Spontaneous Post-COVID-19 Pneumothorax in a Patient with No Prior Respiratory Tract Pathology: A Case Report
Abstract
:1. Introduction
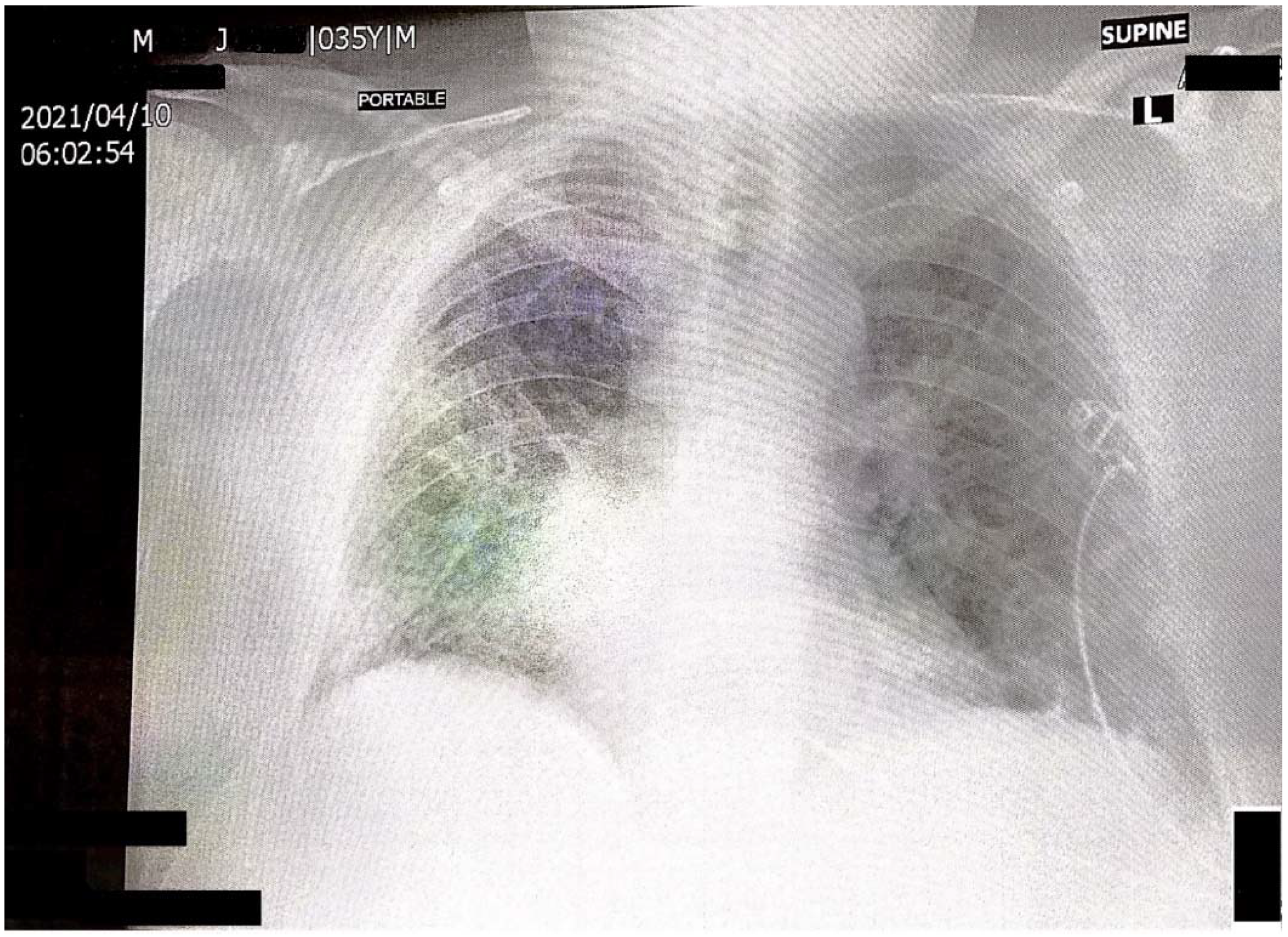
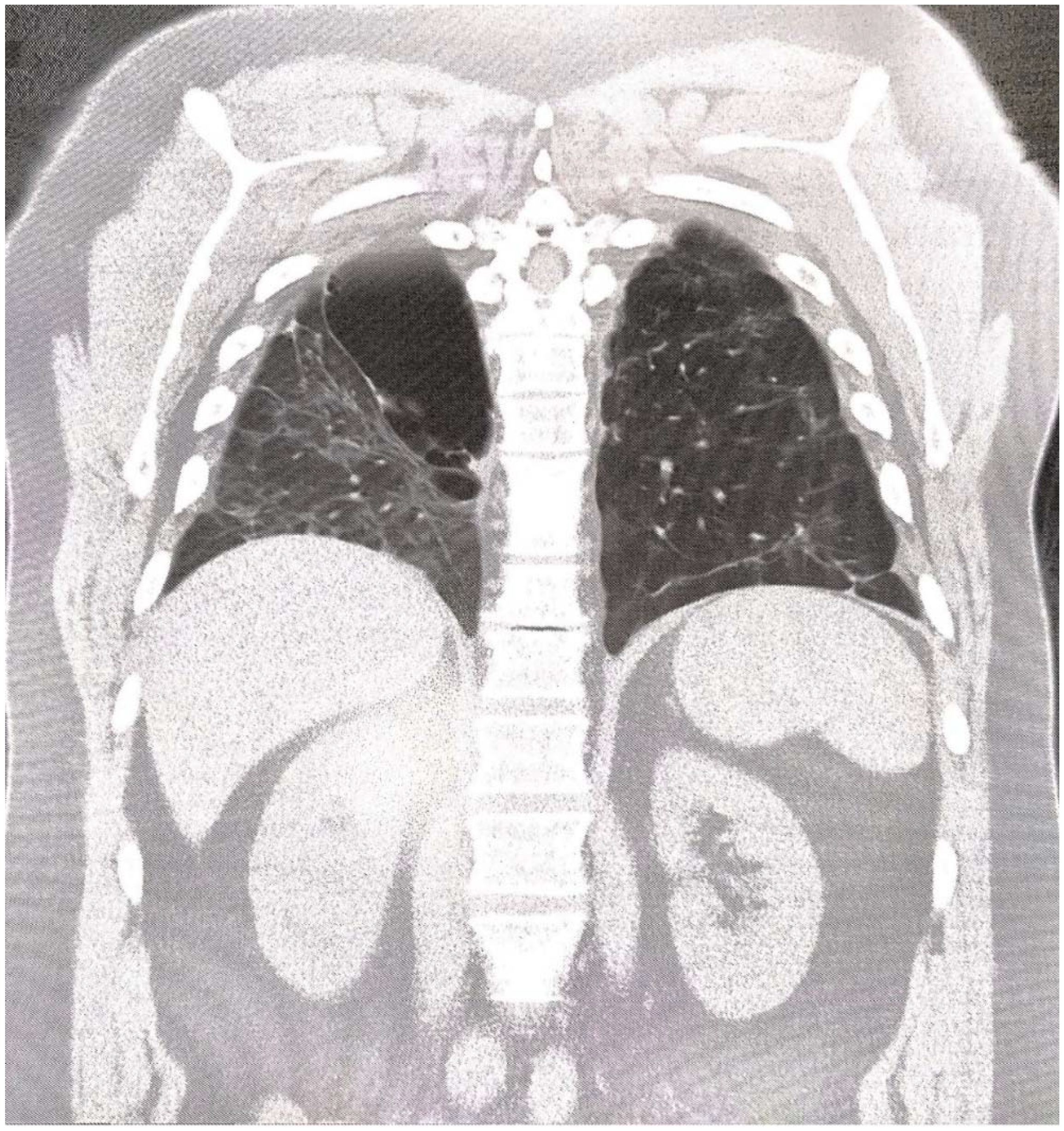
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. Novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, J.C.; Félix, V.B.; Leão, S.A.B.F.; Trindade-Filho, E.M.; Scorza, F.A. New Brazilian variant of the SARS-CoV-2 (P1/Gamma) of COVID-19 in Alagoas state. Braz. J. Infect. Dis. 2021, 25, 101588. [Google Scholar] [CrossRef]
- Da Silva, S.J.R.; Pena, L. Collapse of the public health system and the emergence of new variants during the second wave of the COVID-19 pandemic in Brazil. One Health 2021, 13, 100287. [Google Scholar] [CrossRef]
- Young, B.E.; Ong, S.W.X.; Kalimuddin, S.; Low, J.G.; Tan, S.Y.; Loh, J.; Ng, O.T.; Marimuthu, K.; Ang, L.W.; Mak, T.M.; et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA 2020, 323, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef] [Green Version]
- Ziehr, D.; Alladina, J.; Petri, C.A.; Maley, J.H.; Moskowitz, A.; Medoff, B.D.; Hibbert, K.A.; Thompson, B.T.; Hardin, C.C. Pathophysiology of mechanically ventilated patients with COVID 19 a Cohort study. Am. J. Respir. Crit. Care Med. 2020, 201, 1560–1564. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Quincho-Lopez, A.; Quincho-Lopez, D.L.; Hurtado-Medina, F.D. Case Report: Pneumothorax and Pneumomediastinum as Uncommon Complications of COVID-19 Pneumonia-Literature Review. Am. J. Trop. Med. Hyg. 2020, 103, 1170–1176. [Google Scholar] [CrossRef]
