A Case of COVID-19 with Acute Exacerbation after Anti-Inflammatory Treatment
Abstract
:1. Introduction
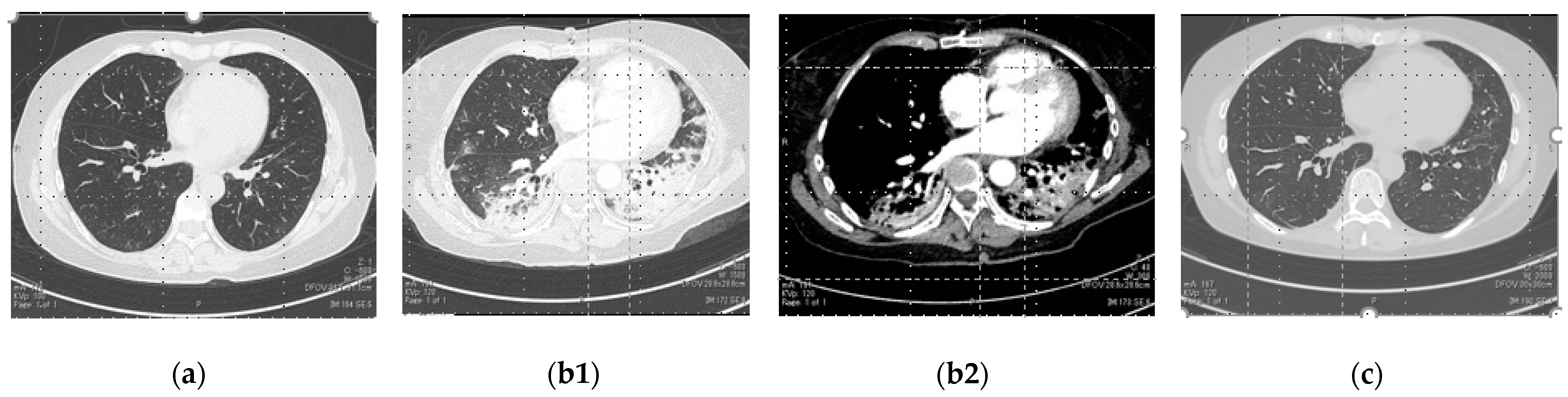
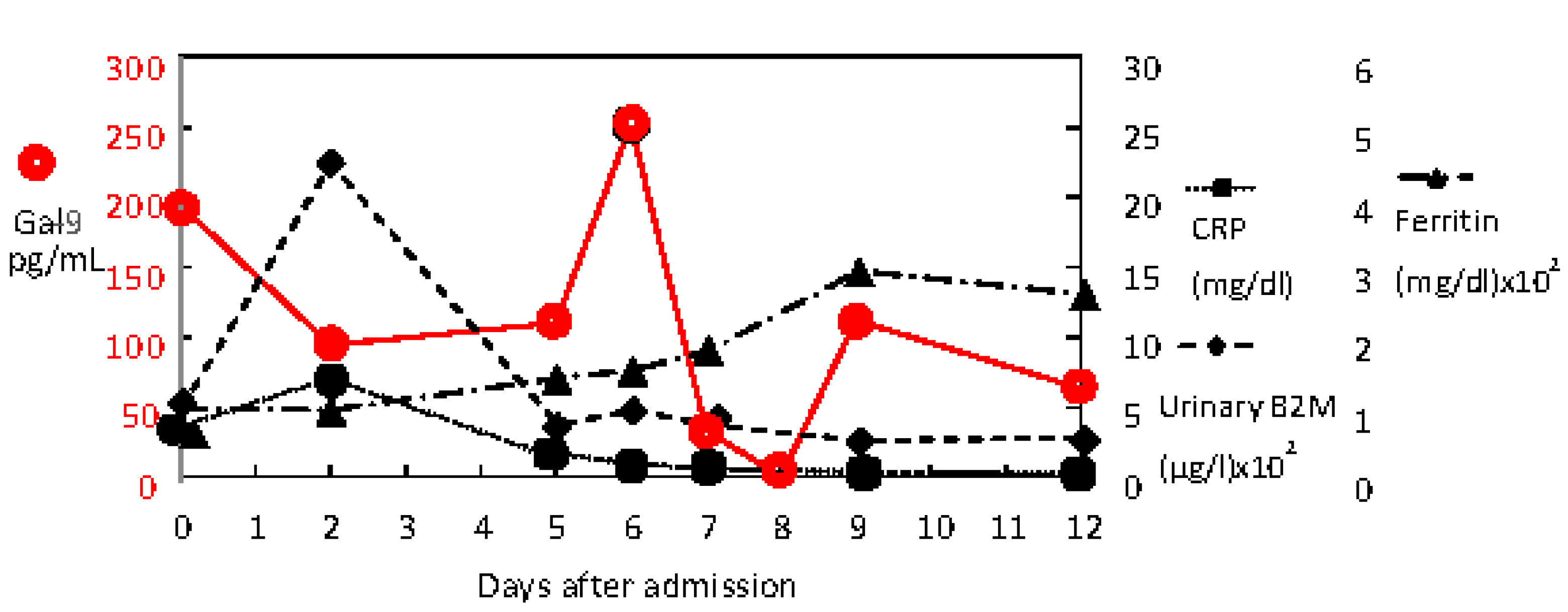
2. Case Presentation
3. Discussion
3.1. Limitations
3.2. Future Direction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripathi, T. One year update on the COVID-19 pandemic: Where are we now? Acta Trop. 2021, 214, 105778. [Google Scholar] [CrossRef]
- Haque, A.; Pant, A.B. Mitigating COVID-19 in the face of emerging virus variants, breakthrough infections and vaccine hesitancy. J. Autoimmun. 2022, 127, 102792. [Google Scholar] [CrossRef]
- Hanff, T.C.; Mohareb, A.M.; Giri, J.; Cohen, J.B.; Chirinos, J.A. Thrombosis in COVID-19. Am. J. Hematol. 2020, 95, 1578–1589. [Google Scholar] [CrossRef]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef]
- Luo, P.; Liu, Y.; Qiu, L.; Liu, X.; Liu, D.; Li, J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020, 92, 814–818. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J.I. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2021, 11, 316–329. [Google Scholar] [CrossRef]
- Ashino, Y.; Chagan-Yasutan, H.; Hatta, M.; Shirato, Y.; Kyogoku, Y.; Komuro, H.; Hattori, T. Successful Treatment of a COVID-19 Case with Pneumonia and Renal Injury Using Tocilizumab. Reports 2020, 3, 29. [Google Scholar] [CrossRef]
- Bai, G.; Furushima, D.; Niki, T.; Matsuba, T.; Maeda, Y.; Takahashi, A.; Hattori, T.; Ashino, Y. High Levels of the Cleaved Form of Galectin-9 and Osteopontin in the Plasma Are Associated with Inflammatory Markers That Reflect the Severity of COVID-19 Pneumonia. Int. J. Mol. Sci. 2021, 22, 4978. [Google Scholar] [CrossRef]
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov (accessed on 2 March 2022).
- Kharat, A.; Simon, M.; Guérin, C. Prone position in COVID 19-associated acute respiratory failure. Curr. Opin. 2022, 28, 57–65. [Google Scholar] [CrossRef]
- Bahloul, M.; Kharrat, S.; Hafdhi, M.; Maalla, A.; Turki, O.; Chtara, K.; Ammar, R.; Suissi, B.; Hamida, C.B.; Chelly, H.; et al. Bouaziz M Impact of prone position on outcomes of COVID-19 patients with spontaneous breathing. Acute Crit. Care 2021, 36, 208–214. [Google Scholar] [CrossRef]
- Nakamura, K.; Ide, S.; Saito, S.; Kinoshita, N.; Kutsuna, S.; Moriyama, Y.; Suzuki, T.; Ota, M.; Nomoto, H.; Mizoue, T.; et al. COVID-19 can suddenly become severe: A case series from Tokyo, Japan. Glob. Health Med. 2020, 2, 174–177. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Copaescu, A.; Smibert, O.; Gibson, A.; Phillips, E.J.; Trubiano, J.A.J. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. Allergy Clin. Immunol. 2020, 146, 518–534. [Google Scholar] [CrossRef]
