Abstract
(1) This study describes the good evolution of a 6-year-old girl genetically diagnosed (R106X) with Rett syndrome (RTT), after having been treated with IGF-I, melatonin (MT), blackcurrant extracts (BC) and rehabilitated for 6 months. (2) The patient stopped normal development in the first year of age. The patient showed short stature and weight and fulfilled the main criteria for typical RTT. Despite her young age, there was pubic hair (Tanner II), very high plasma testosterone, and low levels of plasma gonadotrophins. There were no adrenal enzymatic deficits, and abdominal ultrasound studies were normal. The treatment consisted of IGF-I (0.04 mg/kg/day, 5 days/week, subcutaneous (sc)) for 3 months and then 15 days of rest, MT (50 mg/day, orally, without interruption) and neurorehabilitation. A new blood test, after 3 months of treatment, was absolutely normal and the pubic hair disappeared (Tanner I). Then, a new treatment was started with IGF-I, MT, and BC for another 3 months. In this period, the degree of pubertal development increased to Tanner III (pubic level), without a known cause. (3) The treatment followed led to clear improvements in most of the initial abnormalities, perhaps due to the neurotrophic effect of IGF-I, the antioxidant effects of MT and BC, and the cerebral increase in the cyclic glycine-proline (cGP) achieved with administration of BC. (4) A continuous treatment with IGF-I, MT, and BC appears to be useful in RTT.
1. Introduction
Rett syndrome (RTT), OMIM #312750, was first described by the Austrian doctor Andreas Rett, who gave his name to this serious neurological affectation [1], the main clinical characteristics of which were later described in 1983 [2]. In 1999, it was postulated that RTT was produced by a de novo mutation in the MECP2 gene, an X-linked gene, which encodes methyl-CpG-binding protein 2 [3]. This protein belongs to a family of proteins [4,5] which play an important role in the organization of chromatin and the regulation of transcription after binding to methylated CpG sites [6], although MeCP2 can also bind to methylated CpA [7].
DNA methylation can affect the structure of chromatin and lead to the repression of transcription of several different genes. Therefore, mutations in the MECP2 gene, or its deletion, impede its physiological function as a transcriptional repressor that plays a key role in the maturation of the central nervous system (CNS). This is probably the reason MeCP2 is present mainly in postmitotic neurons, participating in the development and maintenance of synapses [8], although this protein has been found to be widely expressed (at different rates, depending on the tissue) in many human adult tissues [9].
Human neuronal development begins early in the sixth week after conception, a period in which the expression of MeCP2 occurs at a low level [9].
A very important factor in normal brain development is the mechanism of synapse elimination, something that occurs physiologically between early childhood and the onset of puberty [10]; this phenomenon, known as synaptic pruning, can be influenced by environmental factors, leading to neurodevelopmental abnormalities.
This short introduction allows us to understand how the mutation of a gene, such as MECP2, so strongly involved in the maturation of the CNS, can produce very important neurodevelopmental disorders. 95% of patients with classic RTT have mutations in the MECP2 gene [11], suggesting that in 5% of cases with RTT, the cause of this pathology may reside in mutations of other genes [12], which consists mainly of: partial or complete loss of manual skills acquired; partial or complete loss of acquired spoken language; gait abnormalities; and stereotyped hand movements. These are the revised diagnostic criteria for RTT, established by the RettSearch Consortium in 2010 [11]. In addition, the same RettSearch Consortium established supportive criteria for atypical RTT: breathing disturbances when awake; bruxism when awake; impaired sleep pattern; abnormal muscle tone; peripheral vasomotor disturbances; scoliosis/kyphosis; growth retardation; small cold hands and feet; inappropriate laughing/screaming spells; diminished response to pain; and intense eye communication—“eye pointing” [11]. However, as indicated above [12], MECP2 gene alterations are only present in 50–70% of atypical RTT cases, as has been demonstrated in the recent years. This is the case of mutations in the CDKL5 gene [13,14,15], but also in other genes not previously associated with typical RTT, such as the recently described frameshift mutation in the STXBP1 gene in a Japanese girl [16], fully showing the diagnostic criteria for RTT [11], although this kind of mutation had previously been described in one case of atypical RTT [12]. Similar findings were reported in two patients meeting the diagnostic criteria for RTT without showing any mutation in the MECP2 gene; in these cases, mutations were found in the CTNNB1 gene and in the WDR45 gene [17]. Other gene mutations, such as truncating mutations of FOXG1 gene, a gene key for early development of the telencephalon, have been reported to be responsible for the congenital variant of RTT [18], although this is only considered to be a RTT-related disorder [19]. These mutations reflect only a small part of the broad spectrum of genetic heterogeneity that can produce Rett phenotypes. In fact, complete sequencing of the exome in many patients diagnosed with RTT or RTT-like syndrome in whom no mutations were detected in the genes most commonly responsible for this neurodevelopmental disorder, such as MECP2, CDKL5 or FOXG1, revealed that at least 69 different genes could be the cause of the presentation of the RTT phenotype [20].
Given that RTT is transmitted as a dominant trait linked to the X chromosome, it was thought that this syndrome almost exclusively affected females [21], but mutations or deletions of MECP2 can also appear in men, although in this case, severe neonatal encephalopathy occurs, since they have only one copy of the mutated gene, leading to a harsher phenotype that usually causes premature death before 2 years of age [22]. However, it has been reported that a male with classic RTT will remain alive [23], suggesting that many risk factors for premature death can be controlled. In recent years, several reports have described an increasing number of boys with RTT, although the vast majority of them die soon after birth [24]. Therefore, RTT is almost exclusively seen in females. Typically, neurodevelopmental abnormalities begin early in their lives [25,26], although there is a clear regression in acquired fine motor skills and communication between 6 and 18 months of age [1,2].
Since, as previously described, the vast majority of patients with Rett syndrome undergo a mutation in the MECP2 gene, which is key in brain development and normal brain function, given that it affects a large number of genes involved in the formation of synapses and brain plasticity, protein synthesis, mitochondrial function, oxidative stress, lipid metabolism, or the Akt/mTOR cell survival pathway, it is logical that the therapeutic attempts for the treatment of this syndrome are directed primarily towards gene therapy, directly targeting the MECP2 gene or producing recombinant hMeCP2 protein, or acting directly downstream MECP2, using neurotrophic factors, or using drugs which could normalize the neurotransmitter systems affected by MECP2 mutation, or, in the last instance, trying to correct some of the existing disabilities manifested in the syndrome (see [27,28], for a detailed review). Some of these treatments have been tested in clinical trials without significant normalization of the clinical picture of RTT; moreover, some parameters analyzed in a clinical trial in which insulin-like growth factor 1 (IGF-I) was used for the treatment of thirty girls with RTT worsened, and some adverse events were observed [29], although in this trial, improvements in stereotypic behavior and social communication were also observed [29].
The reason for using IGF-I in the treatment of RTT is based on the key role that this peptide plays in the development of the CNS; therefore, it could improve the symptoms of the syndrome at the expense of facilitating brain development [30,31] and increase the small number of dendrites that exist in this pathology [32,33].
Another clinical trial, carried out in 56 adolescent and adult females with RTT, used a chemically modified form of the naturally formed N-terminal IGF-I tripeptide Gly-Pro-Glu (GPE), called Trofinetide, with significant promising results [34], but this peptide is not yet available for medical use, at least in Spain.
In this study, we analyzed the evolution of a young girl with classic RTT [11] during a 6-month treatment with IGF-I, melatonin (MT), blackcurrant extracts (BC) (administered during the last 3 months of treatment) and rehabilitation. IGF-I was given for the reasons explained above; MT was administered due to its important roles in the mitochondria and oxidative stress (in fact many reports suggest that mythochondrial dsyfunction plays a role in the development of RTT [35,36]); for its part, BC was also administered when we were able to use these extracts. The reason for adding BC to the treatment was based on its high content of anthocyanins (antioxidants) and because it also contains the small dipeptide cyclic glycine-proline (cGP), which, as GPE does, plays a very important role in regulating the bioavailability of IGF-I in the brain. In fact, our idea was to use BC (in addition to IGF-I and MT) from the beginning of the treatment, but we did not receive these extracts until the second period of the treatment carried out.
