Astrocyte FABP7 Modulates Seizure Activity-Dependent Protein Expression in Mouse Brain
Abstract
1. Introduction
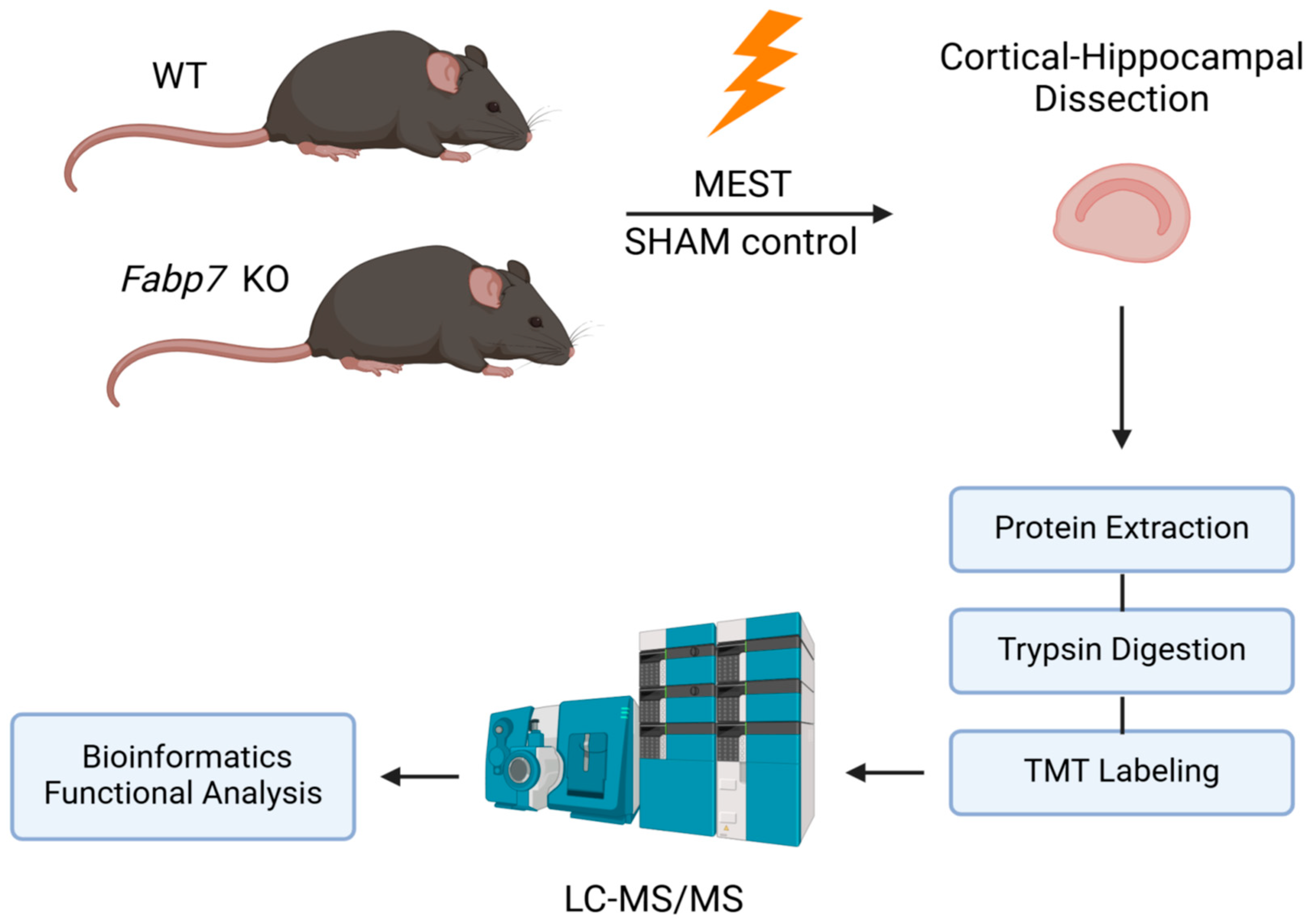
2. Materials and Methods
2.1. Animals and Seizure Threshold
Seizure Tests and Tissue Dissection
2.2. Protein Extraction, Trypsin Digestion, and LC-MS/MS
2.3. Data Analysis
Availability of Data
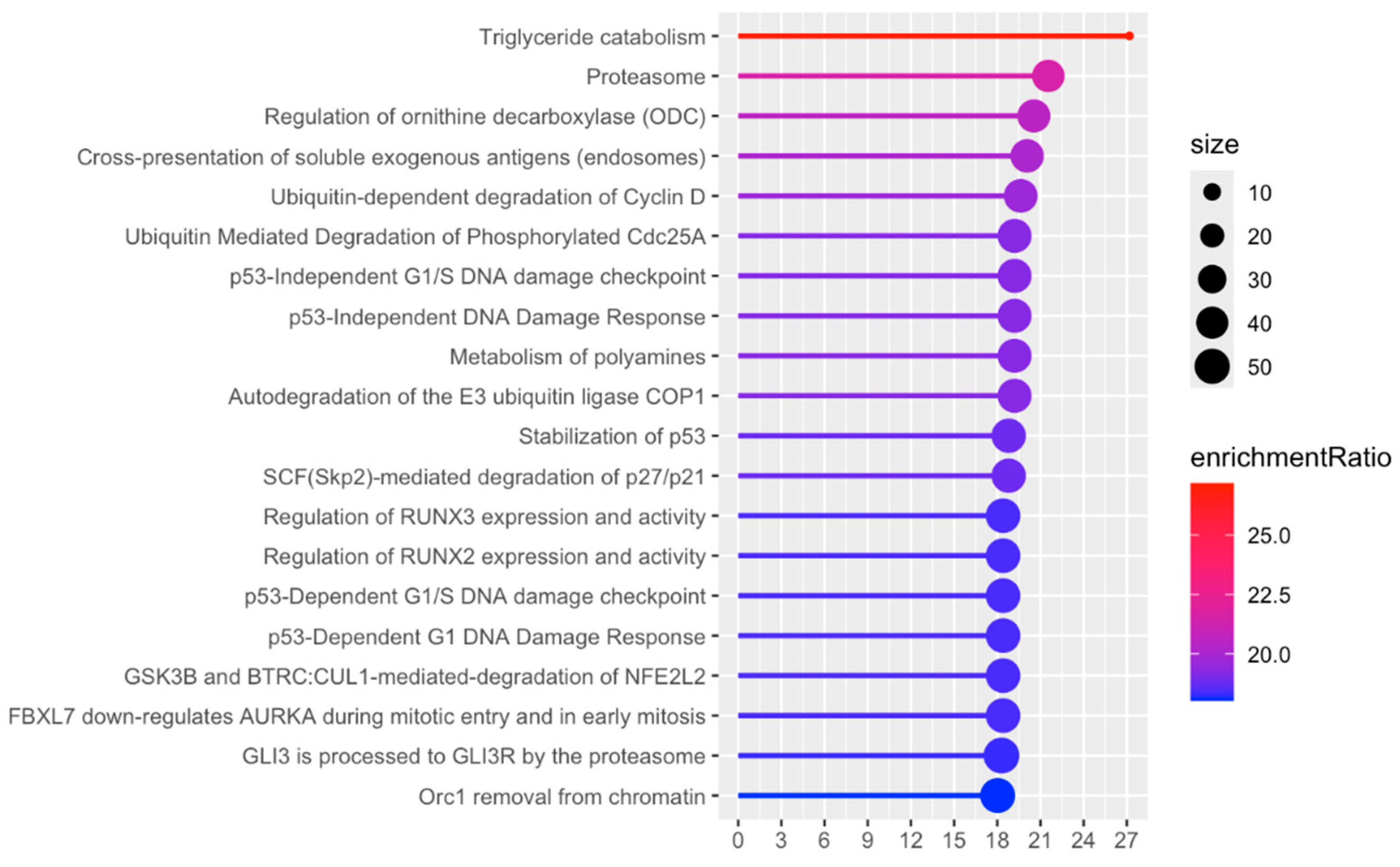
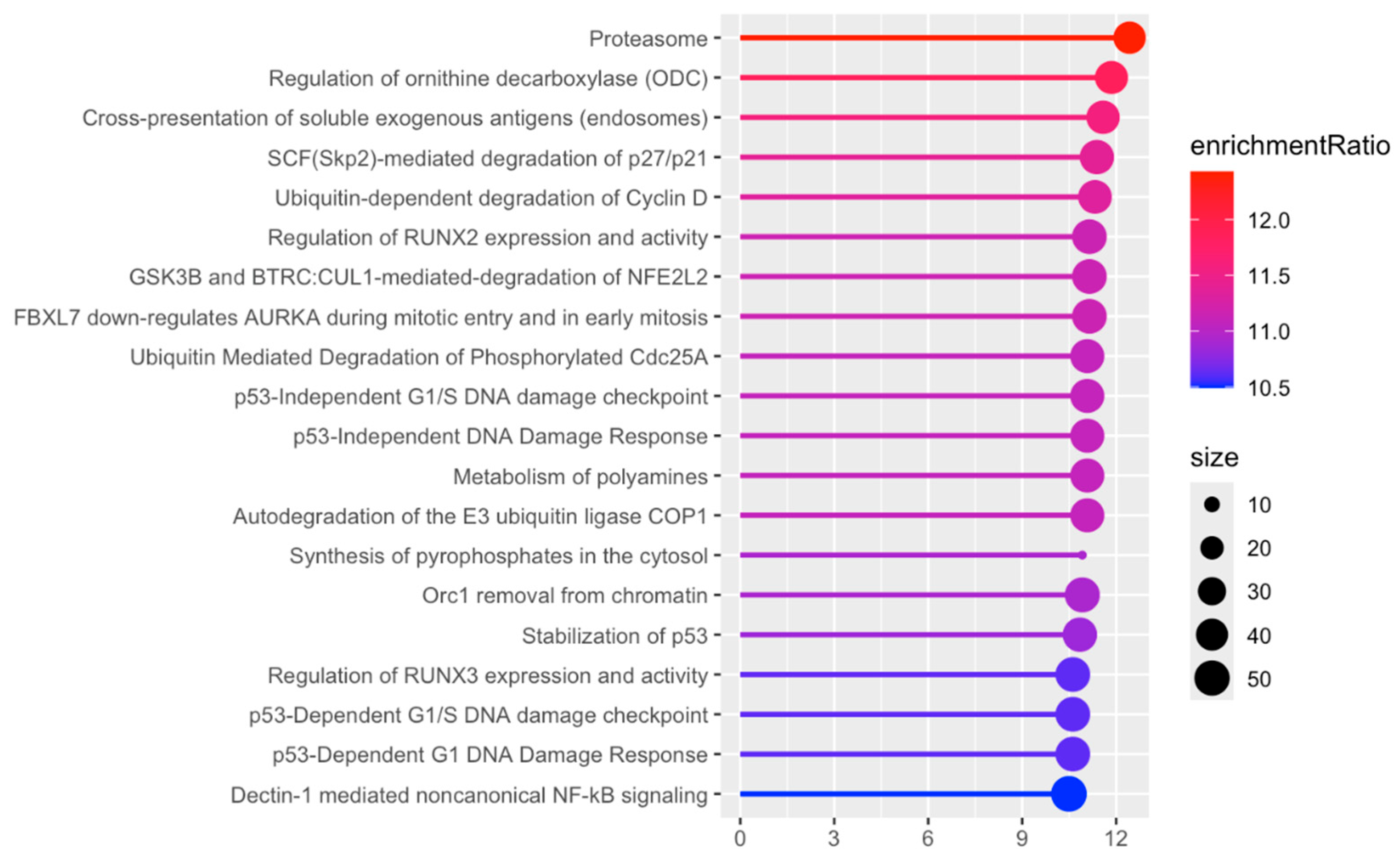
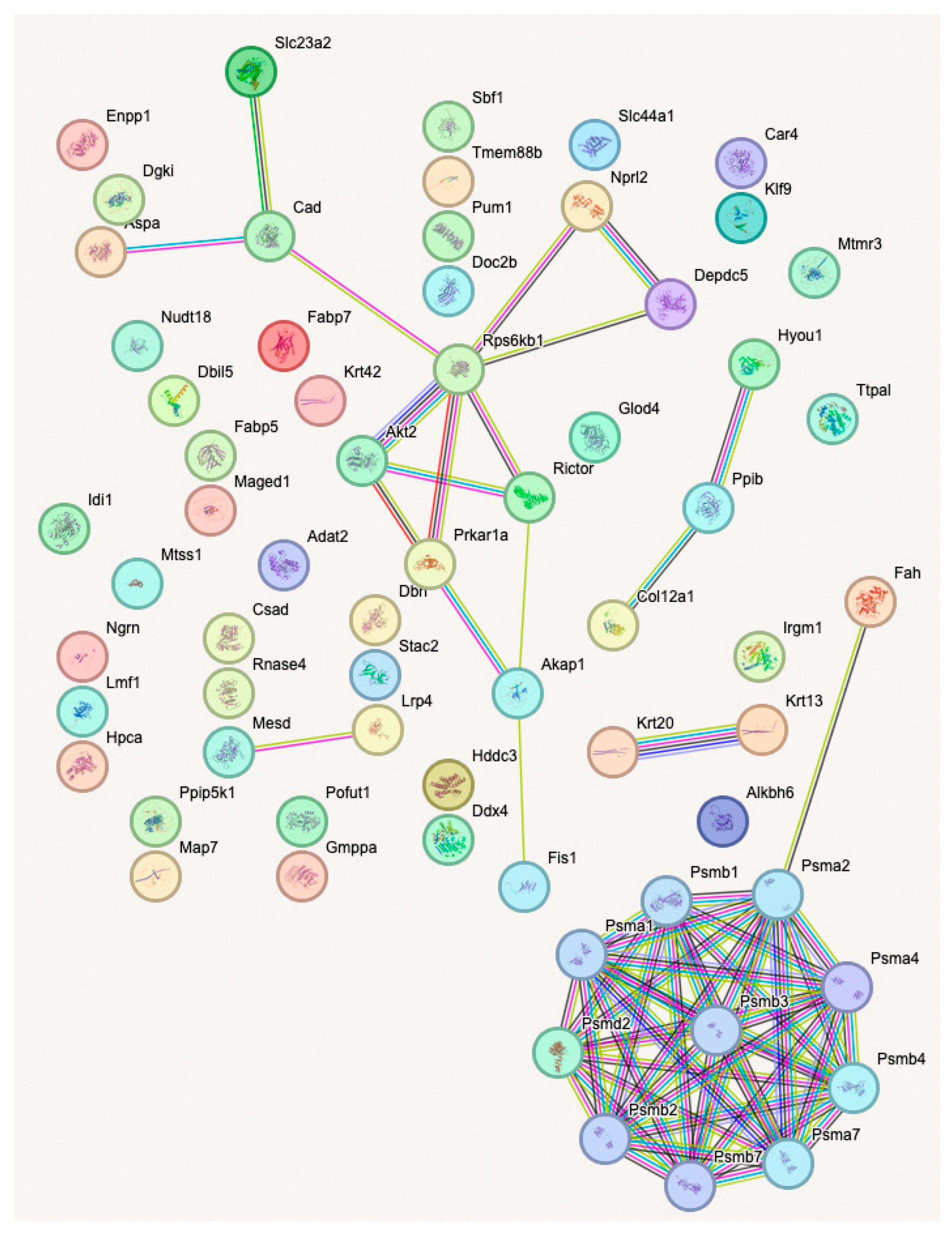
2.4. Bioinformatic Analysis
3. Results
3.1. Changes in MEST Between WT Versus Fabp7 KO
3.2. Proteomic Changes
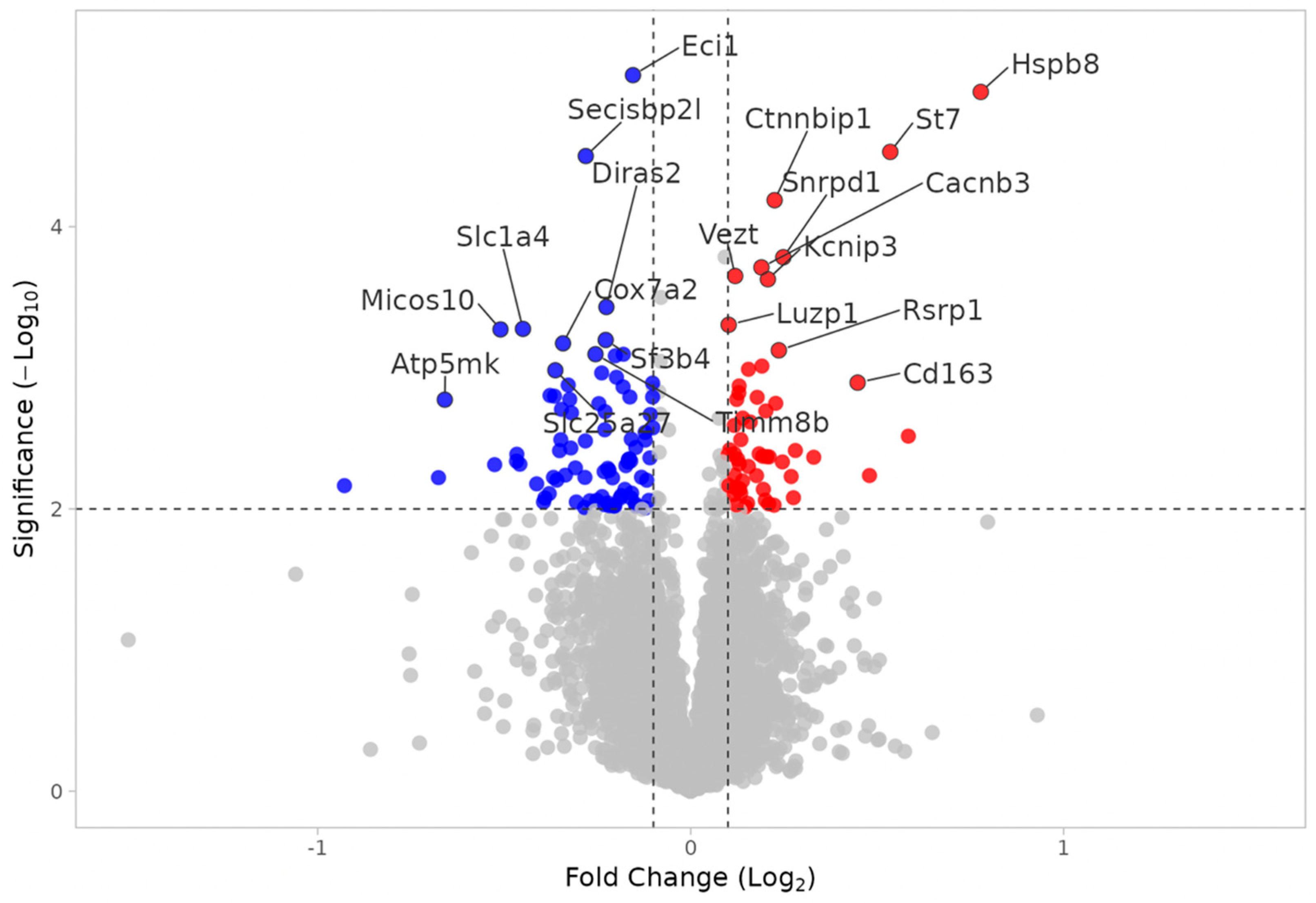
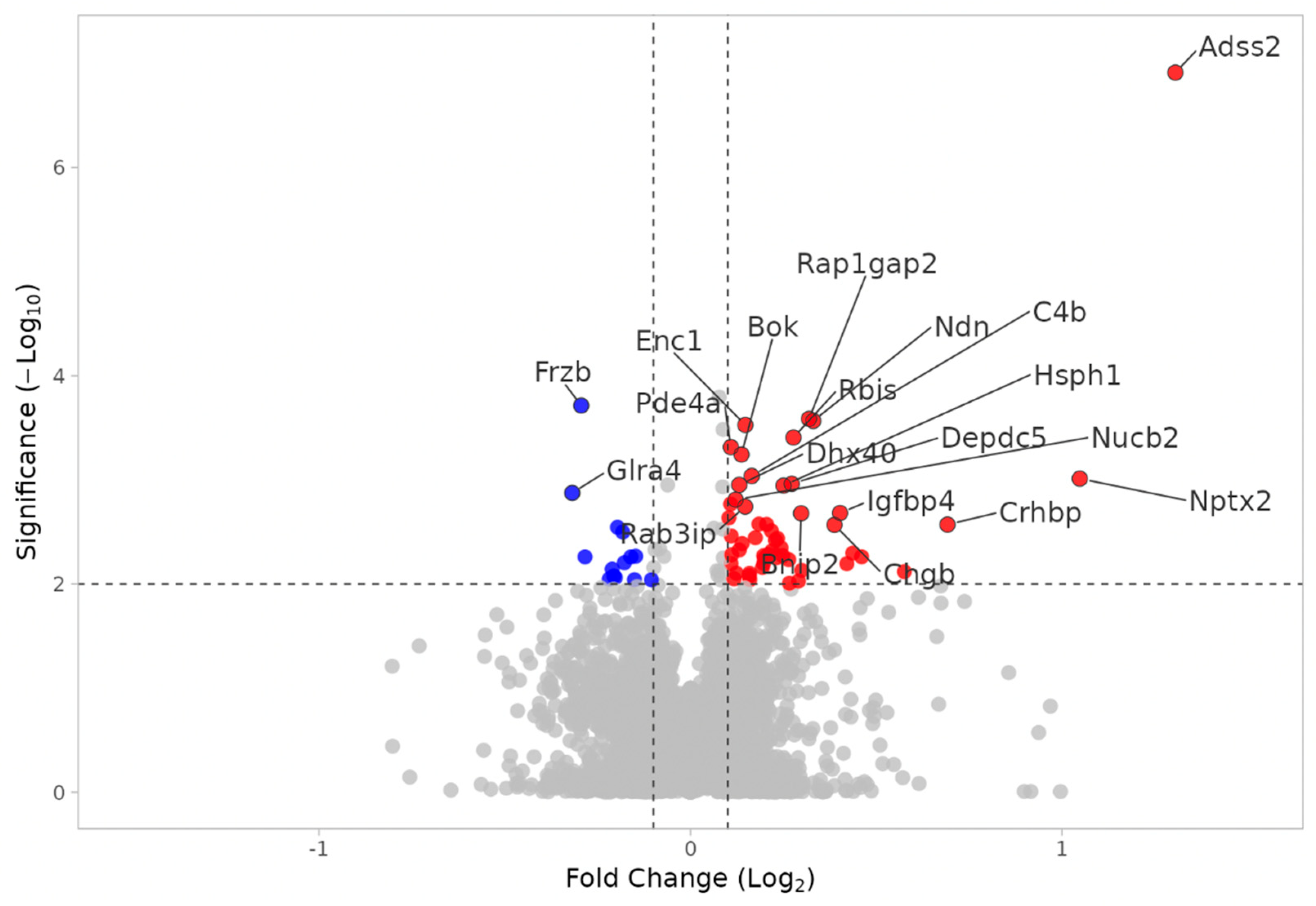
3.2.1. WT MEST vs. WT SHAM
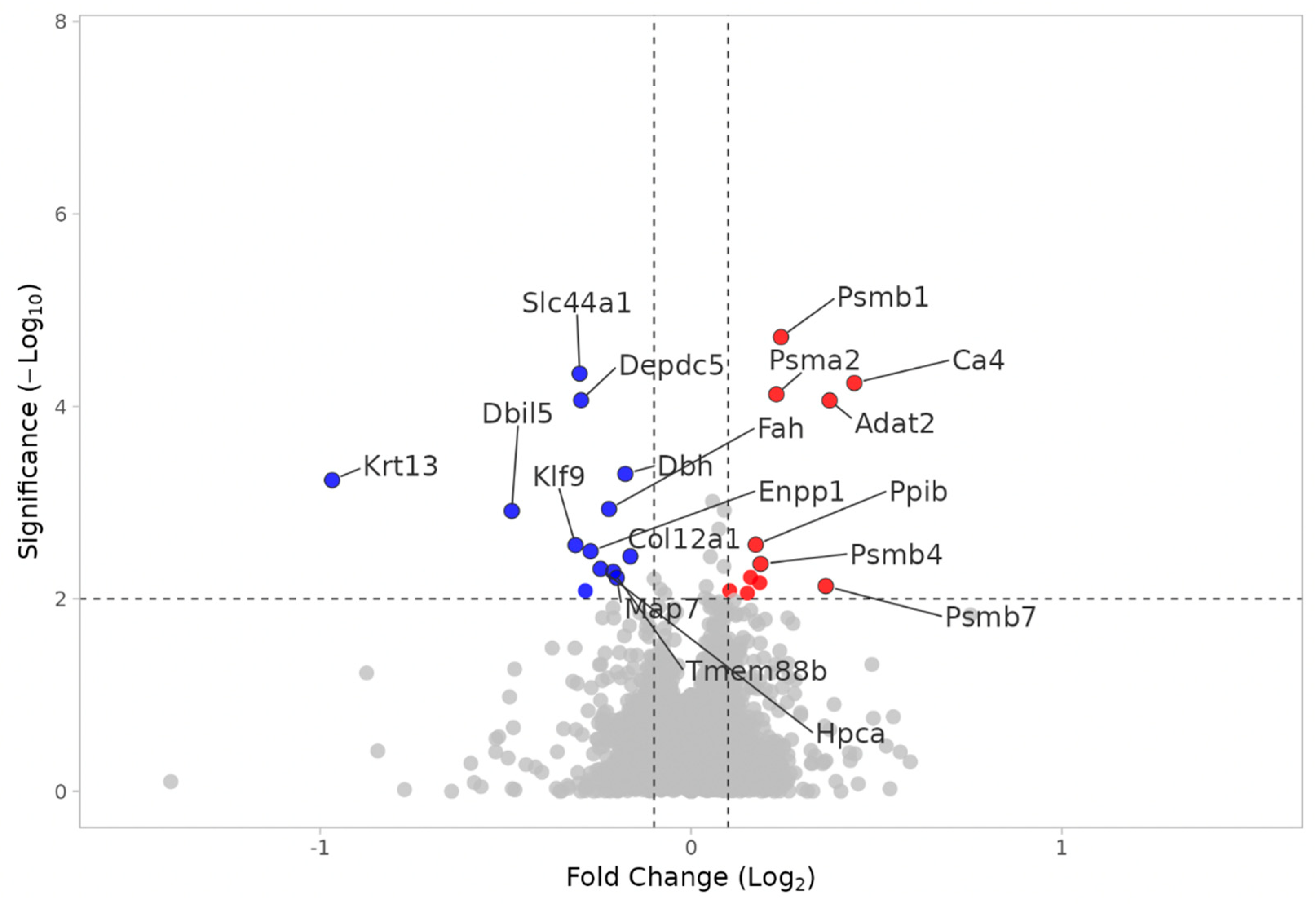
3.2.2. KO MEST vs. KO SHAM
3.2.3. KO SHAM vs. WT SHAM
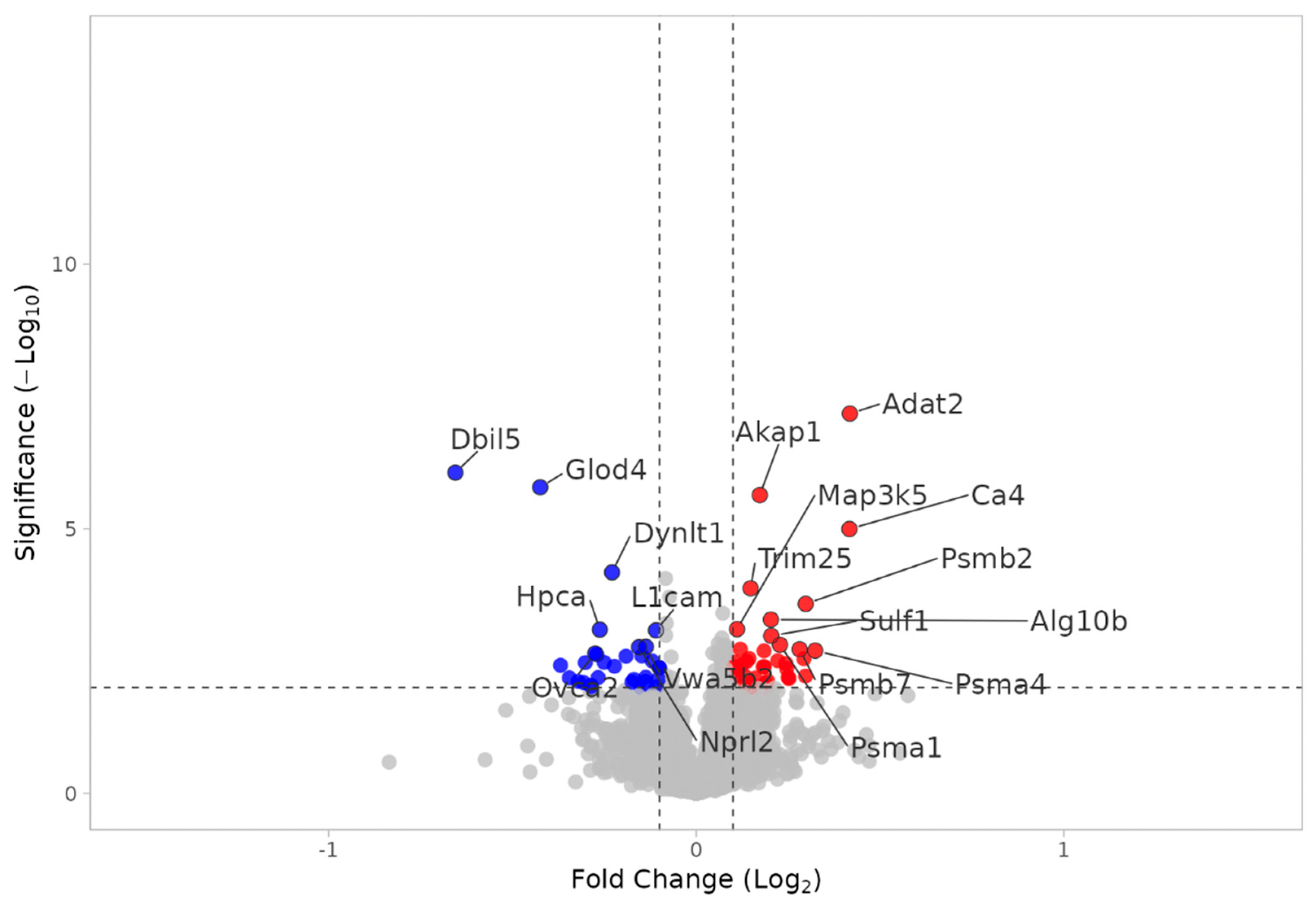
3.2.4. KO MEST vs. WT MEST
3.2.5. Notable DEPs in KO MEST vs. KO SHAM and WT MEST vs. WT SHAM
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B

References
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E. The neurobiology of epilepsy. Curr. Neurol. Neurosci. Rep. 2007, 7, 348–354. [Google Scholar] [CrossRef]
- Pitkänen, A.; Lukasiuk, K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011, 10, 173–186. [Google Scholar] [CrossRef]
- Quigg, M. Circadian rhythms: Interactions with seizures and epilepsy. Epilepsy Res. 2000, 42, 43–55. [Google Scholar] [CrossRef]
- Matos, G.; Andersen, M.L.; do Valle, A.C.; Tufik, S. The relationship between sleep and epilepsy: Evidence from clinical trials and animal models. J. Neurol. Sci. 2010, 295, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Re, C.J.; Batterman, A.I.; Gerstner, J.R.; Buono, R.J.; Ferraro, T.N. The Molecular Genetic Interaction Between Circadian Rhythms and Susceptibility to Seizures and Epilepsy. Front. Neurol. 2020, 11, 520. [Google Scholar] [CrossRef]
- Khan, S.; Nobili, L.; Khatami, R.; Loddenkemper, T.; Cajochen, C.; Dijk, D.J.; Eriksson, S.H. Circadian rhythm and epilepsy. Lancet Neurol. 2018, 17, 1098–1108. [Google Scholar] [CrossRef]
- Loddenkemper, T.; Vendrame, M.; Zarowski, M.; Gregas, M.; Alexopoulos, A.V.; Wyllie, E.; Kothare, S.V. Circadian patterns of pediatric seizures. Neurology 2011, 76, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, T.S.; Spencer, S.S.; Duckrow, R.B.; Novotny, E.J.; Spencer, D.D.; Zaveri, H.P. Temporal distributions of seizure occurrence from various epileptogenic regions. Neurology 2008, 70, 1265–1271. [Google Scholar] [CrossRef]
- Patel, D.C.; Tewari, B.P.; Chaunsali, L.; Sontheimer, H. Neuron-glia interactions in the pathophysiology of epilepsy. Nat. Rev. Neurosci. 2019, 20, 282–297. [Google Scholar] [CrossRef]
