Mexiletine-Induced Esophageal Ulceration: Two Case Reports and a Review of the Literature
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
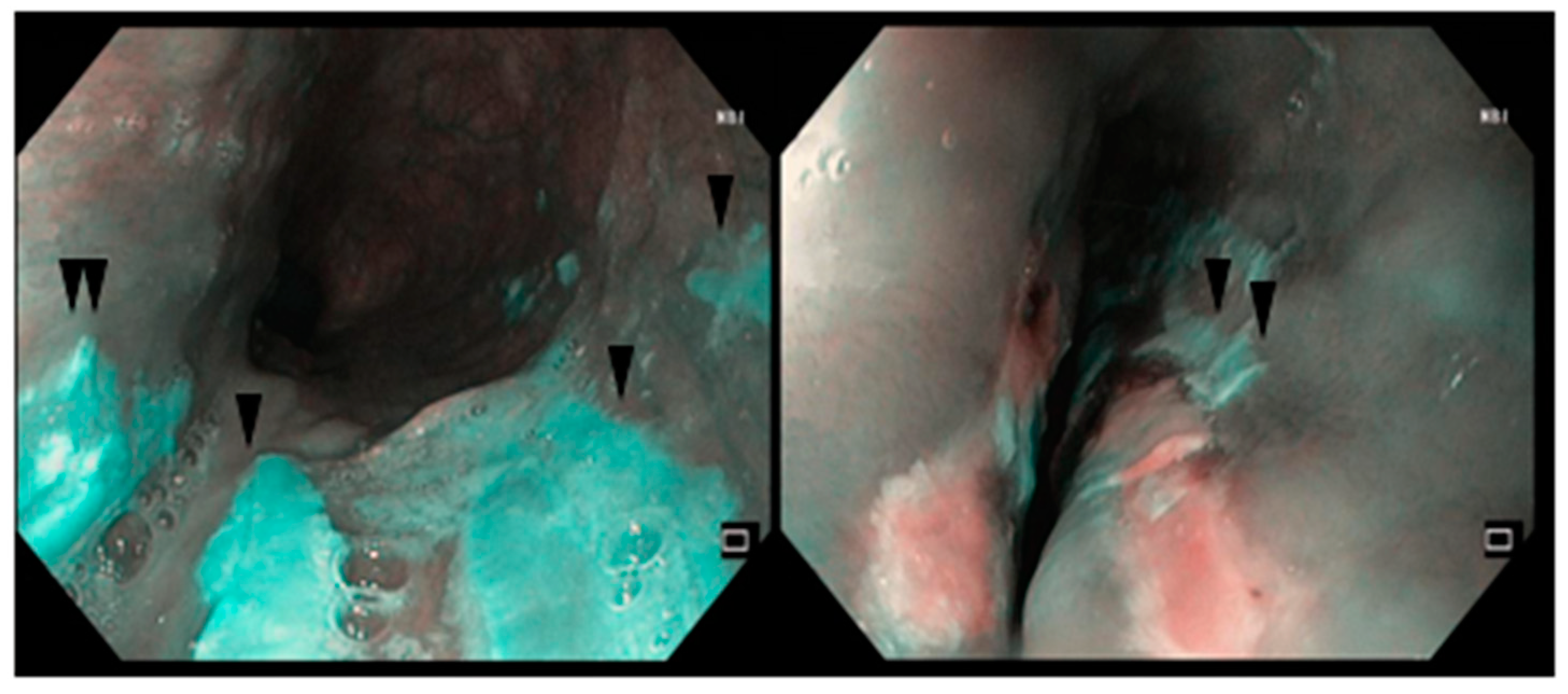
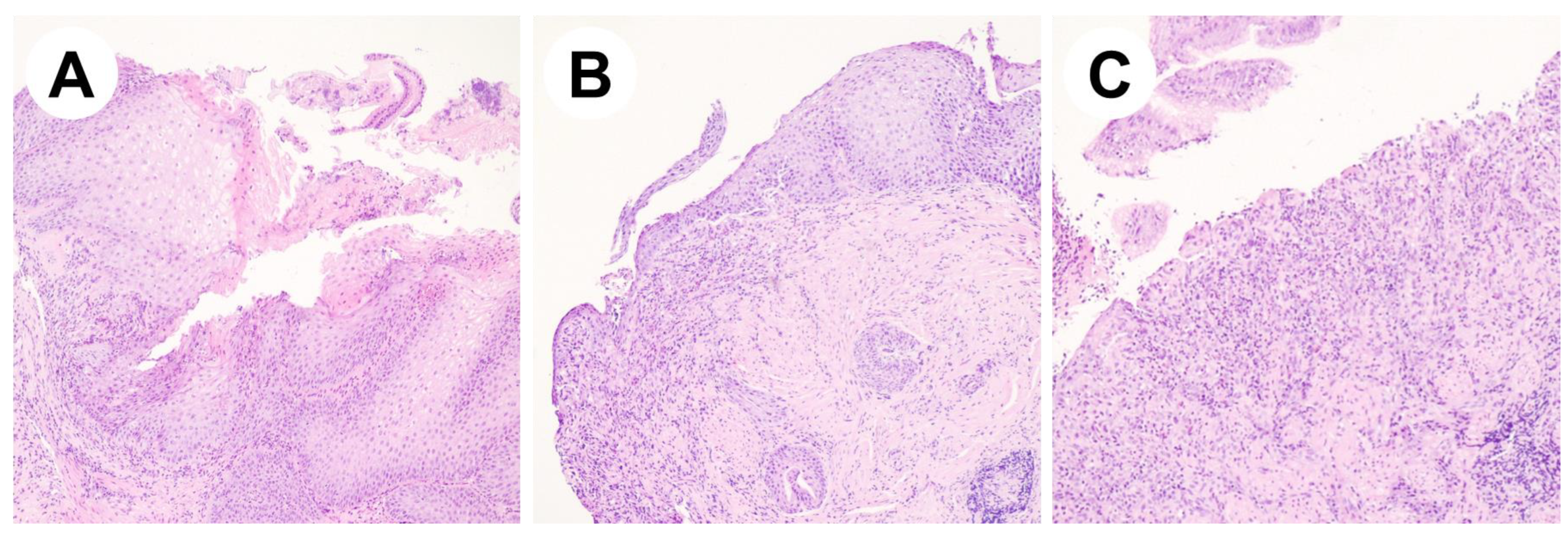
2.1. Case Report #1
2.2. Case Report #2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhtar, M. Practical considerations in the treatment of ventricular arrhythmias with mexiletine. Am. Heart J. 1984, 107, 1086–1090. Available online: https://linkinghub.elsevier.com/retrieve/pii/0002870384901790 (accessed on 17 February 2019). [CrossRef] [PubMed]
- Manolis, A.S.; Deering, T.F.; Cameron, J.; Markestes, N.A., III. Mexiletine: Pharmacology and therapeutic use. Clin. Cardiol. 1990, 13, 349–359. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2189614 (accessed on 17 February 2019). [CrossRef] [PubMed]
- Campbell, R.W.F. Mexiletine. N. Engl. J. Med. 1987, 316, 29–34. [Google Scholar] [CrossRef]
- Sami, M.; Lisbona, R. Mexiletine: Long-term efficacy and hemodynamic actions in patients with ventricular arrhythmia. Can. J. Cardiol. 1985, 1, 251–258. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2413974 (accessed on 17 February 2019).
- Rudolph, R.; Seggewiss, H.; Seckfort, H. Esophageal ulcer caused by mexiletine. Dtsch. Med. Wochenschr. 1983, 108, 1018–1020. Available online: http://www.thieme-connect.de/DOI/DOI?10.1055/s-2008-1069687 (accessed on 16 February 2019). [CrossRef]
- Winstead, N.S.; Bulat, R. Pill esophagitis. Curr. Treat. Options Gastroenterol. 2004, 7, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kadayifci, A.; Gulsen, M.T.; Koruk, M.; Savas, M.C. Doxycycline-induced pill esophagitis. Dis. Esophagus. 2004, 17, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, M.K.; Nayak, H.K.; Samal, S.C. A recent surge of doxycycline-induced pill esophagitis during the corona virus disease 2019 (COVID-19) pandemic. Indian J. Gastroenterol. 