Current Knowledge in Planarian Glia and Its Future Implications in Modeling Neurodegenerative Diseases
Abstract
1. Introduction
2. Planarian Nervous System and Neuronal Subtypes
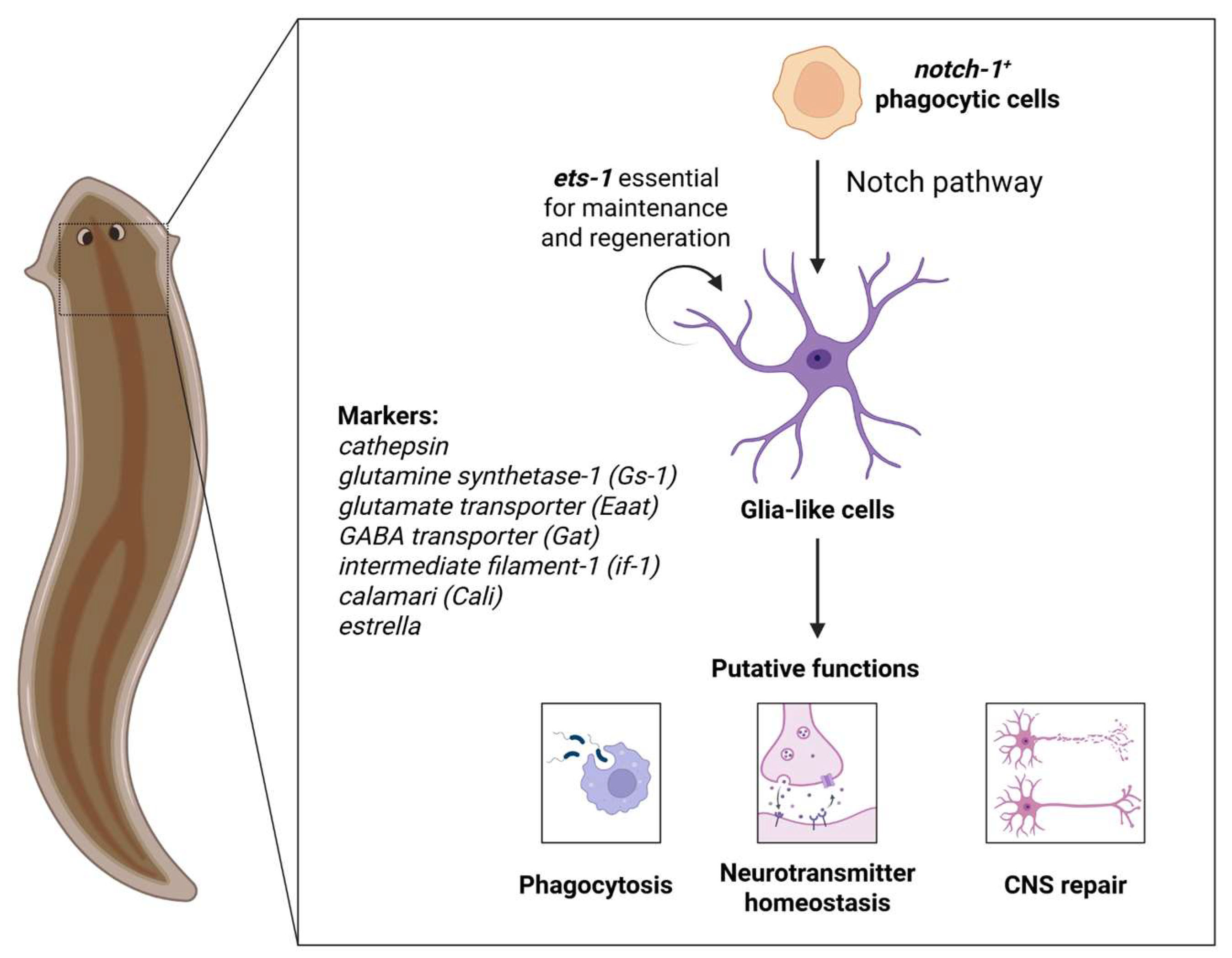
3. Origin and Putative Functions of Planarian Glia
| Feature | Mammalian Glia | Planarian Glia |
|---|---|---|
| Main Types | Astrocytes, oligodendrocytes, microglia, ependymal cells [39] | Glia-like cells (not fully diversified; mostly astrocyte-like and ependymal-like) [40] |
| Origin | Derived from neural progenitors (radial glia), yolk sac precursors [35,41] | Specified from notch-1-expressing mesoderm-like phagocytic progenitors [34] |
| Functions in CNS Support | Metabolic and trophic support, ion and neurotransmitter balance, myelin synthesis, immune response, CNS metabolism [35,36,37,42] | Envelop neuronal somata/processes; regulate neuronal microenvironment [18] |
| Role in Synaptic Function | Modulate synaptic transmission, neurotransmitter uptake (e.g., glutamate, GABA) [43] | Express orthologs of astrocytic transporters (slc1a-5/EAAT, GAT, glutamine synthetase/GS-1) [30] |
| Immune Response | Microglia as CNS-resident immune cells [35,44] | No microglia; innate immunity only [45] |
| Regeneration | Limited (mainly in PNS, poor in CNS) [46] | Extensive: glia-like cells participate in CNS repair [40] |
| Molecular Markers | Astrocytes: GFAP, EAAT1-2, S100β and others. Oligodendrocytes: MBP, MAG, Olig2 and others. Microglia: Iba1, iNOS, TNF-α and others [35,37,47,48] | IF-1 (cytoskeletal component), cali (unknown function), cathepsin (lysosomal protease), estrella (unknown function), GS-1, slc1a-5/EAAT, GAT (neurotransmitter homeostasis) [30,31] |
| Role in Neurodegeneration | Glial dysfunction implicated in AD, PD, ALS, MS and others [49] | Unknown |
4. Planarians as a Model for Studying Neurodegenerative Diseases
5. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| AMD | Age-related macular degeneration |
| cali | Calamari |
| CBS | Corticobasal syndrome |
| CNS | Central nervous system |
| COM | Center of mass tracking |
| DjAADCA | Aromatic amino acid descarboxylase-like gene in Dugesia japonica |
| DjChAT | Choline acetyltransferase gene in Dugesia japonica |
| DjDAT | Dopamine transporter gene in Dugesia japonica |
| DjGAD | Glutamic acid decarboxylase gene in Dugesia japonica |
| DjTH | Tyrosine hydroxylase gene in Dugesia japonica |
| EAAT | Excitatory amino acid transporter |
| FTD | Frontotemporal dementia |
| GABA | Gamma-aminobutyric acid |
| GAT | GABA transporter |
| GFAP | Glial fibrillary acidic protein |
| GS-1 | Glutamine synthetase-1 |
| HD | Huntington’s disease |
| hh | Hedgehog |
| if-1 | Intermediate filament-1 |
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| MAG | Myelin associated glycoprotein |
| MBP | Myelin basic protein |
| MEK/ERK pathway | Mitogen-activated protein kinase/Extracellular signal-regulated kinase pathway |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine |
| MS | Multiple sclerosis |
| MSA | Multiple system atrophy |
| PD | Parkinson’s disease |
| pLMV | Planarian locomotor velocity |
| PNS | Peripheral nervous system |
| PSP | Progressive supranuclear palsy |
| RNAi | Ribonucleic acid interference |
| SMA | Spinal muscular atrophy |
| SOD1 | Superoxide dismutase 1 |
| VNCs | Ventral nerve cords |
References
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Temple, S. Advancing Cell Therapy for Neurodegenerative Diseases. Cell Stem Cell 2023, 30, 512–529. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Ettle, B.; Schlachetzki, J.C.M.; Winkler, J. Oligodendroglia and Myelin in Neurodegenerative Diseases: More Than Just Bystanders? Mol. Neurobiol. 2016, 53, 3046–3062. [Google Scholar] [CrossRef]
- Clement, A.M.; Nguyen, M.D.; Roberts, E.A.; Garcia, M.L.; Boillée, S.; Rule, M.; McMahon, A.P.; Doucette, W.; Siwek, D.; Ferrante, R.J.; et al. Domains, Intracellular Domain Associations Would Be Required to Bring These Groups Together into an Adhesive Patch with a Suf-Ficient Number of Intercellular Bonds to Resist Shear Forces. Science 2003, 302, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Van Harten, A.C.M.; Phatnani, H.; Przedborski, S. Non-Cell-Autonomous Pathogenic Mechanisms in Amyotrophic Lateral Sclerosis. Trends Neurosci. 2021, 44, 658–668. [Google Scholar] [CrossRef]
- Boillée, S.; Yamanaka, K.; Lobsiger, C.S.; Copeland, N.G.; Jenkins, N.A.; Kassiotis, G.; Kollias, G.; Cleveland, D.W. Onset and Progression in Inherited ALS Determined by Motor Neurons and Microglia. Science 2006, 312, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.L.; Leenders, K.L. Neuroinflammation in the Pathophysiology of Parkinson’s Disease: Evidence from Animal Models to Human in Vivo Studies with [11C]-PK11195 PET. Mov. Disord. 2007, 22, 1852–1856. [Google Scholar] [CrossRef] [PubMed]
- Panicker, N.; Ge, P.; Dawson, V.L.; Dawson, T.M. The Cell Biology of Parkinson’s Disease. J. Cell Biol. 2021, 220, e202012095. [Google Scholar] [CrossRef]
- Acioglu, C.; Li, L.; Elkabes, S. Contribution of Astrocytes to Neuropathology of Neurodegenerative Diseases. Brain Res. 2021, 1758, 147291. [Google Scholar] [CrossRef]
- Gurney, M.E.; Pu, H.; Chiu, A.Y.; Dal Canto, M.C.; Polchow, C.Y.; Alexander, D.D.; Caliendo, J.; Hentati, A.; Kwon, Y.W.; Deng, H.X.; et al. Motor Neuron Degeneration in Mice That Express a Human Cu, Zn Superoxide Dismutase Mutation. Science 1994, 264, 1772–1775. [Google Scholar] [CrossRef]
- Borchelt, D.R.; Ratovitski, T. Accelerated Amyloid Deposition in the Brains of Transgenic Mice Coexpressing Mutant Presenilin 1 and Amyloid Precursor Proteins. Neuron 1997, 19, 939–945. [Google Scholar] [CrossRef]
