Abstract
Tarlov Cysts is a pathological condition, with low incidence, characterized by a painful component with a strong impact on quality of life. The therapeutic options are surgery or analgesics and/or anti-inflammatory medications; however, the condition is still without resolution. Herein, we are reporting a case of a woman who expressly followed a low-calorie ketogenic diet program for 3 months. In addition to the change in diet, an appreciable decrease of weight (−5 kg) and body circumferences were recorded; there was also a marked improvement (evident from the questionnaires administered) in the quality of life, of sleep, and in the perception of pain. It is interesting to note how, in conjunction with the Christmas period, upon leaving the ketogenic regime, there was a recurrence of symptoms, confirming the beneficial effect of the low-caloric ketogenic diet at least on the management of pain and, very likely, on inflammation.
1. Introduction
Tarlov Cysts (TCs) are benign, extradural spinal cystic lesions of the nerve root sheath at the dorsal root ganglion, filled by cerebrospinal fluid (CSF), and are usually developed between the endoneurium and perineurium in the lumbosacral spine. TCs etiology is unknown, however, and both a congenital and secondary to trauma or inflammation origin were postulated. The anatomopathological hallmark is that the nerve root is embedded into the membranous connective tissue cyst wall, or its cavity, and often has micro-connections to the subarachnoid space, with liquid circulation between the cyst and spinal CSF. Some of these saccular lesions have a ball-valve mechanism that leads to the expansion of pressure and volume of the cysts. Due to the increase of volume, TCs can compress adjacent nerve fibers, causing pain and other symptoms. Since there are frequently multiple cysts, the clinical presentation can be complex with a multi-nerve compressive symptomatology; in most cases, TCs are fully asymptomatic and their finding is usually accidental when performing lumbosacral spine imaging or postmortem autopsies. In some cases, cysts erode bone structures or induce a caudal syndrome. Conservative treatment by analgesics, anti-inflammatory drugs, and physical therapy is the first therapeutic choice, while the indication of a surgical procedure for symptomatic TCs is still disputed, but it should be attempted to reduce pain, although there is a risk of cyst recurrence [1]. There is no specific, accepted therapy for individuals with symptomatic TCs. Herein, we present a case of a woman who underwent to ketogenic diet and ameliorate chronic pain symptoms linked to this condition.