2022, 41, 206–207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dobrucali, A.; Tobey, N.A.; Awayda, M.S.; Argote, C.; Abdulnour-Nakhoul, S.; Shao, W.; Orlando, R.C. Physiological and morphological effects of alendronate on rabbit esophageal epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G576–G586. [Google Scholar] [CrossRef] [PubMed]
- Morden, N.E.; Munson, J.C.; Smith, J.; Mackenzie, T.A.; Liu, S.K.; Tosteson, A.N. Oral bisphosphonates and upper gastrointestinal toxicity: A study of cancer and early signals of esophageal injury. Osteoporos. Int. 2015, 26, 663–672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Ghisa, M.; Barbuscio, I.; Barberio, B.; Savarino, E.; Senzolo, M. Mexiletine Induced Esophageal Ulceration: Two Case Reports. Dig. Liver Dis. 2020, 52, S82–S83. [Google Scholar]
- Adler, J.B.; Goldberg, R.I. Mexiletine-induced pill esophagitis. Am. J. Gastroenterol. 1990, 85, 629–630. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2337077 (accessed on 16 February 2019).
- Penalba, C. Esophageal ulcerations induced by mexiletine hydrochloride. Ann. Gastroenterol. Hepatol. 1986, 22, 267–268. Available online: http://www.ncbi.nlm.nih.gov/pubmed/3777867 (accessed on 16 February 2019).
- Sakai, Y. Case of Mexiletine-Induced Esophagitis Characteristic of Spongiosis of Squamous Epithelium. Gastroenterol. Endosc. 1992, 34, 2057–2061_1. [Google Scholar]
- Baudinet, G.; Henrard, L.; Quinaux, N.; El Allaf, D.; de Landsheere, C.; Carlier, J.; Dresse, A. Pharmacokinetics of mexiletine in renal insufficiency. Acta Cardiol. Suppl. 1980, 25, 55–65. Available online: http://www.ncbi.nlm.nih.gov/pubmed/6966453 (accessed on 16 February 2019).
