1. Introduction
Central sleep apnea syndrome (CSAS) is a characterized by the cessation of ventilation lasting for ≥10 s due to the transient loss of neural output to the ventilatory muscles. Central sleep apnea (CSA) occurs in less than 5% of subjects admitted to sleep clinics [
1]. A distinction is made between hypercapnic CSA and non-hypercapnic CSA. Hypercapnic CSA is generally seen in neurological disorders with reduced central drive to the respiratory musculature or diminished muscle strength. Non-hypercapnic CSA occurs in connection with congestive heart failure (CHF), high altitudes, hypothyroidism, and the idiopathic form of CSA.
The majority of patients with mild to moderate obstructive sleep apnea (OSA) have more apneic events in the supine position, as compared with the non-supine position. The most commonly used definition for positional OSA stipulates that the apnea–hypopnea-index (AHI) must be at least twice as high in the supine position as compared with the non-supine positions. OSA is a more common and well-described form of sleep-disordered breathing (SDB) than CSA. Indeed, supine-related OSA is a dominant phenotype of OSA with a prevalence of 20–60% in the general population [
2].
Cheyne–Stokes respiration (CSR) is a periodic pattern of waxing and waning of ventilation with episodes of hyperventilation alternating with central apnea/hypopnea. CSR occurs mostly in patients with chronic heart failure and after a stroke. Some studies report that CSR occurs more often in the supine position, probably due to changes in the functional residual capacity [
3]. Positional central sleep apnea (PCSA) has been also reported in a few cases, in whom no underlying disease has been ascertained [
4]. The association between positional sleep apnea (PSA) and CSA remains poorly examined.
Patients with OSA may develop central apneas under the application of positive airway pressure (PAP), especially during the first days or weeks after initiation. This phenomenon has been described as complex sleep apnea syndrome (CompSAS). However, it is of crucial importance to clearly define treatment-induced CSA and separate it from treatment-independent central apneas. CompSAS contributes often to a reduced response or adherence to continuous positive airway pressure (CPAP) and may lead to CPAP failure [
5].
This is a case report of a 78-year-old female with a severe de novo positional CompSAS.
2. Detailed Case Description
A 78-year-old female presented to her primary doctor after a car accident due to unclear syncope in 2016.
A physical exam and basic laboratory investigation, including complete blood count, comprehensive metabolic panel, and thyroid stimulating hormone, were normal. The patient had a history of hypertension and diabetes mellitus Type 2. Also, Scheuermann’s disease had been known since childhood. She had a normal weight with a BMI of 20 kg/m2. The patient denied alcohol or illicit drug use. A full cardiological and neurological examination did not show pathological findings. Also, her mental health history appeared to be noncontributory. As a next step, a diagnostic polysomnography (PSG) was recommended.
The patient presented at the Sleep Medicine Center at the GZO Wetzikon in Switzerland in November 2016. Polysomnography showed a moderate OSA with an average AHI of 20/h. The Epworth sleepiness scale (ESS) showed a score of 3/24, indicating lower normal daytime sleepiness. A maintenance of wakeful test (MWT) was performed the next day and provided no evidence for excessive daytime sleepiness. There were periodic limb movements during sleep (PLMS) found with an average index of 40/h. Otherwise, no other unusual behaviors were noted. Automatic positive airway pressure (APAP) therapy with pressure (9–14 cm H2O) was recommended.
The patient returned 10 months later for follow-up after using APAP. The downloaded data showed an effective treatment of her sleep apnea. Adherence data showed a regular device usage of 6 h on average per night. Therapy data showed an average residual AHI of 1.2/h. She reported a subjective benefit regarding night sweats. The next follow-up visit was in February 2018, which again showed a regular device usage and an average residual AHI of 2.0/h. The ESS showed a score of 3/24, while the patient reported no subjective benefit from the therapy. The therapy data from the next follow-up in September 2019 showed a slight elevated AHI of 9.6/h in spite of the regular usage without significant air leaks. The patient denied the occurrence of excessive daytime sleepiness as well as sleep disturbances. The APAP therapy with pressure (9–14 cm H2O) was continued without any change.
