The Interplay Between Suicidal Behavior and Mental Disorders: Focusing on the Role of Glial Cells
Abstract
1. Introduction
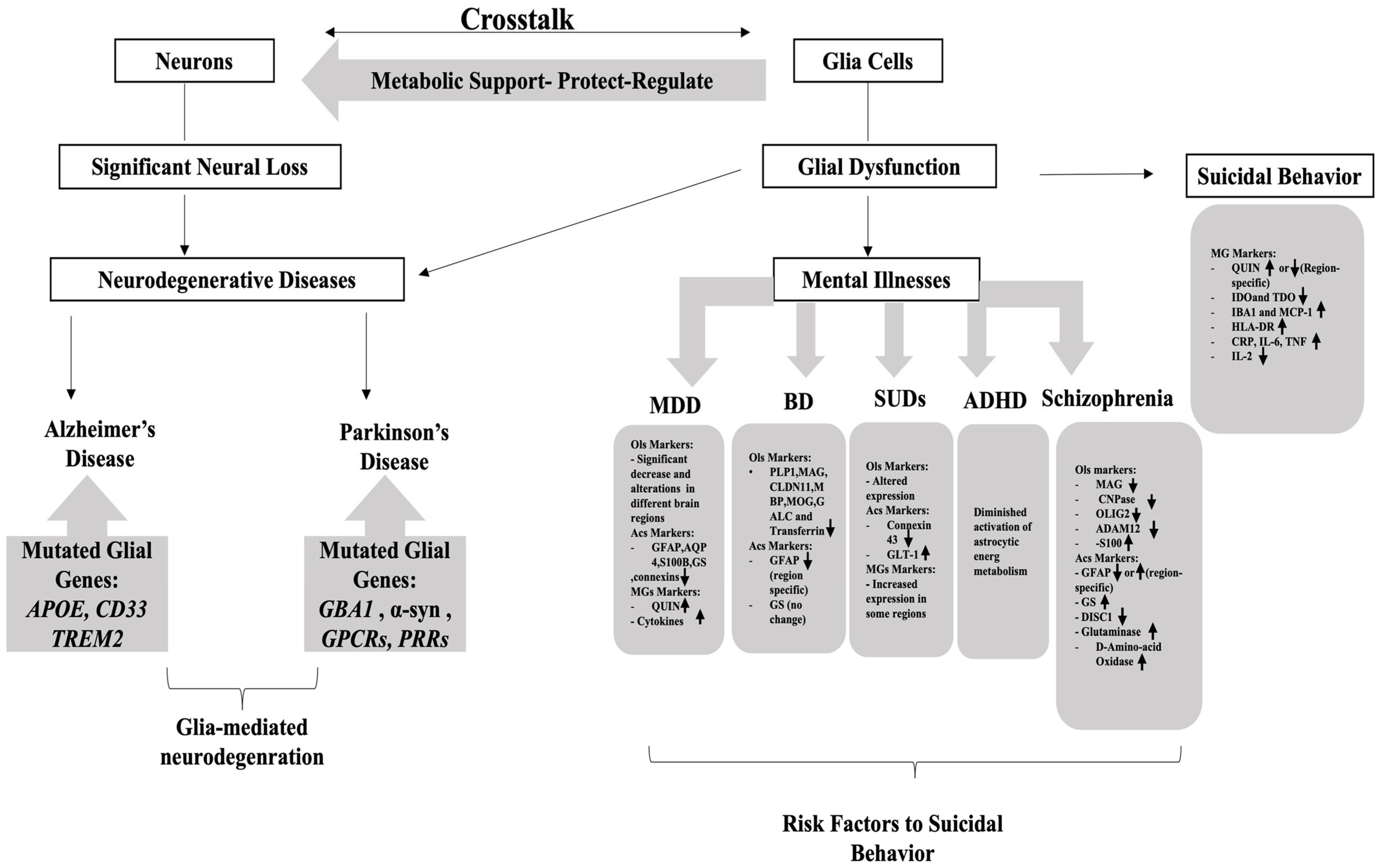
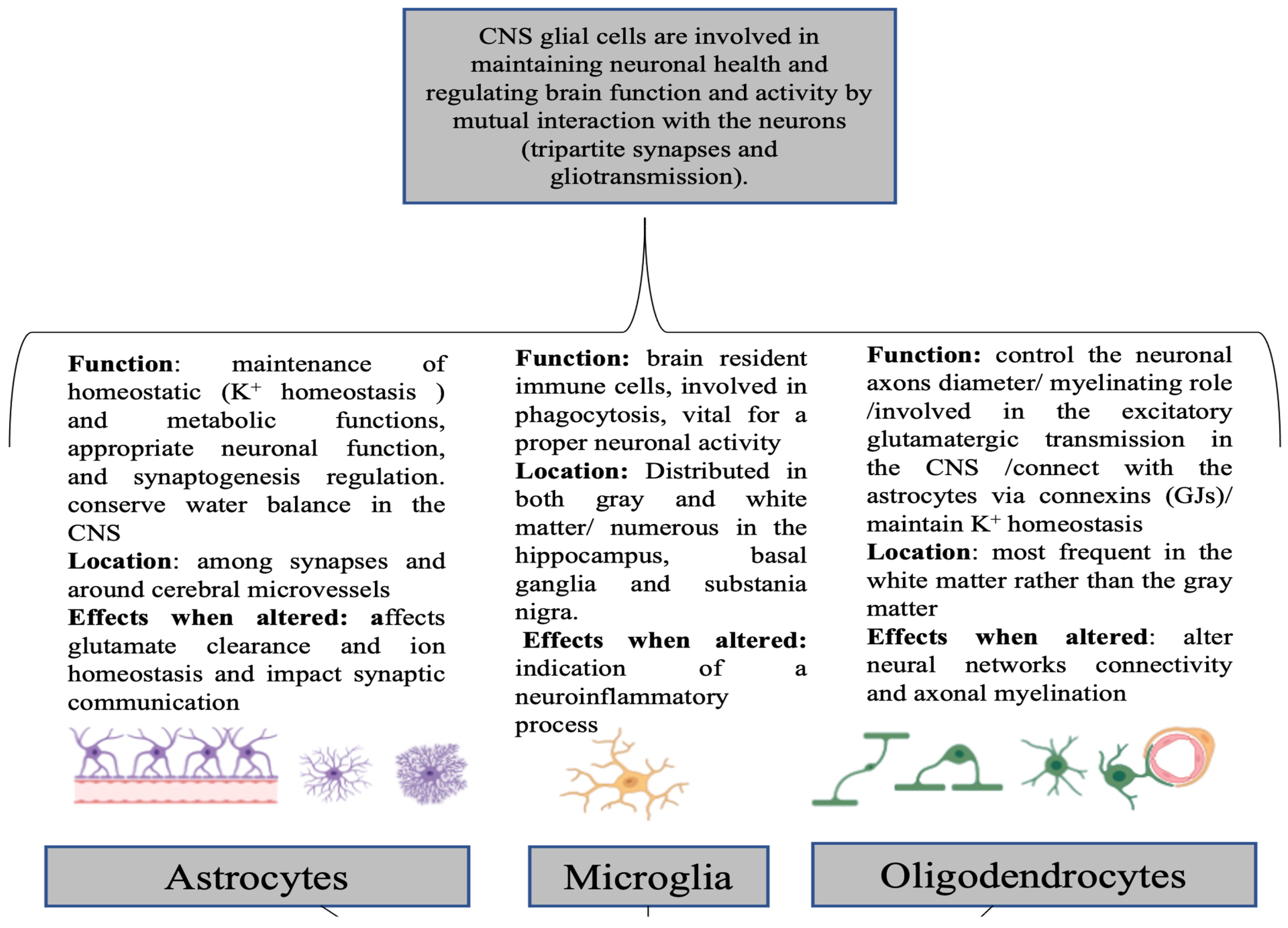
2. Glio-Pathologies
3. Glial Cells and SB
| Mental Disorders | Glial Markers | Impact | References |
|---|---|---|---|
| MDD |
|
| [57,58,59] |
|
| [59,60] | |
|
| [61] | |
|
| [59] | |
|
| [62] | |
|
| [63] | |
|
| [59] | |
| Schizophrenia |
|
| [64] |
|
| [65,66] | |
|
| [67] | |
|
| [68] | |
|
| [69] | |
|
| [70] | |
|
| [71] | |
|
| [71,72] | |
|
| [73] | |
|
| [74] | |
|
| [75,76] | |
| BD |
|
| [77,78] |
|
| [79,80] | |
|
| [81] | |
|
| [77,82] | |
|
| [83] | |
| SUDs |
|
| [84] |
|
| [85] | |
| Suicidal Behavior |
|
| [86,87,88] |
|
| [89] | |
|
| [15] | |
|
| [90,91] | |
|
| [92] | |
|
| [93] | |
|
| [94,95] | |
|
| [96] | |
|
| [96,97] |
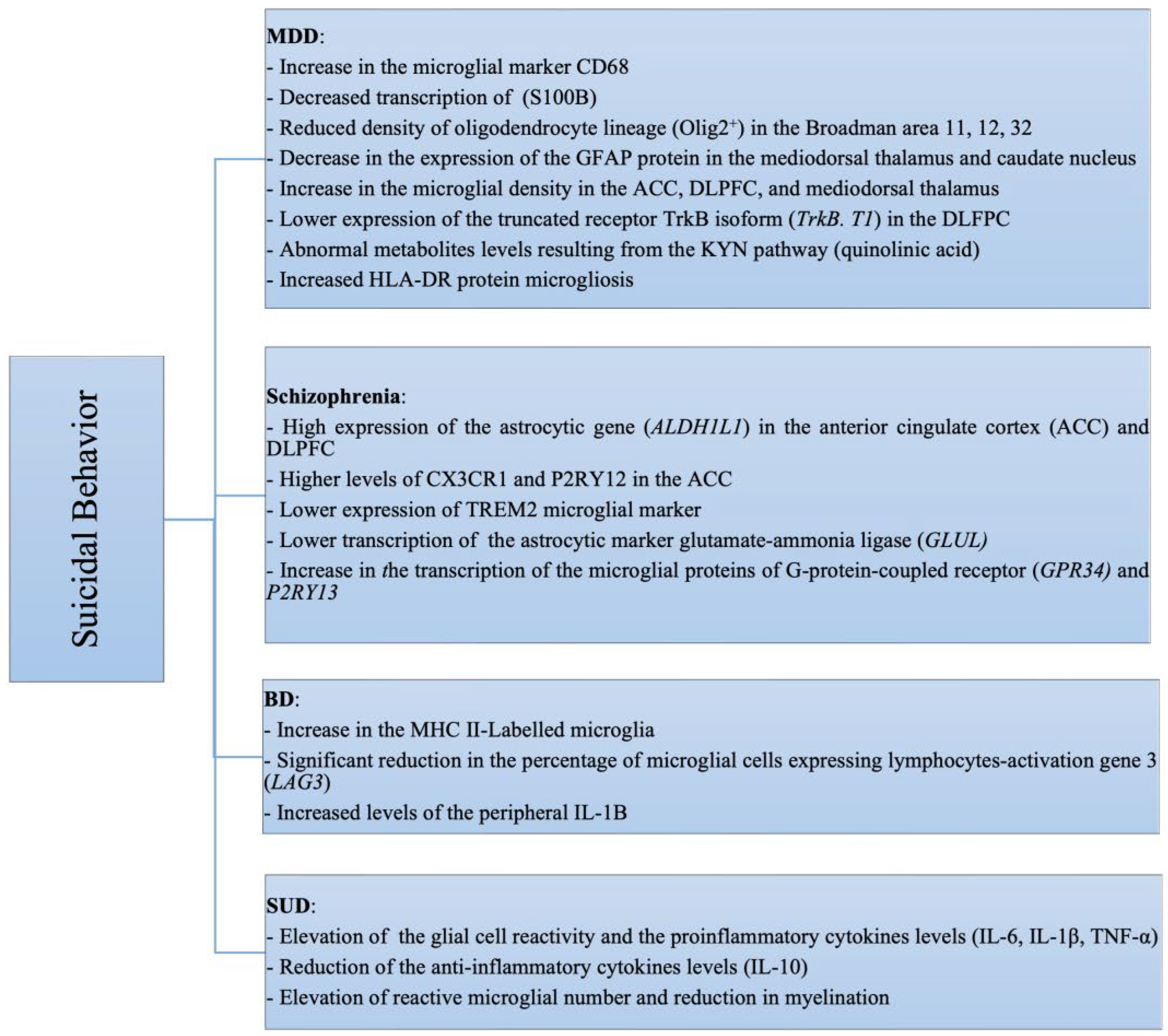
4. Suicidal Behavior and Mental Disorders
4.1. SB and Major Depressive Disorder (MDD)
4.2. SB and Schizophrenia
4.3. SB and Bipolar Disorder (BD)
4.4. SB and Borderline Personality Disorder (BPD)
4.5. SB and Attention Deficit Hyperactivity Disorder (ADHD)
4.6. SB and Post-Traumatic Stress Disorder (PTSD)
4.7. SB and Anxiety Disorders
4.8. SB and Substance Use Disorders (SUDs)
5. Pharmacology
6. Conclusions and Future Perspectives
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parpura, V.; Heneka, M.T.; Montana, V.; Oliet, S.H.R.; Schousboe, A.; Haydon, P.G.; Stout, R.F.; Spray, D.C.; Reichenbach, A.; Pannicke, T.; et al. Glial cells in (patho)physiology. J. Neurochem. 2012, 121, 4–27. [Google Scholar] [CrossRef]
- Jäkel, S.; Dimou, L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell. Neurosci. 2017, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, D.; Murashova, L.; Burzak, N.; Dyachuk, V. Satellite Glial Cells: Morphology, functional heterogeneity, and role in pain. Front. Cell. Neurosci. 2022, 16, 1019449. [Google Scholar] [CrossRef]
- Gazerani, P. Satellite Glial Cells in Pain Research: A Targeted Viewpoint of Potential and Future Directions. Front. Pain. Res. 2021, 2, 646068. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; He, W.; Tang, T.; Qiu, M. Immunological Markers for Central Nervous System Glia. Neurosci. Bull. 2022, 39, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Rasband, M.N. Glial Contributions to Neural Function and Disease. Mol. Cell. Proteom. 2016, 15, 355–361. [Google Scholar] [CrossRef]
- Kato, D.; Eto, K.; Nabekura, J.; Wake, H. Activity-dependent functions of non-electrical glial cells. J. Biochem. 2018, 163, 457–464. [Google Scholar] [CrossRef]
- Um, J.W. Roles of Glial Cells in Sculpting Inhibitory Synapses and Neural Circuits. Front. Mol. Neurosci. 2017, 10, 381. [Google Scholar] [CrossRef]