- Gatti, M.; Fusaroli, M.; Caraceni, P.; Poluzzi, E.; Ponti, D.F.; Raschi, E. Serious adverse events with tocilizumab: Pharmacovigilance asana aid to prioritize monitoring in COVID-19. Br. J. Clin. Pharmacol. 2021, 87, 1533–1540. [Google Scholar] [CrossRef]
- Charan, J.; Dutta, S.; Kaur, R.; Bhardwaj, P.; Sharma, P.; Ambwani, S.; Jahan, I.; Abubakar, A.; Islam, S.; Hardcastle, C.T.; et al. Tocilizumab in COVID-19: A study of adverse drug events reported in the WHO database. Expert Opin. Drug Saf. 2021, 20, 1125–1136. [Google Scholar] [CrossRef]
- Batah, S.S.; Fabro, A.T. Pulmonary pathology of ARDS in COVID-19: A pathological review for clinicians. Respir. Med. 2021, 176, 106239. [Google Scholar] [CrossRef]
- Zeng, Z.; Xiang, M.; Guan, H.; Liu, Y.; Zhang, H.; Xia, L.; Zhan, J.; Hu, Q. Early fibroproliferative signs on high-resolution CT are associated with mortality in COVID-19 pneumonia patients with ARDS: A retrospective study. Ther. Adv. Chronic Dis. 2021, 12, 2040622320982171. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; Li, Y.; Cao, Y.; Gu, J.; Wu, H.; et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 2021, 299, E177–E186. [Google Scholar] [CrossRef]
- Iwasaki-Hozumi, H.; Chagan-Yasutan, H.; Ashino, Y.; Hattori, T. Blood Levels of Galectin-9, an Immuno-Regulating Molecule, Reflect the Severity for the Acute and Chronic Infectious. Dis. Biomol. 2021, 11, 430. [Google Scholar] [CrossRef]
- Toniatia, P.; Pivab, S.; Cattalinid, M.; Garrafaf, E.; Regolaa, F.; Castellie, F.; Franceschinia, F.; Airòa, P.; Bazzania, C.; Beindorfi, E.-A.; et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Nicola Latronicob Autoimmun. Rev. 2020, 19, 102568. [Google Scholar] [CrossRef]
- Hermine, O.; Mariette, X.; Tharaux, P.L.; Resche-Rigon, M.; Porcher, R.; Ravaud, P. Effect of Tocilizumab vs. Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial. CORIMUNO-19 Collaborative Group. JAMA Intern. Med. 2021, 181, 32–40. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Ruano, C.; Riso, N.; Ribeiro, J.C.; Moraes-Fontes, M.F. Paradoxical pulmonary event under tocilizumab treatment for systemic sclerosis-associated usualinterstitial pneumonia. Ann. Rheum. Dis. 2020, 79, e22. [Google Scholar] [CrossRef] [Green Version]
