Abstract
A COVID-19 patient (53-year-old woman from Japan) was admitted to our hospital. She had a high fever (38.3 °C), cough, fatigue, and loss of appetite. She was a smoker and took migraine medication. A thoracic computed tomography (CT) scan showed no evidence of pneumonia. She was treated with antibiotics, protease inhibitors, inhalant corticosteroids, and antivirals. Anti-interleukin-6 receptor antibody tocilizumab (TCZ 400 mg) was added on day 2. On day 4, her temperature decreased, but her vital signs suddenly worsened, with an SpO2 of 70% in ambient air, a blood pressure of 70 mmHg (systolic), loss of consciousness, and tachypnea. Her CT showed bilateral lung consolidation and no pulmonary embolism. She was connected to the ventilator. On day 11, her respiratory condition improved (PaO2/FIO2 400), and she was able to withdraw from the ventilator. Her laboratory data (white cell count, ferritin, d-Dimer, C-reactive protein, and β2-microglobulin) did not increase even at the time of exacerbation, except for Galectin-9 (Gal-9). The plasma Gal-9 levels increased 2.3 times from before the administration of TCZ, followed by a swift decrease associated with improvements in respiratory status. She was discharged on day 16. Patients with TCZ-treated COVID-19 require careful observation.
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly worldwide since 2019. SARS-CoV-2 infection is known as coronavirus 2019 (COVID-19) and causes varying degrees of illness [1]. Today, pandemics do not end since there are repeated mutations of the virus genome [2]. This also makes the treatment of COVID-19 complex. For example, the loss of a vaccine effect or elimination of the effects of antibody treatment has been observed [3].
As one of the characteristics of COVID-19, the sudden onset of lung damage is believed to be caused by thrombotic events and cytokine release.
A pulmonary embolism consists of immune-mediated thrombotic mechanisms, complement activation, macrophage activation syndrome, antiphospholipid antibody syndrome, hyperferritinemia, and renin–angiotensin system dysregulation [4]. On the other hand, the high severity of acute respiratory distress is dependent on a cytokine storm, most likely induced by the interleukin-6 (IL-6) amplifier, which is hyperactivation machinery that regulates the nuclear factor kappa B (NF-κB) pathway [5]. The administration of drugs for anticoagulants and anticytokines is recommended for COVID-19 treatment with these inferences. The anti-interleukin-6 receptor antibody tocilizumab (TCZ) appears to be an effective treatment option in COVID-19 patients with a risk of cytokine storms [6]. Furthermore, patients with COVID-19 and pneumonia showed that TCZ reduced the risk of death by 45% [7]. We have proposed that the early administration of TCZ ameliorated pneumonia and kidney caused by hyperinflammation syndrome in a patient with COVID-19 [8].
However, the exact mechanism by which TCZ improves COVID-19 pneumonia has yet to be clarified. In addition, the biomarkers of COVID-19 pneumonia are in the middle of research. We have already reported that the levels of cleavage forms of plasma osteopontin (OPN) and Galectin-9 (Gal-9) are elevated in COVID-19 patients, and their levels decrease after TCZ administration. These might be used as an indicator of the therapeutic effect and the severity of pathological inflammation [9]. They had a significant association with laboratory markers for lung function, inflammation, coagulopathy, and kidney function in COVID-19 Pneumonia (CP) patients.
New therapeutic strategies recommend the administration of antibodies, which can block the interaction of the RBD (receptor-binding domain) and its ACE2 receptor or neutralize the SARS-CoV-2. However, the patients who were recommended for this treatment were the ones who did not need additional oxygen and were at high risk of progressing to severe COVID-19 [10]. The benefit-risk profile for patients requiring high-flow oxygen or mechanical ventilation was considered unfavorable [10]. This means these agents seemed ineffective in advanced cases, and sole virus control cannot save lives in severe cases. For instance, there was no observed benefit in those on high-flow oxygen, NIV (non-invasive ventilation), MV (mechanical ventilation), or ECMO (extracorporeal membrane oxygenation) in a placebo-controlled, double-blind RCT of Remdesivir in hospitalized patients with COVID-19 [10]. Although the mortality was lower in the TCZ arm than in the usual care arm on day 28, the effect was not marked [10]. It was speculated that lung disease had already progressed in the patients treated with TCZ, and it may have been used too late in previously reported cases. There has been little reporting of the effects of anti-inflammatories before lung damage.
