. Experimental and theoretical diffusivities of Cd and Sr in hydrous ferric oxide. J. Colloid Interface Sci. 1997, 185, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Kinniburgh, D.; Jackson, M.; Syers, J. Adsorption of alkaline earth, transition, and heavy metal cations by hydrous oxide gels of iron and aluminum. Soil Sci. Soc. Am. J. 1976, 40, 796–799. [Google Scholar] [CrossRef]
- McKenzie, R. The adsorption of lead and other heavy metals on oxides of manganese and iron. Soil Res. 1980, 18, 61–73. [Google Scholar] [CrossRef]
- Sposito, G. The Surface Chemistry of Soils; Oxford University Press: Oxford, UK, 1984. [Google Scholar]
- Meng, S.; Wang, H.; Liu, H.; Yang, C.; Wei, Y.; Hou, D. Evaluation of the ability of ferrihydrite to bind heavy metal ions: Based on formation environment, adsorption reversibility and ageing. Appl. Geochem. 2014, 45 (Suppl. C), 114–119. [Google Scholar] [CrossRef]
- Peacock, C.L.; Sherman, D.M. Copper (II) sorption onto goethite, hematite and lepidocrocite: A surface complexation model based on ab initio molecular geometries and EXAFS spectroscopy. Geochim. Cosmochim. Acta 2004, 68, 2623–2637. [Google Scholar] [CrossRef]
- Liu, J.-F.; Zhao, Z.-S.; Jiang, G.-B. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef] [PubMed]
- Kerndorff, H.; Schnitzer, M. Sorption of metals on humic acid. Geochim. Cosmochim. Acta 1980, 44, 1701–1708. [Google Scholar] [CrossRef]
- Schnitzer, M.; Hansen, E. Organo-metallic interactions in soils: 8. An evaluation of methods for the determination of stability constants of metal-fulvic acid complexes. Soil Sci. 1970, 109, 333–340. [Google Scholar] [CrossRef]
- Wei, L.; Li, Y.; Noguera, D.R.; Zhao, N.; Song, Y.; Ding, J.; Zhao, Q.; Cui, F. Adsorption of Cu2+ and Zn2+ by extracellular polymeric substances (EPS) in different sludges: Effect of EPS fractional polarity on binding mechanism. J. Hazard. Mater. 2017, 321, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Gélabert, A.; Spormann, A.M.; Brown, G.E. Role of extracellular polymeric substances in metal ion complexation on Shewanella oneidensis: Batch uptake, thermodynamic modeling, ATR-FTIR, and EXAFS study. Geochim. Cosmochim. Acta 2010, 74, 1–15. [Google Scholar] [CrossRef]