- Volterra, A.; Steinhäuser, C. Glial modulation of synaptic transmission in the hippocampus. Glia 2004, 47, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gonzalo, M.; Losi, G.; Chiavegato, A.; Zonta, M.; Cammarota, M.; Brondi, M.; Vetri, F.; Uva, L.; Pozzan, T.; de Curtis, M.; et al. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol. 2010, 8, e1000352. [Google Scholar] [CrossRef]
- Steinhäuser, C.; Seifert, G.; Bedner, P. Astrocyte dysfunction in temporal lobe epilepsy: K+ channels and gap junction coupling. Glia 2012, 60, 1192–1202. [Google Scholar] [CrossRef]
- Schousboe, A.; Scafidi, S.; Bak, L.K.; Waagepetersen, H.S.; McKenna, M.C. Glutamate metabolism in the brain focusing on astrocytes. Adv. Neurobiol. 2014, 11, 13–30. [Google Scholar]
- Reichenbach, A.; Derouiche, A.; Kirchhoff, F. Morphology and dynamics of perisynaptic glia. Brain Res. Rev. 2010, 63, 11–25. [Google Scholar] [CrossRef]
- Coulter, D.A.; Eid, T. Astrocytic regulation of glutamate homeostasis in epilepsy. Glia 2012, 60, 1215–1226. [Google Scholar] [CrossRef]
- Yang, T.-T.; Qian, F.; Liu, L.; Peng, X.-C.; Huang, J.-R.; Ren, B.-X.; Tang, F.-R. Astroglial connexins in epileptogenesis. Seizure 2021, 84, 122–128. [Google Scholar] [CrossRef]
- Purnell, B.S.; Alves, M.; Boison, D. Astrocyte-neuron circuits in epilepsy. Neurobiol. Dis. 2023, 179, 106058. [Google Scholar] [CrossRef]
- Coulter, D.A.; Steinhäuser, C. Role of astrocytes in epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022434. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Ravizza, T.; Bedner, P.; Aronica, E.; Steinhäuser, C.; Boison, D. Astrocytes in the initiation and progression of epilepsy. Nat. Rev. Neurol. 2022, 18, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, R.; Fu, P.; Mei, Y. Disrupted astrocyte-neuron signaling reshapes brain activity in epilepsy and Alzheimer’s disease. Neuroscience 2025, 570, 132–151. [Google Scholar] [CrossRef]
- Lefton, M.; Flores, C.C.; Owada, Y.; Davis, C.J.; Ferraro, T.N.; Lee, Y.; Schroeder, W.L.; Gerstner, J.R. Astrocyte Fabp7 modulates nocturnal seizure threshold and activity-dependent gene expression in mouse brain. PNAS Nexus 2025, 4, pgaf146. [Google Scholar] [CrossRef]
- Gerstner, J.R.; Smith, G.G.; Lenz, O.; Perron, I.J.; Buono, R.J.; Ferraro, T.N. BMAL1 controls the diurnal rhythm and set point for electrical seizure threshold in mice. Front. Syst. Neurosci. 2014, 8, 121. [Google Scholar] [CrossRef]