- Jankowsky, J.L.; Slunt, H.H.; Gonzales, V.; Jenkins, N.A.; Copeland, N.G.; Borchelt, D.R. APP Processing and Amyloid Deposition in Mice Haplo-Insufficient for Presenilin 1. Neurobiol. Aging 2004, 25, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Giasson, B.I.; Duda, J.E.; Quinn, S.M.; Zhang, B.; Trojanowski, J.Q.; M-Y Lee, V. Neuronal-Synucleinopathy with Severe Movement Disorder in Mice Expressing A53T Human-Synuclein. Neuron 2002, 34, 521–533. [Google Scholar] [CrossRef]
- Pasko, V.I.; Churkina, A.S.; Shakhov, A.S.; Kotlobay, A.A.; Alieva, I.B. Modeling of Neurodegenerative Diseases: ‘Step by Step’ and ‘Network’ Organization of the Complexes of Model Systems. Int. J. Mol. Sci. 2023, 24, 604. [Google Scholar] [CrossRef]
- De Vries, E.J.; Sluys, R. Phylogenetic Relationships of the Genus Dugesia (Platyhelminthes, Tricladida, Paludicola). J. Zool. 1991, 223, 103–116. [Google Scholar] [CrossRef]
- Reho, G.; Lelièvre, V.; Cadiou, H. Planarian Nociception: Lessons from a Scrunching Flatworm. Front. Mol. Neurosci. 2022, 15, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Cebrià, F. Regenerating the Central Nervous System: How Easy for Planarians! Dev. Genes. Evol. 2007, 217, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Kitamura, Y.; Inoue, T.; Umesono, Y.; Sano, S.; Yoshimoto, K.; Inden, M.; Takata, K.; Taniguchi, T.; Shimohama, S.; et al. Reconstruction of Dopaminergic Neural Network and Locomotion Function in Planarian Regenerates. Dev. Neurobiol. 2007, 67, 1059–1078. [Google Scholar] [CrossRef]
- Currie, K.W.; Molinaro, A.M.; Pearson, B.J. Neuronal sources of hedgehog modulate neurogenesis in the adult planarian brain. eLife 2016, 5, e19735. [Google Scholar] [CrossRef]
- Reddien, P.W. The Cellular and Molecular Basis for Planarian Regeneration. Cell 2018, 175, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.Y.; Han, X.; Zhao, Y.L.; Cui, G.S.; Yang, Y.G. An Insight into Planarian Regeneration. Cell Prolif. 2022, 55, e13276. [Google Scholar] [CrossRef]
- Yoshihisa, K.; Jun-ichi, K.; Takashi, T. Protective Effect of Talipexole on MPTP-Treated Planarian, a Unique Parkinsonian Worm Model. Jpn. J. Pharmacol. 1998, 78, 23–29. [Google Scholar] [CrossRef]
- Kitamura, Y.; Inden, M.; Sanada, H.; Takata, K.; Taniguchi, T.; Shimohama, S.; Orii, H.; Mochii, M.; Agata, K.; Watanabe, K. Inhibitory Effects of Antiparkinsonian Drugs and Caspase Inhibitors in a Parkinsonian Flatworm Model. J. Pharmacol. Sci. 2003, 92, 137–142. [Google Scholar] [CrossRef][Green Version]
- Raffa, R.B.; Danah, J.; Tallarida, C.S.; Zimmerman, C.; Gill, G.; Baron, S.J.; Rawls, S.M. Potential of a Planarian Model to Study Certain Aspects of Anti-Parkinsonism Drugs. Adv. Park. Dis. 2013, 2, 70–74. [Google Scholar] [CrossRef]