To find an effective treatment for COVID-19, we need to find a novel biomarker that accurately reflects the heterogeneous host responses after the administration of TCZ. As a new method of treatment, the change in the ventral position is usually accompanied by a marked improvement in the arterial blood gases of both spontaneously breathing and mechanically vented patients [11,12]. Although the survival rate of patients in prone positions tends to have a growing trend, the effects of this procedure on outcomes are still uncertain.
This report describes a case of acute pulmonary exacerbation related to COVID-19 despite the preadministration of anticytokine, anticoagulants, and antiviral therapy drugs during hospitalization. A patient developed lung damage suddenly during treatment and did not show an elevation in inflammatory markers other than the plasma Gal-9 level. The patient was improved by pressurizing mechanical ventilation with dexamethasone and repositioning.
2. Case Presentation
A middle-aged woman with COVID-19 was hospitalized on 9 December 2020.
She had nine days of a history of high fever, arthralgia, anorexia, and dysgeusia before admission. A SARS-CoV-2 infection was confirmed by a PCR assay obtained from the patient’s nasopharyngeal swab as described by us [8].
She had a history of migraine, myasthenia gravis, syringoencephalomyelia, and smoking one pack of cigarettes per day. The patient was not routinely taking any drugs, because she had no symptoms of these diseases. The patient’s lab data showed neutrophilia and lymphopenia. It also showed elevated levels of LDH (342 U/mL), C-reactive protein (CRP; 3.65 mg/dL), fibrinogen (509 mg/dL), and urinary β2-microglobulin (B2M) (510 mg/mL) (Table 1).

Table 1.
Laboratory data from the patient during hospitalization.
A chest computed tomography (CT) scan on admission did not show ground-glass opacities (GGOs) in her lungs (Figure 1a). The vital signs of the patient included a heart rate of 108 beats/min, a respiratory rate of 20 breaths/min, an axillary temperature of 38.3 °C, oxygen saturation (SpO2) in ambient air of 94% (Figure 2), and blood pressure of 120/79 mmHg. These were considered only mild diseases. Azithromycin (500 mg/day), ciclesonide (200 µg inhaler; 2 inhalations per day), nafamostat mesylate (40 mg/day), and favipiravir (3600 mg on the first day, 1600 mg thereafter) were administered. Despite these treatments, the patient’s clinical status was not improved on day 2. Specifically, her high fever and exhaustion persisted. Furthermore, the CRP and urinary B2M levels were elevated. Because of the lack of improvement in clinical outcomes, 400 mg of TCZ was given intravenously. The fever disappeared. However, on day 4, a disturbance of consciousness, associated with tachypnea, appeared suddenly. Her vital signs worsened to an SpO2 of 70% in ambient air and blood pressure of 70 mmHg (systolic). Her enhanced chest CT image showed consolidations in both lower lobes of her lungs and GGOs around the consolidation at this point (Figure 1(b1)). There was no proof of a pulmonary embolism (Figure 1(b2)). The patient was immediately brought to the ICU with the administration of adrenalin for endotracheal intubation and mechanical ventilation. In addition, the drugs already given were modified as follows: levofloxacin at 500 mg (for 4 days), heparin sodium at 15,000 units (for 4 days), dexamethasone at 6.6 mg (for 10 days), and remdesivir (200 mg loading dose on day 1 followed by 100 mg daily for up to 3 additional days). The ventilator was set to positive airway pressure (initial positive inspiratory pressure of 22 cm H2O and an expiratory positive airway pressure of 5 cm H2O) under the condition of the fraction of inspiratory oxygen (FIO2) of 0.6. Arterial blood gas analysis reported an arterial O2 tension (PaO2) of 74.1 Torr and an arterial CO2 tension (PaCO2) of 46.3 Torr.
