Abstract
HIV, primarily targeting CD4 cells, infiltrates the CNS through various mechanisms, including chemokine-mediated signaling and blood–brain barrier disruption, leading to neuroinflammation and neuronal dysfunction. Viral proteins such as gp120, Tat, and Vpr directly induce neurotoxicity, oxidative stress, and mitochondrial dysfunction, exacerbating cognitive deficits and motor impairments observed in HIV-associated neurocognitive disorders (HANDs). Host genetic factors, including CCR5 mutations and HLA alleles, influence susceptibility to HIV-related neurologic complications, shaping disease progression and treatment responses. Advanced molecular and bioinformatics techniques, from genome sequencing to structural modeling and network analysis, provide insights into viral pathogenesis and identify potential therapeutic targets. These findings underscore the future potential of precision medicine approaches tailored to individual genetic profiles to mitigate neurologic complications and improve outcomes in HIV-infected populations. This comprehensive review explores the intricate interplay between HIV infection and neurogenetics, focusing on how the virus impacts the central nervous system (CNS) and contributes to neurocognitive disorders. This report delves into how the virus influences genetic expression, neuroinflammation, and neurodegeneration, offering insights into molecular mechanisms behind HAND.
1. Introduction
1.1. HIV
Human Immunodeficiency Virus (HIV) primarily targets CD4 cells, essential for immune defense, and can progress to acquired immunodeficiency syndrome (AIDS) if untreated. HIV, a retrovirus, has a complex structure with a lipid bilayer derived from host cell membranes, embedding glycoproteins such as gp120, which bind to CD4 receptors, and gp41, facilitating fusion with host cells. Beneath this envelope is the matrix protein p17, crucial for virion integrity, while the cone-shaped capsid, composed of p24, encases the viral RNA and enzymes. HIV encodes nine genes across its two RNA strands, with key enzymes including reverse transcriptase, which converts RNA to DNA, and protease, which processes polyproteins. HIV-1, the more widespread strain causing global pandemics, has the main group M and rarer groups N and O, while HIV-2, less transmissible and primarily found in West Africa, progresses more slowly [1,2]. HIV-1 group M is responsible for the global pandemic and is subdivided into subtypes (clades) A, B, C, D, F, G, H, J, and K, and various Circulating Recombinant Forms (CRFs). Subtype distribution varies geographically. For instance, subtype B predominates in Europe and the Americas, while subtype C is most common in Southern Africa and India. Recombinant forms arise when an individual is co-infected with multiple subtypes, leading to genetic recombination. These strains differ significantly in genetic diversity, transmission rates, and disease progression. The diagrammatic presentation of different types of HIV is shown in Figure 1.
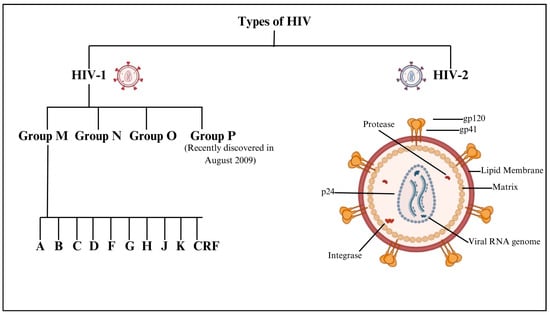
Figure 1.
Diagrammatic presentation of different types of HIV.
HIV’s reverse transcriptase enzyme lacks proofreading ability, resulting in a high mutation rate during replication. This contributes to the rapid evolution of the virus and the emergence of diverse quasispecies within an infected individual. During reverse transcription, the nascent DNA can switch between the two RNA genomes packaged in a single virion, a “copy-choice” recombination process. This mechanism further increases genetic diversity. The host’s immune response and antiretroviral therapies exert selection pressures on HIV, leading to the survival of resistant variants. This dynamic results in a complex, ever-evolving population of viral quasispecies within the host [3,4].
Considering the impact of HIV on the central nervous system (CNS), it was reported to focus on HIV-associated dementia (HAD), a severe neurocognitive disorder seen in the advanced disease stage of HIV-associated neurocognitive disorders (HANDs), which represents a spectrum of cognitive impairments in people living with HIV. HAND includes three clinical categories: asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HAD [5]. While HAD had been more common in the pre-antiretroviral therapy (ART) era, its incidence has significantly declined with widespread ART use. However, milder forms of HAND (ANI and MND) remain prevalent, affecting up to 50% of individuals with HIV [6]. Despite effective viral suppression, HAND persists due to chronic neuroinflammation, neurotoxicity from viral proteins, and ongoing immune activation within the central nervous system. As antiretroviral therapy (ART) improved survival, researchers identified milder forms of cognitive impairment, prompting deeper investigations into viral reservoirs, neuroinflammation, and immune activation [7,8,9].
Modern molecular studies have revealed mechanisms such as viral proteins (e.g., Tat and gp120) disrupting neuronal function, chronic neuroinflammation driven by monocyte infiltration, and altered synaptic signaling. Neuroimaging and biomarker research advances continue to refine our understanding, highlighting persistent low-level CNS inflammation even in virally suppressed individuals [10].
1.2. HIV and Neurogenetics
The subfield of genetics known as “neurogenetics” focuses on the hereditary causes of neurological disorders and the effects of genes on the maturation and operation of the nervous system. It explores how brain health is affected by inherited genetic variables and mutations that occur after conception [11,12]. Considering the therapeutic approach, the current antiretroviral therapy (ART) has significantly reduced the incidence of severe HIV-associated neurocognitive disorders (HANDs). However, milder forms of HAND remain prevalent, partly due to challenges in achieving effective ART concentrations within the central nervous system (CNS). The blood–brain barrier (BBB) restricts the entry of many antiretroviral drugs, leading to suboptimal viral suppression in the CNS. This limited penetration allows HIV to persist in the brain, contributing to ongoing neuroinflammation and cognitive impairment [13]. Researchers have developed the CNS penetration effectiveness (CPE) score to address this issue, which ranks ART drugs based on their ability to penetrate the CNS. Higher CPE scores are associated with better viral suppression in the cerebrospinal fluid (CSF) and improved neurocognitive outcomes. However, some studies have not consistently confirmed these benefits, indicating that factors beyond drug penetration, such as individual patient characteristics and the presence of drug-resistant HIV strains in the CNS, may influence treatment efficacy [14]. Drug resistance within the CNS poses another significant challenge. The CNS can serve as a reservoir for HIV, where the virus may evolve independently from peripheral compartments. This compartmentalization can lead to the development of drug-resistant variants that are not detected in standard blood tests, complicating treatment strategies. Cases of cerebrospinal fluid (CSF) viral escape, where HIV replication persists in the CNS despite effective peripheral viral suppression, have been documented. These cases often involve drug-resistant HIV strains, underscoring the need for tailored ART regimens that effectively target CNS reservoirs [15].