- Nishimura, K.; Kitamura, Y.; Taniguchi, T.; Agata, K. Analysis of Motor Function Modulated by Cholinergic Neurons in Planarian Dugesia Japonica. Neuroscience 2010, 168, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Inoue, T.; Yoshimoto, K.; Taniguchi, T.; Kitamura, Y.; Agata, K. Regeneration of Dopaminergic Neurons after 6-Hydroxydopamine-Induced Lesion in Planarian Brain. J. Neurochem. 2011, 119, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Kitamura, Y.; Umesono, Y.; Takeuchi, K.; Takata, K.; Taniguchi, T.; Agata, K. Identification of Glutamic Acid Decarboxylase Gene and Distribution of GABAergic Nervous System in the Planarian Dugesia Japonica. Neuroscience 2008, 153, 1103–1114. [Google Scholar] [CrossRef]
- Currie, K.W.; Pearson, B.J. Transcription Factors Lhx1/5-1 and Pitx Are Required for the Maintenance and Regeneration of Serotonergic Neurons in Planarians. Development 2013, 140, 3577–3588. [Google Scholar] [CrossRef]
- Roberts-Galbraith, R.H.; Brubacher, J.L.; Newmark, P.A. A Functional Genomics Screen in Planarians Reveals Regulators of Whole-Brain Regeneration. eLife 2016, 5, e17002. [Google Scholar] [CrossRef][Green Version]
- Wang, I.E.; Lapan, S.W.; Scimone, M.L.; Clandinin, T.R.; Reddien, P.W. Hedgehog Signaling Regulates Gene Expression in Planarian Glia. eLife 2016, 5, e16996. [Google Scholar] [CrossRef]
- Chandra, B.; Voas, M.G.; Davies, E.L.; Roberts-Galbraith, R.H. Ets-1 Transcription Factor Regulates Glial Cell Regeneration and Function in Planarians. Development 2023, 150, dev201666. [Google Scholar] [CrossRef] [PubMed]
- Scimone, M.L.; Wurtzel, O.; Malecek, K.; Fincher, C.T.; Oderberg, I.M.; Kravarik, K.M.; Reddien, P.W. FoxF-1 Controls Specification of Non-Body Wall Muscle and Phagocytic Cells in Planarians. Curr. Biol. 2018, 28, 3787–3801.e6. [Google Scholar] [CrossRef] [PubMed]
- Scimone, L.L.; Canales, B.I.I.; Aoude, P.; Atabay, K.D.; Reddien, P.W. Coordinated Neuron-Glia Regeneration through Notch Signaling in Planarians. PLoS Genet. 2025, 21, e1011577. [Google Scholar] [CrossRef]
- Lannes, N.; Eppler, E.; Etemad, S.; Yotovski, P.; Filgueira, L. Microglia at Center Stage: A Comprehensive Review about the Versatile and Unique Residential Macrophages of the Central Nervous System. Oncotarget 2017, 8, 114393–114413. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Dai, X.; Li, X.; Tyshkovskiy, A.; Zuckerman, C.; Cheng, N.; Lin, P.; Paris, D.; Qureshi, S.; Kruglyak, L.; Mao, X.; et al. Regeneration Leads to Global Tissue Rejuvenation in Aging Sexual Planarians. Nat. Aging 2025, 5, 780–798. [Google Scholar] [CrossRef]
- Zuchero, J.B.; Barres, B.A. Glia in Mammalian Development and Disease. Development 2015, 142, 3805–3809. [Google Scholar] [CrossRef]
- Cowles, M.W.; Brown, D.D.R.; Nisperos, S.V.; Stanley, B.N.; Pearson, B.J.; Zayas, R.M. Genome-Wide Analysis of the BHLH Gene Family in Planarians Identifies Factors Required for Adult Neurogenesis and Neuronal Regeneration. Development 2013, 140, 4691–4702. [Google Scholar] [CrossRef]