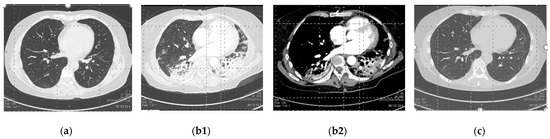
Figure 1.
Computed tomography (CT) images of the patient’s lungs on day 0 (a), day 5 (b1,b2), and day 16 (c) with a: no abnormality; (b1): indicates consolidation and GGOs in bilateral lungs; (b2): indicates pulmonary artery contrast-enhanced findings without defect; and (c): indicates the disappearance of consolidation and GGOs.
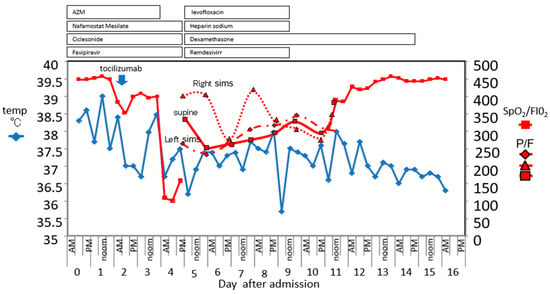
Figure 2.
Fluctuations in the patient’s body temperature and the SpO2/FIO2 and P/F ratio during hospitalization. The drug dosing period is shown at the top of the figure. From day 5 to day 10, the data are from an artificial ventilator in the ICU. The black bordered red square is the SpaO2/FIO2 or P/F in the supine position. The right sims’ position is indicated by the black bordered red triangle. Left sims’ position is indicated by the black bordered red diamond.
Lung compliance was greatly decreased at 24 mL/cm H2O (Figure 3).
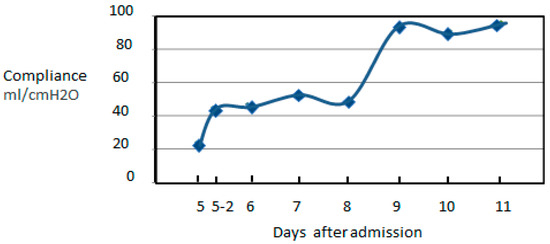
Figure 3.
Lung compliance from the artificial ventilator vita XL (Dräger, Lübec, Germany). The x axis is days after admission. The label 5-2 means the afternoon of day 5.
The change in the right-hand sims’ position was accompanied by a significant improvement in PaO2/FIO2 407, but the left-hand PaO2/FIO2 was 267 (Figure 2). Differences in the respiration status were observed depending on the position. After being placed on a respirator, her respiratory condition had a lasting improvement from day to day. On day 10, PaO2 and PaCO2 were 90.6 and 48.3 Torr, respectively, after adjusting the ventilation to the CPAP mode (5 m H2O FIO2 0.3 setting) in the supine position. Then, she withdrew from the ventilator. Lung compliance just prior to intubation removal was 96 mL/cm H2O (Figure 3).
The consolidation disappeared on the CT after extubation (Figure 1c). The patient’s LDH, CRP, and urinary B2M decreased. The patient’s ferritin and D-dimer levels were not extremely in excess of the normal range in the hospital (Table 1). Only Gal-9 was increased two days (day 2 and 6) after exacerbation. Gal-9 was measured using a human Gal-9 ELISA kit (GalPharma Co., Ltd., Takamatsu, Japan) as described [9]. It was also on the rise when admitted and had a second increase on day 6 (Figure 4). Gal-9 decreased with the improvement in the clinical outcomes. On day 16, the patient was discharged.