- Jung, Y.-J.; Chung, W.-S. Phagocytic Roles of Glial Cells in Healthy and Diseased Brains. Biomol. Ther. 2018, 26, 350–357. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, Y.-K. Neuron-to-microglia Crosstalk in Psychiatric Disorders. Curr. Neuropharmacol. 2020, 18, 84. [Google Scholar] [CrossRef]
- Kong, E.; Li, Y.; Deng, M.; Hua, T.; Yang, M.; Li, J.; Feng, X.; Yuan, H. Glycometabolism Reprogramming of Glial Cells in Central Nervous System: Novel Target for Neuropathic Pain. Front. Immunol. 2022, 13, 861290. [Google Scholar] [CrossRef] [PubMed]
- Hyung, S.; Park, J.-H.; Jung, K. Application of optogenetic glial cells to neuron-glial communication. Front. Cell. Neurosci. 2023, 17, 1249043. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Riboldi, B.; Ceruti, S. Human Glial Cells as Innovative Targets for the Therapy of Central Nervous System Pathologies. Cells 2024, 13, 606. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J.J. Gliogenesis and Glial Pathology in Depression. CNS Neurol. Disord. Drug Targets 2007, 6, 219. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sakai, M.; Yu, Z.; Nakanishi, M.; Yoshii, H. Glial Markers of Suicidal Behavior in the Human Brain-A Systematic Review of Postmortem Studies. Int. J. Mol. Sci. 2024, 25, 5750. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, J.; Yoon, B.-E. Neuron-Glia Interactions in Neurodevelopmental Disorders. Cells 2020, 9, 2176. [Google Scholar] [CrossRef]
- Shen, W.; Pristov, J.B.; Nobili, P.; Nikolić, L. Can glial cells save neurons in epilepsy? Neural Regen. Res. 2023, 18, 1417–1422. [Google Scholar] [CrossRef]
- Ramsey, J.; Martin, E.C.; Purcell, O.M.; Lee, K.M.; MacLean, A.G. Self-injurious behaviours in rhesus macaques: Potential glial mechanisms. J. Intellect. Disabil. Res. 2018, 62, 1008–1017. [Google Scholar] [CrossRef]
- Mayegowda, S.B.; Thomas, C. Glial pathology in neuropsychiatric disorders: A brief review. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 20180120. [Google Scholar] [CrossRef]
- Garden, G.A.; Campbell, B.M. Glial Biomarkers in Human Central Nervous System Disease. Glia 2016, 64, 1755–1771. [Google Scholar] [CrossRef]
- Ochocka, N.; Kaminska, B. Microglia Diversity in Healthy and Diseased Brain: Insights from Single-Cell Omics. Int. J. Mol. Sci. 2021, 22, 3027. [Google Scholar] [CrossRef] [PubMed]
- Zlomuzica, A.; Plank, L.; Kodzaga, I.; Dere, E. A fatal alliance: Glial connexins, myelin pathology and mental disorders. J. Psychiatr. Res. 2023, 159, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Q.R. Convergent epigenetic regulation of glial plasticity in myelin repair and brain tumorigenesis: A focus on histone modifying enzymes. Neurobiol. Dis. 2020, 144, 105040. [Google Scholar] [CrossRef]
- He, C.; Duan, S. Novel Insight into Glial Biology and Diseases. Neurosci. Bull. 2023, 39, 365–367. [Google Scholar] [CrossRef]