Although we did not obtain a complete regression of the symptoms observed at admission, most of them disappeared or decreased clearly during treatment and the patient continues to improve three months after discharge.
2. Case Presentation Section
2.1. Medical History
The patient was a 6-year-old girl, diagnosed with classical Rett syndrome, who came from Bulgaria to the Foltra Medical Center for medical treatment. She was the second daughter of three in the family, with her sisters being completely normal. Her mother did not have any toxic habits. There were no problems during pregnancy, but the birth took place by cesarean, in week 38, due to previous cesarean sections. The birth weight was 2.700 kg, her size was 45 cm and the circumference of the head was 34.5 cm. In the family, there was no medical history of interest. The development of the girl was normal during the first year of life, but from that moment she began to present persistent vomiting for 6 months. This led to a series of medical studies until genetic testing detected the existence of a missense mutation c.316C>T (R106W); p.Arg106Trp in exon 3 of the MECP2 gene, indicating that the patient had Rett syndrome [37,38,39].
The girl had not received any kind of medical treatment; she only received two sessions of physiotherapy per week, as well as equine therapy and music therapy (one session per week in each case).
Upon entering the Foltra Medical Center (6 years of age), both the height (103 cm) and the weight (15.8 kg) of the patient were slightly below the 3rd percentile for her age. Her body mass index (BMI) was 14.9 kg/m2 (underweight). She had microcephaly; she walked alone, without help, but without defined objectives and presenting an increase in the support base (ataxic ambulation), with short steps. At no time did she interact with who was examining her; she continually rubbed her hands, ignored orders, and nothing caught her attention. The girl lacked oral language and did not attempt any kind of communication. There was drooling. She did not chew and only ate semi-solid foods. She only drank hot liquids, which had to be administered through a syringe. She paid no attention to anything, did not pick up objects from the floor, nor look into the eyes of the person speaking to her. She often tightly pressed her lips and stopped breathing. There was a continuous bruxism, day and night. There were no apparent peripheral vasomotor disturbances.
Physically, she had a convergent strabismus of the left eye. The joint range was normal in all 4 extremities, but presented kyphosis and cervico-dorsal scoliosis (15°; the right scapula was higher than the left), and there was mild generalized hypotonia. She had no control of the sphincters. The electrocardiogram was normal (sinus rhythm and frequency of 93 beats per minute). She had never had seizures; however, according to her parents, it was common for her to wake up 2–3 times during the night.
During the physical examination, the existence of incipient pubic hair, Tanner stage II (despite her age), attracted attention, although there was no thelarche (Tanner stage I) or signs of axillary hair. There was also a discrete clitoromegaly. Although it has been postulated that girls with RTT have an abnormal pubertal development [40], given the observed findings, in addition to the routine blood tests that we requested before starting any medical treatment, plasma levels of gonadotrophins and sex hormones were also evaluated in this case.
2.2. Blood Analysis
Upon admission, a blood test was performed. The number of erythrocytes (5.37 × 106/μL), hemoglobin (14.40 g/dL) and hematocrit (42.2%) were at high levels, while leukocytes and platelets were at normal values. Plasma biochemistry was normal, with the exception of creatine phosphokinase (CPK: 315.2 U/L, normal values: 20–195 U/L). Thyroid hormones were in normal values, as was plasma cortisol. Plasma values of IGF-I and IGFBP3 were also normal (IGF-I: 68 ng/mL, normal for their age: 55–248 ng/mL; IGFBP3: 4.8 ng/mL, normal for their age: 2.6–5.8 ng/mL), although the plasma IGF-I was close to the lower limit of normal. Surprisingly, although the plasma levels of FSH and LH were clearly in prepubertal values (FSH: 0.80 mU/mL, LH: <0.1 mU/mL), the plasma levels of testosterone were extremely high (3.98 ng/mL, normal values in adult females: <0.45 ng/mL), equivalent to those of postpubertal male. The plasma estradiol was lower than 5 pg/mL (lower limit of the assay).
Although plasma cortisol levels were normal, we performed a new blood test to rule out a deficit of 3β-hydroxylase or 21-hydroxylase.
However, all adrenal androgens measured (dehydroepiandrosterone sulfate (DHEA-S), and androstenedione) exhibited normal values for the age of the patient. Therefore, we decided to perform an abdominal ultrasound study that ruled out any anomaly; specifically, the existence of no tumor mass was detected, the ovaries were normal for the age, and there was no follicular activity.
The blood test was repeated every 3 months until discharge, seven months after admission. Curiously, 3 months after beginning medical and rehabilitation treatment, the patient performed a blood test in Bulgaria, her country of origin, to which she had returned during the Christmas holidays, and the results of this new analysis completely coincided with those made in our Center a few days before those holidays. Plasma FSH had increased to 3.30 mU/mL, plasma LH was 0.10 mU/mL and, surprisingly, plasma testosterone had decreased to almost undetectable values <0.025 ng/mL. Moreover, the pubic hair had practically disappeared (Tanner stage I). However, 3 months later, just before discharge, the situation had changed again: the pubic hair was now very black and dense (Tanner stage III, only in the pubic area), and there was odorous sweating. Plasma testosterone had increased to 3.2 ng/mL, as did plasma FSH (4.1 mU/mL), while plasma LH was 1.1 mU/mL. The erythrocytes, hemoglobin, and hematocrit also increased reaching values similar to those first observed after admission. Plasma IGF-I was 185 ng/mL, plasma IGFBP3 was 2.7 ng/mL, and CPK had been normalized (195 U/L). Other plasma values were normal. These data are shown in Table 1.

Table 1.
Plasma values (hematology, biochemistry, hormones and Tanner stage), at admission and at 3-month intervals after beginning the treatment, and normal values for the age of the patient. Note the great divergence between the levels of gonadotrophins and those of testosterone and Tanner’s stage. Note also that the levels of adrenal cortisol and adrenal androgens measured do not indicate the existence of any adrenal enzymatic deficit responsible for this great hyperandrogenism. The table also shows the treatment that was carried out in each period analyzed.
2.3. Medical Treatments
Once it was established that the testosterone values and pubertal development were not due to an ovarian or adrenal pathology, or to the existence of a tumor process, the medical treatment was carried out in two phases: (1) Initially, IGF-I (0.04 mg/kg/day, 5 days per week, sc, Increlex, Ipsen Pharma, Barcelona, Spain) was administered for 3 months, followed by a rest period of 15 days, and melatonin (MT) (50 mg/day), prepared by master formula and administered orally, uninterruptedly, before going to bed. (2) In a second phase, in addition to IGF-I and MT, at the same doses and frequency, the patient received capsules containing lyophilized blackcurrant extract powder (BC; obtained from the fresh fruit of Ribes Nigrum L., Vitality Brain Health-Supa, Vitality Wellness Ltd., Christchurch, New Zealand). Each of these capsules contains 35% Anthocyanins. The dose administered, orally, consisted of 3 capsules/day for three days, then 2/day for three days and then 1/day without interruption.
Figure 1 shows a scheme of the treatments carried out.
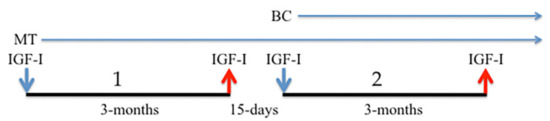
Figure 1.
Medical treatments performed. 1: First phase. IGF-I was administered 5 days/week for 3 months. MT was administered without interruption from the beginning of treatment. 2: Second phase. After 15 days of rest, the administration of IGF-I was resumed for another 3 months. In this second phase, the patient also received BC extracts daily. The blue arrows indicate the beginning of each treatment; red arrows indicate the suspension of IGF-I administration.
This medical treatment was carried out in accordance with the protocols followed in our Medical Center and in compliance with Spanish legislation for the use of GH and MT “off label” and the Code of Ethics of the World Medical Association (Declaration of Helsinki). Signed informed consent to use GH and MT, and subsequently BC, was obtained from the patient’s father (her legal representative). In the figures shown here, the patient’s face has been partially blurred to preserve her privacy. (Spanish legislation: Royal Decree 1015/June 2009; Declaration of Helsinki: June 1964, lastly ammended in October 2013; Ethic Code of Foltra: 2017-06).
