Anti-Diarrheal Effects of Wood Creosote, Seirogan, in Japanese Patients
Abstract
1. Introduction
2. Case Presentation
2.1. Subjects
2.2. Treatments
2.3. Evaluation
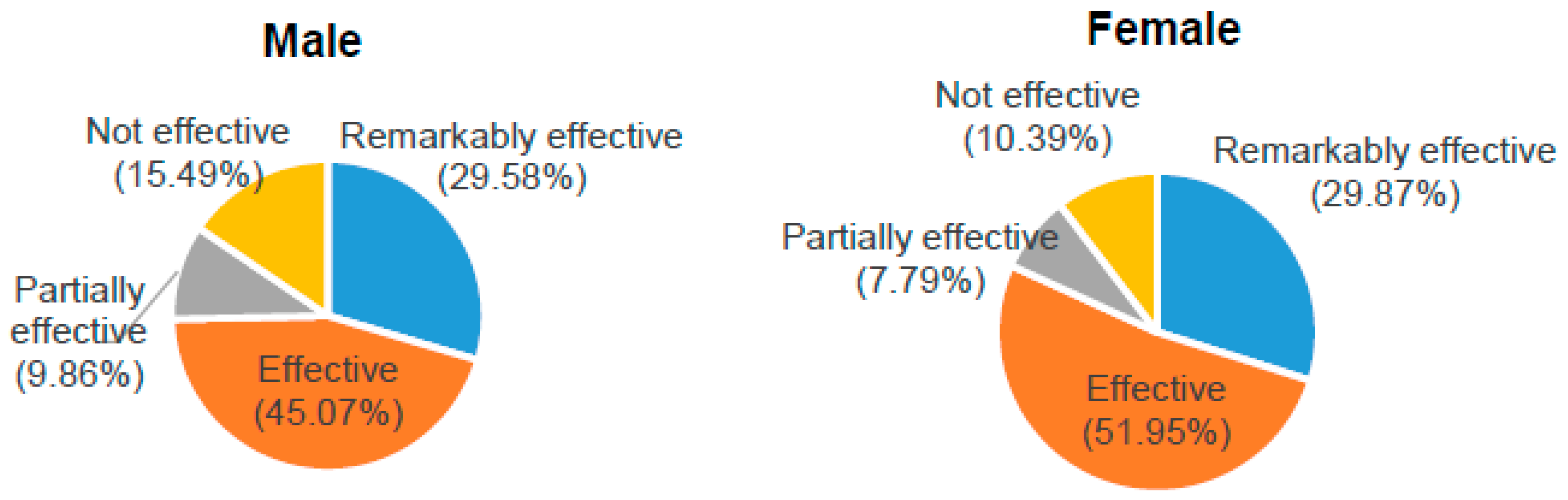
2.4. Results
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kuge, T.; Shibata, T.; Willett, M.S. Wood creosote, the principal active ingredient of seirogan, an herbal antidiarrheal medicine: A single-dose, dose-escalation safety and pharmacokinetic study. Pharmacotherapy 2003, 23, 1391–1400. [Google Scholar] [CrossRef]
- Ogata, N.; Baba, T. Analysis of beechwood creosote by gas chromatography-mass spectrometry and high-performance liquid chromatography. Res. Commun. Chem. Pathol. Pharmacol. 1989, 66, 411–423. [Google Scholar]
- Morino, H.; Ataka, K.; Ito, M.; Kuge, T. Wood creosote inhibits calcium mobilization in Guinea pig colonic smooth muscle. Biol. Pharm. Bull. 2004, 27, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Z.; Sarna, S.K. Gene therapy of Cav1.2 channel with VIP and VIP receptor agonists and antagonists: A novel approach to designing promotility and antimotility agents. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G187–G196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogata, N.; Shibata, T. Inhibition of rat intestinal Cl− secretion by 4,5-dimethylresorcinol. Pharmacology 2004, 72, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Yibcharoenporn, C.; Chusuth, P.; Jakakul, C.; Rungrotmongkol, T.; Chavasiri, W.; Muanprasat, C. Discovery of a novel chalcone derivative inhibiting CFTR chloride channel via AMPK activation and its anti-diarrheal application. J. Pharmacol. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ataka, K.; Kuge, T.; Fujino, K.; Takahashi, T.; Fujimiya, M. Wood creosote prevents CRF-induced motility via 5-HT3 receptors in proximal and 5-HT4 receptors in distal colon in rats. Auton. Neurosci. 2007, 133, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Ataka, K.; Kuge, T.; Venkova, K.; Greenwood-Van Meerveld, B. Seirogan (wood creosote) inhibits stress-induced ion secretion in rat intestinal epithelium. Dig. Dis. Sci. 2003, 48, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Kuge, T.; Shibata, T.; Willett, M.S. Multicenter, double-blind, randomized comparison of wood creosote, the principal active ingredient of Seirogan, an herbal antidiarrheal medication, and loperamide in adults with acute nonspecific diarrhea. Clin. Ther. 2004, 26, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Ogata, N.; Ataka, K.; Morino, H.; Shibata, T. Effect of wood creosote and loperamide on propulsive motility of mouse colon and small intestine. Pharmacology 1999, 59, 212–220. [Google Scholar] [CrossRef] [PubMed]
| Diagnosis. | No. of Patients | Diagnosis | No. of Patients |
|---|---|---|---|
| Acute enteritis | 71 | Colitis | 3 |
| Infectious gastroenteritis | 14 | Chronic gastroenteritis | 2 |
| Uncomplicated diarrhea | 12 | Suspicion of food poisoning | 2 |
| Acute gastroenteritis | 7 | Chronic colitis | 1 |
| Acute colitis | 6 | Chronic diarrhea | 1 |
| Chronic enteritis | 6 | Nervous gastroenteritis | 1 |
| Irritable colitis | 5 | Habitual diarrhea | 1 |
| Gastrogenic diarrhea | 3 | Catarrhal colitis | 1 |