In March 2021, the downloaded data showed ineffective treatment of her sleep apnea with an average residual AHI of 40/h. The patient returned for an overnight titration study at the sleep laboratory. APAP pressures (9–14 cm H
2O) were tested. This study showed a CompSAS with an average residual AHI of 32.1/h. The respiratory events were central with an average central AHI of 32.1/h. Also, the transcutaneous capnography registered a mild hypercapnia with an average transcutaneous CO
2 pressure (PtcCO
2) of 53.1 mmHg and maximum PtcCO
2 of 73.8 mmHg, probably due to Scheuermann’s disease with kyphoscoliosis (
Table 1). According to these findings and also in view of the patient’s clinical condition, we considered a change to adaptive servo-ventilation (ASV) therapy. Echocardiography showed a normal left ventricular ejection fraction (LVEF) of 64%.
Follow-up visits were performed in March, June, and July 2021. The downloaded data of the ASV device showed an ineffective treatment with an average AHI of 18.3/h, 20.2/h, and 15.6/h, respectively. Adherence data showed regular device usage with an average of 5.5 h, 5 h, and 5.5 h of usage per night, respectively. No significant air leaks were noted using the AirTouch Mask F20. Again, the patient reported no subjective benefit from the therapy. Also, the downloaded data from October 2021 showed persistent ineffective treatment with an average residual AHI of 23/h. However, adherence data showed regular device usage with an average of 5.3 h usage per night. As a next step, we considered a device change using an ASV device from another producer. In June 2022, the patient experienced an acute myocardial infarction. Echocardiography showed a reduced LVEF (44%). Therefore, we stopped the ASV therapy according to the recommendation of the American Academy of Sleep Medicine (AASM).