- Robinson, B.S.; Saeedi, B.; Arthur, C.M.; Owens, J.; Naudin, C.; Ahmed, N.; Luo, L.; Jones, R.; Neish, A.; Stowell, S.R. Galectin-9 Is a Novel Regulator of Epithelial Restitution. Am. J. Pathol. 2020, 190, 1657–1666. [Google Scholar] [CrossRef]
- Arikawa, T.; Matsukawa, A.; Watanabe, K.; Sakata, K.M.; Seki, M.; Nagayama, M.; Takeshita, K.; Ito, K.; Niki, T.; Oomizu, S.; et al. Galectin-9 accelerates transforming growth factor beta3-induced differentiation of human mesenchymal stem cells to chondrocytes. Bone 2009, 44, 849–857. [Google Scholar] [CrossRef]
- Hughes, R.C. Galectins as modulators of cell adhesion. Biochimie 2001, 83, 667–676. [Google Scholar] [CrossRef]
- Calkovska, A.; Kolomaznik, M.; Calkovsky, V. Alveolar type II cells and pulmonary surfactant in COVID-19 era. Physiol. Res. 2021, 70, S195–S208. [Google Scholar] [CrossRef]
- Dapat, I.C.; Pascapurnama, D.N.; Iwasaki, H.; Labayo, H.K.; Chagan-Yasutan, H.; Egawa, S.; Hattori, T. Secretion of Galectin-9 as a DAMP during Dengue Virus Infection in THP-1. Cell Int. J. Mol. Sci. 2017, 18, 1644. [Google Scholar] [CrossRef]
- Ashino, Y.; Ying, X.; Dobbs, L.G.; Bhattacharya, J. [Ca2+]i oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L5–L13. [Google Scholar] [CrossRef] [Green Version]
- Takada, H.; Furuya, K.; Sokabe, M. Mechanosensitive ATP release from hemichannels and Ca2⁺ influx through TRPC6 accelerate wound closure in keratinocytes. J. Cell Sci. 2014, 127, 4159–4171. [Google Scholar] [CrossRef] [Green Version]
- Grygorczyk, R.; Furuya, K.; Sokabe, M. Imaging and characterization of stretch-induced ATP release from alveolar A549 cells. J. Physiol. 2013, 591, 1195–1215. [Google Scholar] [CrossRef]


| Laboratory Data | Reference Range | Day 0 | Day 2 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 12 |
|---|---|---|---|---|---|---|---|---|---|
| Complete Blood Cell count and differential | |||||||||
| White cell unit (/µL) | 3700–8500 | 7400 | 5300 | 5700 | 5400 | 4400 | 3900 | 5500 | 6700 |
| Neutrophils (%) | 44.0–68.0 | 76.6 # | 67.2 | 73 | 70.5 | 62.5 | 55 | 61 | 65 |
| Lymphocytes (%)) | 27.0–44.0 | 16 | 23.5 | 13 | 22.5 | 29.5 | 33 | 31.5 | 28 |
| Monocytes (%) | 3.0–12.0 | 6.3 | 7.6 | 4 | 3.5 | 5.5 | 5.5 | 5.5 | 6 |
| Eosinophils (%) | 0.0–10.0 | 0.3 | 1.3 | 4 | 0.5 | 1.5 | 2 | 1.5 | 0 |
| Basophils (%) | 0.0–3.0 | 0.8 | 0.4 | 1 | 0.5 | 1 | 1 | 0 | 0 |
| Hematocrit (%) | 42.0–53 | 43.8 | 38.6 | 39.1 | 34.1 | 30.7 | 31.1 | 33.4 | 37.2 |
| Hemoglobin (g/dL) | 13.5–17.5 | 14.4 | 13 | 13.1 | 11.6 | 10 | 10.2 | 11.1 | 12.2 |
| Platelet count × 103 (/µL) | 150–355 | 268 | 21.8 | 219 | 263 | 251 | 251 | 308 | 381 |