No secondary side effects were observed due to the medical treatments followed. At the time of discharge, both the height (she grew 5 cm) and the weight of the patient had increased, reaching the 5th percentile (p5) in both cases.
2.4. Rehabilitation and Results
Rehabilitation consisted of daily sessions (5 days/week) of Speech Therapy, Neurostimulation and Occupational Therapy, Integrative and Neurosensorial stimulation (EINA), and Physiotherapy (3 days/week).
2.4.1. Speech Therapy
Initially, the patient presented a very important affectation of expressive and receptive language. She did not understand orders and did not respond when she was called by name. She emitted primary sounds to express her mood. The patient did not chew or crush food, which had to be semi-solid. Liquids had to be administered by means of a syringe or bottle, always in supine position, and at a warm or hot temperature. She rejected a series of textures, flavors, and temperatures. There was moderate drooling and frequent voluntary interruptions of breathing.
The main objective in this area was focused on restoring the affected stomatognathic functions using a set of procedures and techniques for the correction of the orofacial muscular imbalance, the creation of new muscular patterns in swallowing, the creation of adequate patterns for the acquisition of linguistic precursors and the articulation of words, as well as the reduction of inappropriate habits.
At the time of discharge, the rejection of a series of solid textures had diminished, the voluntary chewing movement had begun, the labial seal had improved (so there was no more drooling); the frequency of voluntary apneas had diminished considerably. The patient ingested liquids at any temperature with a syringe or spoon and had increased the emission of babbling and guttural sounds. She even imitated the repetition, in form and time, of certain sounds when asked to do so (for example: O-O-O-O......O-O-O-O; U-U-U......U-U-U). The bruxism had already disappeared during the first phase of treatment.

Table 2.
Thomas Stonell and Greenberg scale. At admission, the patient presented a moderate drooling (score 3: wet lips and chin). This score was reduced to 1 at the time of discharge (the patient never drools).
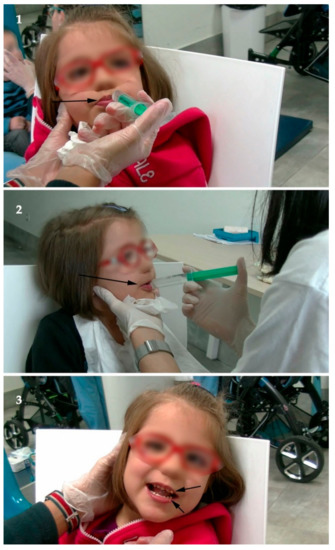
Figure 2.
Speech Therapy. 1. Initially, after admission. Notice (black arrow) how the patient closes her lips to avoid being given cold liquids with a syringe; 2 and 3. Before discharge. In 2, it can be seen how the patient already accepted drinking cold liquids with a syringe (black arrow). In 3, small pieces of a cookie, which the patient had chewed and swallowed, can be seen in the mouth (black arrows). The images have been blurred to avoid identification of the patient.
2.4.2. Neurostimulation and Occupational Therapy
The first examination of the patient showed that there was a marked alteration in attention capacity; there was no visual contact or tracking, horizontal or vertical, of a light. She did not interact with other children or adults, nor did she recognize herself as a causal agent of events; she did not manipulate objects or pay attention to them.
Therefore, the objectives in this area focused on: promoting attention, encouraging communicative intentionality, exploring different objects and the environment, and promoting recognition of the causal agent of events.
At the time of discharge, in the personal and social area, the patient had improved interactions with adults, being able to observe a person’s face during marked periods of time and, in many cases, smile or try to vocalize as an answer. Sometimes she could also react in advance to some activities she had been doing during the treatment. The patient had begun to show greater awareness of her hands, using them as a support when she was unbalanced and responding to her name when she was called. She increased her attention span, observed objects and was already able to follow a light in horizontal and vertical trajectories for relatively long periods of time.
In relation to fine motor skills, she was already able to keep her hands predominantly open and began to perform ulnar-palmar pressure, as well as began to touch and explore objects. As for receptive communication, she began to react to sounds that were outside her field of vision by turning her head towards the source and reacting to different types of voice. At the cognitive level, her mnemonic capacity increased, as did perceptual discrimination, reacting to new situations and beginning to visually explore her environment.
Most of these changes began to appear at the end of the first phase of treatment and clearly improved progressively throughout the second phase of it.

Table 3.
Scores achieved in the Battelle Developmental Inventory Screening Test (BDIST) on admission (PRE-) and discharge (POST-). Note particularly the observed changes in adaptive behavior, receptive communication, and cognitive aspects.

Figure 3.
Interaction with objects. 1. At admission, the patient did not look at the face of the therapist or at any other person. 2A–C and 3. Before discharge, she was already following objects; these images show how the patient follows the cartoons shown on a tablet.
2.4.3. EINA
The objective of this therapy is to stimulate the brain through the ear [41], seeking motor and cognitive improvement and enhancing the results of other therapies undergone by the patient. In this case, given the condition and the lack of speech of the patient the initial and final listening tests could not be performed. Therefore, the changes that could have occurred could not be evaluated graphically. In any case, four different blocks of stimulation were carried out, with a week’s interval between them. In the first two blocks, the patient listened to filtered Mozart music, Gregorian chant, and passing bands. During the third block, only Mozart music and Gregorian chants were used for brain stimulation, while in the fourth block stimulation was carried out with passing bands related to balance and coordination; for this, the sound was filtered, enhancing the frequencies between 125 and 1000 Hz, so that the stimulation occurred at the vestibular level.
2.4.4. Physiotherapy
At the beginning of these sessions, the patient did not make postural changes. When she was sitting in a chair, she remained passively, without moving from it. When she was placed on the floor, she did not move or try to get up. Her defense reactions were poor, as were her straightening responses. She did not crawl, did not climb down or up the stairs. When she walked, she did so by increasing the base of support.
Before discharge, most of these deficiencies had disappeared. The patient could now move from the supine position to the prone position and vice versa. In a supine position, she forced herself to sit down; she was able to go up and down stairs and walked with more balance, without needing to increase the base of support. In addition, upon seeing her therapist, her face showed an expression of joy and approached her.
Some of these changes, observed mainly from the middle of the second phase of treatment, are shown in Figure 4.

Figure 4.
Increase in motor skills observed at discharge. 1. The patient can now climb stairs. 2. The patient can climb onto a bed with the help of her hands.
Table 4 shows the changes observed throughout each phase during the treatment period.

Table 4.
Disorders observed upon admission (Pre-) and during the first phase of the treatment (1) and at the end of the second phase of it (2, before discharge). +: indicates the severity of the affectation (+++ indicate the maximum degree). −: indicates the absence of abnormalities in each item. Although the girl did not reach oral language, she tried to repeat words, therefore the score in this item was represented by −/+. Tanner stage of pubertal development (pubic area only), is indicated by numbers.
3. Discussion
This study analyzes the evolution of a six-year-old girl who met the main criteria required for the typical diagnosis of Rett syndrome, established in 2010 by the RettSearch Consortium [11]. In addition, the patient also showed many of the supportive criteria also established by this group [11], and there was a genetic confirmation of the existence of a mutation in the MECP2 gene, mainly responsible for the development of this syndrome, present in 1:10,000 females and the second known cause of important intellectual disabilities in them. We treated her with IGF-I, MT, BC, and rehabilitation during a short period of time (six months of treatment with IGF-I and MT; BC was also added at the beginning of the second phase of treatment followed). Despite it being only one case, the results obtained should be considered very good in terms of the improvements achieved in the initial disabilities of the patient, and the severity of her genotype, although in this disease, the genotype and phenotype are not always well correlated [37,38]. This may be due to the fact that, as described in the Introduction, other genetic alterations may also be responsible for RTT [42].