The patient returned for an overnight titration study with capnography. Next, a bilevel positive airway pressure-spontaneous/timed (BiPAP-ST) of 16/8 cm H2O was tested. A mean oxygen saturation of 94%, a minimum oxygen saturation of 83%, and an average AHI of 19.3/h were observed at this pressure setting. The transcutaneous capnography registered a normocapnia with an average PtcCO2 of 38.7 mmHg and maximum PtcCO2 of 46 mmHg.
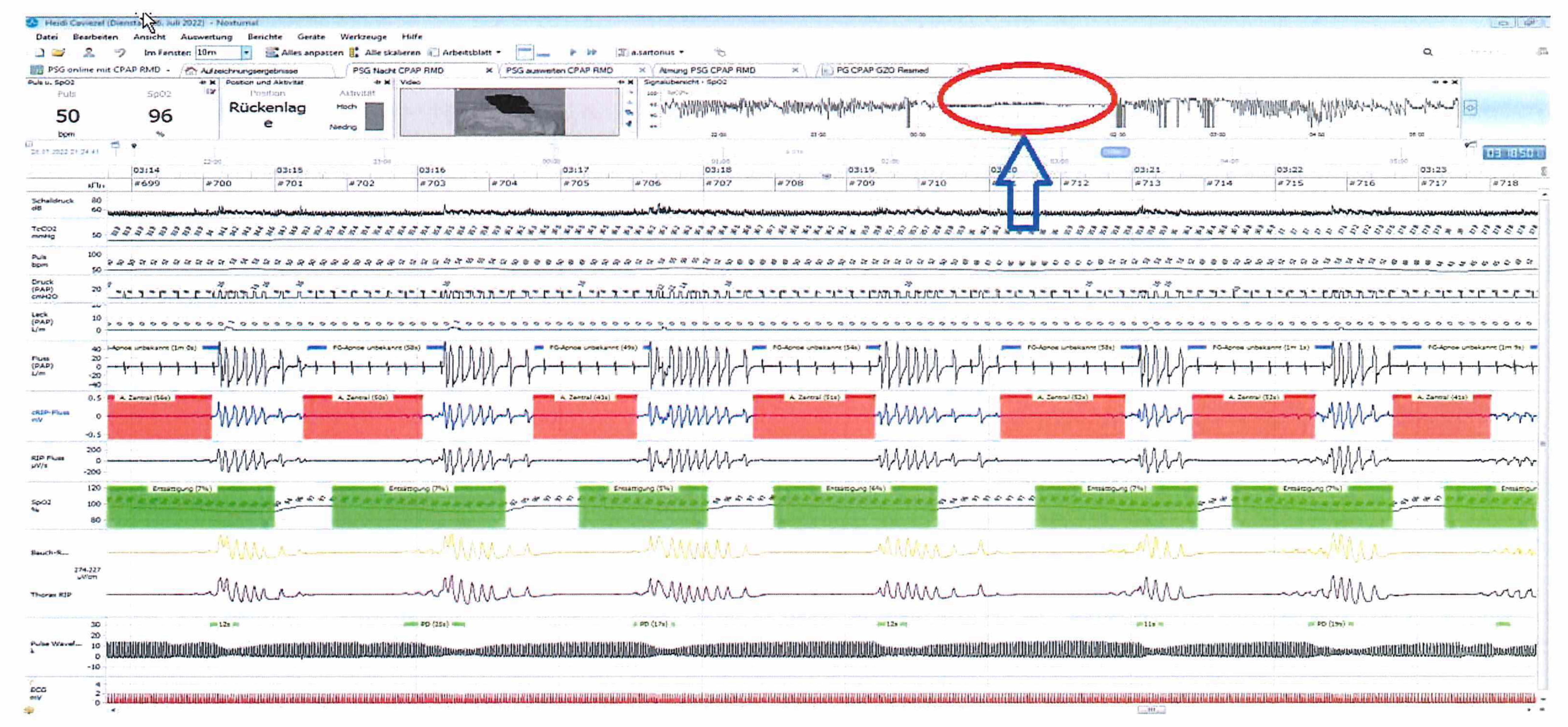
Since the respiratory events were predominantly central events in the supine position (
Figure 1), we considered the initiation of sleep positional therapy (SPT) accompanied by nocturnal oxygen therapy with a flow rate of 2 L/minute.
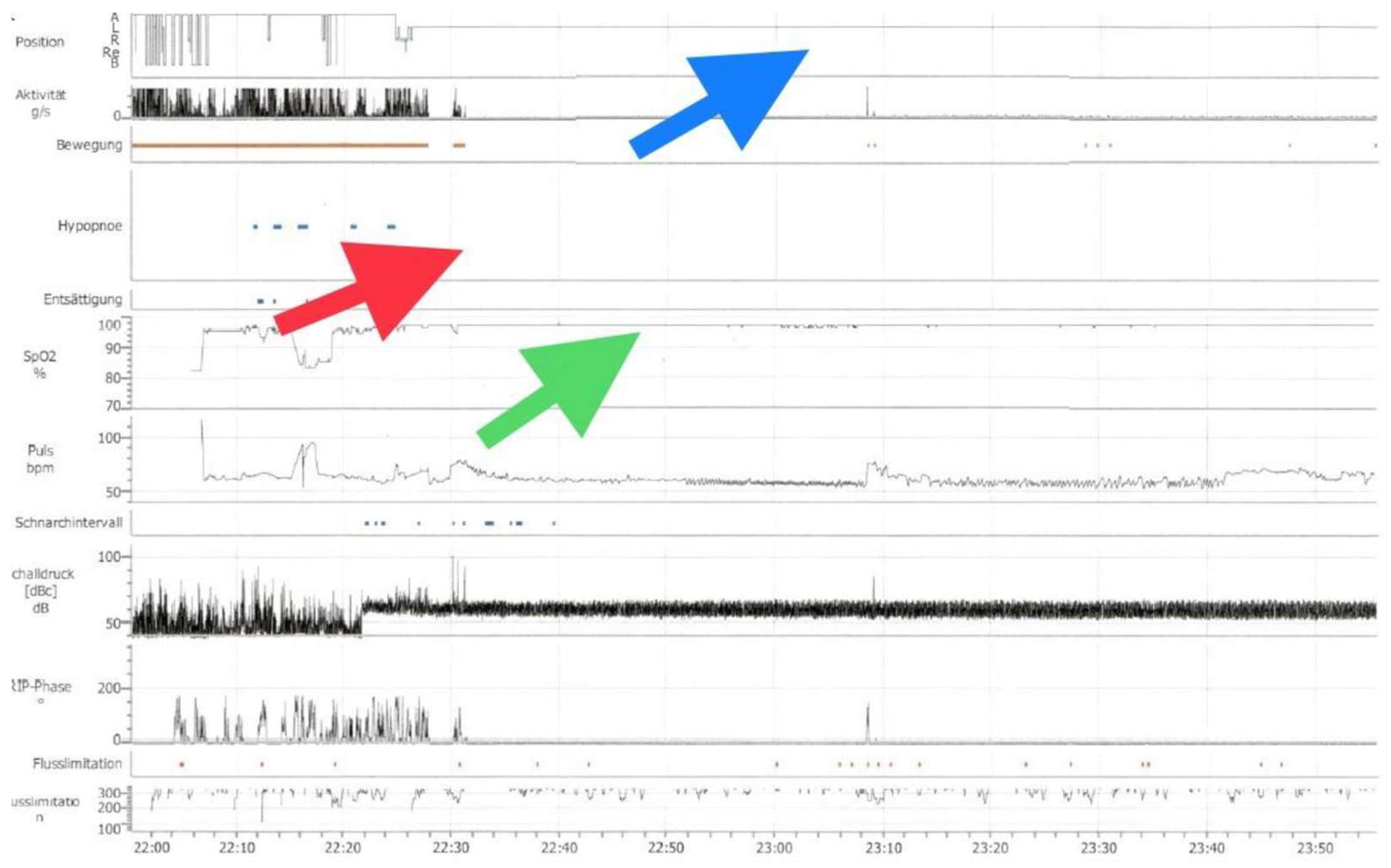
The ambulatory nocturnal polygraphy on SPT and oxygen therapy showed an effective treatment of the respiratory events with an average AHI of 0/h and mean oxygen saturation of 97.8% (
Figure 2). The patient also reported a significant improvement of her sleep quality.
3. Discussion
Sleeping position could be an aggravating factor for sleep apnea. Specifically, sleeping in the supine position can lead to a significant worsening of OSA, which is believed to be related to the relaxation of muscles in the jaw and throat under the influence of gravity, leading to the narrowing of the upper airways. This is frequently seen in patients with less severe OSA and smaller neck circumference.