| Red cell count × 106 (/µL) | 3.90–5.30 | 5.01 | 4.55 | 4.56 | 4.04 | 3.55 | 3.55 | 3.83 | 4.33 |
| Biochemical test | |||||||||
| Urea nitrogen (mg/dL) | 8–20 | 15 | 9 | 7 | 8 | 12 | 12 | 9 | 14 |
| Creatinine (mg/dL) | 0.42–1.07 | 0.64 | 0.6 | 0.53 | 0.45 | 0.47 | 0.48 | 0.42 | 0.41 |
| ALT (U/L) | 3–40 | 23 | 18 | 16 | 14 | 17 | 65 | 70 | 129 |
| AST (U/L) | 8–35 | 25 | 21 | 20 | 19 | 22 | 64 | 109 | 53 |
| LDH (U/L) | 124–222 | 342 | 201 | 295 | 346 | 296 | 302 | 331 | 275 |
| Ferritin (ng/mL) | 14–304 | 104 | 102 | 147 | 158 | 185 | 273 | 298 | 265 |
| CRP (mg/dL) | 0.00–0.3 | 3.67 | 7.33 | 1.64 | 0.88 | 0.48 | 0.31 | 0.25 | 0.09 |
| Total protein (g/dL) | 6.6–8.4 | 7.7 | 6.5 | 6.3 | 5.5 | 5 | 4.9 | 5.5 | 6.2 |
| Albumin (g/dL) | 3.8–5.2 | 4.4 | 3.4 | 3.3 | 2.9 | 2.7 | 2.7 | 3.0 | 3.4 |
| Coagulation test | |||||||||
| PT (s) | 10.0–13.5 | 11.4 | 11.8 | 12.1 | 12.6 | 13.6 | 13.7 | 13.2 | 11.3 |
| PT(%) | 80.0–120.0 | 100.6 | 98.7 | 89.7 | 82.4 | 71.8 | 70.7 | 75.9 | 102.4 |
| APTT (s) | 24.0–39.0 | 33.6 | 37.8 | 33.5 | 41 | 83.1 | 57.5 | 53.6 | 37.9 |
| D-dimer (µg/mL) | 0.00–1.00 | 0.6 | 0.75 | 0.65 | 1.8 | 0.94 | 0.93 | 1.06 | 0.78 |
| Fibrinogen (mg/dL) | 200–400 | 509 | 554 | 389 | 304 | 289 | 253 | 278 | 329 |
| Urine test | |||||||||
| β2-microglobulin (µg/L) | 30–340 | 510 | 2249 | 404 | 510 | 296 | 363 | 570 | 313 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashino, Y.; Shirato, Y.; Yaegashiwa, M.; Yamanouchi, S.; Miyakawa, N.; Ando, K.; Sakurada, Y.; Yasutan, H.C.; Hattori, T. A Case of COVID-19 with Acute Exacerbation after Anti-Inflammatory Treatment. Reports 2022, 5, 24. https://doi.org/10.3390/reports5020024
Ashino Y, Shirato Y, Yaegashiwa M, Yamanouchi S, Miyakawa N, Ando K, Sakurada Y, Yasutan HC, Hattori T. A Case of COVID-19 with Acute Exacerbation after Anti-Inflammatory Treatment. Reports. 2022; 5(2):24. https://doi.org/10.3390/reports5020024
Chicago/Turabian StyleAshino, Yugo, Yoichi Shirato, Masahiro Yaegashiwa, Satoshi Yamanouchi, Noriko Miyakawa, Kokichi Ando, Yumiko Sakurada, Haorile Chagan Yasutan, and Toshio Hattori. 2022. "A Case of COVID-19 with Acute Exacerbation after Anti-Inflammatory Treatment" Reports 5, no. 2: 24. https://doi.org/10.3390/reports5020024
APA StyleAshino, Y., Shirato, Y., Yaegashiwa, M., Yamanouchi, S., Miyakawa, N., Ando, K., Sakurada, Y., Yasutan, H. C., & Hattori, T. (2022). A Case of COVID-19 with Acute Exacerbation after Anti-Inflammatory Treatment. Reports, 5(2), 24. https://doi.org/10.3390/reports5020024