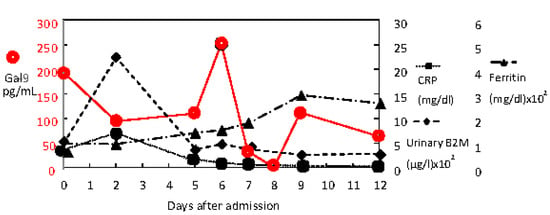
Figure 4.
Changes in Galectin-9 (Gal-9) and other inflammatory biomarkers (CRP, urinary B2M, ferritin) during hospitalization.
3. Discussion
A 53-year-old Japanese COVID-19 patient woman was admitted and given four kinds of drugs (antibiotics, protease inhibitors, inhalant corticosteroids, and antivirals). However, there was no response in terms of symptom alleviation and high fever, cough, fatigue, and loss of appetite persisted. TCZ was administered to improve these clinical findings. Although TCZ demonstrated an antipyretic effect and an improvement in the patient’s laboratory data, the consolidation shadow suddenly appeared in both lungs, and SpO2 was lowered. This was consistent with COVID-19 pneumonia rather than a pulmonary embolism, because the contrast enhancement CT did not show pulmonary artery obstruction. Seven days after she was connected to the ventilator (11th day after hospitalization), her respiratory condition improved.
There are reports that COVID-19 can suddenly become severe [13,14]. The reasons why the case with mild COVID-19 suddenly worsened are not clear. Reports suggest it is more likely related to immune dysregulation or a cytokine storm after SRAS-CoV-2 infection [14], which leads to respiratory diseases. Moreover, interleukin-6 is regarded as the perpetrator of the COVID-19 cytokine storm [15]. In this case, we used TCZ to prevent this transition of COVID-19 to a cytokine storm. Unexpectedly, the onset of respiratory failure and worsening CT findings were observed, though most of the laboratory data did not change other than Gal-9. Since various adverse drug effects (ADEs) were reported in TCZ treatment of COVID-19, including respiratory disorders, it cannot be denied that this deterioration may be due to TCZ [16,17].
After being connected to a ventilator, her respiratory condition immediately recovered. In particular, the right sims’ position resulted in dramatic improvements. The prone position can be used as adjuvant therapy for improving ventilation in patients with acute respiratory distress syndrome (ARDS). Lung damage from the novel coronavirus SRAS-CoV-2 resembles other causes of ARDS [18]. However, this case differed from ARDS caused by lung compliance reduced by vascular permeability. Her chest high-resolution computed tomography (HRCT) findings did not show the traction bronchiolectasis or bronchiectasis seen in COVID-19 ARDS [19]. Lung compliance was low. However, it rapidly recovered to the normal range immediately after intubation. Moreover, 7 days later, the consolidation had disappeared. Six-month follow-up CT showed fibrotic-like changes in the lung in more than one-third of patients who survived severe coronavirus disease 2019 pneumonia [20]. No fibrotic-like changes were observed in this case. After TCZ treatment in COVID-19 cases, the patients might have distinct ARDS, which might be different from COVID-19 ARDS. At least, cytokine storms cannot be positively recognized from a low CRP. It was considered that CRP levels decreased because TCZ blocks the IL-6 receptor. Biomarkers for this morbid condition are unknown. In our case, the levels of CRP, ferritin, D-dimer, and urinary B2M declined or did not increase when she worsened. Only the Gal-9 level was elevated with the deterioration in the respiratory condition and returned to a normal level with its improvement. We already reported that the plasma level of Gal-9 is a representative inflammatory biomarker in COVID-19, tuberculosis, and HIV infections [21]. In addition, Gal-9 may reflect the severity of acute and chronic infectious diseases. It has been discovered that Gal-9 has biological roles in innate and adaptive immune systems. Gal-9 is expressed in endothelial cells, the epithelium of the gastrointestinal tract, and several immune cells, including T cells, B cells, macrophages, mast cells, and dendritic cells. Gal-9 regulates the transduction of intra- and extracellular signals by interacting with several receptors [21]. While the inflammatory marker was not deregulated in this case, only Gal-9 was elevated followed by a decline associated with the deterioration and recovery of the respiratory conditions.