2. Case Presentation Section
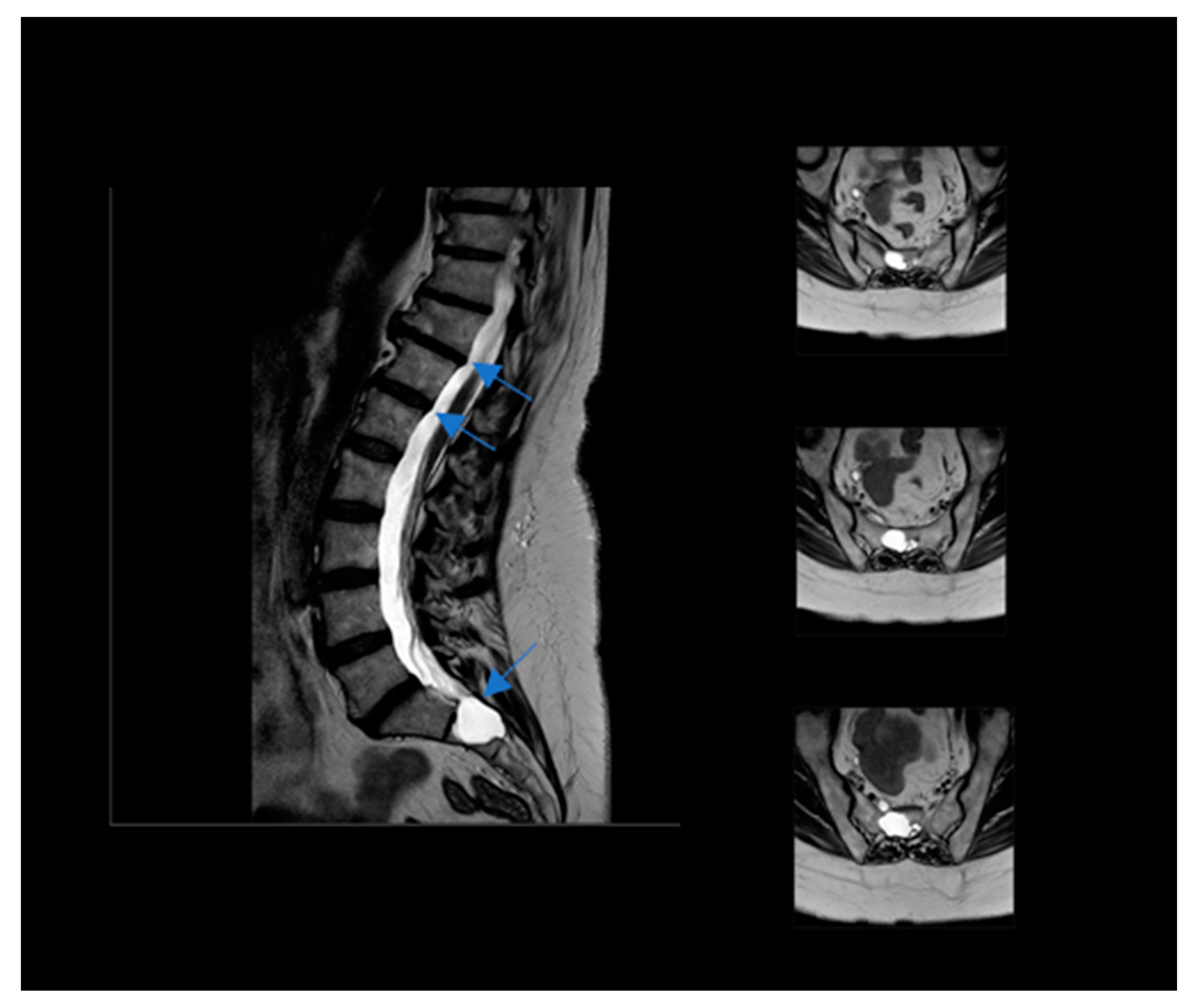
A 56-year-old woman was self-referred to us to start a ketogenic diet for the purpose of weight loss, but she also heard about a possible positive effect on pain related to the diet. At the first examination, her BMI was 35.6 (height 158 cm/weight 89.1 kg). She had an history of major depressive disorder, although it was not pharmacologically treated because of the low compliance to the treatments and the limited benefit she experienced by drugs. Moreover, she was affected by a severe dorsal in the tract between D8 and D11 and lower limb neuropathy due to the presence of multiple bilateral TCs localized in the tract between L4 and S2, with evidence of sacral bone erosive phenomena (see Figure 1). Neurological symptoms included: tingling, numbness, pins and needles, stabbing and shooting pain, stress incontinence, lack of coordination, sleep problems due to night-time pain, muscle weakness, difficulty in limb movements, and episodes of falling. A recent neurological examination evidenced antalgic posture, bilateral steppage gait, and reduced reflexes from the patellar down; Lasègue’s test was bilaterally positive at 45°. Upper motor signs, as clonus and Babinski sign, were absent. Under a therapeutic point of view, the patient was at the beginning addressed to physiotherapy, but with very limited benefits. Moreover, because of organizational issues, the patient was unable to follow treatments regularly, resulting in a low compliance to the treatments and the choice to stop them. In a neurosurgery consultation, she did not receive the indication to undergo the surgical procedure. Therefore, the only available treatment was a pharmacological one. Gabapentin up to 2400 mg/day and pregabalin up to 450 mg/day were ineffective on the symptoms. Duloxetine and amitriptyline were not tolerated by the patient. Additionally, different types of opioids gave her different side effects, inducing their discontinuation. The daily use of indomethacin lead to gastritis and she stopped the treatment. At the time of consultation, she regularly consumed 200 mg/day of celecoxib to reduce the pain, and, on demand, acetaminophen alone or in combination with codeine. Moreover, she was on the waiting list for a further neurosurgical consultation.