- Singh, S.; Zeltser, R. Mexiletine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2018. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30085587 (accessed on 17 February 2019).
- Kerin, N.Z.; Aragon, E.; Marinescu, G.; Faitel, K.; Frumin, H.; Rubenfire, M. Mexiletine. Long-term efficacy and side effects in patients with chronic drug-resistant potentially lethal ventricular arrhythmias. Arch. Intern. Med. 1990, 150, 381–384. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2302013 (accessed on 17 February 2019). [CrossRef]
- Hey, H.; Jørgensen, F.; Sørensen, K.; Hasselbalch, H.; Wamberg, T. Oesophageal transit of six commonly used tablets and capsules. Br. Med. J. (Clin. Res. Ed.) 1982, 285, 1717–1719. Available online: http://www.ncbi.nlm.nih.gov/pubmed/6816343 (accessed on 17 February 2019). [CrossRef] [PubMed]
- Kohjitani, A.; Miyawaki, T.; Funahashi, M.; Mitoh, Y.; Matsuo, R.; Shimada, M. Mexiletine inhibits nonadrenergic noncholinergic lower oesophageal sphincter relaxation in rabbits. Eur. J. Pharmacol. 2003, 465, 145–151. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12650844 (accessed on 6 February 2019). [CrossRef]
- Savarino, E.; Zentilin, P.; Marabotto, E.; Pellegatta, G.; Coppo, C.; Brunacci, M.; Dulbecco, P.; Savarino, V. Drugs for improving esophageal mucosa defense: Where are we now and where are we going? Ann. Gastroenterol. 2017, 30, 585–591. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29118552 (accessed on 17 February 2019). [CrossRef] [PubMed]
- Palmieri, B.; Merighi, A.; Corbascio, D.; Rottigni, V.; Fistetto, G.; Esposito, A. Fixed combination of hyaluronic acid and chondroitin-sulphate oral formulation in a randomized double blind, placebo controlled study for the treatment of symptoms in patients with non-erosive gastroesophageal reflux. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 3272–3278. [Google Scholar] [PubMed]

| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age (yo) | 72 | 63 | 77 | 73 | 67 |
| Sex | woman | man | woman | man | woman |
| Duration of mexiletine treatment | single administration | 3 months | long time (not specified) | 1 month | long time (not specified) |
| Symptoms | severe epigastric pain | hemoptysis | asymptomatic | dysphagia for 2 months | epigastric pain and dysphagia |
| Endoscopy | flat ulcer on the posterior wall of the esophagus with a lilac-pink surface | long ulcer with edematous contours in the right piriform sinus | two 5 mm longer and reddish kissing ulcers, at the cervical esophagus | two 1–1.5 cm large ulcers at the middle esophagus | vertical white striped mass covering the entire surface of the esophagus |
| Histology | diffuse purulent esophagitis, without sign of malignancy | not performed | not performed | exclusion of malignancy or infection | spongiosis of the middle layer of squamous epithelium |
| Therapeutic modification | suspension of mexiletine therapy with chamomile, antacids, and cimetidine | mexiletine suspension | changing modality of assumption of mexiletine (with water and upright) | reduction in mexiletine dosage, starting ranitidine, and suspension of Ascriptin | mexiletine suspension |
| Outcome | symptoms resolution and ulcer healing after 15 days | ulcer healing after 7 days | quick resolution of symptoms. EGD not performed | quick resolution of symptoms. EGD not performed | quick resolution of symptoms. Endoscopic resolution after 4 weeks |
| Reference | [5] Rudolph et al., 1983 (German) | [5] Rudolph et al., 1983 (German) | [13] Penalba, 1986 (French) | [12] Adler et al., 1990 (English) | [14] Sakai et al., 1992 (Japanese) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghisa, M.; Barbuscio, I.; Bonazzi, E.; Fassan, M.; Barberio, B.; Senzolo, M.; Savarino, E.V. Mexiletine-Induced Esophageal Ulceration: Two Case Reports and a Review of the Literature. Reports 2025, 8, 9. https://doi.org/10.3390/reports8010009
Ghisa M, Barbuscio I, Bonazzi E, Fassan M, Barberio B, Senzolo M, Savarino EV. Mexiletine-Induced Esophageal Ulceration: Two Case Reports and a Review of the Literature. Reports. 2025; 8(1):9. https://doi.org/10.3390/reports8010009
Chicago/Turabian StyleGhisa, Matteo, Ilenia Barbuscio, Erica Bonazzi, Matteo Fassan, Brigida Barberio, Marco Senzolo, and Edoardo V. Savarino. 2025. "Mexiletine-Induced Esophageal Ulceration: Two Case Reports and a Review of the Literature" Reports 8, no. 1: 9. https://doi.org/10.3390/reports8010009
APA StyleGhisa, M., Barbuscio, I., Bonazzi, E., Fassan, M., Barberio, B., Senzolo, M., & Savarino, E. V. (2025). Mexiletine-Induced Esophageal Ulceration: Two Case Reports and a Review of the Literature. Reports, 8(1), 9. https://doi.org/10.3390/reports8010009






