Rapid Clinical and Radiological Improvement in a Patient with Severe COVID-19 Infection Treated with Convalescent Plasma
Abstract
:1. Introduction
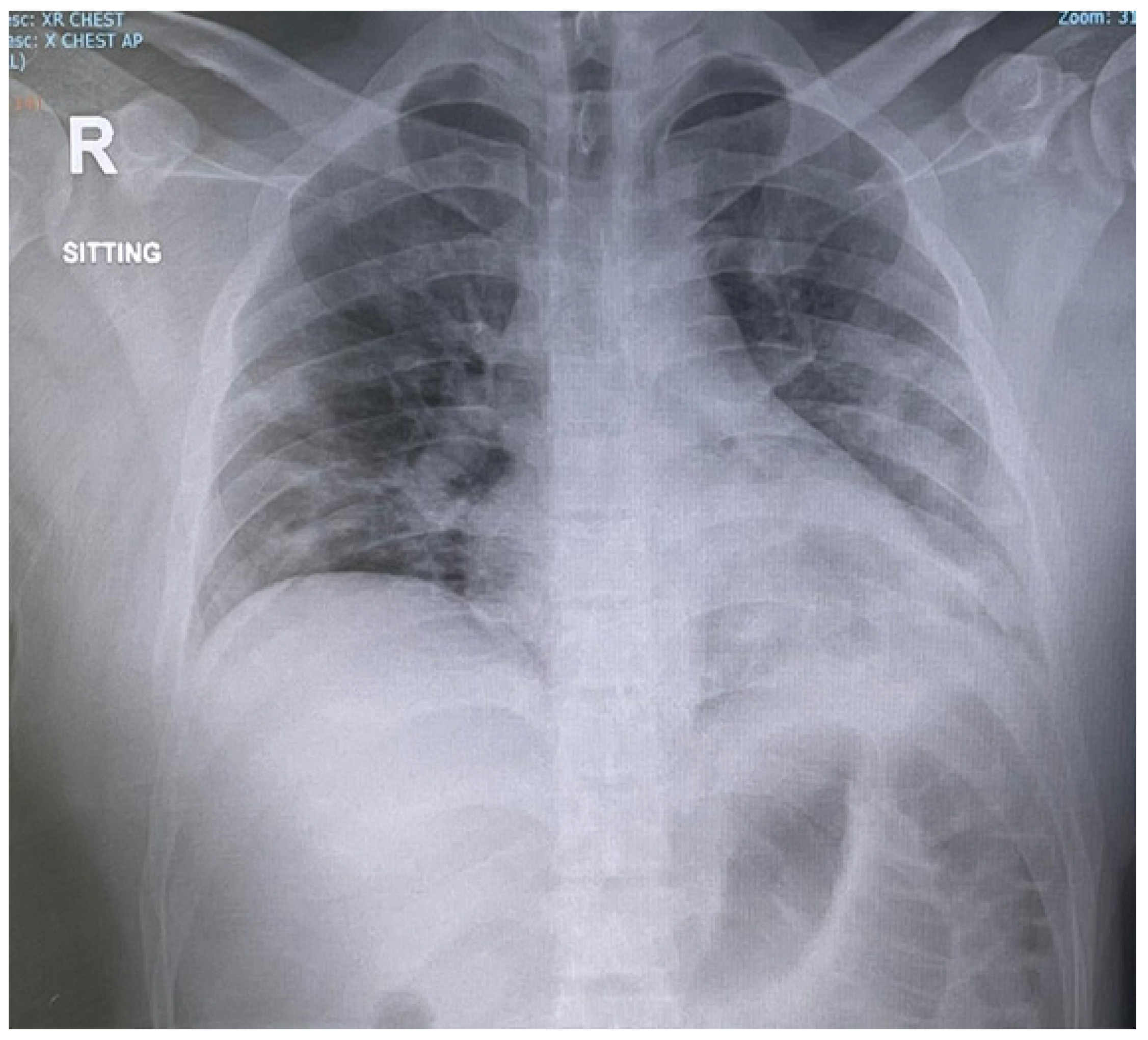
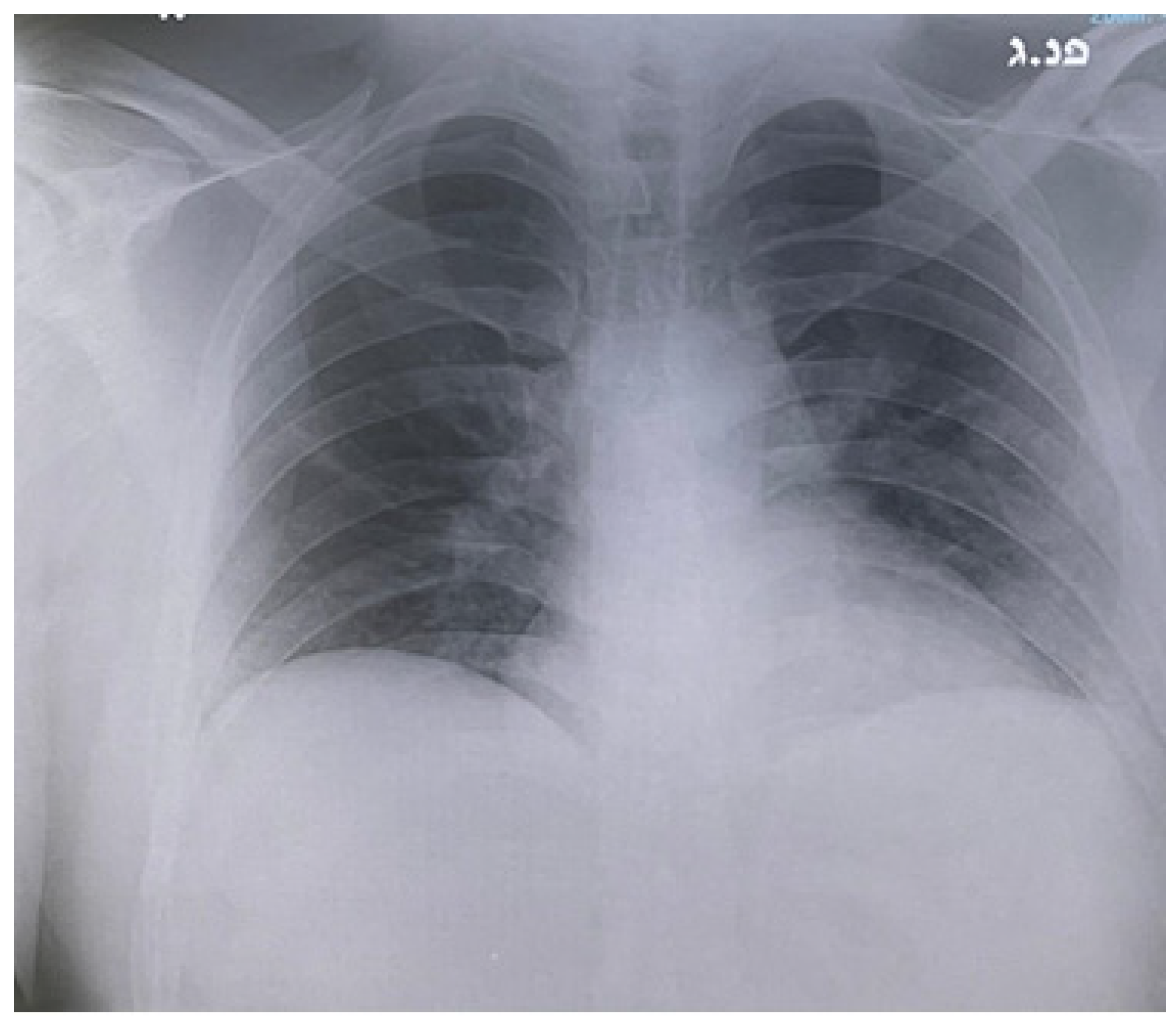
2. Case Report
3. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casadevall, A.; Scharff, M.D. Return to the Past: The Case for Antibody-Based Therapies in Infectious Diseases. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1995, 21, 150–161. [Google Scholar] [CrossRef]
- Joyner, M.J.; Senefeld, J.W.; Klassen, S.A.; Mills, J.R.; Johnson, P.W.; Theel, E.S.; Wiggins, C.C.; Bruno, K.A.; Klompas, A.M.; Lesser, E.R.; et al. Effect of Convalescent Plasma on Mortality among Hospitalized Patients with COVID-19: Initial Three-Month Experience. MedRxiv Preprint Serv. Health Sci. 2020. [Google Scholar] [CrossRef]
- Agarwal, A.; Mukherjee, A.; Kumar, G.; Chatterjee, P.; Bhatnagar, T.; Malhotra, P. Convalescent Plasma in the Management of Moderate Covid-19 in Adults in India: Open Label Phase II Multicentre Randomised Controlled Trial (PLACID Trial). BMJ Clin. Res. Ed. 2020, 371, m3939. [Google Scholar] [CrossRef]
- Joyner, M.J.; Carter, R.E.; Senefeld, J.W.; Klassen, S.A.; Mills, J.R.; Johnson, P.W.; Theel, E.S.; Wiggins, C.C.; Bruno, K.A.; Klompas, A.M.; et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N. Engl. J. Med. 2021, 384, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Simonovich, V.A.; Burgos Pratx, L.D.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vázquez, C.; Savoy, N.; Giunta, D.H.; Pérez, L.G.; Sánchez, M.D.L.; et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N. Engl. J. Med. 2021, 384, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Libster, R.; Pérez