Although ART has transformed HIV into a manageable chronic condition, challenges remain in preventing and treating HAND. Improving the CNS penetration of ART, monitoring for CNS-specific drug resistance, and developing therapies that can effectively cross the BBB are critical steps toward optimizing neurocognitive outcomes for individuals living with HIV. Significant areas of attention include the identification of genes associated with neurodevelopmental disorders, autism spectrum disorders, epilepsy, Parkinson’s disease, and Alzheimer’s disease; this will provide light on the causes of these diseases, including the evaluation of risk and possible treatments [16]. By studying the molecular mechanisms behind processes like neurogenesis and synaptogenesis, neurodevelopmental genetics seeks to understand the impact of genetic differences on the maturation of the nervous system from the embryonic stage into adulthood [17,18]. Crucial in genetic testing and diagnosis, it employs cutting-edge genomic technologies like next-generation sequencing to identify mutations in complicated neurological disorders, allowing targeted treatments. Precision medicine aims to improve therapeutic results while minimizing side effects by customizing therapies according to individual genetic profiles [19]. The field of neurogenetics also investigates novel approaches to treating genetic disorders, such as gene therapy and CRISPR-Cas9 gene editing. Animal models, bioinformatics, and methods for evaluating genomic data, such as whole-exome sequencing and genome-wide association studies, are essential [20]. The future of this discipline holds great promise for more accurate diagnoses and tailored therapies, which might completely transform how people with neurological diseases are cared for [21,22,23]. The overview of neurogenetics is shown in Figure 2.
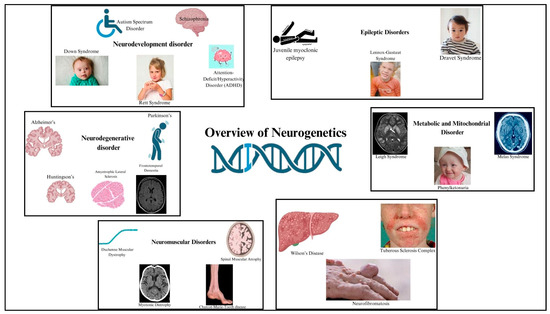
Figure 2.
Illustration of an overview of neurogenetics. Created with BioRender.com (accessed on 21 March 2025).
Furthermore, the efficacy of ART and the persistence of HAND are greatly influenced by host genetic factors [24]. For instance, people with the CCR5\u039432 mutation exhibit resistance to R5-tropic HIV strains, therefore affecting ART results and lowering HAND risk [25]. While the APOE ε4 allele relates to worse cognitive results in HAND, HLA-B57 and B27 alleles are linked to better viral control [26]. Moreover, the CNS penetration effectiveness (CPE) score lets ART regimens be ranked according to their CNS activity; combining genetic information with CPE profiles could help individualized ART plans going forward [27].
1.3. HAND
HIV-associated neurocognitive disorders (HANDs) encompass a range of cognitive, motor, and behavioral impairments that arise as complications of HIV infection, varying from mild cognitive deficits to severe dementia. These conditions significantly impact daily life and quality of life for affected individuals. HAND includes asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV-associated dementia (HAD). Pathophysiologically, HIV enters the central nervous system early in infection, infecting microglia and macrophages, leading to neuroinflammation and neuronal dysfunction through viral toxicity and neurotransmitter disruption [28]. Clinical manifestations include cognitive deficits in attention, memory, and executive function, alongside behavioral changes like mood disorders and motor impairments. Diagnosis involves comprehensive neuropsychological assessments, clinical evaluations, and sometimes neuroimaging. Management strategies include early and adherent antiretroviral therapy (ART) to reduce viral load and inflammation and symptomatic treatments such as cognitive rehabilitation and psychiatric interventions [29]. Ongoing research aims to understand HAND mechanisms better, develop targeted therapies, and improve diagnostic and monitoring tools to enhance outcomes and support for those living with HAND [27,30]. The difference between a healthy brain and a HAND brain is shown in Figure 3.
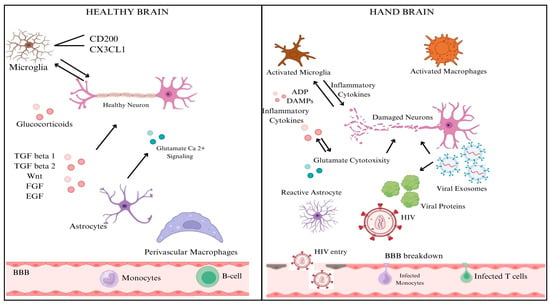
Figure 3.
A diagrammatic representation of the difference between a healthy and HAND brain. Created with BioRender.com.
2. Neuropathogenesis
2.1. Impact of HIV on Neurogenetics
The numerous processes by which HIV penetrates the central nervous system (CNS) include both direct and indirect channels. In most cases, access to the central nervous system is limited by the blood–brain barrier (BBB), which consists of endothelial cells, pericytes, and astrocytic end feet. In contrast, inflammatory reactions and chemokine-mediated signaling allow HIV-infected immune cells, such as monocytes/macrophages and CD4+ T cells, to penetrate the BBB. Once within the central nervous system, HIV uses this sly tactic to set up shop in perivascular macrophages and microglia [31]. Gp120 and Tat are viral proteins that indirectly worsen neuronal damage by interfering with glutamatergic and dopaminergic pathways, which in turn cause neurocognitive disorders and cognitive deficiencies. Systemic inflammation maintains neurotoxicity and oxidative stress, and microglial activation and astrocyte dysfunction cause neuroinflammation, exacerbating this damage [32]. HIV-1 proteins gp120 and Tat contribute to neurotoxicity and neuroinflammation, playing key roles in HIV-associated neurocognitive disorders (HANDs). gp120 binds to CXCR4/CCR5 receptors, inducing neuronal apoptosis, excitotoxicity, and blood–brain barrier (BBB) dysfunction while also activating astrocytes and microglia to release proinflammatory cytokines (TNF-α, IL-1β, and IL-6). Tat exacerbates neurodegeneration by disrupting BDNF signaling, mitochondrial function, and glutamate uptake, leading to excessive excitotoxicity. Both proteins promote chronic inflammation via NF-κB activation and enhance neurotoxicity when combined with drug abuse or co-infections. These mechanisms drive synaptic damage, cognitive decline, and neurodegeneration, even in ART-treated individuals [33,34]. Genetic variables, such as CCR5 mutations and HLA alleles, impact neurocognitive effects and vulnerability to HIV infection. Gene expression related to neuroinflammation and neuronal survival is regulated by epigenetic alterations, which include changes in DNA methylation and histone acetylation. To better control antiretroviral treatment and reduce the risk of neurologic problems, it is essential to understand the underlying processes at work [31]. The neuropathogenesis of HIV in the CNS is shown in Figure 4.