| Nervous diarrhea | 3 | Hemorrhagic gastritis | 1 |
| Gastroenteritis | 3 | Gastric ulcer | 1 |
| Ulcerous colitis | 3 | Acute pancreatitis | 1 |
| Total | 148 |
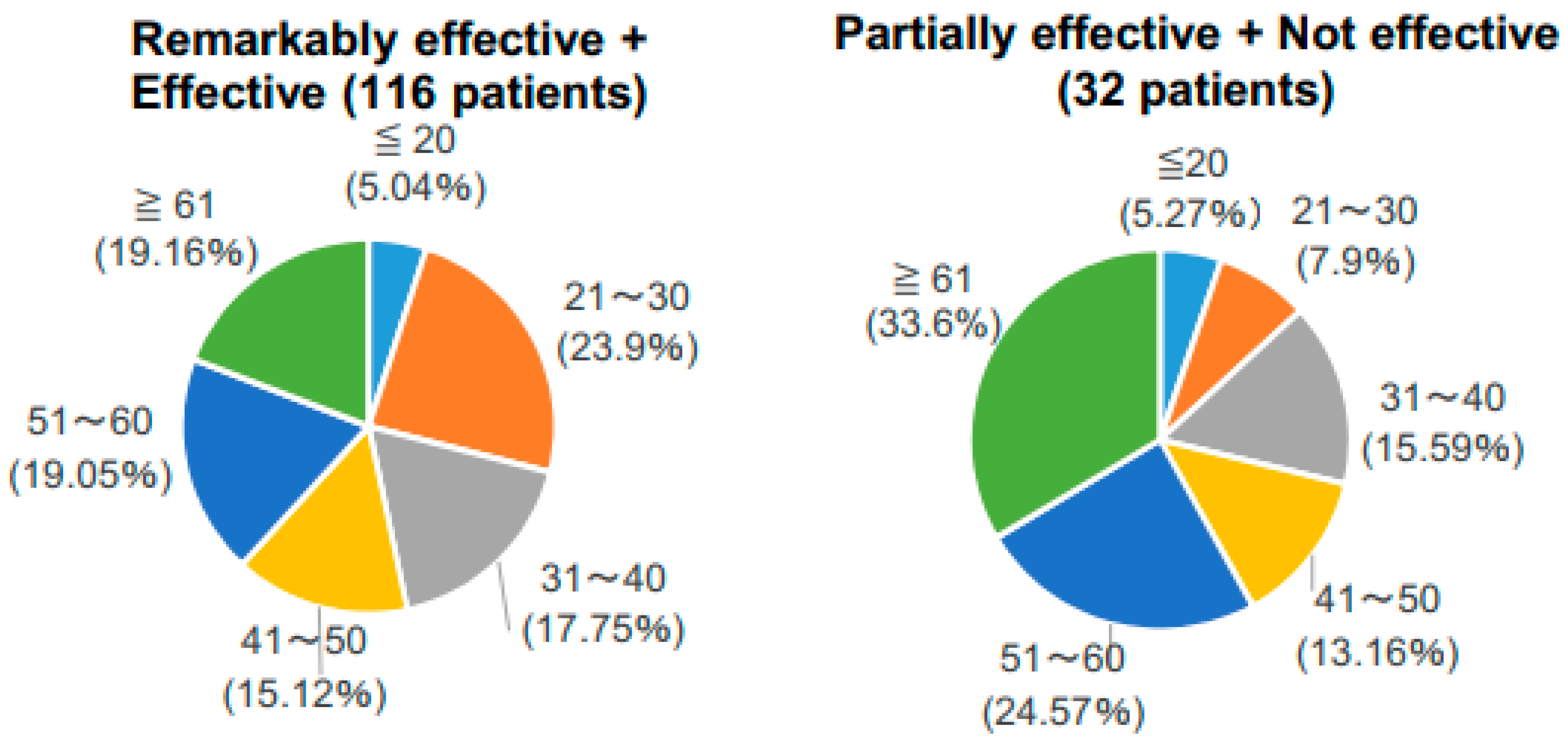
| Age (Years) | Number |
|---|---|
| ≤20 | 6 |
| 21–30 | 30 |
| 31–40 | 23 |
| 41–50 | 22 |
| 51–60 | 31 |
| ≥61 | 36 |
| Total | 148 |
| Number | % | |
|---|---|---|
| Remarkably effective | 44 | 29.73 |
| Effective | 72 | 48.65 |
| Partially effective | 13 | 8.78 |
| Not effective | 19 | 12.84 |
| Total | 148 | 100 |
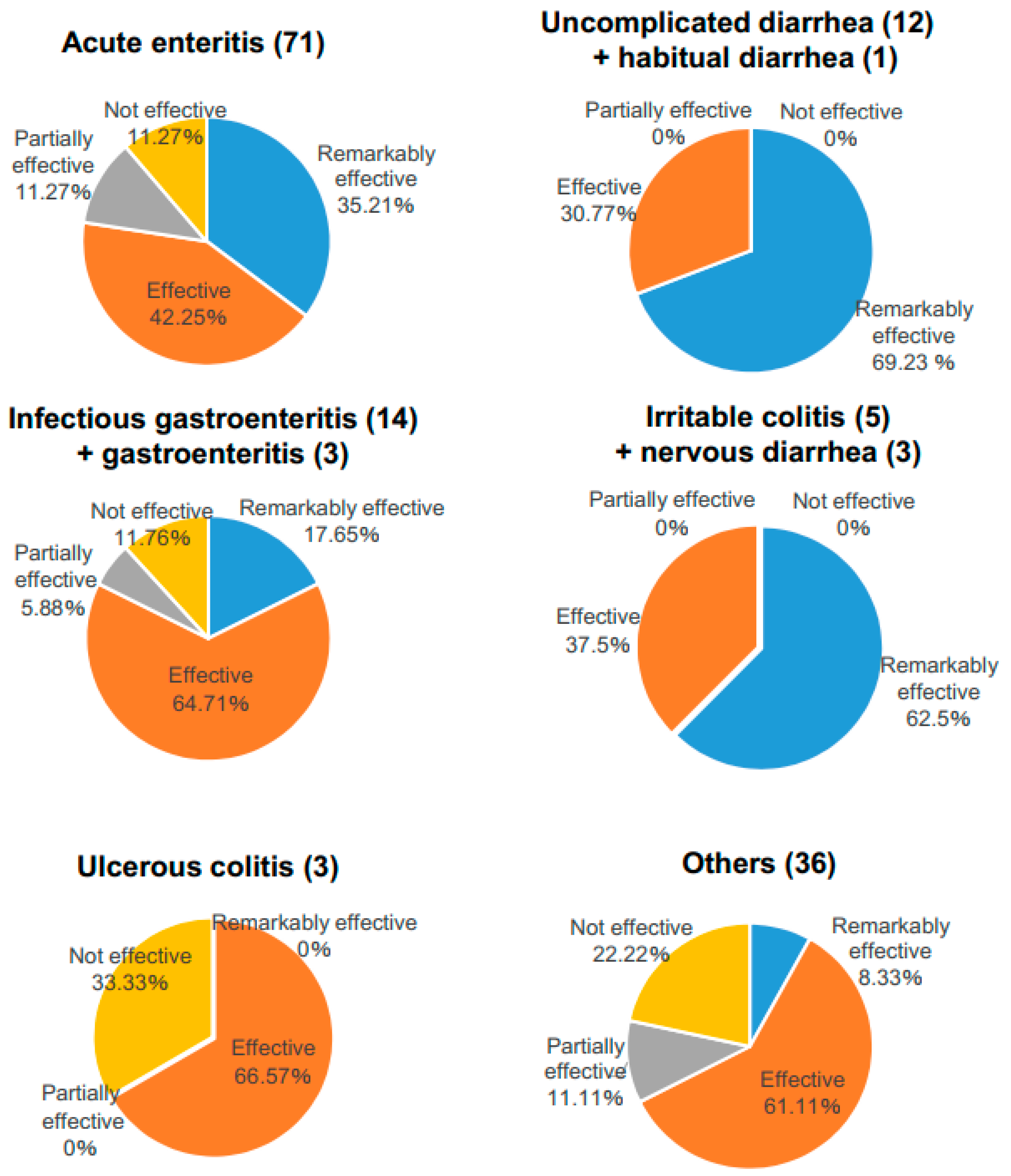
| Number of Patients | ||||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis | Remarkably Effective | (%) | Effective | (%) | Partially Effective | (%) | Not Effective | (%) |
| Acute enteritis | 25 | 35.21 | 30 | 42.25 | 8 | 11.27 | 8 | 11.27 |
| Acute gastroenteritis | 1 | 14.29 | 5 | 71.43 | 0 | 0 | 1 | 41.29 |
| Acute colitis | 1 | 16.67 | 5 | 83.33 | 0 | 0 | 0 | 0 |
| Chronic enteritis | 0 | 0 | 3 | 50 | 2 | 33.33 | 1 | 16.67 |
| Chronic gastroenteritis | 0 | 0 | 0 | 0 | 1 | 50 | 1 | 50 |
| Chronic colitis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 100 |
| Chronic diarrhea | 0 | 0 | 1 | 100 | 0 | 0 | 0 | 0 |
| Infectious gastroenteritis | 2 | 14.29 | 10 | 71.43 | 1 | 7.14 | 1 | 7.14 |
| Uncomplicated diarrhea | 9 | 75 | 3 | 25 | 0 | 0 | 0 | 0 |
| Irritable colitis | 3 | 60 | 2 | 40 | 0 | 0 | 0 | 0 |
| Gastrogenic diarrhea | 0 | 0 | 3 | 100 | 0 | 0 | 0 | 0 |
| Nervous diarrhea | 2 | 66.67 | 1 | 33.33 | 0 | 0 | 0 | 0 |
| Gastroenteritis | 1 | 33.33 | 1 | 33.33 | 0 | 0 | 1 | 33.33 |