- Li, P.; Fu, X.; Smith, N.A.; Ziobro, J.; Curiel, J.; Tenga, M.J.; Martin, B.; Freedman, S.; Cea-Del Rio, C.A.; Oboti, L.; et al. Loss of CLOCK Results in Dysfunction of Brain Circuits Underlying Focal Epilepsy. Neuron 2017, 96, 387–401.e386. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, F.; Xu, H.; Chen, M.; Chen, X.; Guo, L.; Zhou, C.; Xu, Y.; Wang, F.; Yu, J.; et al. Dysregulation of REV-ERBα impairs GABAergic function and promotes epileptic seizures in preclinical models. Nat. Commun. 2021, 12, 1216. [Google Scholar] [CrossRef] [PubMed]
- Mita, R.; Coles, J.E.; Glubrecht, D.D.; Sung, R.; Sun, X.; Godbout, R. B-FABP-expressing radial glial cells: The malignant glioma cell of origin? Neoplasia 2007, 9, 734–744. [Google Scholar] [CrossRef]
- Feng, L.; Hatten, M.E.; Heintz, N. Brain lipid-binding protein (BLBP): A novel signaling system in the developing mammalian CNS. Neuron 1994, 12, 895–908. [Google Scholar] [CrossRef]
- Schnell, A.; Chappuis, S.; Schmutz, I.; Brai, E.; Ripperger, J.A.; Schaad, O.; Welzl, H.; Descombes, P.; Alberi, L.; Albrecht, U. The nuclear receptor REV-ERBα regulates Fabp7 and modulates adult hippocampal neurogenesis. PLoS ONE 2014, 9, e99883. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, J.R.; Paschos, G.K. Circadian expression of Fabp7 mRNA is disrupted in Bmal1 KO mice. Mol. Brain 2020, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, J.R.; Vanderheyden, W.M.; LaVaute, T.; Westmark, C.J.; Rouhana, L.; Pack, A.I.; Wickens, M.; Landry, C.F. Time of day regulates subcellular trafficking, tripartite synaptic localization, and polyadenylation of the astrocytic Fabp7 mRNA. J. Neurosci. 2012, 32, 1383–1394. [Google Scholar] [CrossRef]
- Gerstner, J.R.; Bremer, Q.Z.; Vander Heyden, W.M.; Lavaute, T.M.; Yin, J.C.; Landry, C.F. Brain fatty acid binding protein (Fabp7) is diurnally regulated in astrocytes and hippocampal granule cell precursors in adult rodent brain. PLoS ONE 2008, 3, e1631. [Google Scholar] [CrossRef]
- O’Connell, J.D.; Paulo, J.A.; O’Brien, J.J.; Gygi, S.P. Proteome-Wide Evaluation of Two Common Protein Quantification Methods. J. Proteome Res. 2018, 17, 1934–1942. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Gritsenko, M.A.; Wang, Y.; Clauss, T.; Liu, T.; Shen, Y.; Monroe, M.E.; Lopez-Ferrer, D.; Reno, T.; et al. Reversed-phase chromatography with multiple fraction concatenation strategy for proteome profiling of human MCF10A cells. Proteomics 2011, 11, 2019–2026. [Google Scholar] [CrossRef]