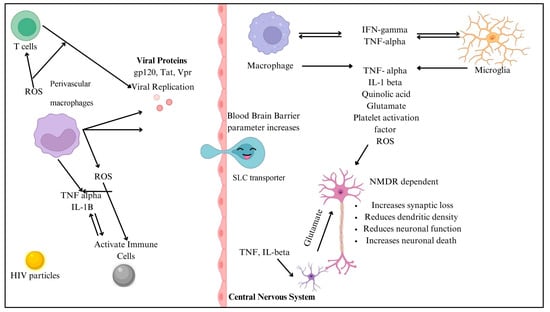
Figure 4.
Illustration of the pathway by which HIV enters the blood–brain barrier. Created with BioRender.com.
2.2. Impact of Proteins of HIV on Neurogenetics
Multiple viral proteins directly affect neurons when HIV reaches the CNS, leading to neurotoxicity. When gp120 binds to CD4 receptors on neurons, it sets off a cascade of events that includes microglia and astrocyte activation, oxidative stress due to the formation of reactive oxygen species (ROS), and excitotoxicity caused by the overactivation of NMDA receptors [35]. HIV-1 Nef protein also plays a significant role in neuroinflammation by promoting microglial activation, astrocyte dysfunction, and immune cell infiltration into the CNS. Nef disrupts the blood–brain barrier (BBB), facilitating the entry of infected monocytes and amplifying neuroinflammation. It induces the release of proinflammatory cytokines such as TNF-α, IL-6, and IL-1β, leading to chronic neurotoxicity.
Additionally, Nef promotes oxidative stress and mitochondrial dysfunction in neurons, contributing to synaptic damage and neuronal apoptosis. By activating the NF-κB and MAPK signaling pathways, Nef sustains neuroinflammatory responses, exacerbating HIV-associated neurocognitive disorders (HANDs) and neuronal dysfunction, even in ART-treated individuals [36]. The HIV replication-essential protein Tat infiltrates neurons and interferes with mitochondrial function, which in turn causes neuroinflammation by triggering the production of cytokines, poor energy metabolism, and death via the activation of the apoptotic pathway [37]. Neurotoxicity is worsened when Vpr, a viral replication factor, infects neurons and triggers cell cycle arrest, DNA damage, and proinflammatory responses. Because of their central roles in HIV-related neurocognitive disorders (HANDs), inflammatory processes, and neuronal health, gp120, Tat, and Vpr need specific treatment strategies to reduce central nervous system (CNS) difficulties in HIV-infected individuals [38,39]. The proteins involved in the process of neuropathogenesis are shown in Figure 5.
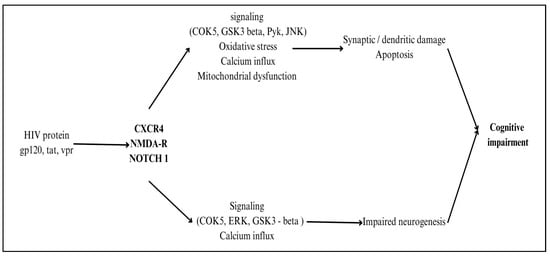
Figure 5.
Illustration of proteins involved in the process of neuropathogenesis. Created with BioRender.com.
2.3. Host Genes Along with HIV Affecting Neurons
Host genes interact with HIV proteins to exacerbate neuroinflammation and neuronal damage in HIV-associated neurocognitive disorders (HANDs). CCR5 and CXCR4, key co-receptors for HIV entry, facilitate viral invasion into the CNS and trigger inflammatory signaling cascades. TNF-α, IL-1β, and IL-6, upregulated in response to HIV proteins (gp120, Tat, and Nef), promote chronic neuroinflammation and neurotoxicity [36]. The NF-κB and MAPK pathways, activated by both HIV and host immune responses, enhance the production of inflammatory mediators, contributing to neuronal apoptosis and synaptic dysfunction [36]. Additionally, BDNF and CREB signaling, critical for neuronal survival and plasticity, are disrupted by HIV, leading to cognitive impairments. Genetic variations in MCP-1 (CCL2) and APOE have been linked to increased neuroinflammatory responses and a higher risk of HAND [40]. These interactions create a vicious cycle of inflammation, oxidative stress, and excitotoxicity, driving progressive neuronal damage even in individuals receiving ART [41].
Particularly in neurogenetics and central nervous system functioning, chemokine receptors are vital in HIV infection. HIV uses gp120 to connect to CCR5 or CXCR4 co-receptors and CD4 receptors, so invading cells mainly employing CCR5, R5-tropic HIV strains target macrophages and microglia, hence influencing neuroinflammation and neuronal damage. They also cause the first central nervous system invasion and primary infection. Variations in CCR5, especially the beneficial CCR5 Delta32 mutation, influence receptor function and might reduce the risk of HIV-associated neurocognitive impairment. HIV damages neurons and reduces cognitive ability by infecting the central nervous system CCR5-expressing microglia and macrophages, generating inflammatory mediators [35]. Investigated for their capacity to block viral entry and lower neuroinflammation, maraviroc and other CCR5 antagonists help to enable tailored treatment based on genetic testing. Research is in progress to clarify the roles of chemokine receptors in HIV neuropathogenesis and their impact on neuronal gene expression and functioning, and to develop creative treatments meant to protect neurons and enhance neurocognitive outcomes in patients with HIV [42,43].