As stated in the Introduction, MeCP2 plays a key role in the development of the brain, mainly acting on the formation and maintenance of synaptic connections and their functionality throughout life. Therefore, it is foreseeable that any mutation in the gene that encodes the expression of this protein will produce devastating neurological disorders, due to the lack of synapses and their dysfunction, as occurs in RTT [43], although, as indicated, the genotypes and phenotypes of these patients are not always well correlated. Most likely this is due to the fact that in patients with RTT, as in other disorders related to autism (ASD), the presence of the mutation may affect different brain regions or different neuronal types, while in other neurons the genotype is normal; even in the same area of the brain, different mixtures of excitation and inhibition can coexist, leading to an imbalance in the relationship between neuronal excitation and inhibition that would affect the expression of many genes, including Brain-Derived Nerve Factor (BDNF), that depend on neuronal activity. In this situation, the functional homeostasis of the brain would be affected (see [44] for a detailed review). Therefore, in RTT, as in other ASD, any phenotype could be secondary to the development of other compensatory adaptive mechanisms, which in fact are rather maladaptive, in terms of brain plasticity, because they would contribute to the appearance of the symptoms in each case.
It has been reported that a mutation like that existing in our patient leads to a 100-fold reduction in the binding of MeCP2 to methylated DNA, thus strongly decreasing the physiological transcriptional repressor functions of MeCP2 [45]. This could explain the finding that reduced BDNF signaling, a peptide with important neurotrophic activities (including the induction of synaptic plasticity) that is transcriptionally regulated by MeCP2 [46], accelerates the appearance of the symptoms of MECP2 KO mice, while BDNF overexpression improves them [47]. For a more detailed understanding of the relationships between MeCP2 and BDNF, see references [48,49,50].
The administration of IGF-I recovers dendritic spines in mice deficient in MeCP2, although this treatment must begin very early when the phenotype of the syndrome is still not too severe [51]. The positive effects of the administration of IGF-I in RTT have been confirmed in mouse models [52,53], and in human patients [29,30,31,54,55,56], corroborating our results, although in those studies only IGF-I was used, and the treatment time and dose of this peptide were lower than in our case.
There could be many reasons for which IGF-I exerts positive effects on RTT. IGF-I expression has been found in neural stem cells derived from fetal human forebrains [57], and most likely it is induced by GH in the human adult brain [58]. The IGF-I receptor (IGF-IR) maintains a stable expression in the brain throughout life, at least in rats [59], but it is likely that it also occurs in human adults to bind to free IGF-I, which reaches the brain from plasma [60]. Activation of IGF-IR triggers a series of signaling pathways [61] that play a very important role in the normal neurodevelopment [62]. Plasma levels of IGF-I have been found to be decreased in 8 of 23 RTT patients [63], and the GH/IGF-I axis has been shown to function abnormally in RTT [64], although this may be a consequence of poor nutritional status in many of these patients with RTT, since hepatic IGF-I production is strongly dependent on nutritional status [65]. This coincides with the short stature of our patient and with the data of another study in a large population of patients with RTT, in whom classical RTT is associated with poor growth [66]. The fact that our patient grew normally during the whole treatment with IGF-I suggests that the low growth in some patients with RTT is caused by an abnormal functioning of the GH/IGF-I axis that leads to a deficient production of IGF-I; in our case, this was most likely due to the patient’s low weight, since the plasma levels of IGFBP3 were normal values before starting treatment, and this GH-binding protein is dependent on GH [67].
Muscle hypotonia is frequently observed in RTT girls and MeCP2-null mice, and occurs as a consequence of deficiencies in muscle signaling by the GH/IGF-I system [68]. This is in agreement with our data in this study; the initial hypotonia disappeared progressively during treatment with IGF-I until the muscle tone at discharge was practically normal. Most likely, this muscle involvement may explain the finding in our patient of elevated plasma levels of CPK at admission and its normalization before discharge. Similarly high CPK values in plasma were described in a girl with RTT, relating them to mitochondrial anomalies and abnormalities in the enzymes responsible for oxidative phosphorylation [69].
As described previously, oxidative stress (OS) exists in RTT patients [70], although there are doubts about whether this increase in OS occurs due to the disease or if is the factor responsible [71]. Studies in animals showed a clear increase in the production of harmful reactive oxygen species in the brain of mice deficient in MeCP2, apparently produced by a defective functioning of the mitochondrial respiratory chain complex II, which could be recovered with the administration of a bacterial protein, CNF1, which has also been shown to improve the affected neural phenotype of these animals [72]. Although it has been suggested that RTT could be a mitochondrial disease [35,36], it has been discovered that mutations of the MECP2 gene precede mitochondrial dysfunction [36]. In any case, it is clear that OS plays a very important role in the expression and severity of symptoms in this syndrome; therefore, the treatment of mitochondrial dysfunction or the use of reactive oxygen species scavengers should be useful in patients with RTT or other neurodegenerative diseases [73].
This was one of the reasons why we have used MT in our patient. MT exerts potent scavenging effects on toxic reactive oxygen species [74,75]. In addition, this hormone has anti-inflammatory properties, and it is a mitochondrial protector, besides playing multiple important functions in the human body [76,77,78,79,80,81].
In RTT, as in many other pathologies of the central nervous system, neuroinflammation is the most frequent finding. It occurs mainly as a result of the excessive production of inflammatory cytokines that lead to greater stress in the brain cells and a strong activation of the microglia. Microglia play a prominent role in the maintenance of synapses and pruning of dendrites, but all this is lost when overactivated. In this sense, an interesting fact is that MT levels are greatly diminished in a series of neurodegenerative pathologies [76], which favors the progression of inflammation in the brain. Therefore, the administration of MT must have played an important role in the positive evolution of our patient, decreasing neuroinflammation by reducing the formation of free radicals, preventing the activation of NADPH oxidase and suppressing inducible nitric oxide synthase, as well as regulating negatively proinflammatory cytokines [76]. In addition, chronic treatment with MT completely prevents mitochondrial damage in multiple organs, including the heart [81]. However, we do not know any case of RTT treated with MT; therefore, we cannot compare our results with those of other studies in this syndrome.
In 1989, it was identified that in the brain, IGF-I underwent a specific proteolytic cleavage that led to the generation of two fragments: des-N-(1-3)-IGF-I, and the tripeptide N-terminal Gly-Pro-Glu (GPE) [82]. This mechanism of selective IGF-I breakage was also identified in rat serum [83], and GPE was detected in human urine [84]. It was soon discovered that this peptide was not only a mere product of IGF-I degradation, but also exerted important activities at the brain level, acting as a neuromodulator, neuroprotector and even inducing proliferation and migration of neural stem cells [85]. Moreover, GPE acts on neurons through the same signaling pathways as IGF-I [85].
However, the short life of the GPE in plasma when administered intravenously limits its therapeutic use. Currently, a chemically modified form of GPE, called Trofinetide, makes the peptide suitable for oral administration, with a longer half-life in plasma and an easy passage to the brain. These are the reasons why Trofinetide is being used in clinical trials in human patients with RTT with significantly promising results [34]. Trofinetide is safe and well tolerated, and the highest dose used (70 mg/kg/day, twice a day, for 14 or 28 days) improved the main characteristics of this syndrome (communication and speech, behavior, respiratory abnormalities, hand stereotypies and muscle dysfunction) compared with placebo, despite the advanced age of the patients and the short time of administration of the peptide [34].
Another metabolite of IGF-I, possibly derived from GPE, is the dipeptide cyclic glycine-proline (cGP). It has been shown that this small peptide acts, as does GPE, by modulating the amount of free IGF-I, the biologically active fraction, by regulating the binding of IGF-I to its binding proteins, particularly to IGFBP3; therefore, it normalizes the function of IGF-I in pathological conditions [86,87]. In this sense, it has been shown that cGP increases the availability of free IGF-I when it is low (as occurs in the brain of RTT patients), but inhibits it when free IGF-I is in high values; this may explain why IGF-I can show such different effects as improving the recovery of brain lesions in rats and decreasing or inhibiting the growth of some tumors in mice [87], but also why cGP improves memory in adult rats [88].