- Mertins, P.; Tang, L.C.; Krug, K.; Clark, D.J.; Gritsenko, M.A.; Chen, L.; Clauser, K.R.; Clauss, T.R.; Shah, P.; Gillette, M.A.; et al. Reproducible workflow for multiplexed deep-scale proteome and phosphoproteome analysis of tumor tissues by liquid chromatography-mass spectrometry. Nat. Protoc. 2018, 13, 1632–1661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, T.; Zhang, Z.; Payne, S.H.; Zhang, B.; McDermott, J.E.; Zhou, J.Y.; Petyuk, V.A.; Chen, L.; Ray, D.; et al. Integrated Proteogenomic Characterization of Human High-Grade Serous Ovarian Cancer. Cell 2016, 166, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Gupta, N.; Pevzner, P.A. Spectral probabilities and generating functions of tandem mass spectra: A strike against decoy databases. J. Proteome Res. 2008, 7, 3354–3363. [Google Scholar] [CrossRef]
- Elias, J.E.; Gygi, S.P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 2007, 4, 207–214. [Google Scholar] [CrossRef]
- Qian, W.J.; Liu, T.; Monroe, M.E.; Strittmatter, E.F.; Jacobs, J.M.; Kangas, L.J.; Petritis, K.; Camp, D.G., 2nd; Smith, R.D. Probability-based evaluation of peptide and protein identifications from tandem mass spectrometry and SEQUEST analysis: The human proteome. J. Proteome Res. 2005, 4, 53–62. [Google Scholar] [CrossRef]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR is a web app for creating, exploring, labeling and sharing volcano plots. Sci. Rep. 2020, 10, 20560. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Abi Habib, J.; Lesenfants, J.; Vigneron, N.; Van den Eynde, B.J. Functional Differences between Proteasome Subtypes. Cells 2022, 11, 421. [Google Scholar] [CrossRef]
- Sahu, I.; Mali, S.M.; Sulkshane, P.; Xu, C.; Rozenberg, A.; Morag, R.; Sahoo, M.P.; Singh, S.K.; Ding, Z.; Wang, Y.; et al. The 20S as a stand-alone proteasome in cells can degrade the ubiquitin tag. Nat. Commun. 2021, 12, 6173. [Google Scholar] [CrossRef]
- Davidson, K.; Pickering, A.M. The proteasome: A key modulator of nervous system function, brain aging, and neurodegenerative disease. Front. Cell Dev. Biol. 2023, 11, 1124907. [Google Scholar] [CrossRef]
- Pires, G.; Leitner, D.; Drummond, E.; Kanshin, E.; Nayak, S.; Askenazi, M.; Faustin, A.; Friedman, D.; Debure, L.; Ueberheide, B.; et al. Proteomic differences in the hippocampus and cortex of epilepsy brain tissue. Brain Commun. 2021, 3, fcab021. [Google Scholar] [CrossRef]
- Huttner, W.B.; Gerdes, H.H.; Rosa, P. The granin (chromogranin/secretogranin) family. Trends Biochem. Sci. 1991, 16, 27–30. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Possenti, R.; Mahata, S.K.; Fischer-Colbrie, R.; Loh, Y.P.; Salton, S.R. The extended granin family: Structure, function, and biomedical implications. Endocr. Rev. 2011, 32, 755–797. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Matteoli, M.; Parpura, V.; Mothet, J.P.; Zorec, R. Astrocytes as secretory cells of the central nervous system: Idiosyncrasies of vesicular secretion. EMBO J. 2016, 35, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Hur, Y.S.; Kim, K.D.; Paek, S.H.; Yoo, S.H. Evidence for the existence of secretory granule (dense-core vesicle)-based inositol 1,4,5-trisphosphate-dependent Ca2+ signaling system in astrocytes. PLoS ONE 2010, 5, e11973. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, M.; Watanabe, T.; Sakai, Y.; Kato, T.; Takeuchi, T. Interaction between secretogranin III and carboxypeptidase E facilitates prohormone sorting within secretory granules. J. Cell Sci. 2005, 118, 4785–4795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Siletti, K.; Hodge, R.; Mossi Albiach, A.; Lee, K.W.; Ding, S.L.; Hu, L.; Lönnerberg, P.; Bakken, T.; Casper, T.; Clark, M.; et al. Transcriptomic diversity of cell types across the adult human brain. Science 2023, 382, eadd7046. [Google Scholar] [CrossRef]
- Guicherit, O.M.; Rudolph, F.B.; Kellems, R.E.; Cooper, B.F. Molecular cloning and expression of a mouse muscle cDNA encoding adenylosuccinate synthetase. J. Biol. Chem. 1991, 266, 22582–22587. [Google Scholar] [CrossRef]
- Guicherit, O.M.; Cooper, B.F.; Rudolph, F.B.; Kellems, R.E. Amplification of an adenylosuccinate synthetase gene in alanosine-resistant murine T-lymphoma cells. Molecular cloning of a cDNA encoding the “non-muscle” isozyme. J. Biol. Chem. 1994, 269, 4488–4496. [Google Scholar] [CrossRef]
- Honzatko, R.B.; Stayton, M.M.; Fromm, H.J. Adenylosuccinate synthetase: Recent developments. Adv. Enzym. Relat. Areas Mol. Biol. 1999, 73, 57–102, ix–x. [Google Scholar]
- Stayton, M.M.; Rudolph, F.B.; Fromm, H.J. Regulation, genetics, and properties of adenylosuccinate synthetase: A review. Curr. Top. Cell Regul. 1983, 22, 103–141. [Google Scholar] [PubMed]
- Xia, Y.; McMillin, J.B.; Lewis, A.; Moore, M.; Zhu, W.G.; Williams, R.S.; Kellems, R.E. Electrical stimulation of neonatal cardiac myocytes activates the NFAT3 and GATA4 pathways and up-regulates the adenylosuccinate synthetase 1 gene. J. Biol. Chem. 2000, 275, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Rybalka, E.; Park, H.J.; Nalini, A.; Baskar, D.; Polavarapu, K.; Durmus, H.; Xia, Y.; Wan, L.; Shieh, P.B.; Moghadaszadeh, B.; et al. Current insights in ultra-rare adenylosuccinate synthetase 1 myopathy—Meeting report on the First Clinical and Scientific Conference. 3 June 2024, National Centre for Advancing Translational Science, Rockville, Maryland, the United States of America. Orphanet J. Rare Dis. 2024, 19, 438. [Google Scholar] [CrossRef]
- Lovatt, D.; Xu, Q.; Liu, W.; Takano, T.; Smith, N.A.; Schnermann, J.; Tieu, K.; Nedergaard, M. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc. Natl. Acad. Sci. USA 2012, 109, 6265–6270. [Google Scholar] [CrossRef]