Host genetic variables influence the interaction between HIV and neurogenetics. HLA alleles determine the transport of viral antigens to T cells; HLA-B57 and HLA-B27 are linked with less disease progression and less neurological sequelae in patients with HIV [44,45]. The CCR5 Delta32 mutation reduces the CCR5 receptor required for cellular entrance, rendering resistance to CCR5-tropic strains and highlighting the effect of genetic variants on susceptibility to HIV-related neurogenetic disorders [46]. Genetic polymorphisms in immune response genes, like cytokines and chemokines, may increase immune activation and neuroinflammation, aggravating brain injury and cognitive impairment. Genetic susceptibility is vital in neurons, as specific genetic variants in neuronal genes linked to survival and neurotransmission might cause neurocognitive impairments in HIV infection. Host genes and epigenetic changes, including DNA methylation and histone acetylation, may influence gene expression in response to HIV. Furthermore, it can cause neuroinflammation, oxidative stress, and mitochondrial malfunction, resulting in neurocognitive impairment [47]. Developing focused medications and treatments meant to reduce neurological problems in HIV-positive individuals depends on an understanding of these genetic and epigenetic mechanisms. Under HIV-associated neurocognitive diseases (HANDs), neurodegeneration results from neuronal death. Neuronal loss in regions important for motor and cognitive abilities might cause functional disability and cognitive decline [39,46].
Usually occurring at CpG dinucleotides, DNA methylation is the process by which DNA methyltransferase enzymes (DNMTs) add methyl groups to DNA. Through changes in chromatin shape and transcription factor accessibility, this epigenetic process affects gene expression. Within the framework of HIV and neurogenetics, HIV infection may cause changes in DNA methylation patterns in immune cells and central nervous system tissues [48,49]. At specific genomic locations, inflammatory cytokines and viral proteins such as Tat and gp120 might cause hypermethylation or hypomethylation. These changes activate inflammatory pathways or repress essential genes, influencing neuronal genes linked with survival, inflammation, and death [50]. Gene control via chromatin accessibility depends on histone modifications, including acetylation and methylation. HIV proteins interact with enzymes, including histone acetyltransferases (HATs) and histone methyltransferases (HMTs), therefore affecting histone modification patterns that control immune response genes and neuronal survival [51]. By generating a proinflammatory milieu in the central nervous system, HIV’s disturbance of histone alterations may aggravate neuronal malfunction and neurocognitive loss. Targeting histone-modulating enzymes to restore normal gene expression patterns and lower neuroinflammation might open therapeutic doors to help with HIV-associated neurological problems [32,52].
By invading neurons, the HIV Tat protein reduces mitochondrial activity, lowering membrane potential, raising reactive oxygen species production, and slowing ATP synthesis. This causes mitochondrial oxidative stress and DNA damage and activates death-causing pathways inside neurons, producing neuronal death. Dependent on mitochondrial ATP generation for energy, neurons have lowered ATP levels resulting from HIV-induced mitochondrial dysfunction, limiting their survival and usefulness [53]. Mitochondrial failure aggravates neuroinflammation and neuronal damage by controlling oxidative stress and inflammation. Genetic differences in mitochondrial genes, particularly those related to antioxidant defense like SOD2 or the preservation of mitochondrial DNA integrity, might influence sensitivity to HIV-induced mitochondrial dysfunction and accompanying neurological consequences. Antioxidants or stimulators of mitochondrial biogenesis are two possible treatment approaches aiming at mitochondrial activity that show great potential to reduce neuronal damage in people with HIV. Moreover, the genetic screening of mitochondrial genes may make people prone to mitochondrial malfunction, guiding individualized treatment plans to protect neural function [54].
3. Molecular and Computational Strategies for Neurogenetic Study
Polymerase chain reaction (PCR) is often used to detect pathogens of infectious diseases. Establishing a minimum blood pathogen load, therefore, might result in false-negative findings. Laboratory results could also be distorted by repeated pathogen-based studies increasing sensitivity and specificity. But, host response-based immunodiagnostic techniques tailor treatments to specific individuals, therefore increasing their accuracy and individualization. In urgent situations, new omics technologies have identified molecular host biomarkers as potential rapid diagnostics. Unlike pathogen-based testing, host immunodiagnostics can differentiate infectious from non-infectious immune responses such as sterile inflammation, autoimmune diseases, and malignancies. Diagnostic precision is enhanced by RT-PCR, RNA sequencing, host gene expression profiling, and metabolic and protein biomarker detection. Prediction models with several biomarkers have been developed using genomes, transcriptomics, proteomics, epigenomics, lipidomics, and metabolomics. Though untested and unapproved, these creative ideas could enhance diagnoses of infectious diseases. Bioinformatics in neurogenetic research has changed our understanding of neurological disease genetics. By means of large amounts of genomic, transcriptomic, and proteomic data, bioinformatics enables researchers to identify disease-associated genes, pathways, and molecular processes. High-throughput sequencing technologies such as NGS, WGS, WES, and RNA-seq are evolving; bioinformatics is, therefore, crucial for handling and comprehending complicated neurogenetic data. These techniques find genetic variations, mutations, and expression patterns related to neurodevelopmental and neurodegenerative diseases such as Alzheimer’s, Parkinson’s, Huntington’s, epilepsy, schizophrenia, and ASD [7].
Among the key bioinformatics tools in neurogenetics are GWAS, which reveal SNPs and genetic risk factors for neurological disorders. To identify gene–disease links, bioinformatics pipelines offer quality control, imputation, statistical analysis, and functional annotation on GWAS datasets. Functional genomic techniques such as ChIP-seq and ATAC-seq expose gene control mechanisms and epigenetic alterations influencing neurodevelopment and neurodegeneration. GWAS and bioinformatics allow for precision medicine biomarker discovery and therapeutic target identification [53]. Apart from genomics, bioinformatics is crucial in transcriptomics for exploring gene expression patterns in neurological disorders. RNA sequencing and scRNA-seq let scientists examine gene expression variations in specific neuronal populations. DESeq2, edgeR, and Seurat assess differential gene expression, pathway enrichment, and cell-type-specific gene regulatory networks. Knowing these biological fingerprints helps to identify new treatment targets and define variations in neurodegenerative diseases [54].
In neurological diseases, proteomics and metabolomics provide complementary perspectives on the functional consequences of genetic changes. While metabolomics shows metabolic processes connected to neurological diseases, mass spectrometry-based proteomics finds and measures changes in protein expression in affected neurons. Big datasets are managed and understood using bioinformatics tools such as MaxQuant, Perseus, and MetaboAnalyst. Systems biology methods that combine genomes, transcriptomics, proteomics, and metabolomics data enhance disease pathophysiology and tailored therapy [55]. In neurogenetics, artificial intelligence and machine learning are powerful bioinformatics tools. Using complex genetic and clinical data, artificial intelligence models forecast disease susceptibility, progression, and treatment outcomes. Deep learning systems support drug discovery, find neurodegenerative genetic patterns, and classify neuroimaging data. While artificial intelligence-driven integrative systems like DeepVariant and AlphaFold annotate neurologic disease-associated mutations, computer technologies such as PolyPhen, SIFT, and MutationTaster forecast genetic variant pathogenicity.