Interestingly, the positive evolution of our patient was even greater when she received BC, in addition to IGF-I and MT. Studies carried out years ago, and recently, show that anthocyanins from BC act as strong antioxidants, anti-inflammatory and have anti-neurodegenerative properties [89,90,91,92,93]. In addition, it has been shown that administration of BC for 28 days increases the concentration of cGP in cerebrospinal fluid samples of 11 patients with Parkinson’s disease; this increase in cerebral cGP correlates with both the plasma concentration of cGP and the cGP/IGF-I ratio in plasma, without modifying the concentration of IGF-I and IGFBP3, both in plasma and cerebrospinal fluid [94]. In the same study, it has been shown that cGP is present in extracts of BC, and that the administration of these leads to an increase in brain concentration of this small peptide [94]. Therefore, it is likely that the extracts of BC have contributed significantly, acting as antioxidants, anti-inflammatories, but also increasing the concentration of cGP in the brain and, therefore, the bioavailability of brain IGF-I, to the additional positive evolution of our patient with RTT.
What seems surprising, at least for us, are the changes observed in the pubertal development of the patient throughout the treatment followed.
Precocious puberty in RTT was first described in 2013 in a 6-year-old girl [95]. This patient was in stage 3 of Tanner at 6 years of age, had low gonadotrophins levels and showed an exaggerated response of LH and FSH to stimulation with GnRH (gonadotrophin-releasing hormone). A larger study carried out in 802 women with classic RTT [40], indicated that a significant percentage of patients had premature breast development (Tanner stage 2) and premature appearance of pubic hair (Tanner stage 2). In normal girls, there is usually a synchronic transition between thelarche and pubarche, while the data from that RTT study indicate that in RTT girls there is an inversion of the normal pattern, so that pubarche precedes thelarche by a ratio of 3:1, as happened in our patient. The reasons for this are still unknown.
It has been shown that MECP2-null mouse has hypothalamic alterations [96]; among these, an altered expression of the estrogen receptor has been demonstrated [97]. Therefore, an early hyperactivity of the hypothalamic-pituitary-gonadal axis (HPG) could be a reason explaining precocious puberty in girls with RTT. However, this is not consistent with the plasma levels of gonadotrophins, testosterone and estradiol detected in our patient at admission; there was pubarche (Tanner stage 2), and plasma testosterone was extremely high, but plasma gonadotrophins and estradiol were at very low or undetectable values. The low levels of LH could be explained by the inhibitory effect of testosterone on the pituitary secretion of LH; but in the hypothalamus, as in most areas of the brain, testosterone is aromatized to estradiol [98], which in turn affects the pulsatile pattern of GnRH. In addition, given that LH stimulates the production of testosterone by the cells of the ovarian teak, our data do not support the hypothesis of hyperactivity of HPG to explain the results that we found in our patient at admission, although the values of FSH and LH were similar to those described in the first case of precocious puberty detected in a girl with RTT of the same age [95], and in another younger RTT girl with premature pubarche [99].
Another possibility, which does not exclude an abnormal functioning of HPG in RTT girls, could be an increase in the secretion of adrenal androgenic hormones, due to excessive production of corticotropin-releasing hormone (CRH) and ACTH [40], as described in an RTT mouse model [100]. However, plasma adrenal steroids were normal in our patient, while it would be expected that the adrenal precursors of testosterone were in high values, and cortisol in low values, but this did not occur. In addition, the abdominal ultrasound study did not reveal adrenal or ovarian abnormalities. Perhaps if we had performed a stimulation with GnRH and another one with ACTH we could have clarified the data found, but we did not.
Even more intriguing were the results, clinical and analytical, after three months of treatment with IGF-I and MT. Plasma testosterone had decreased to undetectable values, and Tanner’s stage was now 1. However, these data changed again during the second phase of treatment, when BC was added to her medication. Plasma testosterone again reached very high values (even for an adult normal female) and pubarche was even more evident (Tanner stage 3).
We do not know the reasons for these changes, nor have we seen that they have been described in other cases of RTT; more studies are needed to try to find an explanation for them, as well as to explain how such high values of testosterone in plasma could be reached in a girl with this syndrome of RTT.
In summary, in this study, we showed that a treatment with IGF-I, MT, and BC, together with neurorehabilitation, has been useful to improve the neurological disabilities existing in the patient with RTT that we treated. In addition, since the extracts of BC increase the levels of cGP, the mitogenic potential of IGF-I can be counteracted, so that the treatments with this hormone can be prolonged for a longer time. However, it is necessary to investigate whether the changes in the androgenic anomalies observed in our patient could have been produced by any of the treatments administered.
It is difficult to compare our results with those found in other studies using only IGF-I or Trofinetide due to the different treatments and times used, including neurorehabilitation, and the fact that this is a case report in which we only treated one patient with classic RTT.
It is more than probable that the administration of IGF-I was the main inducer of the improvements observed in the patient, but it seems to be clear that the course of neurorehabilitation followed greatly helped the effects of this hormone. On the other hand, both melatonin and BC, which reduce oxidative stress and neuroinflammation, must have contributed to the positive effects observed. Furthermore, since it has been shown that the administration of BC increases brain levels of free IGF-I, it is reasonable to assume that BC has led to an increase in the actions of IGF-I in the patient’s brain.
A limitation of this study is the fact that the girl was from Bulgaria, where the language is very different from the Spanish used by her therapists, as well as the fact that she could only receive treatment for six months due to the work of her parents in her country of origin. Currently, she is still treated with MT (100 mg/day) and BC extracts there (one capsule/day), but without IGF-I, which is not marketed in her home country. However, the last report sent by her parents, three months after discharge, indicate that the improvements achieved here are not only still maintained, but also continue to increase. Tanner stage did not change.
Most likely, in the near future, gene therapy with MECP2 will optimize and perhaps end the syndrome. In fact, a series of studies are being carried out in preclinical models, but the results are not yet conclusive.
Author Contributions
Conceptualization, J.D.; Methodology, O.D., M.C., N.C., A.D., D.L., C.G. and J.D.; Validation, J.D.; Formal Analysis, O.D., M.C., N.C., A.D., D.L., C.G. and J.D.; Investigation, J.D.; Writing-Review & Editing, J.D.; Supervision, J.D.
Funding
This research received no external funding.