- Kinniburgh, D.; Jackson, M. Concentration and pH dependence of calcium and zinc adsorption by iron hydrous oxide gel. Soil Sci. Soc. Am. J. 1982, 46, 56–61. [Google Scholar] [CrossRef]
- Templeton, A.S.; Ostergren, J.D.; Trainor, T.P.; Foster, A.L.; Traina, S.; Spormann, A.; Brown, G. XAFS and XSW study of the distribution of Pb(II) sorbed to biofilms on α-Al2O3 and α-FeOOH surfaces. J. Synchrotron Radiat. 1999, 6, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Boonfueng, T.; Axe, L.; Maeng, S.; Tyson, T. Surface complexation of Pb(II) on amorphous iron oxide and manganese oxide: Spectroscopic and time studies. J. Colloid Interface Sci. 2006, 299, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Manceau, A.; Lanson, B.; Drits, V.A. Structure of heavy metal sorbed birnessite. Part III: Results from powder and polarized extended X-ray absorption fine structure spectroscopy. Geochim. Cosmochim. Acta 2002, 66, 2639–2663. [Google Scholar] [CrossRef]
- Xu, T.; Catalano, J.G. Effects of Ionic Strength on Arsenate Adsorption at Aluminum Hydroxide-Water Interfaces. Soil Syst. 2018, 2, 1. [Google Scholar]
- Peak, D.; Regier, T. Direct observation of tetrahedrally coordinated Fe(III) in ferrihydrite. Environ. Sci. Technol. 2012, 46, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gélabert, A.; Michel, F.M.; Choi, Y.; Gescher, J.; Ona-Nguema, G.; Eng, P.J.; Bargar, J.R.; Farges, F.; Spormann, A.M.; et al. Effect of biofilm coatings at metal-oxide/water interfaces I: Pb(II) and Zn(II) partitioning and speciation at Shewanella oneidensis/metal-oxide/water interfaces. Geochim. Cosmochim. Acta 2016, 188 (Suppl. C), 368–392. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Althoff, H.W.; Bartel, H.; Jekel, M. The effect of groundwater composition on uranium (VI) sorption onto bacteriogenic iron oxides. Water Res. 2006, 40, 3646–3652. [Google Scholar] [CrossRef] [PubMed]