Neurogenetic studies gain from biorepositories and neuroinformatics databases as well. Substantial datasets on gene expression, regulatory elements, and neuroimaging-genetics correlations are provided by GEO, ENCODE, dbSNP, and NeuroVault. These tools could help researchers validate independent cohorts, conduct hypothesis testing, and mine large amounts of data. Though bioinformatics in neurogenetics has developed, ethical concerns, data heterogeneity, and computational complexity still exist. Multi-omics dataset integration calls for robust computational frameworks and consistent workflows for reproducibility and accuracy [56].
Evaluating genetic variations in clinical practice is a complex process that requires cooperation among geneticists, bioinformaticians, neurologists, and data scientists. Transforming neurogenetic research, bioinformatics enables the large-scale processing of metabolomic, proteomic, transcriptomic, and genomic data. Advanced computer technologies and AI-driven strategies help find genetic risk factors, biological pathways, and treatment targets for neurological diseases. Although obstacles remain, bioinformatics and interdisciplinarity will increase our understanding of neurogenetic diseases, therefore enabling precision medicine and tailored therapies [57].
The molecular, cellular, and computational techniques shown in Table 1 are central to identifying how HIV affects neuronal gene regulation and brain function. By integrating genetic, transcriptomic, and proteomic data, researchers can pinpoint biomarkers for HAND, map neuropathogenic mechanisms, and identify therapeutic targets. In the future, these methods may support clinical decision making through predictive diagnostics and personalized interventions.

Table 1.
Diagrammatic representation of molecular, cellular, and computational techniques and their relevance in HAND.
4. Clinical Relevance
The future clinical relevance of understanding how HIV impacts neurogenetics encompasses several critical areas poised to advance diagnosis, treatment, and care for HIV-infected individuals. Key advancements include early detection and monitoring through the genetic and epigenetic biomarkers of neurocognitive impairment, alongside future neuroimaging innovations for the precise visualization of HIV-induced brain changes [55,58]. Personalized medicine approaches could optimize treatment regimens by integrating genetic profiling and targeting epigenetic modifications to mitigate neuronal damage marking a significant step toward precision medicine in neuroHIV management. Novel therapeutic targets may emerge from insights into genetic and molecular pathways, potentially leading to neuroprotective agents and immune modulation strategies to bolster CNS resilience. Integrative care models, incorporating neurogenetic insights, aim to enhance overall health outcomes and quality of life through tailored rehabilitation and psychosocial support [56,57]. Advances in research tools and longitudinal studies are pivotal, offering a deeper understanding of HIV–neurogenetics interactions and paving the way for innovative therapeutic discoveries and clinical applications in HIV care [59]. Neurogenetic insights may also lead to the discovery of new therapeutic targets aimed at modulating glial activation, mitochondrial dysfunction, or synaptic plasticity in the context of HAND [60].
Bioinformatics plays a crucial role in HIV neurogenetics by facilitating precision medicine through the identification of genetic markers and molecular signatures linked to neurocognitive impairment in patients with HIV, which informs personalized treatment strategies. It also supports drug discovery efforts by pinpointing specific pathways and molecular targets for developing new therapies targeting HIV-associated neurocognitive disorders (HANDs) [56,61]. Additionally, bioinformatics enables the early detection of neurologic complications in HIV-infected individuals by uncovering biomarkers and developing diagnostic tools based on genomic, transcriptomic, and epigenetic data [62,63]. Moreover, it provides valuable systems biology insights by integrating multi-omics data, enhancing our understanding of HIV-associated transcriptional and epigenetic alterations across multiple cell types, exploring intricate interactions between HIV and the central nervous system (CNS), and advancing knowledge of disease mechanisms and pathophysiology [64].
5. Conclusions and Future Aspects
There is immense potential in advancing personalized medicine and improving therapeutic strategies for HIV-related neurological complications. As technologies like single-cell sequencing, advanced proteomics, and machine learning continue to evolve, they will enable more precise mapping of the molecular interactions between HIV and the CND. This will enhance our ability to identify novel biomarkers, uncover genetic variations that influence susceptibility and disease progression, and develop targeted treatments that address the underlying neuroinflammation and neurodegeneration caused by the virus. Moreover, integrating these findings into clinical practice could lead to better diagnostic tools, optimized antiretroviral therapies, and interventions tailored to individual genetic profiles, ultimately improving the quality of life for those living with HIV-related neurocognitive disorders.
Addressing knowledge gaps in HIV-associated neurocognitive disorders (HANDs) requires a multifaceted future research approach. Longitudinal studies are essential to track HAND progression, distinguishing between reversible and permanent cognitive decline and identifying factors contributing to disease worsening despite viral suppression. Additionally, developing more accurate animal models for HAND is critical, as the current models often fail to fully replicate the complexity of HIV’s effects on the human brain, particularly regarding chronic inflammation and synaptic dysfunction. Furthermore, with the aging HIV-positive population, studies on the impact of aging on HAND are increasingly important. Aging-related neurodegenerative processes may interact with HIV-associated neuroinflammation, exacerbating cognitive decline. Understanding these interactions can inform tailored interventions to improve cognitive outcomes and quality of life in older adults with HIV.
The development of new diagnostic and therapeutic tools is crucial for improving the management of HIV-associated neurocognitive disorders (HANDs). Identifying reliable biomarkers for early HAND detection could enable timely intervention before a significant cognitive decline occurs. Advances in neuroimaging, cerebrospinal fluid (CSF) analysis, and blood-based biomarkers are helping to refine diagnostic accuracy. Additionally, artificial intelligence (AI) and machine learning (ML) are transforming HAND research by evaluating complex datasets to identify patterns in cognitive decline, predict disease progression, and optimize personalized treatment strategies. On the therapeutic front, a significant challenge remains the delivery of effective treatments across the blood–brain barrier (BBB). Developing novel drug formulations, such as nanoparticles and small-molecule therapies, that can efficiently penetrate the CNS while minimizing systemic toxicity could revolutionize HAND treatment and improve long-term outcomes for people living with HIV.