- Malatesta, P.; Hartfuss, E.; Götz, M. Isolation of Radial Glial Cells by Fluorescent-Activated Cell Sorting Reveals a Neural Lineage. Development 2000, 127, 5253–5263. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Gan, L.; Liu, C.; Xu, T.; Zhou, S.; Guo, Y.; Zhang, Z.; Yang, G.Y.; Tian, H.; Tang, Y. Roles of Ependymal Cells in the Physiology and Pathology of the Central Nervous System. Aging Dis. 2023, 14, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.R.; Robitaille, R.; Volterra, A. Gliotransmitters Travel in Time and Space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef]
- Kangale, L.J.; Raoult, D.; Fournier, P.E.; Abnave, P.; Ghigo, E. Planarians (Platyhelminthes)—An Emerging Model Organism for Investigating Innate Immune Mechanisms. Front. Cell. Infect. Microbiol. 2021, 11, 619081. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the Glial Scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef]
- Mirarchi, A.; Albi, E.; Arcuri, C. Microglia Signatures: A Cause or Consequence of Microglia-Related Brain Disorders? Int. J. Mol. Sci. 2024, 25, 10951. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Ho, M.S.; Zorec, R.; Parpura, V. Physiology of astroglia. In Neuroglia in Neurodegenerative Diseases; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Inoue, T.; Hoshino, H.; Yamashita, T.; Shimoyama, S.; Agata, K. Planarian Shows Decision-Making Behavior in Response to Multiple Stimuli by Integrative Brain Function. Zool. Lett. 2015, 1, 7. [Google Scholar] [CrossRef]
- Inoue, T.; Agata, K. Quantification of Planarian Behaviors. Dev. Growth Differ. 2022, 64, 16–37. [Google Scholar] [CrossRef]
- Hagstrom, D.; Cochet-Escartin, O.; Collins, E.S. Planarian Brain Regeneration as a Model System for Developmental Neurotoxicology. Regeneration 2016, 3, 65–77. [Google Scholar] [CrossRef]
- Buttarelli, F.R.; Pontieri, F.E.; Margotta, V.; Palladini, G. Acetylcholine/Dopamine Interaction in Planaria. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 2000, 125, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, N.; Nishimura, K.; Daido, K.; Oka, T.; Todo, M.; Toshikawa, A.; Tsushima, J.; Takata, K.; Ashihara, E.; Yoshimoto, K.; et al. Pharmacological Assessment of Methamphetamine-Induced Behavioral Hyperactivity Mediated by Dopaminergic Transmission in Planarian Dugesia Japonica. Biochem. Biophys. Res. Commun. 2014, 449, 412–418. [Google Scholar] [CrossRef]
- Hijioka, M.; Ikemoto, Y.; Fukao, K.; Inoue, T.; Kobayakawa, T.; Nishimura, K.; Takata, K.; Agata, K.; Kitamura, Y. MEK/ERK Signaling Regulates Reconstitution of the Dopaminergic Nerve Circuit in the Planarian Dugesia Japonica. Neurochem. Res. 2022, 47, 2558–2567. [Google Scholar] [CrossRef]
- Miller, T.; Vu, J.; Tran, C.J.; Ritvik, A.; Cruz-Ham, K.; Haq, K.; Musto, B.; Musto, A.E. Planarian as an Animal Model for Experimental Acute Seizure. J. Vis. Exp. 2025, 216, e67307. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, D.; Cochet-Escartin, O.; Zhang, S.; Khuu, C.; Collins, E.M. Freshwater Planarians as an Alternative Animal Model for Neurotoxicology. Toxicol. Sci. 2015, 147, 270–285. [Google Scholar] [CrossRef]