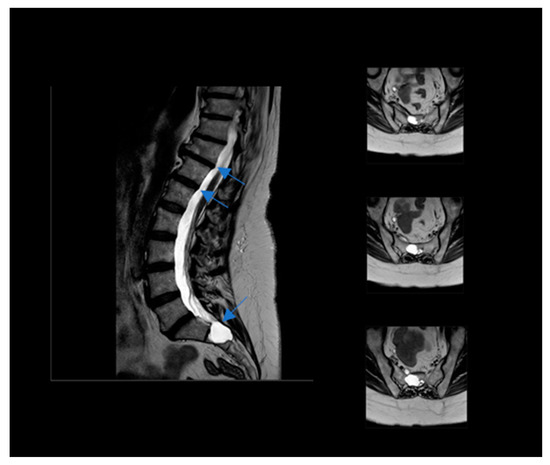
Figure 1.
Nuclear Magnetic Resonance of the patient Tarlov’s Cyst indicated by blue arrow.
2.1. Data Collection and Questionnaires
A few days before our first evaluation, she completed a self-reported Hamilton Depression Rating Scale (HDRS) [2], Western Ontario and McMaster Universities Index (WOMAC) [3], Sleep Quality Scale (SQS) [4], RAND-36 [5], and VAS [6]; the questionnaires were administered every month.
2.2. Bioimpedance Analysis
The bioimpedance analyzer used for bioimpedance analysis (BIA) was a BIA 101 Anniversary, Akern, Florence, Italy [7,8], and was used with alternating current at a frequency of 50 kHz. The accuracy of the BIA instrument was validated before each test session, following the manufacturer’s instructions. Measurements were made on a medical bed isolated from electrical conductors. Briefly, the patient was in the supine position with legs (45° compared to the median line of the body) and arms (30° from the trunk) abducted. Alcohol was used to clean the skin and two electrodes were placed on the right hand and two on the right foot. Resistance (R) and reactance (Xc) parameters were divided by standing body height in meters. Phase angle (PhA)was calculated as the arctangent of Xc/R × 180°/π. Body fat percentage was calculated via Bodygram™ software (Akern, Florence, Italy).
2.3. Ketogenic Program
The subject had asked to follow a low-calorie ketogenic diet (KD) program, which was largely considered favorable by us; in fact, a low-caloric ketogenic nutritional plan is used not only for the purpose of slimming, but also to support the therapy of various pathologies such as neurological ones [9]; in particular, it has recently been proposed that KDs can help to improve migraine [10], lipedema [11], polycystic ovary [12], sarcopenia [13], and even some cancers [14]. The program generally provides a very limited intake of carbohydrates, less than 30 g per day or less than 5% of total calories. We choose a caloric deficit of 200–250 kcal that comes from a 14-day food diary where we applied a similar protocol in previous papers [11] to better adapt the nutritional program; the nutritional program was set to have a carbohydrate amount that was less than 20 g per day, where the ratio between proteins and fats is between 1:1 and 1:2, and we chose a moderate-high protein intake to preserve muscle mass as much as possible. We did not exclude any kind of food, so milk and dairy products, meat, and even gluten are included (the latter in any case in a reduced amount being linked to cereals or keto-specific food); we introduced the following as nutritional supplements: omega3 fish oil (4 g per day of supplement, giving around 2.4 g of DHA + EPA), ascorbic acid (1 g per day into 2 times), and vitamin D (2000 IU per day) as we noted a deficiency. All supplements are from 4+ Nutrition, Padua—Italy. Table 1 details the daily plan.

Table 1.
Scheme of KD plan.
The patient was instructed on how to prepare food, but also, in this case, with few limitations; for example, even fried foods were not forbidden. The caloric intake was set at 1200 kcal, where 25–33% of that was proteins, 58–65% was fats, and 5% or less was carbohydrates. Protein sources included every kind of meat (veal, beef, pork, chicken, turkey), fish and seafood, and eggs, particularly egg white and milk derivates. We suggested to limit the intake of tomatoes, eggplant, and sweet peppers due to the not-negligible carbohydrate amount; beyond that, we strongly suggested to choose seasonal vegetables. As expected, we completely prohibited tubers, squash, legumes, and any kind of cereal or similar grain (excepting the very small amount at breakfast). The main source of fat was EVO (butter or mayonnaise was permitted as a substitute) and nuts or seeds. The patient had continuous support via text messaging via mobile phone, and we saw her every month in our facility. Few dietary supplements have been used in a targeted way as too often they are used improperly and/or are not scientifically justified, which can sometimes lead to important side effects [15]. In particular, the omega3 fish oil, in our opinion, has a beneficial action, given its proven anti-inflammatory action [16].