- Poggi, G.; Wennström, M.; Müller, M.B.; Treccani, G. NG2-glia: Rising stars in stress-related mental disorders? Mol. Psychiatry 2023, 28, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Abou Chahla, M.N.; Khalil, M.I.; Comai, S.; Brundin, L.; Erhardt, S.; Guillemin, G.J. Biological Factors Underpinning Suicidal Behaviour: An Update. Brain Sci. 2023, 13, 505. [Google Scholar] [CrossRef]
- Baharikhoob, P.; Kolla, N.J. Microglial Dysregulation and Suicidality: A Stress-Diathesis Perspective. Front. Psychiatry 2020, 11, 781. [Google Scholar] [CrossRef]
- Aydin, M.; Hacimusalar, Y.; Hocaoğlu, Ç. İntihar Davranışının Nörobiyolojisi. Psikiyatr. Güncel Yaklaşımlar 2019, 11, 1–23. [Google Scholar] [CrossRef]
- Pereira, C.A.; Reis-de-Oliveira, G.; Pierone, B.C.; Martins-de-Souza, D.; Kaster, M.P. Depicting the molecular features of suicidal behavior: A review from an “omics” perspective. Psychiatry Res. 2024, 332, 115682. [Google Scholar] [CrossRef]
- Supakul, S.; Murakami, R.; Oyama, C.; Shindo, T.; Hatakeyama, Y.; Itsuno, M.; Bannai, H.; Shibata, S.; Maeda, S.; Okano, H. Mutual interaction of neurons and astrocytes derived from iPSCs with APP V717L mutation developed the astrocytic phenotypes of Alzheimer’s disease. Inflamm. Regen. 2024, 44, 8. [Google Scholar] [CrossRef]
- Sočan, V.; Dolinar, K.; Kržan, M. Transporters involved in adult rat cortical astrocyte dopamine uptake: Kinetics, expression and pharmacological modulation. Eur. J. Neurosci. 2024, 59, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.I.; Ryan, M.A.; McNabb, M.C.; Kamasawa, N.; Scholl, B. Astrocyte coverage of excitatory synapses correlates to measures of synapse structure and function in ferret primary visual cortex. Glia 2024, 72, 1785–1800. [Google Scholar] [CrossRef]
- Doliwa, M.; Kuzniewska, B.; Nader, K.; Reniewicz, P.; Kaczmarek, L.; Michaluk, P.; Kalita, K. Astrocyte-Secreted Lcn2 Modulates Dendritic Spine Morphology. Cells 2025, 14, 159. [Google Scholar] [CrossRef]
- Nozawa, O.; Miyata, M.; Shiotani, H.; Kameyama, T.; Komaki, R.; Shimizu, T.; Kuriu, T.; Kashiwagi, Y.; Sato, Y.; Koebisu, M.; et al. Necl2/3-mediated mechanism for tripartite synapse formation. Development 2023, 150, dev200931. [Google Scholar] [CrossRef]
- Garcia, D.W.; Jacquir, S. Astrocyte-mediated neuronal irregularities and dynamics: The complexity of the tripartite synapse. Biol. Cybern. 2024, 118, 249–266. [Google Scholar] [CrossRef]
- Chahinian, H.; Yahi, N.; Fantini, J. Glutamate, Gangliosides, and the Synapse: Electrostatics at Work in the Brain. Int. J. Mol. Sci. 2024, 25, 8583. [Google Scholar] [CrossRef] [PubMed]
- Bhoi, R.; Mitra, T.; Tejaswi, K.; Manoj, V.; Ghatak, S. Role of Ion Channels in Alzheimer’s Disease Pathophysiology. J. Membr. Biol. 2025, 258, 187–212. [Google Scholar] [CrossRef] [PubMed]
- Vitureira, N.; Rafael, A.; Abudara, V. P2X7 receptors and pannexin1 hemichannels shape presynaptic transmission. Purinergic Signal. 2024, 20, 223–236. [Google Scholar] [CrossRef]