Author Contributions
As the first author, S.J. conducted a literature survey about the review topic and wrote a draft of the manuscript. S.N. contributed to writing the manuscript and drawing the figures. V.N. is the corresponding author who had taken leadership in conceptualizing, supervising, and finalizing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This narrative review synthesizes information from previously published studies, which are appropriately cited within the manuscript. No new data were generated or analyzed in this study. Therefore, data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANI | Asymptomatic Neurocognitive Impairment |
| ART | Antiretroviral Therapy |
| ATAC-seq | Assay for Transposase-Accessible Chromatin Using Sequencing |
| BBB | Blood–Brain Barrier |
| CCR5 | chemokine receptor 5 |
| CNS | Central Nervous System |
| CND | Central Nervous Diseases |
| CpG | Cytosine phosphate Guanine |
| CRISPR-Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) CRISPR-associated protein 9 (Cas9). |
| CXCR4 | C-X-C motif chemokine receptor 4 |
| DNMT | DNA Methyltransferase enzyme |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| fMRI | Functional Magnetic Resonance Imaging |
| HAD | HIV-Associated Dementia |
| HAT | Histone Acetyltransferases |
| HAND | HIV-Associated Neurocognitive Diseases |
| HIV | Human Immunodeficiency Virus |
| HMT | Histone Methyltransferases |
| HLA | Human Leukocyte Antigen |
| ISH | In situ Hybridization |
| MND | Mild Neurocognitive Disorder |
| MRI | Magnetic Resonance Imaging |
| NMDA | N-methyl-D-aspartate |
| PET | Positron Emission Tomography |
| ROS | Reactive Oxygen Species |
| scRNA-seq | Single-Cell RNA sequencing |
| scDNA-seq | Single-Cell DNA sequencing |
| SOD | Superoxide Dismutase |
| SMRT | Single-Molecule Real Time Sequencing |
| SLC | Solute Carrier |
References
- Takehisa, J.; Zekeng, L.; Ido, E.; Mboudjeka, I.; Moriyama, H.; Miura, T.; Yamashita, M.; Gürtler, L.G.; Hayami, M.; Kaptué, L. Various Types of HIV Mixed Infections in Cameroon. Virology 1998, 245, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abongwa, L.E.; Nyamache, A.K.; Torimiro, J.N.; Okemo, P.; Charles, F. Human immunodeficiency virus type 1 ((HIV-1) subtypes in the northwest region, Cameroon. Virol. J. 2019, 16, 103. [Google Scholar] [CrossRef]
- Eberle, J.; Gürtler, L. HIV Types, Groups, Subtypes and Recombinant Forms: Errors in Replication, Selection Pressure and Quasispecies. Intervirology 2012, 55, 79–83. [Google Scholar] [CrossRef]
- Hemelaar, J.; Elangovan, R.; Yun, J.; Dickson-Tetteh, L.; Fleminger, I.; Kirtley, S.; Williams, B.; Gouws-Williams, E.; Ghys, P.D.; Abimiku, A.G.; et al. Global and regional molecular epidemiology of HIV-1, 1990–2015: A systematic review, global survey, and trend analysis. Lancet Infect. Dis. 2019, 19, 143–155. [Google Scholar] [CrossRef]
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef]
- Rumbaugh, J.A.; Tyor, W. HIV-associated neurocognitive disorders: Five new things. Neurol. Clin. Pract. 2015, 5, 224–231. [Google Scholar] [CrossRef]
- For the CHARTER and HNRC Groups; Heaton, R.K.; Franklin, D.R.; Ellis, R.J.; McCutchan, J.A.; Letendre, S.L.; LeBlanc, S.; Corkran, S.H.; Duarte, N.A.; Clifford, D.B.; et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J. Neurovirol. 2011, 17, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Saylor, D.; Dickens, A.M.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, J.C. HIV-associated neurocognitive disorder—Pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016, 12, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Fadel, A.; Garcia, E.; Michel, G.; Saadoon, Z.F.; Fernandes, A.; Jarrett, O.; Naseer, L.; Abellard, R.-B.; Dalgado, P. HIV and dementia. Microbe 2024, 2, 100052. [Google Scholar] [CrossRef]
- Avdoshina, V.; Mocchetti, I. Recent Advances in the Molecular and Cellular Mechanisms of gp120-Mediated Neurotoxicity. Cells 2022, 11, 1599. [Google Scholar] [CrossRef]
- Firdaus, Z.; Li, X. Unraveling the Genetic Landscape of Neurological Disorders: Insights into Pathogenesis, Techniques for Variant Identification, and Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 2320. [Google Scholar] [CrossRef] [PubMed]
- Houlden, H.; Cortese, A.; Wild, E. Neurogenetics. In Neurology, 1st ed.; Howard, R., Kullmann, D., Werring, D., Zandi, M., Eds.; Wiley: New York, NY, USA, 2024; pp. 79–89. [Google Scholar] [CrossRef]
- Varatharajan, L.; Thomas, S.A. The transport of anti-HIV drugs across blood–CNS interfaces: Summary of current knowledge and recommendations for further research. Antivir. Res. 2009, 82, A99–A109. [Google Scholar] [CrossRef] [PubMed]
- Decloedt, E.H.; Rosenkranz, B.; Maartens, G.; Joska, J. Central Nervous System Penetration of Antiretroviral Drugs: Pharmacokinetic, Pharmacodynamic and Pharmacogenomic Considerations. Clin. Pharmacokinet. 2015, 54, 581–598. [Google Scholar] [CrossRef]
- Kang, J.; Wang, Z.; Zhou, Y.; Wang, W.; Wen, Y. Learning from cerebrospinal fluid drug-resistant HIV escape-associated encephalitis: A case report. Virol. J. 2023, 20, 292. [Google Scholar] [CrossRef] [PubMed]
- Osborne, O.; Peyravian, N.; Nair, M.; Daunert, S.; Toborek, M. The Paradox of HIV Blood–Brain Barrier Penetrance and Antiretroviral Drug Delivery Deficiencies. Trends Neurosci. 2020, 43, 695–708. [Google Scholar] [CrossRef]