- River, M.; Richardson, C.J. Stream transport of iron and phosphorus by authigenic nanoparticles in the Southern Piedmont of the U.S. Water Res. 2018, 130, 312–321. [Google Scholar] [CrossRef] [PubMed]
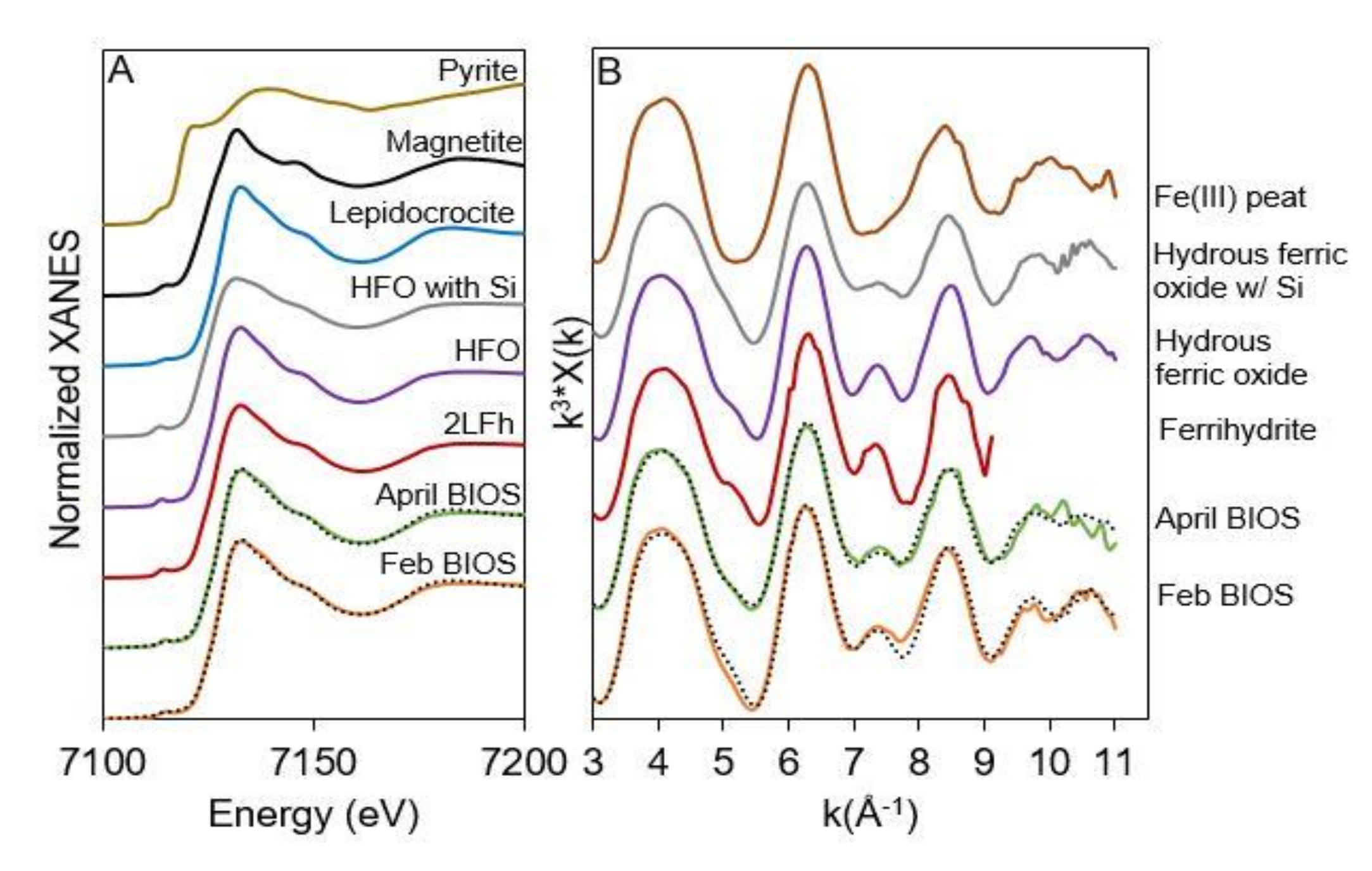
| Sample ID | Component | % Contribution | R-Value |
|---|---|---|---|
| Feb BIOS | Hydrous ferric oxide | 46 ± 7 | 0.0282353 |
| Hydrous ferric oxide with Si | 54 ± 8 | ||
| April BIOS | Hydrous ferric oxide | 42 ± 7 | 0.0203070 |
| Fe(III) peat | 35 ± 3 | ||
| Hydrous ferric oxide with Si | 23 ± 8 |
| Adsorbate | Adsorbent | Kf (µmol Cu/Pb/Zn g−1) | n | E |
|---|---|---|---|---|
| Cu(II) | 2LFh | 18 ± 3.0 | 0.44 ± 0.02 | 0.979 |
| Cu(II) | BIOS | 160 ± 30 | 0.46 ± 0.03 | 0.986 |
| Pb(II) | 2LFh | 100 ± 20 | 0.31 ± 0.05 | 0.871 |
| Pb(II) | BIOS | 470 ± 20 | 0.29 ± 0.02 | 0.943 |
| Zn(II) | 2LFh | 0.4 ± 0.3 | 0.80 ± 0.08 | 0.888 |
| Zn(II) | BIOS | 13 ± 3 | 0.47 ± 0.03 | 0.949 |
| Sample | Path | N a | R(Å) b | σ2 (Å2) c | ΔE0 (eV) d | R-Factor e |
|---|---|---|---|---|---|---|
| 2LFh Cu Low | Cu-Oeq | 4.1 (0.2) | 1.93 (0.01) | 0.006 | −5.0 (1.4) | 0.0204 |
| Cu-Cu/Fe | 0.6 (0.3) | 2.99 (0.03) | 0.01 | |||
| Cu-O-O | 4 | 3.87 | 0.012 | |||
| BIOS Cu Low | Cu-Oeq | 4.2 (0.2) | 1.93 (0.01) | 0.006 | −5.0 (1.4) | 0.0198 |
| Cu-Cu/Fe | 0.4 (0.3) | 2.99 (0.04) | 0.01 | |||
| Cu-O-O | 4 | 3.87 | 0.012 | |||
| 2LFh Cu High | Cu-Oeq | 4.1 (0.2) | 1.93 (0.01) | 0.006 | −5.4 (1.1) | 0.0124 |
| Cu-Cu/Fe | 0.7 (0.2) | 2.99 (0.02) | 0.01 | |||
| Cu-O-O | 4 | 3.86 | 0.012 | |||
| BIOS Cu High | Cu-Oeq | 4.1 (0.2) | 1.93 (0.01) | 0.006 | −5.4 (1.3) | 0.0166 |
| Cu-Cu/Fe | 0.6 (0.2) | 2.98 (0.03) | 0.01 | |||
| Cu-O-O | 4 | 3.86 | 0.012 | |||
| 2LFh Pb Low | Pb-O | 2.7 (0.4) | 2.35 (0.03) | 0.01 | 1.1 (4.0) | 0.0604 |
| Pb-Fe1 | 1.0 (0.4) | 3.41 (0.05) | 0.01 | |||
| Pb-Fe2 | 1.3 (0.7) | 4.03 (0.07) | 0.01 | |||
| BIOS Pb Low | Pb-O | 2.5 (0.4) | 2.35 (0.03) | 0.01 | 0.4 (4.0) | 0.0581 |
| Pb-Fe1 | 1.2 (0.4) | 3.42 (0.04) | 0.01 | |||
| Pb-Fe2 | 1.0 (0.7) | 4.05 (0.07) | 0.01 | |||
| 2LFh Pb High | Pb-O | 2.6 (0.2) | 2.35 (0.01) | 0.01 | 2.9 (2.0) | 0.0398 |