- Yasuda, R.; Hayashi, Y.; Hell, J.W. CaMKII: A central molecular organizer of synaptic plasticity, learning and memory. Nat. Rev. Neurosci. 2022, 23, 666–682. [Google Scholar] [CrossRef]
- Bingol, B.; Wang, C.F.; Arnott, D.; Cheng, D.; Peng, J.; Sheng, M. Autophosphorylated CaMKIIalpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell 2010, 140, 567–578. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Yamamoto, Y.; Sharifi, K.; Kida, H.; Kagawa, Y.; Yasumoto, Y.; Islam, A.; Miyazaki, H.; Shimamoto, C.; Maekawa, M.; et al. Astrocyte-expressed FABP7 regulates dendritic morphology and excitatory synaptic function of cortical neurons. Glia 2016, 64, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Z.; Sanchez, R.; Sali, A.; Heintz, N. Ligand specificity of brain lipid-binding protein. J. Biol. Chem. 1996, 271, 24711–24719. [Google Scholar] [CrossRef]
- Owada, Y.; Abdelwahab, S.A.; Kitanaka, N.; Sakagami, H.; Takano, H.; Sugitani, Y.; Sugawara, M.; Kawashima, H.; Kiso, Y.; Mobarakeh, J.I.; et al. Altered emotional behavioral responses in mice lacking brain-type fatty acid-binding protein gene. Eur. J. Neurosci. 2006, 24, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Toyota, T.; Owada, Y.; Hayashi, T.; Iwayama, Y.; Matsumata, M.; Ishitsuka, Y.; Nakaya, A.; Maekawa, M.; Ohnishi, T.; et al. Fabp7 maps to a quantitative trait locus for a schizophrenia endophenotype. PLoS Biol. 2007, 5, e297. [Google Scholar] [CrossRef]
- Schultess, J.; Danielewski, O.; Smolenski, A.P. Rap1GAP2 is a new GTPase-activating protein of Rap1 expressed in human platelets. Blood 2005, 105, 3185–3192. [Google Scholar] [CrossRef]
- Neumüller, O.; Hoffmeister, M.; Babica, J.; Prelle, C.; Gegenbauer, K.; Smolenski, A.P. Synaptotagmin-like protein 1 interacts with the GTPase-activating protein Rap1GAP2 and regulates dense granule secretion in platelets. Blood 2009, 114, 1396–1404. [Google Scholar] [CrossRef]
- Sjöstedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 2020, 367, eaay5947. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Sadrian, B.; Cheng, T.W.; Shull, O.; Gong, Q. Rap1gap2 regulates axon outgrowth in olfactory sensory neurons. Mol. Cell Neurosci. 2012, 50, 272–282. [Google Scholar] [CrossRef]
- Swanson, A.; Wolf, T.; Sitzmann, A.; Willette, A.A. Neuroinflammation in Alzheimer’s disease: Pleiotropic roles for cytokines and neuronal pentraxins. Behav. Brain Res. 2018, 347, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Pelkey, K.A.; Barksdale, E.; Craig, M.T.; Yuan, X.; Sukumaran, M.; Vargish, G.A.; Mitchell, R.M.; Wyeth, M.S.; Petralia, R.S.; Chittajallu, R.; et al. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron 2015, 85, 1257–1272. [Google Scholar] [CrossRef]