TCZ treatment was found to be associated with rapid, sustained, and significant clinical improvement [22]. However, there was an inconsistency between this fact and the changes in the CT image and SpO2. The other reports indicated that fewer patients needed NIV or MV or died in the TCZ group than the usual-care-alone group [23]. These facts implicate the heterogenous host responses against TCZ. Additionally, a patient who is not COVID-19-infected but have CT findings with ground-glass opacities and clinical courses to this case was reported after TCZ administration [24].
It is of note that this patient recovered in 6 days after connection to a ventilator. Gal-9 can be an indicator for pulmonary regeneration [25,26]. Gal-9 has also been reported to regulate cell–cell and cell–matrix adhesion [27]. As SARS-CoV-2 targets various cell types of the proximal airways and the alveolar type 2 cells of the gas exchange region of the distal lung, the surfactant might be decreased in the lung. The decrease in the surfactant caused alveolar cell damage [28] and cell to cell adhesion was disabled. The increasing Gal-9 in our case indicates tissue destruction. Gal-9 was proposed to be one of the danger-associated molecules in dengue virus infection [29]. This tissue destruction was different from traditional ARDS caused by permeable pulmonary edema, because the immediate resuscitated lung conformance and CT after recovery showed no changes in fibrosis. TCZ terminates the IL-6-dependent inflammatory reaction. The released Gal-9 may modify the recovery process of COVID-19 pneumonia because this case recovered swiftly and there was no lung fibrosis as a sequela. A larger study is necessary for conclusions to be made regarding the clinical significance of Gal-9 in detecting ADEs in TCZ-treated COVID-19 patients.
In this case, the sims’ position was useful for improving the respiratory state. Prone position pronation can also recruit the dorsal lung regions and drain airway secretions, improving gas exchange. The blowing of the decreasing area of the surfactant made the collapsed alveoli swell and encouraged surfactant secretion [30]. ATP secretion and [Ca(2+)] (i) oscillations induced by lung stretch could lead to tissue repair [31,32]. After virus infection, when the suppression of inflammation alone does not cure tissue destruction, etc., we may need to adopt another treatment method to recover from this destroyed lung damage.
This is a single-case report; to generate evidence, long-term follow-up studies with a large sample size will enlighten medical science about unknown ADEs associated with TCZ in COVID-19 patients.
3.1. Limitations
Since this is a rare case in which the condition of this COVID-19 case changed suddenly after TCZ administration, a systemic search with similar cases could not be performed.
3.2. Future Direction
It is necessary to also note the involvement of Gal-9 in cases of lung disorders other than patients with lung disorders related to COVID-19.
4. Conclusions
A 53-year-old COVID-19 patient without pneumonia was treated with TCZ. Her laboratory findings were improved, but acute exacerbation occurred on day 4. Gal-9 increased simultaneously with the appearance of symptoms; nevertheless, the other biomarkers did not increase. After positive pressure ventilation, the patient showed a remarkable recovery. We reported unexpected respiratory failure after TCZ treatment. Careful monitoring, including Gal-9, would be useful for identifying these patients.
Author Contributions
Conceptualization, Y.A. and T.H.; methodology, Y.A.; formal analysis, H.C.Y.; resources and data curation, Y.S. (Yoichi Shirato), M.Y., S.Y., N.M., K.A. and Y.S. (Yumiko Sakurada); writing—original draft preparation, Y.A.; writing—review and editing, H.C.Y. and T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI), Grant Number JP17H01690 and Sendai city hospital Medical Science Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent was obtained from the patient to publish this paper.