Acknowledgments
We acknowledge David Eder (Vitality Wellness Ltd., Christchurch, New Zealand) for his generous gift of Vitality Brain Health-Supa capsules. We also acknowledge Jian Guan (Department of Pharmacology and Clinical Pharmacology; Faculty of Medical and Health Science, Auckland, New Zealand) for her advices and explanations about cGP-IGF-I relationships and effects on the central nervous system.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rett, A. On an unusual brain atropic syndrome with hyperammonemia in childhood. Wien Med. Wochenschr. 1966, 116, 723–726. [Google Scholar] [PubMed]
- Hagberg, B.; Aicardi, J.; Dias, K.; Ramos, O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: Report of 35 cases. Ann. Neurol. 1983, 14, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Meehan, R.R.; Henzel, W.J.; Maurer-Fogy, I.; Jeppesen, P.; Klein, F.; et al. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 1992, 69, 905–914. [Google Scholar] [CrossRef]
- D’Esposito, M.; Quaderi, N.A.; Ciccodicola, A.; Bruni, P.; Esposito, T.; D’Urso, M.; Brown, S.D. Isolation, physical mapping, and northern analysis of the X-linked human gene encoding methyl CpG-binding protein, MECP2. Mamm. Genome 1996, 7, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Galvão, T.C.; Thomas, J.O. Structure-specific binding of MeCP2 to four-way junction DNA through its methyl CpG-binding domain. Nucleic Acids Res. 2005, 33, 6603–6609. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.U.; Su, Y.; Shin, J.H.; Shin, J.; Li, H.; Xie, B.; Zhong, C.; Hu, S.; Le, T.; Fan, G. Distribution, recognition and regulation of non-CpG methylation in the adult mammalian brain. Nat. Neurosci. 2014, 17, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Shahbazian, M.D.; Antalffy, B.; Armstrong, D.L.; Zoghbi, H.Y. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum. Mol. Genet. 2002, 11, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Meehan, R.R.; Lewis, J.D.; Bird, A.P. Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA. Nucleic Acids Res. 1992, 20, 5085–5092. [Google Scholar] [CrossRef] [PubMed]
- Chechnik, G.; Meilijson, I.; Ruppin, E. Synaptic pruning in development: A computational account. Neural Comput. 1998, 10, 1759–1777. [Google Scholar] [CrossRef]
- Neul, J.L.; Kaufmann, W.E.; Glaze, D.G.; Christodolou, J.; Clarke, A.J.; Bahi-Buisson, N.; Leonard, H.; Bailey, M.E.; Schanen, N.C.; Zapella, M.; et al. Rett Syndrome: Revised Diagnostic Criteria and Nomenclature. Ann. Neurol. 2010, 68, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.E.; Tambunan, D.; LaCoursiere, C.; Godenberg, M.; Pinsky, R.; Martin, E.; Ho, E.; Khwaja, O.; Kaufmann, W.E.; Poduri, A. Mutations in Epilepsy and Intellectual Disability in Patients with Features of Rett Syndrome. Am. J. Med. Genet. A 2015, 167, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, R.; Parrini, E. Epilepsy in Rett syndrome, and CDL5- and FOXG1-gene-related encephalopathies. Epilepsia 2012, 53, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Hagebeuk, E.E.; van den Bossche, R.A.; de Weerd, A.W. Respiratory and sleep disorders in female children with atypical Rett syndrome caused by mutations in the CDKL5 gene. Dev. Med. Child Neurol. 2013, 55, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.; Mehta, B.; Menon, S.R.; Raha, S.; Udani, V. Novel mutations in cyclin-dependent kinase-like 5 (CDKL5) gene in Indian cases of Rett syndrome. Neuromol. Med. 2013, 15, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Yuge, K.; Iwama, K.; Yonee, C.; Matsufuji, M.; Sano, N.; Saikusa, T.; Yae, Y.; Yamashita, Y.; Mizuguchi, T.; Matsumoto, N.; et al. A novel STXBP1 mutation causes typical Rett syndrome in a Japanese girl. Brain Dev. 2018, 40, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Percy, A.K.; Lane, J.; Annese, F.; Warren, H.; Skinner, S.A.; Neul, J.L. When Rett syndrome is due to genes other than MECP2. Transl. Sci. Rare Dis. 2018, 13, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Ariani, F.; Hayek, G.; Rondinella, D.; Artuso, R.; Mencarelli, M.A.; Spanhol-Rosetto, A.; Pollazon, M.; Buoni, S.; Spiga, O.; Ricciardi, S.; et al. FOXG1 is responsible for the congenital variant of Rett syndrome. Am. J. Hum. Genet. 2008, 83, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.; Johnston, M.V. Neurodevelopmental disorders: Clinical criteria for Rett syndrome. Nat. Rev. Neurol. 2011, 7, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart, F.; Sangani, N.B.; Curfs, L.M.G. Current developments in the genetics of Rett and Rett-like syndrome. Curr. Opin. Psychiatry 2018, 3, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Neul, J.L.; Lane, J.B.; Lee, H.S.; Geerts, S.; Barrish, J.O.; Annese, F.; Baggett, L.M.; Barnes, K.; Skinner, S.M.; Motil, K.J.; et al. Developmental delay in Rett syndrome: Data from the natural history study. J. Neurodev. Disord. 2014, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Tokaji, N.; Ito, H.; Kohmoto, T.; Naruto, T.; Takahashi, R.; Goji, A.; Mori, T.; Toda, Y.; Saito, M.; Tange, S.; et al. A rare male patient with classic Rett syndrome cause by MeCP2_e1 mutation. Am. J. Med. Genet. 2018, 176, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Tarquinio, D.C.; Hou, W.; Neul, J.L.; Kaufmann, W.E.; Glaze, D.G.; Motil, K.J.; Skinner, S.A.; Lee, H.S. The Changing Face of Survival in Rett Syndrome and MECP2-Related Disorders. Pediatr. Neurol. 2015, 53, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Shioda, T.; Takahashi, S.; Kaname, T.; Yamauchi, T.; Fukuoka, T. MECP2 mutation in a boy with severe apnea and sick sinus syndrome. Brain Dev. 2018, 40, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Leonard, H.; Bower, C. Is the girl with Rett syndrome normal at birth? Dev. Med. Child Neurol. 1988, 40, 115–121. [Google Scholar]
- Kerr, A.M. Early clinical signs in the Rett disorder. Neuropediatrics 1995, 26, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.M.; Bird, A.; Coenraads, M.; Gray, S.J.; Menon, D.U.; Philpot, B.D.; Tarquinio, D.C. Rett Syndrome: Crossing the Treshold to Clinical Translation. Trends Neurosci. 2016, 39, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Lonard, H.; Cobb, S.; Downs, J. Clinical and biological progress over 50 years in Rett syndrome. Nat. Rev. Neurol. 2017, 13, 37–51. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, H.M.; Kauffmann, W.E.; Barnes, K.V.; Rakesh, K.; Kapur, K.; Tarquinio, D.C.; Cantwell, N.G.; Roche, K.J.; Rose, S.A.; Walco, A.C.; et al. Placebo-controlled crossover assessment of mecasermin for the treatment of Rett syndrome. Ann. Clin. Transl. Neurol. 2018, 5, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Pini, G.; Scusa, M.F.; Congiu, L.; Benincassa, A.; Morescalchi, P.; Bottiglioni, I.; Di Marco, P.; Borelli, P.; Bonuccelli, U.; Della-Chiesa, A.; et al. IGF1 as a Potential Treatment for Rett Syndrome: Safety Assessment in Six Rett Patients. Autism Res. Treat. 2012, 2012, 679801. [Google Scholar] [CrossRef] [PubMed]
- Pini, G.; Scusa, M.F.; Benincassa, A.; Bottiglioni, I.; Congiu, L.; Vadhatpour, C.; Romanelli, A.M.; Gemo, I.; Puccetti, C.; McNamara, R.; et al. Repeated insulin-like growth factor 1 treatment in a patient with Rett syndrome: A single case study. Front. Pediatr. 2014, 2, 52. [Google Scholar] [CrossRef] [PubMed]
- Belichenko, P.V.; Wright, E.E.; Belichenko, N.P.; Masliah, E.; Li, H.H.; Mobley, W.C.; Francke, U. Widespread changes in dendritic and axonal morphology in Mecpp2-mutant mouse models of Rett syndrome: Evidence for disruption of neuronal networks. J. Comp. Neurol. 2009, 514, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cerdeño, V. Dendrite and spine modifications in autism and related neurodevelopmental disorders in patients and animal models. Dev. Neurobiol. 2017, 77, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Glaze, D.G.; Neul, J.L.; Percy, A.; Feyma, T.; Beisang, A.; Yaroshinsky, A.; Zuchero, D.; Horrigan, J.; Glass, L.; Jones, N.E. A Double-Blind, Randomized, Placebo-Controlled Clinical Study of Trofinetide in the Treatment of Rett Syndrome. Pediatr. Neurol. 2017, 76, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Eeg-Olofsson, O.; Al-Zuhair, A.G.; Teebi, A.S.; Al-Essa, M.M. Abnormal mitochondria in the Rett syndrome. Brain Dev. 1988, 10, 260–262. [Google Scholar] [CrossRef]