- Bernstein, H.-G.; Nussbaumer, M.; Vasilevska, V.; Dobrowolny, H.; Nickl-Jockschat, T.; Guest, P.C.; Steiner, J. Glial cell deficits are a key feature of schizophrenia: Implications for neuronal circuit maintenance and histological differentiation from classical neurodegeneration. Mol. Psychiatry 2025, 30, 1102–1116. [Google Scholar] [CrossRef]
- Czéh, B.; Nagy, S.A. Clinical Findings Documenting Cellular and Molecular Abnormalities of Glia in Depressive Disorders. Front. Mol. Neurosci. 2018, 11, 56. [Google Scholar] [CrossRef]
- Griciuc, A.; Serrano-Pozo, A.; Parrado, A.R.; Lesinski, A.N.; Asselin, C.N.; Mullin, K.; Hooli, B.; Choi, S.H.; Hyman, B.T.; Tanzi, R.E. Alzheimer’s Disease Risk Gene CD33 Inhibits Microglial Uptake of Amyloid Beta. Neuron 2013, 78, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Pinarbasi, E.S.; Barmada, S.J. Glia in FTLD-GRN: From supporting cast to leading role. J. Clin. Investig. 2025, 133, e168215. [Google Scholar] [CrossRef] [PubMed]
- Marsan, E.; Velmeshev, D.; Ramsey, A.; Patel, R.K.; Zhang, J.; Koontz, M.; Andrews, M.G.; Majo, M.d.; Mora, C.; Blumenfeld, J.; et al. Astroglial toxicity promotes synaptic degeneration in the thalamocortical circuit in frontotemporal dementia with GRN mutations. J. Clin. Investig. 2023, 133, e164919. [Google Scholar] [CrossRef]
- Vidović, M.; Rikalovic, M.G. Alpha-Synuclein Aggregation Pathway in Parkinson’s Disease: Current Status and Novel Therapeutic Approaches. Cells 2022, 11, 1732. [Google Scholar] [CrossRef]
- Komatsu, H. Novel Therapeutic GPCRs for Psychiatric Disorders. Int. J. Mol. Sci. 2015, 16, 14109–14121. [Google Scholar] [CrossRef] [PubMed]
- Catapano, L.A.; Manji, H.K. G protein-coupled receptors in major psychiatric disorders. Biochim. Biophys. Acta (BBA)—Biomembr. 2007, 1768, 976–993. [Google Scholar] [CrossRef]
- Saleki, K.; Alijanizadeh, P.; Javanmehr, N.; Rezaei, N. The role of Toll-like receptors in neuropsychiatric disorders: Immunopathology, treatment, and management. Med. Res. Rev. 2024, 44, 1267–1325. [Google Scholar] [CrossRef]
- D’Egidio, F.; Castelli, V.; d’Angelo, M.; Ammannito, F.; Quintiliani, M.; Cimini, A. Brain incoming call from glia during neuroinflammation: Roles of extracellular vesicles. Neurobiol. Dis. 2024, 201, 106663. [Google Scholar] [CrossRef]
- Melzer, L.; Freiman, T.M.; Derouiche, A. Rab6A as a Pan-Astrocytic Marker in Mouse and Human Brain, and Comparison with Other Glial Markers (GFAP, GS, Aldh1L1, SOX9). Cells 2021, 10, 72. [Google Scholar] [CrossRef]
- Wang, R.; Ren, H.; Gao, Y.; Wang, G. Editorial: Role of Glial Cells of the Central and Peripheral Nervous System in the Pathogenesis of Neurodegenerative Disorders. Front. Aging Neurosci. 2022, 14, 920861. [Google Scholar] [CrossRef]
- Ludwig, P.E.; Das, J.M. Histology, Glial Cells. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK441945/ (accessed on 2 June 2025).