In the literature, there are only a few reports of PCSA in patients without cardiac history or CHF. The change in the pattern of the sleep-disordered breathing (SDB) from obstructive to central or mixed respiratory events with the positional change to a supine one has already been described in a study which included eight patients in whom no underlying cardiac disease was known. Two patients already had a history of cerebrovascular events (one with cerebral hemorrhage and the other with cerebral ischemia) [
6]. A significant worsening of the treatment-emergent CSA/CompSAS while employing the CPAP/ASV in supine sleep has also been reported in another study [
7]. Zaharna et al. also reported a case of idiopathic CSA in an otherwise healthy young man with significant worsening in supine sleep [
4].
Benosit L. et al. published a prospective multicenter trial of 16 patients, in whom 4 patients suffered from hypertension and arrhythmia, which showed that SPT was effective and can be considered a new treatment modality in PCSA. Long-term follow-up and compliance monitoring is ongoing [
8].
This case report showed a CompSAS that was significantly worse in the supine position. While ASV and BiPAP-ST are the first choice in such cases, no significant improvement in residual AHI was seen in this case. Therefore, we have considered SPT using a sleep pillow. An additional therapy with oxygen was recommended. The ambulatory nocturnal polygraphy after nearly 6 weeks showed a significant improvement of the average AHI without significant oxygen desaturations. The patient reported a better sleep quality without any excessive daytime sleepiness.