- Shulyakova, N.; Andreazza, A.C.; Mills, L.R.; Eubanks, J.H. Mitochondrial Dysfunction in the Pathogenesis of Rett syndrome: Implications for Mitochondria-Targeted Therapies. Front. Cell Neurosci. 2017, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Hoffbuhr, K.; Devaney, J.M.; LaFleur, B.; Sirianni, N.; Scacheri, C.; Giron, J.; Schuette, J.; Innis, J.; Marino, M.; Philippart, M.; et al. MeCP2 mutations in children with and without the phenotype of Rett syndrome. Neurology 2001, 56, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Matijevic, T.; Knezevic, J.; Slavica, M.; Pavelic, J. Rett syndrome: From the gene to the disease. Eur. Neurol. 2009, 61, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.W.; He, X.L.; Lin, J.; Wu, G.F.; Yue, X.; Bi, B.; Hu, J.S.; Liu, Z.S. Clinical features and MECP2 mutations in children with Rett syndrome. Zhongguo Dang Dai Er Ke Za Zhi 2014, 16, 393–396. [Google Scholar] [PubMed]
- Killian, J.T.; Lane, J.B.; Cutter, G.R.; Skinner, S.A.; Kaufmann, W.E.; Tarquinio, D.C.; Glaze, D.G.; Motil, K.J.; Neul, J.L.; Percy, A.K. Pubertal Development in Rett Syndrome Deviates from Typical Females. Pediatr. Neurol. 2014, 51, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Quintana, A.; Agra, C.; Outeiral, L.; Devesa, A.; Llorente, D.; Devesa, J. Cognitive evolution of a patient who suffered a Subarachnoid Haemorrhage eight years ago, after being treated with Growth Hormone, Melatonin and Neurorehabilitation. Reports 2018, 1, 2. [Google Scholar] [CrossRef]
- Hadzsiev, K.; Polgar, N.; Bene, J.; Komlosi, K.; Karteszi, J.; Hollody, K.; Kosztolanyi, G.; Renieri, A.; Melegh, B. Analysis of Hungarian patients with Rett syndrome phenotype for MECP2, CDKL5 and FOXG1 gene mutations. J. Hum. Genet. 2011, 56, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.E.; Johnston, M.V.; Blue, M.E. MeCP2 expression and function during brain development: Implications for Rett syndrome’s pathogenesis and clinical evolution. Brain Dev. 2005, 27 (Suppl. 1), 77–87. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.B.; Valakh, V. Excitatory/Inbitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron 2015, 87, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Ballestar, E.; Yusufzai, T.M.; Wolffe, A.P. Effects of Rett syndrome mutations of the methyl-CpG binding domain of the transcriptional repressor MeCP2 on selectivity for association with methylated DNA. Biochemistry 2000, 39, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Miller, E.C.; Pozzo-Miller, L. Dendritic spine dysgenesis in Rett syndrome. Front. Neuroanat. 2014, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Khare, G.; Dani, V.; Nelson, S.; Jaenisch, R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron 2006, 49, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Abuhatzira, L.; Makendoski, K.; Kaufman, Y.; Razin, A.; Shemer, R. MeCP2 deficiency in the brain decreases BDNF levels by REST/CoREST-mediated repression and increases TRKB production. Epigenetics 2007, 2, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, E.; Luikenhuis, S.; Beard, C.; Jaenisch, R. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proc. Natl. Acad. Sci. USA 2007, 104, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.; Gan, J.; Selfridge, J.; Cobb, S.; Bird, A. Reversal of neurological defects in a mouse model of Rett syndrome. Science 2007, 315, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Landi, S.; Putignano, E.; Boggio, E.M.; Giustetto, M.; Pizzorusso, T.; Ratto, G.M. The short-time structural plasticity of dendritic spines is altered in a model of Rett syndrome. Sci. Rep. 2011, 1, 45. [Google Scholar] [CrossRef] [PubMed]
- Tropea, D.; Giacometti, E.; Wilson, N.R.; Beard, C.; McCurry, C.; Fu, D.D.; Flannery, R.; Jaenisch, R.; Sur, M. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc. Natl. Acad. Sci. USA 2009, 106, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; García, R.I.; Kwok, S.; Banerjee, A.; Petravicz, J.; Woodson, J.; Mellios, N.; Tropea, D.; Sur, M. Functional recovery with recombinant human IGF1 treatment in a mouse model of Rett Syndrome. Proc. Natl. Acad. Sci. USA 2014, 111, 9941–9946. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, O.S.; Ho, E.; Barnes, K.V.; O’Leary, H.M.; Pereira, L.M.; Finkelstein, Y.; Nelson, C.A., 3rd; Vogel-Farley, V.; DeGregorio, G.; Holm., I.A.; et al. Safety, pharmacokinetics, and preliminary assessment of efficacy of mecasermin (recombinant human IGF-1) for the treatment of Rett syndrome. Proc. Natl. Acad. Sci. USA 2014, 111, 4596–4601. [Google Scholar] [CrossRef] [PubMed]
- Pini, G.; Congiu, L.; Benincasa, A.; DiMarco, P.; Bigoni, S.; Dyer, A.H.; Mortimer, N.; Della-Chiesa, A.; O'Leary, S.; McNamara, R.; et al. Illness Severity, Social and Cognitive Analysis, and EEG Analysis of Ten Patients with Rett Syndrome Treated with Mecasermin (Recombinant Human IGF-1). Autism Res. Treat. 2016, 2016, 5073078. [Google Scholar] [CrossRef] [PubMed]
- Riikonen, R. Treatment of autistic spectrum disorder with insulin-like growth factors. Eur. J. Paediatr. Neurol. 2016, 20, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Pathipati, P.; Gorba, T.; Scheepens, A.; Goffin, V.; Sun, Y.; Fraser, M. Growth hormone and prolactin regulate human neural stem cell regenerative activity. Neuroscience 2011, 190, 409–427. [Google Scholar] [CrossRef] [PubMed]
- McGinley, L.M.; Sims, E.; Lunn, J.S.; Kashlan, O.N.; Chen, K.S.; Bruno, E.S.; Pacut, C.M.; Hazel, T.; Johe, K.; Sakowski, S.A.; et al. Human cortical neural stem cells expressing Insulin-Like Growth Factor-I: A Novel Cellular Therapy for Alzheimer’s Disease. Stem Cells Transl. Med. 2016, 5, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Bondy, C.; Werner, H.; Roberts, C.T., Jr.; LeRoith, D. Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: Comparison with insulin-like growth factors I and II. Neuroscience 1992, 46, 909–923. [Google Scholar] [CrossRef]
- Fernández, A.M.; Torres-Alemán, I. The many faces of insulin-like peptide signaling in the brain. Nat. Rev. Neurosci. 2012, 13, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Costales, J.; Kolevzon, A. The Therapeutic Potential of Insulin-Like Growth Factor-1 in Central Nervous System Disorders. Neurosci. Biobehav. Rev. 2016, 63, 207–222. [Google Scholar] [CrossRef] [PubMed]
- O’Kusky, J.; Ye, P. Neurodevelopmental effects of insulin-like growth factor signaling. Front. Neuroendocrinol. 2012, 33, 230–251. [Google Scholar] [CrossRef] [PubMed]
- Huppke, P.; Roth, C.; Christen, H.J.; Brockmann, K.; Hanefeld, F. Endocrinological study on growth retardation in Rett syndrome. Acta Paediatr. 2001, 90, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Nishi, Y.; Yamashita, Y.; Hirata, R.; Takahashi, S.; Nagamitsu, S.; Hosoda, H.; Kangawa, K.; Kojima, M.; Matsuishi, T. Relation between circulating levels of GH, IGF-1, ghrelin and somatic growth in Rett syndrome. Brain Dev. 2014, 36, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Almengló, C.; Devesa, P. Multiple Effects of Growth hormone in the Body: Is it Really the Hormone for Growth? Clin. Med. Insights Endocrinol. Diabetes 2016, 9, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Tarquinio, D.C.; Motil, K.J.; Hou, W.; Lee, H.S.; Glaze, D.G.; Skinner, S.A.; Neul, J.L.; Annese, F.; McNair, L.; Barrish, J.O.; et al. Growth failure and outcome in Rett syndrome: Specific growth references. Neurology 2012, 76, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Ranke, M.B. Insulin-like growth factor binding-protein-3 (IGFBP-3). Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Gandaglia, A.; Galli, F.; Tirone, M.; Bellini, E.; Campana, L.; Kilstrup-Nielsen, C.; Rovere-Querini, P.; Brunelli, S.; Landsberger, N. MeCP2 Affects Skeletal Muscle Growth and Morphology through Non Cell-Autonomous Mechanisms. PLoS ONE 2015, 10, e0130183. [Google Scholar] [CrossRef] [PubMed]