2.4. Ketosis Assessment and Unwanted Effects of the KD
Ketosis status was checked weekly in urine (self-administered) via Bayer Keto-diastix (Bayer—Germany) and monthly in blood via a Wellion Galileo Glu/Ket blood glucose meter (Wellion—Austria). With the latter, we recorded a value that was always more than 0.7–0.9 mmol/L at every test; only after Christmas time was it at 0.1 mmol/L. We checked the usual side effects reported following a ketogenic diet: frequent urination, fatigue, hunger, confusion, anxiety and/or irritability, and sweating. Sometimes she reported: constipation, difficulty in focusing, and muscle soreness. However, none of these occurred frequently.
2.5. Physical Exercise
After a careful functional evaluation of the range of motion and proprioceptive capacity, a training program was started. The goal of the first phase was to improve aerobic capacity, with a particular focus on proprioception and simple motor screens, where the loads were extremely limited. After the first five weeks, the program increased to a greater intensity, both from the aerobic and strength point of view. For the entire duration of the program, fundamental importance was given to stretching and the ability to coordinate movements. An example of the training patterns, can be found in the Supplementary Materials of the first mesocycle (Tables S1 and S2) and second mesocycle (Tables S3 and S4).
3. Results
In terms of weight, the result is not striking: there was a loss of 3.1 kg. It is noted, however, that a clear improvement in body composition, as can be seen from the percentage of fat and body circumferences (See Table 2), occurred; this is certainly due to the nutritional program rich in proteins, but, above all, to the effectiveness of the training, which can be seen is in positive progression (just consider the second mesocycle which certainly has a greater intensity and level of difficulty than the first, but was well-tolerated by the subject).

Table 2.
Anthropometric parameters.
This result is also confirmed by the decrease in body circumferences, which would suggest a greater decrease in weight, compensated by an improvement in the muscular condition, despite a low-calorie program. The most important result, in our opinion, comes from the questionnaires that show a clear improvement. In fact, at the third administration, there is an improvement in sleep quality by 57%, the WOMAC and VAS scales relating to pain are improved by 64% and 54%, respectively, the HDRS scale with a score of 8 returns to normal, as illustrated in Table 3.

Table 3.
Pain assessment and quality of life.
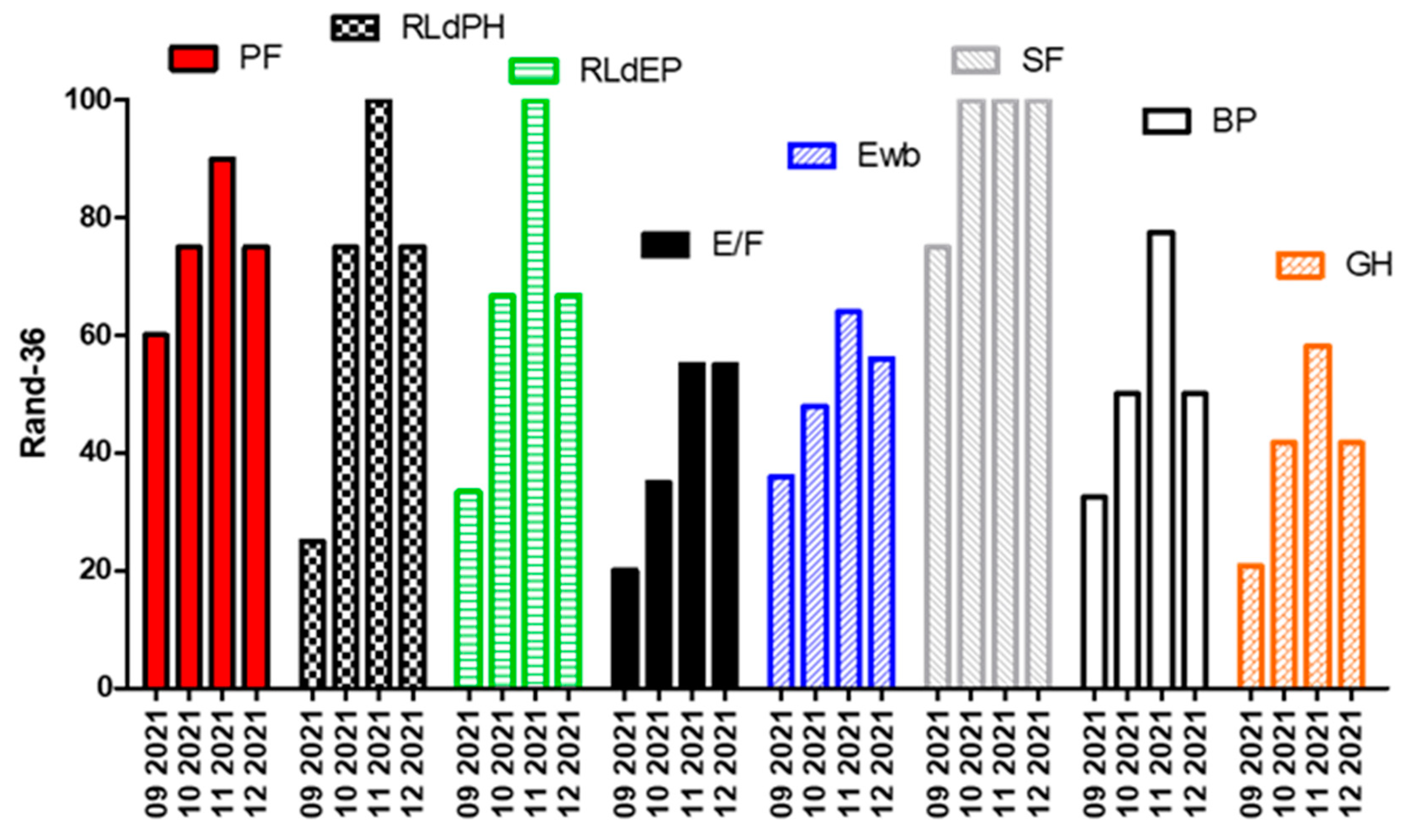
This means a significant impact on the quality of life in general (see result from RAND-36 in Figure 2). It is very important to note that the fourth administration, operated on 27 December and, therefore, happening after an intake of carbohydrates and thus stopping the state of ketosis, shows a rapid deterioration in the general conditions.
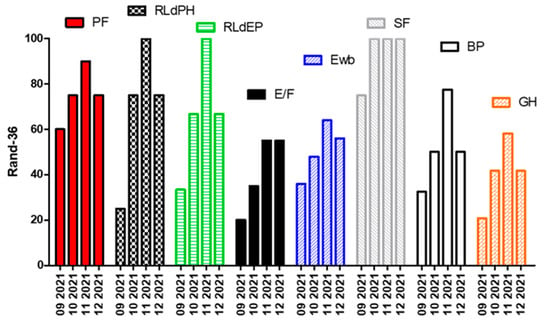
Figure 2.
Rand-36 questionnair results: PF, Physical Functioning; RLdPH, Role Limitations due to Physical Health; RLdEP, Role Limitations due to Emotional problems; E/F, Energy/Fatigue; Ewb, Emotional well-being; SF, Social Functioning; BP, Body Pain; GH, General Health.