Treatment of CSA is generally based on the underlying cause. In our case, we assumed that the central apneas were not due to CHF, even with a mildly reduced LVEF of 44% after the myocardial infarction in June 2022. However, CSAs were reported already in APAP overnight titration in 2021 followed by echocardiography showing a normal LVEF of 64% before the beginning of ASV therapy. Additionally, a CSR pattern and periodic breathing were absent on both the diagnostic and titration studies. Also, the patient did not tolerate opioids and was receiving nonsteroidal anti-inflammatory drugs (NSAIDs) for her chronic pain.
The individual response of the ventilatory system to changes in the CO2 level can be described as loop gain. This represents the ratio of the ventilatory response to any breathing disturbances. Stimulation of ventilation by arousals increases tidal volumes or breathing frequency and reduces the actual PaCO2. This executive part of the loop gain represents the plant gain. High loop gain results in a ventilatory overshoot with hyperventilation and hypocapnia leading to apneas and hypoxemia.
The peripheral and central chemoreceptors measure increases or decreases in PaCO2 and function as the feedback gain in a biological system. Cardiovascular failure may be associated with increased circulation time, which delays the perception of the metabolic variations at the chemoreceptors.
Sleeping in the supine position reduces cardiac output and functional residual capacity, which consequently enhances plant gain [
9].
Although CSA is usually associated with hypocapnia, it is not compulsory. This patient’s PtcCO2 was slightly elevated (mean 53.1 mmHg) in the capnography measurement during the overnight titration study employing APAP therapy in March 2021 and was normal (mean 38.1 mmHg) during the BiPAP-ST overnight titration study in July 2022. The proximity of the central apnea threshold to the carbon dioxide (CO2) level was more important. The slightly elevated PtcCO2 corresponds to the fact that hypoventilation is the predominant SDB in kyphoscoliosis patients. Eupneic patients with CHF may also have CSA.
The prevalence of CompSAS seems to vary among different studies, ranging between 18% and 56%. However, it is not easy to assume the prevalence of CompSAS in a clinical setting, because of the dynamic nature of this condition, with improvement or disappearance of the central respiratory events during sleep in some patients and its de novo appearance in others. CompSAS may occur de novo in 4% of patients with OSA under CPAP during follow-up [
10]. Only those central apneas that do not disappear under CPAP fulfill the criteria of treatment-induced CSA (CompSAS).
We assume that our patient represented a case of de novo positional CompSAS, which improved significantly with nocturnal oxygen therapy (NOT) and SPT. NOT is still indicated for CSA-CSR. While the exact mode of action is unknown, it is believed that supplemental oxygen dampens the respiratory drive, thus reducing the minute ventilation and increasing partial pressure of carbon dioxide. However, the possibility of coexisting obstructive respiratory events in the overnight titrations studies under APAP and BiPAP-ST cannot be ruled out due to the absence of esophageal pressure, which is seen as a gold standard in identification of obstructive and central respiratory events during sleep [
11]. Other causes for worsening of OSA like reduced adherence, significant air leaks, and weight gain had already been excluded.
4. Conclusions
The present case suggests that SPT should be taken into consideration as a treatment option of CSA or CompSAS, especially in those patients who have difficulties in tolerating other treatment options for CSA including CPAP, BiPAP, and ASV therapy. In some cases, patients view SPT as a more tolerable alternative treatment. However, high scientific evidence for SPT as viable treatment option for CSA has been lacking until now.
5. Patents
This research was performed in the Sleep Medicine Center (GZO Wetzikon) in Zurich Oberland, Switzerland.