- Bogdan, R.; Hyde, L.W.; Hariri, A.R. A neurogenetics approach to understanding individual differences in brain, behavior, and risk for psychopathology. Mol. Psychiatry 2013, 18, 288–299. [Google Scholar] [CrossRef]
- Ferrell, D.; Giunta, B. The impact of HIV-1 on neurogenesis: Implications for HAND. Cell. Mol. Life Sci. 2014, 71, 4387–4392. [Google Scholar] [CrossRef]
- Lindl, K.A.; Marks, D.R.; Kolson, D.L.; Jordan-Sciutto, K.L. HIV-Associated Neurocognitive Disorder: Pathogenesis and Therapeutic Opportunities. J. Neuroimmune Pharmacol. 2010, 5, 294–309. [Google Scholar] [CrossRef]
- Kolanu, N.D. CRISPR–Cas9 Gene Editing: Curing Genetic Diseases by Inherited Epigenetic Modifications. Glob. Med. Genet. 2024, 11, 113–122. [Google Scholar] [CrossRef]
- Jha, N.K.; Chen, W.-C.; Kumar, S.; Dubey, R.; Tsai, L.-W.; Kar, R.; Jha, S.K.; Gupta, P.K.; Sharma, A.; Gundamaraju, R.; et al. Molecular mechanisms of developmental pathways in neurological disorders: A pharmacological and therapeutic review. Open Biol. 2022, 12, 210289. [Google Scholar] [CrossRef]
- Notaras, M.; Lodhi, A.; Dündar, F.; Collier, P.; Sayles, N.M.; Tilgner, H.; Greening, D.; Colak, D. Schizophrenia is defined by cell-specific neuropathology and multiple neurodevelopmental mechanisms in patient-derived cerebral organoids. Mol. Psychiatry 2022, 27, 1416–1434. [Google Scholar] [CrossRef] [PubMed]
- Haorah, J.; Malaroviyam, S.; Iyappan, H.; Samikkannu, T. Neurological impact of HIV/AIDS and substance use alters brain function and structure. Front. Med. 2025, 11, 1505440. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, J.; Teng, Z.; Liu, Q. Role of ART and PrEP treatments in a stochastic HIV/AIDS epidemic model. Math. Comput. Simul. 2024, 221, 337–357. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Chang, L.; Ji, H.; Sun, H.; Yan, Y.; Xu, J.; Wang, L. Optimizing HIV testing: Comparing diagnostic signatures and assay performance in ART-treated and general screening populations. Clin. Chim. Acta 2025, 570, 120207. [Google Scholar] [CrossRef] [PubMed]
- Braibant, M.; Xie, J.; Samri, A.; Agut, H.; Autran, B.; Barin, F. Disease progression due to dual infection in an HLA-B57-positive asymptomatic long-term nonprogressor infected with a nef-defective HIV-1 strain. Virology 2010, 405, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, K.; Banerjee, A.; Sarkar, R.; Banerjee, R.; Chowdhury, S.R.; Ganguly, K.; Karak, P. HIV-associated neurocognitive disorders (HAND): Optimal diagnosis, antiviral therapy, pharmacological treatment, management, and future scopes. J. Neurol. Sci. 2025, 470, 123410. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Musselman, D.; Jayaweera, D.; Da Fonseca Ferreira, A.; Marzouka, G.; Dong, C. HIV-Associated Neurocognitive Disorder (HAND) and Alzheimer’s Disease Pathogenesis: Future Directions for Diagnosis and Treatment. Int. J. Mol. Sci. 2024, 25, 11170. [Google Scholar] [CrossRef]
- Norcini-Pala, A.; Stringer, K.L.; Kempf, M.-C.; Konkle-Parker, D.; Wilson, T.E.; Tien, P.C.; Wingood, G.; Neilands, T.B.; Johnson, M.O.; Weiser, S.D.; et al. Longitudinal associations between intersectional stigmas, antiretroviral therapy adherence, and viral load among women living with HIV using multidimensional latent transition item response analysis. Soc. Sci. Med. 2025, 366, 117643. [Google Scholar] [CrossRef]
- Putatunda, R.; Ho, W.-Z.; Hu, W. HIV-1 and Compromised Adult Neurogenesis: Emerging Evidence for a New Paradigm of HAND Persistence. Aids Rev. 2019, 21, 1908. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Sreeram, S.; Ye, F.; Garcia-Mesa, Y.; Nguyen, K.; El Sayed, A.; Leskov, K.; Karn, J. The potential role of HIV-1 latency in promoting neuroinflammation and HIV-1-associated neurocognitive disorder. Trends Immunol. 2022, 43, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Graur, A.; Erickson, N.; Sinclair, P.; Nusir, A.; Kabbani, N. HIV-1 gp120 Interactions with Nicotine Modulate Mitochondrial Network Properties and Amyloid Release in Microglia. Neurochem. Res. 2025, 50, 103. [Google Scholar] [CrossRef] [PubMed]
- Kandanearatchi, A.; Nath, A.; Lipton, S.A.; Masliah, E.; Everall, I.P. Protection against HIV-1 gp120 and HIV-1 Tat neurotoxicity. In Neurol AIDS, 2nd ed.; Gendelman, H.E., Grant, I., Everall, I.P., Lipton, S.A., Swindells, S., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 201–210. [Google Scholar] [CrossRef]
- Smith, L.K.; Babcock, I.W.; Minamide, L.S.; Shaw, A.E.; Bamburg, J.R.; Kuhn, T.B. Direct interaction of HIV gp120 with neuronal CXCR4 and CCR5 receptors induces cofilin-actin rod pathology via a cellular prion protein- and NOX-dependent mechanism. PLoS ONE 2021, 16, e0248309. [Google Scholar] [CrossRef]
- Jadhav, S.; Makar, P.; Nema, V. The NeuroinflammatoryPotential of HIV-1 NefVariants in Modulating the Gene Expression Profile of Astrocytes. Cells 2022, 11, 3256. [Google Scholar] [CrossRef]
- Siddiqui, A.; He, C.; Lee, G.; Figueroa, A.; Slaughter, A.; Robinson-Papp, J. Neuropathogenesis of HIV and emerging therapeutic targets. Expert Opin. Ther. Targets 2022, 26, 603–615. [Google Scholar] [CrossRef] [PubMed]
- McRae, M. HIV and viral protein effects on the blood brain barrier. Tissue Barriers 2016, 4, e1143543. [Google Scholar] [CrossRef]
- Jadhav, S.; Nema, V. HIV-Associated Neurotoxicity: The Interplay of Host and Viral Proteins. Mediat. Inflamm. 2021, 2021, 1267041. [Google Scholar] [CrossRef]