Acknowledgments
We are grateful to T. Niki for measurement of plasma Gal-9 in this patient. All individuals included in this section have consented to the acknowledgement.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripathi, T. One year update on the COVID-19 pandemic: Where are we now? Acta Trop. 2021, 214, 105778. [Google Scholar] [CrossRef]
- Haque, A.; Pant, A.B. Mitigating COVID-19 in the face of emerging virus variants, breakthrough infections and vaccine hesitancy. J. Autoimmun. 2022, 127, 102792. [Google Scholar] [CrossRef]
- Hanff, T.C.; Mohareb, A.M.; Giri, J.; Cohen, J.B.; Chirinos, J.A. Thrombosis in COVID-19. Am. J. Hematol. 2020, 95, 1578–1589. [Google Scholar] [CrossRef]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef]
- Luo, P.; Liu, Y.; Qiu, L.; Liu, X.; Liu, D.; Li, J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020, 92, 814–818. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J.I. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2021, 11, 316–329. [Google Scholar] [CrossRef]
- Ashino, Y.; Chagan-Yasutan, H.; Hatta, M.; Shirato, Y.; Kyogoku, Y.; Komuro, H.; Hattori, T. Successful Treatment of a COVID-19 Case with Pneumonia and Renal Injury Using Tocilizumab. Reports 2020, 3, 29. [Google Scholar] [CrossRef]
- Bai, G.; Furushima, D.; Niki, T.; Matsuba, T.; Maeda, Y.; Takahashi, A.; Hattori, T.; Ashino, Y. High Levels of the Cleaved Form of Galectin-9 and Osteopontin in the Plasma Are Associated with Inflammatory Markers That Reflect the Severity of COVID-19 Pneumonia. Int. J. Mol. Sci. 2021, 22, 4978. [Google Scholar] [CrossRef]
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov (accessed on 2 March 2022).
- Kharat, A.; Simon, M.; Guérin, C. Prone position in COVID 19-associated acute respiratory failure. Curr. Opin. 2022, 28, 57–65. [Google Scholar] [CrossRef]
- Bahloul, M.; Kharrat, S.; Hafdhi, M.; Maalla, A.; Turki, O.; Chtara, K.; Ammar, R.; Suissi, B.; Hamida, C.B.; Chelly, H.; et al. Bouaziz M Impact of prone position on outcomes of COVID-19 patients with spontaneous breathing. Acute Crit. Care 2021, 36, 208–214. [Google Scholar] [CrossRef]
- Nakamura, K.; Ide, S.; Saito, S.; Kinoshita, N.; Kutsuna, S.; Moriyama, Y.; Suzuki, T.; Ota, M.; Nomoto, H.; Mizoue, T.; et al. COVID-19 can suddenly become severe: A case series from Tokyo, Japan. Glob. Health Med. 2020, 2, 174–177. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Copaescu, A.; Smibert, O.; Gibson, A.; Phillips, E.J.; Trubiano, J.A.J. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. Allergy Clin. Immunol. 2020, 146, 518–534. [Google Scholar] [CrossRef]
- Gatti, M.; Fusaroli, M.; Caraceni, P.; Poluzzi, E.; Ponti, D.F.; Raschi, E. Serious adverse events with tocilizumab: Pharmacovigilance asana aid to prioritize monitoring in COVID-19. Br. J. Clin. Pharmacol. 2021, 87, 1533–1540. [Google Scholar] [CrossRef]
- Charan, J.; Dutta, S.; Kaur, R.; Bhardwaj, P.; Sharma, P.; Ambwani, S.; Jahan, I.; Abubakar, A.; Islam, S.; Hardcastle, C.T.; et al. Tocilizumab in COVID-19: A study of adverse drug events reported in the WHO database. Expert Opin. Drug Saf. 2021, 20, 1125–1136. [Google Scholar] [CrossRef]
- Batah, S.S.; Fabro, A.T. Pulmonary pathology of ARDS in COVID-19: A pathological review for clinicians. Respir. Med. 2021, 176, 106239. [Google Scholar] [CrossRef]