Author Contributions
Conceptualization, A.K.; methodology A.K.; investigation, A.K. and M.S.; resources, A.K. and M.S.; data curation, A.K.; writing—original draft preparation, A.K.; writing—review and editing, A.K. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was determined by the Institutional Review Board of GZO, Spital Wetzikon, Zurich, to meet criteria for the waiver due to the absence of patient-related data as well as the absence of experiments on biological specimens or medications.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
We sincerely thank our patient for her willingness to share the details of her sleep breathing disorder in this case report.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AASM | American Academy of Sleep Medicine |
| AHI | Apnea–hypopnea index |
| APAP | Automatic positive airway pressure |
| ASV | Adaptive servo-ventilation |
| BiPAP-ST | Bilevel positive airway pressure-spontaneous/timed |
| CHF | Congestive heart failure |
| CO2 | Carbon dioxide |
| CompSAS | Complex sleep apnea syndrome |
| CPAP | Continuous positive airway pressure |
| CSA | Central sleep apnea |
| CSAS | Central sleep apnea syndrome |
| CSR | Cheyne–Stokes respiration |
| ESS | Epworth sleepiness scale |
| LVEF | Left ventricular ejection fraction |
| MWT | Maintenance of wakeful test |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| NOT | Nocturnal oxygen therapy |
| OSA | Obstructive sleep apnea |
| PAP | Positive airway pressure |
| PaCO2 | Carbon dioxide tension |
| PCSA | Positional central sleep apneas |
| PLMS | Periodic limb movements during sleep |
| PSA | Positional sleep apnea |
| PSG | Polysomnography |
| PtcCO2 | Transcutaneous CO2 pressure |
| SBD | Sleep-disordered breathing |
| SPT | Sleep positional therapy |
References
- Bradley, T.D.; Floras, J.S. Sleep apnea and heart failure: Part II: Central sleep apnea. Circulation 2003, 107, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Subramani, Y.; Singh, M.; Wong, J.; Kushida, C.A.; Malhotra, A.; Chung, F. Understanding phenotypes of obstructive sleep apnea: Applications in anesthesia, surgery, and perioperative medicine. Anesth. Analg. 2017, 124, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Joho, S.; Oda, Y.; Hirai, T.; Inoue, H. Impact of sleeping position on central sleep apnea/Cheyne–Stokes respiration in patients with heart failure. Sleep Med. 2010, 11, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Zaharna, M.; Rama, A.; Chan, R.; Kushida, C. A Case of Positional Central Sleep Apnea. J. Clin. Sleep Med. 2013, 9, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Naughton, M.T.; Benard, D.C.; Rutherford, R.; Bradley, T.D. Effect of continuous positive airway pressure on central sleep apnea and nocturnal PCO2 in heart failure. Am. J. Respir. Crit. Care Med. 1994, 150, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Issa, F.G.; Sullivan, C.E. Reversal of Central Sleep Apnea Using Nasal CPAP. Chest 1986, 90, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Allam, J.S.; Olson, E.J.; Gay, P.C.; Morgenthaler, T.I. Efficacy of Adaptive Servoventilation in Treatment of Complex and Central Sleep Apnea Syndromes. Chest 2007, 132, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Benosit, L.; Verbraecken, J.; Hennine, J.; de Vried, N. Positional therapy as a treatment for central sleep apnoea: A prospective multicentre trial. Eur. Respir. J. 2016, 48 (Suppl. S60), PA2388. [Google Scholar]
- Orr, J.E.; Malhotra, A.; Sands, S.A. Pathogenesis of central and complex sleep apnoea. Respirology 2016, 22, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Cassel, W.; Canisius, S.; Becker, H.F.; Leistner, S.; Ploch, T.; Jerrentrup, A.; Vogelmeier, C.; Koehler, U.; Heitmann, J. A prospective polysomnographic study on the evolution of complex sleep apnoea. Eur. Respir. J. 2011, 38, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, C.; Schwaibold, M.; Randerath, W.J.; Bolz, A.; Jané, R. An Invasive and a Noninvasive Approach for the Automatic Differentiation of Obstructive and Central Hypopneas. IEEE Trans. Biomed. Eng. 2010, 57, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).