4. Discussion
Overall, the ketogenic diet was well-tolerated and the classic side effects were practically absent. Although the period is limited to three months, the result in terms of improved quality of life is important; this could be due to the anti-inflammatory and pain-modulating effect of the KD. The case we report highlights a beneficial effect of the KD on a particular pain condition, being the TCs. It is well established that KDs can be useful for several neurological conditions [9]; however, the pathophysiological basis of pain is fully different, since in migraine it has been proposed that pain comes from the sterile vascular inflammation induced by different neural and metabolic triggers [17], whereas in TCs, pain seems to be due to a mechanical compression of nerve roots, a mechanism that appears to not be modifiable by ketones. However, it has been observed that a KD can exert an anti-inflammatory action that can explain the observed outcome [11]. A further ancillary mechanism of action calls into cause two observed changes in the clinical phenotype of the patient’s syndrome. In fact, she experienced an improvement of sleep and mood. There is a bidirectional relationship between chronic pain and sleep disorders [18], and it has been observed that there is an ameliorative effect of a KD on sleep disorders [19]. We can hypothesize that the KD-induced sleep improvement played an additive role on the general benefit experienced by our patient. Likewise, mood disorders and pain conditions are also in a very close relationship [20], and KDs can improve the emotional disorder by different mechanisms of action [21]. Thus, for the KD induced improvement of depressive symptoms, we can also suppose an additional benefit on the patient’s global condition. Tempting to speculate about an alternative way to explain the unexpected effect we observe, we can hypothesize that the KD can lead to a modification in cortical pain processing of the patient. Consistent with the literature [22,23,24], a KD can influence the cognitive cortical response to painful stimulation. Besides that, thanks to the supervised and progressive practice of a physical activity that includes, in particular, proprioceptive aspects, joint mobilization, and stretching, as well as a component of strength, there is an improvement in daily functions; for example, the subject reports much more ease in doing housework, which contributes to the overall positive result. The BIA data and the measurement of body circumferences show an improvement in muscle mass, which is very important in the general management of the condition. It is interesting to note that concomitant with the Christmas period, when the patient, for obvious reasons, did not follow the ketogenic program for three days (ketonemia at 0.1 mmol/L), recorded a recurrence of symptoms, which decreased when returning to ketosis (ketonemia 0.7 mmol/L)
5. Conclusions
The effect of a KD should be investigated more in depth while monitoring classic inflammatory markers, such as CRP or interleukins, or miRNAs linked to the inflammatory state [25]. Similarly, the application of the KD should be evaluated on a larger sample, to evaluate not only any side effects, but also compliance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/reports5020012/s1. Table S1. First mesocycle day 1, Table S2. First mesocycle day 2, Table S3. Second mesocycle day 1, Table S4. Second mesocycle day 2.
Author Contributions
R.C. and E.C. drafted the main text; C.D.L. and R.C. reviewed the literature and contributed to the concept of the manuscript; C.D.L. and G.D.S. wrote the neurological part; M.I. supervised and administered the physical activity; R.C. supervised and administered the nutritional plan. M.C.C. and L.G. facilitate the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Ethic Committe Calabria Centro 120/2018, approval date 17 May 2018.
Informed Consent Statement
Informed consent was obtained from the subject involved in the study.
Data Availability Statement
Data is contained within the article and in the supplementary material. The data presented in this study are available in our hardware.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lucantoni, C.; Than, K.D.; Wang, A.C.; Valdivia-Valdivia, J.M.; Maher, C.O.; La Marca, F.; Park, P. Tarlov cysts: A controversial lesion of the sacral spine. Neurosurg. Focus 2011, 31, 14. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, M.; Martinez, J.H.; Young, D.; Chelminski, I.; Dalrymple, K. Severity classification on the Hamilton Depression Rating Scale. J. Affect. Disord. 2013, 150, 384–388. [Google Scholar] [CrossRef] [PubMed]
- American College of Rheumatology. Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)—General Description; ACR: Atlanta, GA, USA, 2012. [Google Scholar]