- Martinelli, A.W.; Ingle, T.; Newman, J.; Nadeem, I.; Jackson, K.; Lane, N.D.; Melhorn, J.; Davies, H.E.; Rostron, A.J.; Adeni, A.; et al. COVID-19 and pneumothorax: A multicentre retrospective case series. Eur. Respir. J. 2020, 56, 2002697. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Belletti, A.; Palumbo, D.; Zangrillo, A.; Fominskiy, E.V.; Franchini, S.; Dell’Acqua, A.; Marinosci, A.; Monti, G.; Vitali, G.; Colombo, S.; et al. Predictors of Pneumothorax/Pneumomediastinum in Mechanically Ventilated COVID-19 Patients. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3642–3651. [Google Scholar] [CrossRef] [PubMed]
- Schiller, M.; Wunsch, A.; Fisahn, J.; Gschwendtner, A.; Huebner, U.; Kick, W. Pneumothorax with bullous lesions as a late complication of COVID-19 pneumonia—A report on two clinical cases. J. Emerg. Med. 2021, 61, 581–586. [Google Scholar] [CrossRef]
- Sun, R.; Liu, H.; Sang, X. Mediastinal emphysema, giant bulla and pneumothorax developed during the course of COVID-19 pneumonia. Korean J. Radiol. 2020, 21, 541–544. [Google Scholar] [CrossRef] [Green Version]
- Tucker, L.; Patel, S.; Vatsis, C.; Poma, A.; Ammar, A.; Nasser, W.; Mukkera, S.; Vo, M.; Khan, R.; Carlan, S. Pneumothorax and Pneumomediastinum Secondary to COVID-19 Disease Unrelated to Mechanical Ventilation. Case Rep. Crit. Care 2020, 2020, 6655428. [Google Scholar] [CrossRef] [PubMed]
- Elhakim, T.S.; Abdul, H.S.; Pelaez Romero, C.; Rodriguez-Fuentes, Y. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19 pneumonia: A rare case and literature review. BMJ Case Rep. 2020, 13, e239489. [Google Scholar] [CrossRef]
- Noppen, M. Spontaneos pneumothorax. Epiemiology, pathophysiology and cause. Eur. Respir. Rev. 2010, 19, 217–219. [Google Scholar] [CrossRef] [Green Version]
- Schramel, F.; Meyer, C.J.; Postmus, P.E. Inflammation as a cause of spontaneous pneumothorax and empyema like changes. Results of bronchoalveolar lavage. Eur. Respir. Rev. 1995, 8, 397s. [Google Scholar]