- You, S.; Su, X.; Ying, J.; Li, S.; Qu, Y.; Mu, D. Research Progress on the Role of RNA m6A Modification in Glial Cells in the Regulation of Neurological Diseases. Biomolecules 2022, 12, 1158. [Google Scholar] [CrossRef]
- Laricchiuta, D.; Papi, M.; Decandia, D.; Panuccio, A.; Cutuli, D.; Peciccia, M.; Mazzeschi, C.; Petrosini, L. The role of glial cells in mental illness: A systematic review on astroglia and microglia as potential players in schizophrenia and its cognitive and emotional aspects. Front. Cell. Neurosci. 2024, 18, 1358450. [Google Scholar] [CrossRef]
- Zhang, L.; Lucassen, P.J.; Salta, E.; Verhaert, P.D.E.M.; Swaab, D.F. Hippocampal neuropathology in suicide: Gaps in our knowledge and opportunities for a breakthrough. Neurosci. Biobehav. Rev. 2022, 132, 542–552. [Google Scholar] [CrossRef]
- Turecki, G.; Brent, D.A.; Gunnell, D.; O’Connor, R.C.; Oquendo, M.A.; Pirkis, J.; Stanley, B.H. Suicide and suicide risk. Nat. Rev. Dis. Primers 2019, 5, 74. [Google Scholar] [CrossRef]
- Turecki, G.; Brent, D.A. Suicide and suicidal behaviour. Lancet 2016, 387, 1227–1239. [Google Scholar] [CrossRef]
- Rahimian, R.; Perlman, K.; Fakhfouri, G.; Mpai, R.; Richard, V.R.; Hercher, C.; Penney, L.; Davoli, M.A.; Nagy, C.; Zahedi, R.P.; et al. Proteomic evidence of depression-associated astrocytic dysfunction in the human male olfactory bulb. bioRxiv 2024. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef]
- Rajkowska, G.; Stockmeier, C.A. Astrocyte pathology in major depressive disorder: Insights from human postmortem brain tissue. Curr. Drug Targets 2013, 14, 1225–1236. [Google Scholar] [CrossRef]
- Schroeter, M.L.; Sacher, J.; Steiner, J.; Schoenknecht, P.; Mueller, K. Serum S100B represents a new biomarker for mood disorders. Curr. Drug Targets 2013, 14, 1237–1248. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, H.; Baek, J.H.; Song, M.; Park, H.; Jeong, W.; Chung, H.J.; Yoo, D.Y.; Lee, D.K.; Park, S.W.; et al. Activation of glutamine synthetase (GS) as a new strategy for the treatment of major depressive disorder and other GS-related diseases. Acta Pharmacol. Sin. 2025, 46, 880–891. [Google Scholar] [CrossRef]
- Öztürk, M.; Yalın Sapmaz, Ş.; Kandemir, H.; Taneli, F.; Aydemir, Ö. The role of the kynurenine pathway and quinolinic acid in adolescent major depressive disorder. Int. J. Clin. Pract. 2021, 75, e13739. [Google Scholar] [CrossRef]
- Kouba, B.R.; de Araujo Borba, L.; Borges de Souza, P.; Gil-Mohapel, J.; Rodrigues, A.L.S. Role of Inflammatory Mechanisms in Major Depressive Disorder: From Etiology to Potential Pharmacological Targets. Cells 2024, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.-Y.; Jin, R.-Y.; Wu, S.-S.; Yuan, W.; Wu, Y.-W.; Xue, S.-M.; Yang, X.-H.; Qiao, H.-F. AQP4 is upregulated in schizophrenia and Its inhibition attenuates MK-801-induced schizophrenia-like behaviors in mice. Behav. Brain Res. 2024, 475, 115220. [Google Scholar] [CrossRef] [PubMed]
- Felsky, D.; Voineskos, A.N.; Lerch, J.P.; Nazeri, A.; Shaikh, S.A.; Rajji, T.K.; Mulsant, B.H.; Kennedy, J. Myelin-Associated Glycoprotein Gene and Brain Morphometry in Schizophrenia. Front. Psychiatry 2012, 3, 40. [Google Scholar] [CrossRef]
- Fessel, J. Abnormal oligodendrocyte function in schizophrenia explains the long latent interval in some patients. Transl. Psychiatry 2022, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Burnet, P.; Eastwood, S.; Bristow, G.; Godlewska, B.; Sikka, P.; Walker, M.; Harrison, P. D-Amino acid oxidase (DAO) activity and expression are increased in schizophrenia. Mol. Psychiatry 2008, 13, 658–660. [Google Scholar] [CrossRef]
- Bustillo, J.R.; Chen, H.; Jones, T.; Lemke, N.; Abbott, C.; Qualls, C.; Canive, J.; Gasparovic, C. Increased Glutamine in Patients Undergoing Long-term Treatment for Schizophrenia. JAMA Psychiatry 2014, 71, 265–272. [Google Scholar] [CrossRef]
- Dahoun, T.; Trossbach, S.V.; Brandon, N.J.; Korth, C.; Howes, O.D. The impact of Disrupted-in-Schizophrenia 1 (DISC1) on the dopaminergic system: A systematic review. Transl. Psychiatry 2017, 7, e1015. [Google Scholar] [CrossRef]
- Boksha, I.S.; Tereshkina, E.B.; Savushkina, O.K.; Prokhorova, T.A.; Vorobyeva, E.A.; Burbaeva, G.S. Comparative Studies of Glutamine Synthetase Levels in the Brains of Patients with Schizophrenia and Mentally Healthy People. Neurochem. J. 2018, 12, 95–101. [Google Scholar] [CrossRef]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Sologova, S.S.; Mukhortova, P.; Levushkin, D.; Somasundaram, S.G.; Kirkland, C.E.; Bachurin, S.O.; Aliev, G. Alterations of Astrocytes in the Context of Schizophrenic Dementia. Front. Pharmacol. 2020, 10, 1612. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, Y.; Yang, Y.; Zhang, H.; Li, W.; Zhong, Z.; Lv, L. Serum S100B protein and white matter changes in schizophrenia before and after medication. Brain Res. Bull. 2024, 210, 110927. [Google Scholar] [CrossRef] [PubMed]
- Farkas, N.; Lendeckel, U.; Dobrowolny, H.; Funke, S.; Steiner, J.; Keilhoff, G.; Schmitt, A.; Bogerts, B.; Bernstein, H.-G. Reduced density of ADAM 12-immunoreactive oligodendrocytes in the anterior cingulate white matter of patients with schizophrenia. World J. Biol. Psychiatry 2010, 11, 556–566. [Google Scholar] [CrossRef]