- Mu, T.; Wei, J.; Sun, J.; Jin, J.; Zhang, T.; Wu, H.; Su, B. Association of apolipoprotein E epsilon 4 and cognitive impairment in adults living with human immunodeficiency virus: A meta-analysis. Chin. Med. J. 2022, 135, 2677–2686. [Google Scholar] [CrossRef]
- Pisani, A.; Paciello, F.; Del Vecchio, V.; Malesci, R.; De Corso, E.; Cantone, E.; Fetoni, A.R. The Role of BDNF as a Biomarker in Cognitive and Sensory Neurodegeneration. J. Pers. Med. 2023, 13, 652. [Google Scholar] [CrossRef]
- Nickoloff-Bybel, E.A.; Festa, L.; Meucci, O.; Gaskill, P.J. Co-receptor signaling in the pathogenesis of neuroHIV. Retrovirology 2021, 18, 24. [Google Scholar] [CrossRef]
- Freedman, B.D.; Liu, Q.-H.; Del Corno, M.; Collman, R.G. HIV-1 gp120 Chemokine Receptor-Mediated Signaling in Human Macrophages. Immunol. Res. 2003, 27, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, L.W.; Bragatte, M.A.D.S.; Vieira, G.F. The influence of HLA/HIV genetics on the occurrence of elite controllers and a need for therapeutics geotargeting view. Braz. J. Infect. Dis. 2021, 25, 101619. [Google Scholar] [CrossRef]
- Lobos, C.A.; Downing, J.; D’Orsogna, L.J.; Chatzileontiadou, D.S.M.; Gras, S. Protective HLA-B57: T cell and natural killer cell recognition in HIV infection. Biochem. Soc. Trans. 2022, 50, 1329–1339. [Google Scholar] [CrossRef]
- Jadhav, S.; Nema, V. Association of Viral and Host Genetic Architecture with the Status of Neurocognitive Disorder in HIV-Infected Individuals. AIDS Res. Hum. Retroviruses 2023, 39, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Jennings, A.; Akbarian, S. Genomic Exploration of the Brain in People Infected with HIV—Recent Progress and the Road Ahead. Curr. HIV/AIDS Rep. 2023, 20, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Overk, C.; Achim, C.L.; Masliah, E. Pathogenesis of age-related HIV neurodegeneration. J. Neurovirol. 2019, 25, 622–633. [Google Scholar] [CrossRef]
- Ehrlich, M. DNA hypermethylation in disease: Mechanisms and clinical relevance. Epigenetics 2019, 14, 1141–1163. [Google Scholar] [CrossRef]
- Nehme, Z.; Pasquereau, S.; Herbein, G. Control of viral infections by epigenetic-targeted therapy. Clin. Epigenetics 2019, 11, 55. [Google Scholar] [CrossRef]
- Kong, W.; Frouard, J.; Xie, G.; Corley, M.J.; Helmy, E.; Zhang, G.; Schwarzer, R.; Montano, M.; Sohn, P.; Roan, N.R.; et al. Neuroinflammation generated by HIV-infected microglia promotes dysfunction and death of neurons in human brain organoids. PNAS Nexus 2024, 3, 179. [Google Scholar] [CrossRef]
- El-Amine, R.; Germini, D.; Zakharova, V.V.; Tsfasman, T.; Sheval, E.V.; Louzada, R.A.N.; Dupuy, C.; Bilhou-Nabera, C.; Hamade, A.; Najjar, F.; et al. HIV-1 Tat protein induces DNA damage in human peripheral blood B-lymphocytes via mitochondrial ROS production. Redox Biol. 2018, 15, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.W.; Norman, J.P.; Litzburg, A.; Zhang, D.; Dewhurst, S.; Gelbard, H.A. HIV-1 Transactivator of Transcription Protein Induces Mitochondrial Hyperpolarization and Synaptic Stress Leading to Apoptosis. J. Immunol. 2005, 174, 4333–4344. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Sharma, D.; Kumar, S.; Puri, B. Understanding HIV-associated neurocognitive and neurodegenerative disorders (neuroAIDS): Enroute to achieve the 95-95-95 target and sustainable development goal for HIV/AIDS response. VirusDisease 2023, 34, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Guerrero, A.; Pallàs, M. Advancing personalized medicine in neurodegenerative diseases: The role of epigenetics and pharmacoepigenomics in pharmacotherapy. Pharmacol. Res. 2024, 205, 107247. [Google Scholar] [CrossRef]
- Tonti, E.; Dell’Omo, R.; Filippelli, M.; Spadea, L.; Salati, C.; Gagliano, C.; Musa, M.; Zeppieri, M. Exploring Epigenetic Modifications as Potential Biomarkers and Therapeutic Targets in Glaucoma. Int. J. Mol. Sci. 2024, 25, 2822. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiu, X.; Wang, Z.; Zhang, Y. Neuroimaging advances in neurocognitive disorders among HIV-infected individuals. Front. Neurol. 2025, 16, 1479183. [Google Scholar] [CrossRef]
- Zhao, Y. Advances in HIV Research: Mechanisms, Transmission Routes, and Emerging Therapeutic Strategies. Highlights Sci. Eng. Technol. 2024, 123, 505–509. [Google Scholar] [CrossRef]
- Ojeda-Juárez, D.; Kaul, M. Transcriptomic and Genetic Profiling of HIV-Associated Neurocognitive Disorders. Front. Mol. Biosci. 2021, 8, 721954. [Google Scholar] [CrossRef]
- Borrajo, A.; Pérez-Rodríguez, D.; Fernández-Pereira, C.; Prieto-González, J.M.; Agís-Balboa, R.C. Genomic Factors and Therapeutic Approaches in HIV-Associated Neurocognitive Disorders: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 14364. [Google Scholar] [CrossRef]
- Jang, W.-J.; Lee, S.; Jeong, C.-H. Uncovering transcriptomic biomarkers for enhanced diagnosis of methamphetamine use disorder: A comprehensive review. Front. Psychiatry 2024, 14, 1302994. [Google Scholar] [CrossRef]
- Du, P.; Fan, R.; Zhang, N.; Wu, C.; Zhang, Y. Advances in Integrated Multi-omics Analysis for Drug-Target Identification. Biomolecules 2024, 14, 692. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Agrawal, K.; Stanley, J.; Li, R.; Jacobs, N.; Wang, H.; Lu, C.; Qu, R.; Clarke, D.; Chen, Y.; et al. Multi-omic Characterization of HIV Effects at Single Cell Level across Human Brain Regions. bioRxiv 2025. bioRxiv:8:2025.02.05.636707. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).