- Yi, H.; Shin, K.; Shin, C. Development of the sleep quality scale. J. Sleep Res. 2006, 15, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Sherbourne, C.D.; Mazel, R.M. The RAND 36-Item Health Survey 1.0. Health Econ. 1993, 2, 217–227. [Google Scholar] [CrossRef]
- Bijur, P.E.; Silver, W.; Gallagher, E.J. Reliability of the visual analog scale for measurement of acute pain. Acad. Emerg. Med. 2001, 8, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef] [Green Version]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Composition of the ESPEN Working Group. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Ballerini, G.; Barbanti, P.; Bernardini, A.; D’Arrigo, G.; Egeo, G.; Frediani, F.; Garbo, R.; Pierangeli, G.; Prudenzano, M.P.; et al. Applications of Ketogenic Diets in Patients with Headache: Clinical Recommendations. Nutrients 2021, 13, 2307. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, M.C.; Wood, S.; Bagary, M.; Balabanov, A.; Bercovici, E.; Brown, M.G.; Devinsky, O.; Di Lorenzo, C.; Doherty, C.P.; Felton, E.; et al. International Recommendations for the Management of Adults Treated with Ketogenic Diet Therapies. Neurol. Clin. Pract. 2021, 11, 385–397. [Google Scholar] [CrossRef]
- Cannataro, R.; Michelini, S.; Ricolfi, L.; Caroleo, M.C.; Gallelli, L.; De Sarro, G.; Onorato, A.; Cione, E. Management of Lipedema with Ketogenic Diet: 22-Month Follow-Up. Life 2021, 11, 1402. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Giacona, M.C.; Bianco, A.; Caprio, M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J. Transl. Med. 2020, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, R.; Carbone, L.; Petro, J.L.; Cione, E.; Vargas, S.; Angulo, H.; Forero, D.A.; Odriozola-Martínez, A.; Kreider, R.B.; Bonilla, D.A. Sarcopenia: Etiology, Nutritional Approaches, and miRNAs. Int. J. Mol. Sci. 2021, 22, 9724. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.L.; Mattingly, S.; Schirrmacher, R.; Sawyer, M.B.; Fine, E.J.; Prado, C.M. A Nutritional Perspective of Ketogenic Diet in Cancer: A Narrative Review. J. Acad. Nutr. Diet. 2018, 118, 668–688. [Google Scholar] [CrossRef]
- Russo, R.; Gallelli, L.; Cannataro, R.; Perri, M.; Calignano, A.; Citraro, R.; Russo, E.; Gareri, P.; Corsonello, A.; De Sarro, G. When Nutraceuticals Reinforce Drugs Side Effects: A Case Report. Curr. Drug Saf. 2016, 11, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Layé, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Di Lorenzo, C.; Serrao, M.; Parisi, V.; Schoenen, J.; Pierelli, F. Pathophysiological targets for non-pharmacological treatment of migraine. Cephalalgia 2016, 36, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Laksono, I.; Selvanathan, J.; Saripella, A.; Nagappa, M.; Pham, C.; Englesakis, M.; Peng, P.; Morin, C.M.; Chung, F. Prevalence of sleep disturbances in patients with chronic non-cancer pain: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 57, 101467. [Google Scholar] [CrossRef]
- Barrea, L.; Pugliese, G.; Frias-Toral, E.; Napolitano, B.; Laudisio, D.; Aprano, S.; Ceriani, F.; Savastano, S.; Colao, A.; Muscogiuri, G. Is there a relationship between the ketogenic diet and sleep disorders? Int. J. Food Sci. Nutr. 2021, 1–11. [Google Scholar] [CrossRef]
- Campos, A.C.P.; Antunes, G.F.; Matsumoto, M.; Pagano, R.L.; Martinez, R.C.R. Neuroinflammation, Pain and Depression: An Overview of the Main Findings. Front. Psychol. 2020, 11, 1825. [Google Scholar] [CrossRef]
- Brietzke, E.; Mansur, R.B.; Subramaniapillai, M.; Balanzá-Martínez, V.; Vinberg, M.; González-Pinto, A.; Rosenblat, J.D.; Ho, R.; McIntyre, R.S. Ketogenic diet as a metabolic therapy for mood disorders: Evidence and developments. Neurosci. Biobehav. Rev. 2018, 94, 11–16. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Coppola, G.; Bracaglia, M.; Di Lenola, D.; Sirianni, G.; Rossi, P.; Di Lorenzo, G.; Parisi, V.; Serrao, M.; Cervenka, M.C.; et al. A ketogenic diet normalizes interictal cortical but not subcortical responsivity in migraineurs. BMC Neurol. 2019, 19, 136. [Google Scholar] [CrossRef] [PubMed]
- Carito, V.; Pingitore, A.; Cione, E.; Perrotta, I.; Mancuso, D.; Russo, A.; Genchi, G.; Caroleo, M.C. Localization of nerve growth factor (NGF) receptors in the mitochondrial compartment: Characterization and putative role. Biochim. Biophys. Acta. 2012, 1820, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, R.; Di Maio, L.; Malorgio, A.; Levi Micheli, M.; Cione, E. Spondyloarthritis and Strength Training: A 4-Year Report. J. Funct. Morphol. Kinesiol. 2021, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, R.; Perri, M.; Gallelli, L.; Caroleo, M.C.; De Sarro, G.; Cione, E. Ketogenic Diet Acts on Body Remodeling and MicroRNAs Expression Profile. MicroRNA 2019, 8, 116–126. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).