- Liu, H.; Zhai, J.; Wang, B.; Fang, M. Olig2 Silence Ameliorates Cuprizone-Induced Schizophrenia-Like Symptoms in Mice. Med. Sci. Monit. 2017, 23, 4834–4840. [Google Scholar] [CrossRef] [PubMed]
- Peirce, T.R.; Bray, N.J.; Williams, N.M.; Norton, N.; Moskvina, V.; Preece, A.; Haroutunian, V.; Buxbaum, J.D.; Owen, M.J.; O’Donovan, M.C. Convergent evidence for 2′,3′-cyclic nucleotide 3′-phosphodiesterase as a possible susceptibility gene for schizophrenia. Arch. Gen. Psychiatry 2006, 63, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Cassoli, J.S.; Guest, P.C.; Malchow, B.; Schmitt, A.; Falkai, P.; Martins-de-Souza, D. Disturbed macro-connectivity in schizophrenia linked to oligodendrocyte dysfunction: From structural findings to molecules. npj Schizophr. 2015, 1, 15034. [Google Scholar] [CrossRef]
- Valdés-Tovar, M.; Rodríguez-Ramírez, A.M.; Rodríguez-Cárdenas, L.; Sotelo-Ramírez, C.E.; Camarena, B.; Sanabrais-Jiménez, M.A.; Solís-Chagoyán, H.; Argueta, J.; López-Riquelme, G.O. Insights into myelin dysfunction in schizophrenia and bipolar disorder. World J. Psychiatry 2022, 12, 264–285. [Google Scholar] [CrossRef]
- Dong, X.; Zhen, X. Glial Pathology in Bipolar Disorder: Potential Therapeutic Implications. CNS Neurosci. Ther. 2015, 21, 393–397. [Google Scholar] [CrossRef]
- Çetin, İ.; Demirel, Ö.F.; Sağlam, T.; Yıldız, N.; Duran, A. Decreased serum levels of glial markers and their relation with clinical parameters in patients with schizophrenia. J. Clin. Psychiatry 2023, 26, 155–162. [Google Scholar] [CrossRef]
- Plasma Glial Fibrillary Acidic Protein and Neurofilament Light Are Elevated in Bipolar Disorder: Evidence for Neuroprogression and Astrocytic Activation|medRxiv. Available online: https://www.medrxiv.org/content/10.1101/2024.07.30.24311203v1 (accessed on 2 June 2025).
- Potential Candidates for Biomarkers in Bipolar Disorder: A Proteomic Approach Through Systems Biology. Available online: https://www.cpn.or.kr/journal/view.html?doi=10.9758/cpn.2022.20.2.211 (accessed on 2 June 2025).
- Singh, B.; Cruz-Flores, S.; Chaudhry, M.R.; Piriyawat, P.; Ponce, C.P. Psychiatric manifestations of anti-MOG antibody disease. Neuroimmunol. Rep. 2022, 2, 100073. [Google Scholar] [CrossRef]
- Kamaeva, D.A.; Smirnova, L.P.; Vasilieva, S.N.; Kazantseva, D.V.; Vasilieva, A.R.; Ivanova, S.A. Catalytic Antibodies in Bipolar Disorder: Serum IgGs Hydrolyze Myelin Basic Protein. Int. J. Mol. Sci. 2022, 23, 7397. [Google Scholar] [CrossRef]
- Darvishmolla, M.; Heysieattalab, S.; Saeedi, N.; Hosseinmardi, N.; Janahmadi, M. Involvement of Hippocampal Astrocytic Connexin-43 in Morphine dependence. Physiol. Behav. 2022, 247, 113710. [Google Scholar] [CrossRef]
- Roberts-Wolfe, D.J.; Kalivas, P.W. Glutamate Transporter GLT-1 as a Therapeutic Target for Substance Use Disorders. CNS Neurol. Disord. Drug Targets 2015, 14, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Bryleva, E.Y.; Brundin, L. Kynurenine pathway metabolites and suicidality. Neuropharmacology 2017, 112, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Wisłowska-Stanek, A.; Kołosowska, K.; Maciejak, P. Neurobiological Basis of Increased Risk for Suicidal Behaviour. Cells 2021, 10, 2519. [Google Scholar] [CrossRef] [PubMed]
- Brundin, L.; Sellgren, C.M.; Lim, C.K.; Grit, J.; Pålsson, E.; Landén, M.; Samuelsson, M.; Lundgren, K.; Brundin, P.; Fuchs, D.; et al. An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl. Psychiatry 2016, 6, e865. [Google Scholar] [CrossRef]
- Tamimou, R.; Montout, C.; Mura, T.; Conejero, I.; Evrard, A.; Courtet, P.; Bonilla-Escribano, P.; Riaza, C.; Vaquero-Lorenzo, C.; Baca-Garcia, E.; et al. Genetic association of the kynurenine pathway to suicidal behavior. Brain Behav. Immun.—Health 2024, 42, 100903. [Google Scholar] [CrossRef]
- Janelidze, S.; Ventorp, F.; Erhardt, S.; Hansson, O.; Minthon, L.; Flax, J.; Samuelsson, M.; Traskman-Bendz, L.; Brundin, L. Altered chemokine levels in the cerebrospinal fluid and plasma of suicide attempters. Psychoneuroendocrinology 2013, 38, 853–862. [Google Scholar] [CrossRef]
- Serafini, G.; Parisi, V.M.; Aguglia, A.; Amerio, A.; Sampogna, G.; Fiorillo, A.; Pompili, M.; Amore, M. A Specific Inflammatory Profile Underlying Suicide Risk? Systematic Review of the Main Literature Findings. Int. J. Environ. Res. Public Health 2020, 17, 2393. [Google Scholar] [CrossRef]
- Vuscan, M.; Vica, M.; Balici, S.; Nicula, G.; Rusu, S.; Siserman, C.; Coman, H.; Matei, H. Association of HLA class II alleles with suicidal behavior in a Transylvanian population. Rev. Romana De Med. De Lab. 2023, 31, 15–24. [Google Scholar] [CrossRef]
- Fatemian, H.; Moslemi, H.; Hosseini, Y.; Moshfeghinia, R. C-reactive protein (CRP) level in depressed patients with suicidal behavior: A systematic review and meta-analysis. J. Affect. Disord. 2024, 366, 423–433. [Google Scholar] [CrossRef]