- Zeng, Z.; Xiang, M.; Guan, H.; Liu, Y.; Zhang, H.; Xia, L.; Zhan, J.; Hu, Q. Early fibroproliferative signs on high-resolution CT are associated with mortality in COVID-19 pneumonia patients with ARDS: A retrospective study. Ther. Adv. Chronic Dis. 2021, 12, 2040622320982171. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; Li, Y.; Cao, Y.; Gu, J.; Wu, H.; et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 2021, 299, E177–E186. [Google Scholar] [CrossRef]
- Iwasaki-Hozumi, H.; Chagan-Yasutan, H.; Ashino, Y.; Hattori, T. Blood Levels of Galectin-9, an Immuno-Regulating Molecule, Reflect the Severity for the Acute and Chronic Infectious. Dis. Biomol. 2021, 11, 430. [Google Scholar] [CrossRef]
- Toniatia, P.; Pivab, S.; Cattalinid, M.; Garrafaf, E.; Regolaa, F.; Castellie, F.; Franceschinia, F.; Airòa, P.; Bazzania, C.; Beindorfi, E.-A.; et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Nicola Latronicob Autoimmun. Rev. 2020, 19, 102568. [Google Scholar] [CrossRef]
- Hermine, O.; Mariette, X.; Tharaux, P.L.; Resche-Rigon, M.; Porcher, R.; Ravaud, P. Effect of Tocilizumab vs. Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial. CORIMUNO-19 Collaborative Group. JAMA Intern. Med. 2021, 181, 32–40. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Ruano, C.; Riso, N.; Ribeiro, J.C.; Moraes-Fontes, M.F. Paradoxical pulmonary event under tocilizumab treatment for systemic sclerosis-associated usualinterstitial pneumonia. Ann. Rheum. Dis. 2020, 79, e22. [Google Scholar] [CrossRef] [Green Version]
- Robinson, B.S.; Saeedi, B.; Arthur, C.M.; Owens, J.; Naudin, C.; Ahmed, N.; Luo, L.; Jones, R.; Neish, A.; Stowell, S.R. Galectin-9 Is a Novel Regulator of Epithelial Restitution. Am. J. Pathol. 2020, 190, 1657–1666. [Google Scholar] [CrossRef]
- Arikawa, T.; Matsukawa, A.; Watanabe, K.; Sakata, K.M.; Seki, M.; Nagayama, M.; Takeshita, K.; Ito, K.; Niki, T.; Oomizu, S.; et al. Galectin-9 accelerates transforming growth factor beta3-induced differentiation of human mesenchymal stem cells to chondrocytes. Bone 2009, 44, 849–857. [Google Scholar] [CrossRef]
- Hughes, R.C. Galectins as modulators of cell adhesion. Biochimie 2001, 83, 667–676. [Google Scholar] [CrossRef]
- Calkovska, A.; Kolomaznik, M.; Calkovsky, V. Alveolar type II cells and pulmonary surfactant in COVID-19 era. Physiol. Res. 2021, 70, S195–S208. [Google Scholar] [CrossRef]
- Dapat, I.C.; Pascapurnama, D.N.; Iwasaki, H.; Labayo, H.K.; Chagan-Yasutan, H.; Egawa, S.; Hattori, T. Secretion of Galectin-9 as a DAMP during Dengue Virus Infection in THP-1. Cell Int. J. Mol. Sci. 2017, 18, 1644. [Google Scholar] [CrossRef]
- Ashino, Y.; Ying, X.; Dobbs, L.G.; Bhattacharya, J. [Ca2+]i oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L5–L13. [Google Scholar] [CrossRef] [Green Version]
- Takada, H.; Furuya, K.; Sokabe, M. Mechanosensitive ATP release from hemichannels and Ca2⁺ influx through TRPC6 accelerate wound closure in keratinocytes. J. Cell Sci. 2014, 127, 4159–4171. [Google Scholar] [CrossRef] [Green Version]
- Grygorczyk, R.; Furuya, K.; Sokabe, M. Imaging and characterization of stretch-induced ATP release from alveolar A549 cells. J. Physiol. 2013, 591, 1195–1215. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).