- Connors, J.M.; Levy, J.H. Covid 19 and its implication for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Poissy, J.; Goutay, J.; Caplan, M.; Parmentier-Decrucq, E.; Duburcq, T.; Lassalle, F.; Jeanpierre, E.; Rauch, A.; Labreuche, J.; Susen, S.; et al. Pulmonary Embolism in Patients with COVID-19: Awareness of an Increased Prevalence. Circulation 2020, 142, 184–186. [Google Scholar] [CrossRef] [PubMed]
| Laboratory Evaluation | Patient’s Result | Normal Range |
|---|---|---|
| CRP | 106 mg/L | <5 mg/L |
| PCT | 0.08 ng/mL | <0.5 ng/mL |
| Crea | 0.8 mg/dL | 0.7–1.2 mg/dL |
| LDH | 367 U/L | <250 U/L |
| d-dimer | 310 ng/mL | <250 ng/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorov, V.; Grigorov, M.; Grigorov, E.; Nocheva, H. Spontaneous Post-COVID-19 Pneumothorax in a Patient with No Prior Respiratory Tract Pathology: A Case Report. Reports 2022, 5, 11. https://doi.org/10.3390/reports5010011
Grigorov V, Grigorov M, Grigorov E, Nocheva H. Spontaneous Post-COVID-19 Pneumothorax in a Patient with No Prior Respiratory Tract Pathology: A Case Report. Reports. 2022; 5(1):11. https://doi.org/10.3390/reports5010011
Chicago/Turabian StyleGrigorov, Vladimir, Mladen Grigorov, Evgeni Grigorov, and Hristina Nocheva. 2022. "Spontaneous Post-COVID-19 Pneumothorax in a Patient with No Prior Respiratory Tract Pathology: A Case Report" Reports 5, no. 1: 11. https://doi.org/10.3390/reports5010011
APA StyleGrigorov, V., Grigorov, M., Grigorov, E., & Nocheva, H. (2022). Spontaneous Post-COVID-19 Pneumothorax in a Patient with No Prior Respiratory Tract Pathology: A Case Report. Reports, 5(1), 11. https://doi.org/10.3390/reports5010011