- Bokor, J.; Sutori, S.; Torok, D.; Gal, Z.; Eszlari, N.; Gyorik, D.; Baksa, D.; Petschner, P.; Serafini, G.; Pompili, M.; et al. Inflamed Mind: Multiple Genetic Variants of IL6 Influence Suicide Risk Phenotypes in Interaction with Early and Recent Adversities in a Linkage Disequilibrium-Based Clumping Analysis. Front. Psychiatry 2021, 12, 746206. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Avila, R.G.; Genis-Mendoza, A.D.; Juárez-Rojop, I.E.; López-Narváez, M.L.; Dionisio-García, D.M.; Nolasco-Rosales, G.A.; Ramos-Méndez, M.Á.; Hernández-Díaz, Y.; Tovilla-Zárate, C.A.; González-Castro, T.B.; et al. High Serum Levels of IL-6 Are Associated with Suicide Attempt but Not with High Lethality Suicide Attempts: A Preliminary Case-Control Study. Int. J. Environ. Res. Public Health 2022, 19, 14735. [Google Scholar] [CrossRef]
- Hoprekstad, G.E.; Skrede, S.; Bartz-Johannessen, C.; Joa, I.; Reitan, S.K.; Steen, V.M.; Torsvik, A.; Johnsen, E.; Kroken, R.A.; Rettenbacher, M. Association between cytokines and suicidality in patients with psychosis: A multicentre longitudinal analysis. Brain Behav. Immun.—Health 2024, 37, 100756. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Trihn, T.H.; Wu, H.E.; Tong, Y.; Xiu, M.; Zhang, X.Y. Association between TNF-alpha polymorphism and the age of first suicide attempt in chronic patients with schizophrenia. Aging 2020, 12, 1433–1445. [Google Scholar] [CrossRef]
- Mann, J.J.; Rizk, M.M. A Brain-Centric Model of Suicidal Behavior. Am. J. Psychiatry 2020, 177, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Brisch, R.; Wojtylak, S.; Saniotis, A.; Steiner, J.; Gos, T.; Kumaratilake, J.; Henneberg, M.; Wolf, R. The role of microglia in neuropsychiatric disorders and suicide. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 929–945. [Google Scholar] [CrossRef]
- Elsayed, M.; Magistretti, P.J. A New Outlook on Mental Illnesses: Glial Involvement Beyond the Glue. Front. Cell. Neurosci. 2015, 9, 468. [Google Scholar] [CrossRef]
- Baldessarini, R.J.; Tondo, L.; Pinna, M.; Nuñez, N.; Vázquez, G.H. Suicidal risk factors in major affective disorders. Br. J. Psychiatry 2019, 215, 621–626. [Google Scholar] [CrossRef]
- Cabrera-Mendoza, B.; Fresno, C.; Monroy-Jaramillo, N.; Fries, G.R.; Walss-Bass, C.; Glahn, D.C.; Ostrosky-Wegman, P.; Genis-Mendoza, A.D.; Martínez-Magaña, J.J.; Romero-Pimentel, A.L.; et al. Brain Gene Expression Profiling of Individuals with Dual Diagnosis Who Died by Suicide. J. Dual Diagn. 2020, 16, 177–190. [Google Scholar] [CrossRef]
- Almeida, P.G.C.; Nani, J.V.; Oses, J.P.; Brietzke, E.; Hayashi, M.A.F. Neuroinflammation and glial cell activation in mental disorders. Brain Behav. Immun.—Health 2020, 2, 100034. [Google Scholar] [CrossRef]
- Sanadgol, N.; Miraki Feriz, A.; Lisboa, S.F.; Joca, S.R.L. Putative role of glial cells in treatment resistance depression: An updated critical literation review and evaluation of single-nuclei transcriptomics data. Life Sci. 2023, 331, 122025. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, R.P. Do enteric glial cells play a role in the pathophysiology of major depression? Explor. Neurosci. 2024, 3, 156–174. [Google Scholar] [CrossRef]
- Evrensel, A.; Tarhan, N. The Role of Glial Pathology in Pathophysiology and Treatment of Major Depression: Clinical and Preclinical Evidence. In Translational Research Methods for Major Depressive Disorder; Kim, Y.-K., Amidfar, M., Eds.; Springer: New York, NY, USA, 2022; pp. 21–34. ISBN 978-1-07-162083-0. [Google Scholar]
- Zhang, L.; Verwer, R.W.H.; Zhao, J.; Huitinga, I.; Lucassen, P.J.; Swaab, D.F. Changes in glial gene expression in the prefrontal cortex in relation to major depressive disorder, suicide and psychotic features. J. Affect. Disord. 2021, 295, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.A.; O’Neill, K.; Milner, J.; Mahajan, G.J.; Lawrence, T.J.; May, W.L.; Miguel-Hidalgo, J.; Rajkowska, G.; Stockmeier, C.A. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience 2016, 316, 209–220. [Google Scholar] [CrossRef]
- Qi, X.-R.; Kamphuis, W.; Shan, L. Astrocyte Changes in the Prefrontal Cortex from Aged Non-suicidal Depressed Patients. Front. Cell. Neurosci. 2019, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T. Progress in Monoamine Oxidase (MAO) Research in Relation to Genetic Engineering. NeuroToxicology 2004, 25, 11–20. [Google Scholar] [CrossRef]
- Toledo-Lozano, C.G.; López-Hernández, L.B.; Suárez-Cuenca, J.A.; Villalobos-Gallegos, L.; Jiménez-Hernández, D.A.; Alcaraz-Estrada, S.L.; Mondragón-Terán, P.; Joya-Laureano, L.; Coral-Vázquez, R.M.; García, S. Individual and Combined Effect of MAO-A/MAO-B Gene Variants and Adverse Childhood Experiences on the Severity of Major Depressive Disorder. Behav. Sci. 2023, 13, 795. [Google Scholar] [CrossRef]
- Jaisa-aad, M.; Connors, T.; Hyman, B.; Serrano-Pozo, A. Characterization of the Monoamine Oxidase-B (MAO-B) Expression in Postmortem Normal and Alzheimer’s Disease Brains (S39.006). Neurology 2023, 100 (Suppl. S2), 2357. [Google Scholar] [CrossRef]
- Jaisa-Aad, M.; Muñoz-Castro, C.; Healey, M.A.; Hyman, B.T.; Serrano-Pozo, A. Characterization of monoamine oxidase-B (MAO-B) as a biomarker of reactive astrogliosis in Alzheimer’s disease and related dementias. Acta Neuropathol. 2024, 147, 66. [Google Scholar] [CrossRef]
- Ekblom, J.; Jossan, S.S.; Bergström, M.; Oreland, L.; Walum, E.; Aquilonius, S.M. Monoamine oxidase-B in astrocytes. Glia 1993, 8, 122–132. [Google Scholar] [CrossRef]