- Poling, J.S.; Frye, R.E.; Shoffner, J.; Zimmerman, A.W. Developmental regression and Mitochondrial Dysfunction in a Child with Autism. J. Child Neurol. 2006, 21, 170–172. [Google Scholar] [CrossRef] [PubMed]
- De Felice, C.; Signorini, C.; Leoncini, S.; Pecorelli, A.; Durand, T.; Valacchi, G.; Ciccoli, L.; Hayek, J. Oxidative stress and Rett syndrome. Minerva Pediatr. 2014, 66, 41–62. [Google Scholar] [PubMed]
- Filosa, S.; Pecorelli, A.; D’Esposito, M.; Valacchi, G.; Hajek, J. Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome. Free Radic. Biol. Med. 2015, 88, 81–90. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, B.; Valenti, D.; de Bari, L.; De Rasmo, D.; Musto, M.; Fabbri, A.; Ricceri, L.; Fiorentini, C.; Laviola, G.; Vacca, R.A. Mitochondrial free radical overproduction due to respiratory chain impairment in the brain of a mouse model of Rett syndrome: Protective effect of CNF1. Free Radic. Biol. Med. 2015, 83, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Functional aspects of the pineal hormone melatonin in combating cell and tissue damage induced by free radicals. Eur. J. Endocrinol. 1996, 134, 412–430. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Antioxidant actions of melatonin. Adv. Pharmacol. 1997, 38, 103–117. [Google Scholar] [PubMed]
- Sánchez, A.; Calpena, A.C.; Clares, B. Evaluating the Oxidative Stress in Inflammation. Int. J. Mol. Sci. 2015, 16, 16981–17004. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin and brain inflammaging. Prog. Neurobiol. 2015, 127–128, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Fan, C.; Hu, W.; Jiang, S.; Ma, Z.; Yan, X.; Deng, C.; Di, S.; Xin, Z.; Wu, G.; et al. Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signaling. J. Pineal Res. 2016, 60, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Vigo, D.E. Melatonin, mitochondria, and the metabolic syndrome. Cell Mol. Sci. 2017, 74, 3941–3954. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Franceschetti, L.; Bonomini, F.; Rodella, L.F.; Rezzani, R. Melatonin as an Anti-Inflammatory Agent Modulating Inflammasome Activation. Int. J. Endocrinol. 2017, 2017, 1835195. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, L.; Nunez-Abades, P.; Ayala, A.; Cano, M. Role of melatonin in the inflammatory process and its therapeutic potential. Curr. Pharm. Des. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.I.; Carretero, M.; Escames, G.; López, L.C.; Maldonado, M.D.; Tan, D.X.; Reiter, R.J.; Acuña-Castroviejo, D. Chronic melatonin treatment prevents age-dependent cardiac mitochondrial dysfunction in senescence-accelerated mice. Free Radic. Res. 2007, 41, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Sara, V.R.; Carlsson-Skwirut, C.; Bergman, T.; Jörnvall, H.; Roberts, P.J.; Crawford, M.; Hakansson, L.N.; Civalero, I.; Nordberg, A. Identification of Gly-Pro-Glu (GPE), the aminoterminal tripeptide of insulin-like growth factor 1 which is truncated in brain, as a novel neuroactive peptide. Biochem. Biophys. Res. Commun. 1989, 165, 766–771. [Google Scholar] [CrossRef]
- Yamamoto, H.; Murphy, L.J. Enzymatic conversion of IGF-I to des (1–3) IGF-I in rat serum and tissues: A further potential site of growth hormone regulation of IGF-I action. J. Endocrinol. 1995, 146, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Murphy, L.J. N-terminal truncated insulin-like growth factor-I in human urine. J. Clin. Endocrinol. Metab. 1995, 80, 1179–1183. [Google Scholar] [PubMed]
- Almengló, C.; Devesa, P.; Devesa, J.; Arce, V. GPE promotes the proliferation and migration of mouse embryonic neural stem cells and their progeny in vitro. Int. J. Mol. Sci. 2017, 18, 1280. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Gluckman, P.; Yang, P.; Krissansen, G.; Sun, X.; Zhou, Y.; Wen, J.; Phillips, G.; Shorten, P.R.; McMahon, C.D.; et al. Cyclic glycine-proline regulates IGF-1 homeostasis by altering the binding of IGFBP-3 to IGF-1. Sci. Rep. 2014, 4, 4388. [Google Scholar] [CrossRef] [PubMed]
- Sing-Mallah, G.; McMahon, C.D.; Guan, J.; Singh, K. Cyclic-glycine-proline accelerates mammary involution by promoting apoptosis and inhibiting IGF-1 function. J. Cell Physiol. 2017, 232, 3369–3383. [Google Scholar] [CrossRef] [PubMed]
- Singh-Mallah, G.; Sing, K.; McMahon, C.D.; Harris, P.; Brimble, M.A.; Thorstensen, E.; Guan, J. Maternally administered Cyclic Glycine-Proline increases Insulin-Like Growth Factor-1 Bioavailability and Novelty Recognition in Developing Offspring. Endocrinology 2016, 157, 3130–3139. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Tew, K.D.; Ba, G.N.; Mathe, G. Polyphenols: Do they play a role in the prevention of human pathologies? Biomed. Pharmacother. 2002, 56, 200–207. [Google Scholar] [CrossRef]
- Ghosh, D.K. Anthocyanins and anthocyanin-rich extracts in biology and medicine: Biochemical, cellular and medicinal properties. Curr. Top. Nutraceut. Res. 2005, 3, 113–124. [Google Scholar]
- Kim, M.J.; Rehman, S.U.; Amin, F.U.; Kim, M.O. Enhanced neuroprotection of anthocyanin-loaded PEG-gold nanoparticles against Aß1-42-induced neuroinflammation and neurodegeneration via the NF-KB/JNK/GSK3ß signaling pathway. Nanomedicine 2017, 13, 2533–2544. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Ross, E.K.; Wilkins, H.M.; Stankiewicz, T.R.; Wallace, T.; Miller, K.; Linseman, D.A. An anthocyanin-enriched extract from strawberries delays disease onset and extends survival in the hSOD1G93A mouse model of amyotrophic lateral sclerosis. Nutr. Neurosci. 2018, 21, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Chung, J.I.; Kim, M.O. Anthocyanins Improve Hippocampus-Dependent Memory Function and Prevent Neurodegeneration via JNK/Akt/GSK3ß Signaling in LPS-Treated Adult Mice. Mol. Neurobiol. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Alamri, Y.; Liu, K.; MacAskill, M.; Harris, P.; Brimble, M.; Dalrympe-Alford, J.; Prickett, T.; Menzies, O.; Laurenson, A.; et al. Supplementation of Blackcurrant Anthocyanins Increased Cyclic Glicine-Proline in the Cerebrospinal Fluid of Parkinson Patients: Potential Treatment to Improve Insulin-Like Growth Factor-1 Function. Nutrients 2018, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Bas, V.N.; Çetinkaya, S.; Ağladıoğlu, S.Y.; Aksoy, A.; Gülpınar, B.; Aycan, Z. Report of the first case of precocious puberty in Rett syndrome. J. Pediatr. Endocrinol. Metab. 2013, 26, 937–939. [Google Scholar] [CrossRef] [PubMed]
- Ben-Schachar, S.; Chahrour, M.; Thaller, C.; Shaw, C.A.; Zoghbi, H.Y. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum. Mol. Genet. 2009, 18, 2431–2442. [Google Scholar] [CrossRef] [PubMed]
- Westberry, J.M.; Trout, A.L.; Wilson, M.E. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology 2010, 151, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Lima, L.; Tresguerres, J.A. Neuroendocrine control of growth hormone secretion in humans. Trends Endocrinol. Metab. 1992, 3, 175–183. [Google Scholar] [CrossRef]
- Keshin, M.; Erdeve, S.S.; Aycan, Z. Rett syndrome and precocius puberty association. J. Pediatr. Endocrinol. Metab. 2015, 28, 1197. [Google Scholar]
- McGill, B.E.; Bundle, S.F.; Yaylaoglu, M.B.; Carson, J.P.; Thaller, C.; Zoghbi, H.Y. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 18267–18272. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).