| Pb-Fe1 | 0.9 (0.2) | 3.42 (0.02) | 0.01 | |||
| Pb-Fe2 | 0.7 (0.4) | 4.02 (0.04) | 0.01 | |||
| BIOS Pb High | Pb-O | 2.6 (0.3) | 2.34 (0.02) | 0.01 | 1.1 (2.8) | 0.0293 |
| Pb-Fe1 | 0.9 (0.3) | 3.42 (0.04) | 0.01 | |||
| Pb-Fe2 | 1.3 (0.5) | 4.02 (0.05) | 0.01 | |||
| 2LFh Zn Low | ZnIV-O | 4.2 (0.3) | 1.97 (0.01) | 0.006 | 3.4 (2.3) | 0.0463 |
| ZnVI-O | - | - | - | |||
| Zn-Zn/Fe1 | 1.3 (0.9) | 3.21 (0.05) | 0.01 | |||
| Zn-Zn/Fe2 | 2.1 (1.1) | 3.43 (0.04) | 0.01 | |||
| BIOS Zn Low | ZnIV-O | 3.7 (0.5) | 1.97 (0.02) | 0.006 | 5.3 (1.5) | 0.0112 |
| ZnVI-O | 2.6 (0.4) | 2.12 (0.04) | 0.01 | |||
| Zn-Zn/Fe1 | 1.3 (0.5) | 3.27 (0.03) | 0.01 | |||
| Zn-Zn/Fe2 | 1.5 (0.6) | 3.47 (0.03) | 0.01 | |||
| 2LFh Zn High | ZnIV-O | 4.3 (0.3) | 1.97 (0.01) | 0.006 | 5.9 (1.8) | 0.0168 |
| ZnVI-O | 2.0 (0.6) | 2.16 (0.04) | 0.01 | |||
| Zn-Zn/Fe1 | 1.1 (0.6) | 3.27 (0.04) | 0.01 | |||
| Zn-Zn/Fe2 | 1.6 (0.7) | 3.48 (0.03) | 0.01 | |||
| BIOS Zn High | ZnIV-O | 4.1 (0.3) | 1.99 (0.01) | 0.006 | 6.2 (1.7) | 0.015 |
| ZnVI-O | 1.9 (0.5) | 2.18 (0.04) | 0.01 | |||
| Zn-Zn/Fe1 | 0.8 (0.5) | 3.23 (0.06) | 0.01 | |||
| Zn-Zn/Fe2 | 1.3 (0.6) | 3.42 (0.05) | 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitaker, A.H.; Duckworth, O.W. Cu, Pb, and Zn Sorption to Biogenic Iron (Oxyhydr)Oxides Formed in Circumneutral Environments. Soil Syst. 2018, 2, 18. https://doi.org/10.3390/soilsystems2020018
Whitaker AH, Duckworth OW. Cu, Pb, and Zn Sorption to Biogenic Iron (Oxyhydr)Oxides Formed in Circumneutral Environments. Soil Systems. 2018; 2(2):18. https://doi.org/10.3390/soilsystems2020018
Chicago/Turabian StyleWhitaker, Andrew H., and Owen W. Duckworth. 2018. "Cu, Pb, and Zn Sorption to Biogenic Iron (Oxyhydr)Oxides Formed in Circumneutral Environments" Soil Systems 2, no. 2: 18. https://doi.org/10.3390/soilsystems2020018
APA StyleWhitaker, A. H., & Duckworth, O. W. (2018). Cu, Pb, and Zn Sorption to Biogenic Iron (Oxyhydr)Oxides Formed in Circumneutral Environments. Soil Systems, 2(2), 18. https://doi.org/10.3390/soilsystems2020018