| Nervous gastroenteritis | 0 | 0 | 1 | 100 | 0 | 0 | 0 | 0 |
| Ulcerous colitis | 0 | 0 | 2 | 66.57 | 0 | 0 | 1 | 33.3 |
| Habitual diarrhea | 0 | 0 | 1 | 100 | 0 | 0 | 0 | 0 |
| Catarrhal colitis | 0 | 0 | 1 | 100 | 0 | 0 | 0 | 0 |
| Colitis | 0 | 0 | 1 | 33.33 | 0 | 0 | 2 | 66.67 |
| Hemorrhagic gastritis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 100 |
| Gastric ulcer | 0 | 0 | 0 | 0 | 1 | 100 | 0 | 0 |
| Acute pancreatitis | 0 | 0 | 1 | 100 | 0 | 0 | 0 | 0 |
| Suspicion of food poisoning | 0 | 0 | 1 | 50 | 0 | 0 | 1 | 50 |
| Total number | 44 | 72 | 13 | 19 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takagi, M.; Ito, M.; Morino, H.; Miura, T.; Oshida, K.; Suzuki, M.; Takemori, H.; Shibata, T. Anti-Diarrheal Effects of Wood Creosote, Seirogan, in Japanese Patients. Reports 2019, 2, 28. https://doi.org/10.3390/reports2040028
Takagi M, Ito M, Morino H, Miura T, Oshida K, Suzuki M, Takemori H, Shibata T. Anti-Diarrheal Effects of Wood Creosote, Seirogan, in Japanese Patients. Reports. 2019; 2(4):28. https://doi.org/10.3390/reports2040028
Chicago/Turabian StyleTakagi, Masanori, Masafumi Ito, Hirofumi Morino, Takanori Miura, Kyoichi Oshida, Mayu Suzuki, Hiroshi Takemori, and Takashi Shibata. 2019. "Anti-Diarrheal Effects of Wood Creosote, Seirogan, in Japanese Patients" Reports 2, no. 4: 28. https://doi.org/10.3390/reports2040028
APA StyleTakagi, M., Ito, M., Morino, H., Miura, T., Oshida, K., Suzuki, M., Takemori, H., & Shibata, T. (2019). Anti-Diarrheal Effects of Wood Creosote, Seirogan, in Japanese Patients. Reports, 2(4), 28. https://doi.org/10.3390/reports2040028