- Tsui, C.C.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Barnes, C.; Worley, P.F. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J. Neurosci. 1996, 16, 2463–2478. [Google Scholar] [CrossRef]
- Xing, M.; Yang, X.; Jin, S.; Xu, X. Inhibition of neuronal pentraxin 2 relieved epileptic seizure via reducing GluA1 phosphorylation. Cell Biochem. Funct. 2024, 42, e4003. [Google Scholar] [CrossRef]
- Owada, Y.; Yoshimoto, T.; Kondo, H. Increased expression of the mRNA for brain- and skin-type but not heart-type fatty acid binding proteins following kainic acid systemic administration in the hippocampal glia of adult rats. Brain Res. Mol. Brain Res. 1996, 42, 156–160. [Google Scholar] [CrossRef]
- Gerstner, J.R.; Perron, I.J.; Riedy, S.M.; Yoshikawa, T.; Kadotani, H.; Owada, Y.; Van Dongen, H.P.A.; Galante, R.J.; Dickinson, K.; Yin, J.C.P.; et al. Normal sleep requires the astrocyte brain-type fatty acid binding protein FABP7. Sci. Adv. 2017, 3, e1602663. [Google Scholar] [CrossRef]
- Ioannou, M.S.; Jackson, J.; Sheu, S.H.; Chang, C.L.; Weigel, A.V.; Liu, H.; Pasolli, H.A.; Xu, C.S.; Pang, S.; Matthies, D.; et al. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 2019, 177, 1522–1535.e14. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Haynes, P.R.; Pyfrom, E.S.; Li, Y.; Stein, C.; Cuddapah, V.A.; Jacobs, J.A.; Yue, Z.; Sehgal, A. A neuron-glia lipid metabolic cycle couples daily sleep to mitochondrial homeostasis. Nat. Neurosci. 2024, 27, 666–678. [Google Scholar] [CrossRef]
- Gerstner, J.R.; Flores, C.C.; Lefton, M.; Rogers, B.; Davis, C.J. FABP7: A glial integrator of sleep, circadian rhythms, plasticity, and metabolic function. Front. Syst. Neurosci. 2023, 17, 1212213. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Florian, C.; Fellin, T.; Munoz, J.R.; Lee, S.Y.; Abel, T.; Haydon, P.G.; Frank, M.G. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 2009, 61, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Dal Maschio, M.; Beltramo, R.; Haydon, P.G.; Benfenati, F.; Fellin, T. Integrated brain circuits: Neuron-astrocyte interaction in sleep-related rhythmogenesis. Sci. World J. 2010, 10, 1634–1645. [Google Scholar] [CrossRef]
- Schmitt, L.I.; Sims, R.E.; Dale, N.; Haydon, P.G. Wakefulness affects synaptic and network activity by increasing extracellular astrocyte-derived adenosine. J. Neurosci. 2012, 32, 4417–4425. [Google Scholar] [CrossRef] [PubMed]
- Rorsman, H.O.; Müller, M.A.; Liu, P.Z.; Sanchez, L.G.; Kempf, A.; Gerbig, S.; Spengler, B.; Miesenböck, G. Sleep pressure accumulates in a voltage-gated lipid peroxidation memory. Nature 2025, 641, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Aalling, N.N.; Nedergaard, M.; DiNuzzo, M. Cerebral Metabolic Changes During Sleep. Curr. Neurol. Neurosci. Rep. 2018, 18, 57. [Google Scholar] [CrossRef]