Marc, G.; Wappner, D.; Coviello, S.; Bianchi, A.; Braem, V.; Esteban, I.; Caballero, M.T.; Wood, C.; Berrueta, M.; et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N. Engl. J. Med. 2021, 384, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Salazar, E.; Christensen, P.A.; Graviss, E.A.; Nguyen, D.T.; Castillo, B.; Chen, J.; Lopez, B.V.; Eagar, T.N.; Yi, X.; Zhao, P.; et al. Significantly Decreased Mortality in a Large Cohort of Coronavirus Disease 2019 (COVID-19) Patients Transfused Early with Convalescent Plasma Containing High-Titer Anti-Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Protein IgG. Am. J. Pathol. 2021, 191, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Piechotta, V.; Chai, K.L.; Valk, S.J.; Doree, C.; Monsef, I.; Wood, E.M.; Lamikanra, A.; Kimber, C.; McQuilten, Z.; So-Osman, C.; et al. Convalescent Plasma or Hyperimmune Immunoglobulin for People with COVID-19: A Living Systematic Review. Cochrane Database Syst. Rev. 2020, 7, CD013600. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.L.; Alin, P.; Malnick, S. The Evidence for High-Titer Convalescent Plasma in SARS-CoV-2. SN Compr. Clin. Med. 2021, 3, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Cohn, C.S.; Estcourt, L.; Grossman, B.J.; Pagano, M.B.; Allen, E.S.; Bloch, E.M.; Casadevall, A.; Devine, D.V.; Dunbar, N.M.; Foroutan, F.; et al. Covid-19. Convalescent plasma: Interim recommendations from the AABB. Transfusion 2021, 61, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Gue, Y.X.; Tennyson, M.; Gao, J.; Ren, S.; Kanji, R.; Gorog, D.A. Development of a Novel Risk Score to Predict Mortality in Patients Admitted to Hospital with COVID-19. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.; Emberson, J.