- Zhang, S.; Hagstrom, D.; Hayes, P.; Graham, A.; Collins, E.M. Multi-Behavioral Endpoint Testing of an 87-Chemical Compound Library in Freshwater Planarians. Toxicol. Sci. 2019, 167, 26–44. [Google Scholar] [CrossRef]
- Livengood, E.J.; Fong, R.A.M.V.; Pratt, A.M.; Alinskas, V.O.; Van Gorder, G.; Mezzio, M.; Mulligan, M.E.; Voura, E.B. Taurine stimulation of planarian motility: A role for the dopamine receptor pathway. PeerJ 2024, 12, e18671. [Google Scholar] [CrossRef]
- Talbot, J.; Schötz, E.M. Quantitative Characterization of Planaria Wild-Type Behavior as a Platform for Screening Locomotion Phenotypes. J. Exp. Biol. 2011, 214, 1063–1067. [Google Scholar] [CrossRef]
- Cochet-Escartin, O.; Mickolajczyk, K.; Collins, E.M. Scrunching: A Novel Escape Gait in Planarians. Phys. Biol. 2015, 12, 056010. [Google Scholar] [CrossRef] [PubMed]
- Ireland, D.; Collins, E.M. New Worm on the Block: Planarians in (Neuro) toxicology. Curr. Protoc. 2022, 2, e637. [Google Scholar] [CrossRef] [PubMed]
- Ireland, D.; Word, L.J.; Collins, E.S. Statistical analysis of multi-endpoint phenotypic screening increases sensitivity of planarian neurotoxicity testing. Toxicol. Sci. 2025, kfaf117. [Google Scholar] [CrossRef] [PubMed]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Hodebourg, R.; Scofield, M.D.; Kalivas, P.W.; Kuhn, B.N. Nonneuronal contributions to synaptic function. Neuron 2025, 113, 2399–2415. [Google Scholar] [CrossRef]
- Collins, E.M.; Hessel, E.V.S.; Hughes, S. How Neurobehavior and Brain Development in Alternative Whole-Organism Models Can Contribute to Prediction of Developmental Neurotoxicity. Neurotoxicology 2024, 102, 48–57. [Google Scholar] [CrossRef]
- Ireland, D.; Rabeler, C.; Rao, S.; Richardson, R.J.; Collins, E.S. Distinguishing classes of neuroactive drugs based on computational physicochemical properties and experimental phenotypic profiling in planarians. PLoS ONE 2025, 20, e0315394. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, D.; Alarcón, V.; Vásquez-Doorman, C. Current Knowledge in Planarian Glia and Its Future Implications in Modeling Neurodegenerative Diseases. Neuroglia 2025, 6, 37. https://doi.org/10.3390/neuroglia6040037
Gonzalez D, Alarcón V, Vásquez-Doorman C. Current Knowledge in Planarian Glia and Its Future Implications in Modeling Neurodegenerative Diseases. Neuroglia. 2025; 6(4):37. https://doi.org/10.3390/neuroglia6040037
Chicago/Turabian StyleGonzalez, David, Víctor Alarcón, and Constanza Vásquez-Doorman. 2025. "Current Knowledge in Planarian Glia and Its Future Implications in Modeling Neurodegenerative Diseases" Neuroglia 6, no. 4: 37. https://doi.org/10.3390/neuroglia6040037
APA StyleGonzalez, D., Alarcón, V., & Vásquez-Doorman, C. (2025). Current Knowledge in Planarian Glia and Its Future Implications in Modeling Neurodegenerative Diseases. Neuroglia, 6(4), 37. https://doi.org/10.3390/neuroglia6040037