- Chen, Z.P.; Wang, S.; Zhao, X.; Fang, W.; Wang, Z.; Ye, H.; Wang, M.J.; Ke, L.; Huang, T.; Lv, P.; et al. Lipid-accumulated reactive astrocytes promote disease progression in epilepsy. Nat. Neurosci. 2023, 26, 542–554. [Google Scholar] [CrossRef] [PubMed]
- McCauley, J.P.; Petroccione, M.A.; D’Brant, L.Y.; Todd, G.C.; Affinnih, N.; Wisnoski, J.J.; Zahid, S.; Shree, S.; Sousa, A.A.; De Guzman, R.M.; et al. Circadian Modulation of Neurons and Astrocytes Controls Synaptic Plasticity in Hippocampal Area CA1. Cell Rep. 2020, 33, 108255. [Google Scholar] [CrossRef]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Bernlohr, D.A. Metabolic functions of FABPs—Mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 2015, 11, 592–605. [Google Scholar] [CrossRef]




| KO MEST v KO SHAM | WT MEST v WT SHAM | |||
|---|---|---|---|---|
| DEP | Log2FC | p-Val/d-Val | Log2FC | p-Val/d-Val |
| C4b | 0.1646 | 0.0009/0.4465 | 0.1173 | 0.0041/0.3468 |
| Kcnip3 | 0.2272 | 0.0011/0.3029 | 0.2070 | 0.0002/0.5864 |
| Rap1gap2 | 0.3190 | 0.0003/0.3680 | 0.1394 | 0.0023/0.3705 |
| Hsph1 | 0.2502 | 0.0011/0.2712 | 0.2016 | 0.0020/0.2688 |
| Nptx2 | 1.048 | 0.0010/1.9672 | 0.3300 | 0.0043/0.4552 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berg, A.P.; Tariq, S.H.; Flores, C.C.; Lefton, M.; Owada, Y.; Davis, C.J.; Ferraro, T.N.; Jacobs, J.M.; Gritsenko, M.A.; Lee, Y.; et al. Astrocyte FABP7 Modulates Seizure Activity-Dependent Protein Expression in Mouse Brain. Neuroglia 2025, 6, 33. https://doi.org/10.3390/neuroglia6030033
Berg AP, Tariq SH, Flores CC, Lefton M, Owada Y, Davis CJ, Ferraro TN, Jacobs JM, Gritsenko MA, Lee Y, et al. Astrocyte FABP7 Modulates Seizure Activity-Dependent Protein Expression in Mouse Brain. Neuroglia. 2025; 6(3):33. https://doi.org/10.3390/neuroglia6030033
Chicago/Turabian StyleBerg, Adam P., Shahroz H. Tariq, Carlos C. Flores, Micah Lefton, Yuji Owada, Christopher J. Davis, Thomas N. Ferraro, Jon M. Jacobs, Marina A. Gritsenko, Yool Lee, and et al. 2025. "Astrocyte FABP7 Modulates Seizure Activity-Dependent Protein Expression in Mouse Brain" Neuroglia 6, no. 3: 33. https://doi.org/10.3390/neuroglia6030033
APA StyleBerg, A. P., Tariq, S. H., Flores, C. C., Lefton, M., Owada, Y., Davis, C. J., Ferraro, T. N., Jacobs, J. M., Gritsenko, M. A., Lee, Y., Schroeder, W. L., & Gerstner, J. R. (2025). Astrocyte FABP7 Modulates Seizure Activity-Dependent Protein Expression in Mouse Brain. Neuroglia, 6(3), 33. https://doi.org/10.3390/neuroglia6030033