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowaski, A.; Elmahi, E.; et al. Dexamethasone I hospitalized patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.M.; Fatak, D.F.; Hashim, H.A.; Maulood, M.F.; Kabah, K.K.; Almusawi, Y.A.; Abdulamir, A.S. The therapeutic potential of convalescent plasma therapy on treating critically ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. Infez. Med. 2020, 28, 357–366. [Google Scholar] [PubMed]
- Salman, O.H.; Mohamed, H.S.A. Safety and efficacy of transfusing plasma from COVID-19 survivors to COVID-19 victims with severe illness. A double-blinded controlled preliminary study. Egypt J. Anesth. 2020, 36, 264–272. [Google Scholar]
- Fisher, D.L.; Pavel, A.; Malnick, S. Rapid Recovery of Taste and Smell in a Patient with SARS-CoV-2 Following Convalescent Plasma Therapy. QJM Mon. J. Assoc. Phys. 2021, hcaa341. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malnick, S.; Ghannam, W.; Sharb, A.A.; Alin, P. Rapid Clinical and Radiological Improvement in a Patient with Severe COVID-19 Infection Treated with Convalescent Plasma. Reports 2021, 4, 15. https://doi.org/10.3390/reports4020015
Malnick S, Ghannam W, Sharb AA, Alin P. Rapid Clinical and Radiological Improvement in a Patient with Severe COVID-19 Infection Treated with Convalescent Plasma. Reports. 2021; 4(2):15. https://doi.org/10.3390/reports4020015
Chicago/Turabian StyleMalnick, Stephen, Waleed Ghannam, Adam Abu Sharb, and Pavel Alin. 2021. "Rapid Clinical and Radiological Improvement in a Patient with Severe COVID-19 Infection Treated with Convalescent Plasma" Reports 4, no. 2: 15. https://doi.org/10.3390/reports4020015
APA StyleMalnick, S., Ghannam, W., Sharb, A. A., & Alin, P. (2021). Rapid Clinical and Radiological Improvement in a Patient with Severe COVID-19 Infection Treated with Convalescent Plasma. Reports, 4(2), 15. https://doi.org/10.3390/reports4020015