- Tong, J.; Rathitharan, G.; Meyer, J.H.; Furukawa, Y.; Ang, L.-C.; Boileau, I.; Guttman, M.; Hornykiewicz, O.; Kish, S.J. Brain monoamine oxidase B and A in human parkinsonian dopamine deficiency disorders. Brain 2017, 140, 2460–2474. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.C.; Sawa, A.; Pomper, M.G. Glia and immune cell signaling in bipolar disorder: Insights from neuropharmacology and molecular imaging to clinical application. Transl. Psychiatry 2014, 4, e350. [Google Scholar] [CrossRef]
- Naggan, L.; Robinson, E.; Dinur, E.; Goldenberg, H.; Kozela, E.; Yirmiya, R. Suicide in bipolar disorder patients is associated with hippocampal microglia activation and reduction of lymphocytes-activation gene 3 (LAG3) microglial checkpoint expression. Brain Behav. Immun. 2023, 110, 185–194. [Google Scholar] [CrossRef]
- Serra, G.; De Crescenzo, F.; Maisto, F.; Galante, J.R.; Iannoni, M.E.; Trasolini, M.; Maglio, G.; Tondo, L.; Baldessarini, R.J.; Vicari, S. Suicidal behavior in juvenile bipolar disorder and major depressive disorder patients: Systematic review and meta-analysis. J. Affect. Disord. 2022, 311, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Sher, L. Testosterone and Suicidal Behavior in Bipolar Disorder. Int. J. Environ. Res. Public Health 2023, 20, 2502. [Google Scholar] [CrossRef]
- Leichsenring, F.; Heim, N.; Leweke, F.; Spitzer, C.; Steinert, C.; Kernberg, O.F. Borderline Personality Disorder: A Review. JAMA 2023, 329, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Isaeva, E.R.; Ryzhova, D.M.; Stepanova, A.V.; Mitrev, I.N. Assessment of Suicide Risk in Patients with Depressive Episodes Due to Affective Disorders and Borderline Personality Disorder: A Pilot Comparative Study. Brain Sci. 2024, 14, 463. [Google Scholar] [CrossRef]
- Paris, J. Suicidality in Borderline Personality Disorder. Medicina 2019, 55, 223. [Google Scholar] [CrossRef]
- Shahnovsky, O.; Apter, A.; Barzilay, S. The Association between Hyperactivity and Suicidal Behavior and Attempts among Children Referred from Emergency Departments. Eur. J. Investig. Health Psychol. Educ. 2024, 14, 2616–2627. [Google Scholar] [CrossRef]
- Olsson, P.; Wiktorsson, S.; Strömsten, L.M.J.; Salander Renberg, E.; Runeson, B.; Waern, M. Attention deficit hyperactivity disorder in adults who present with self-harm: A comparative 6-month follow-up study. BMC Psychiatry 2022, 22, 428. [Google Scholar] [CrossRef]
- Taylor, M.R.; Boden, J.M.; Rucklidge, J.J. The relationship between ADHD symptomatology and self-harm, suicidal ideation, and suicidal behaviours in adults: A pilot study. Atten. Defic. Hyperact. Disord. 2014, 6, 303–312. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.S.; Grevet, E.H.; Silva, L.C.F.; Ramos, J.K.N.; Rovaris, D.L.; Bau, C.H.D. An overview on neurobiology and therapeutics of attention-deficit/hyperactivity disorder. Discov. Ment. Health 2023, 3, 2. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Dalsgaard, S.; Nordentoft, M.; Erlangsen, A. Suicidal behaviour among persons with attention-deficit hyperactivity disorder. Br. J. Psychiatry 2019, 215, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, G.; Perotti, C.; Filippo, L.; Garrone, C.; Rosso, G.; Maina, G. Assessing suicidality in adult ADHD patients: Prevalence and related factors. Ann. Gen. Psychiatry 2024, 23, 42. [Google Scholar] [CrossRef]
- Oades, R.D.; Dauvermann, M.R.; Schimmelmann, B.G.; Schwarz, M.J.; Myint, A.-M. Attention-deficit hyperactivity disorder (ADHD) and glial integrity: S100B, cytokines and kynurenine metabolism—Effects of medication. Behav. Brain Funct. 2010, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Kritikos, M.; Kuan, P.-F.; Carr, M.A.; Yang, X.; Yang, Y.; Kotov, R.; Bromet, E.J.; Clouston, S.A.P.; Luft, B.J. Glial suppression and post-traumatic stress disorder: A cross-sectional study of 1520 world trade center responders. Brain Behav. Immun. Health 2023, 30, 100631. [Google Scholar] [CrossRef]
- Gidzgier, P.A.; Bari, M.; López-Atanes, M.; Lotzin, A.; Grundmann, J.; Hiller, P.; Schneider, B.; Schäfer, I. Improving care for SUD patients with complex trauma-relationships between childhood trauma, dissociation, and suicidal behavior in female patients with PTSD and SUD. Front. Psychiatry 2022, 13, 1047274. [Google Scholar] [CrossRef]
- McRae, E.; Stoppelbein, L.; O’Kelley, S.; Smith, S.; Fite, P. Pathways to Suicidal Behavior in Children and Adolescents: Examination of Child Maltreatment and Post-Traumatic Symptoms. J. Child. Adolesc. Trauma. 2022, 15, 715–725. [Google Scholar] [CrossRef]
- Bansal, Y.; Codeluppi, S.A.; Banasr, M. Astroglial Dysfunctions in Mood Disorders and Rodent Stress Models: Consequences on Behavior and Potential as Treatment Target. Int. J. Mol. Sci. 2024, 25, 6357. [Google Scholar] [CrossRef]
- Valenza, M.; Facchinetti, R.; Torazza, C.; Ciarla, C.; Bronzuoli, M.R.; Balbi, M.; Bonanno, G.; Popoli, M.; Steardo, L.; Milanese, M.; et al. Molecular signatures of astrocytes and microglia maladaptive responses to acute stress are rescued by a single administration of ketamine in a rodent model of PTSD. Transl. Psychiatry 2024, 14, 209. [Google Scholar] [CrossRef]
- Kopacz, M.S.; Currier, J.M.; Drescher, K.D.; Pigeon, W.R. Suicidal behavior and spiritual functioning in a sample of Veterans diagnosed with PTSD. J. Inj. Violence Res. 2016, 8, 6–14. [Google Scholar] [CrossRef][Green Version]
- Lee, D.-H.; Lee, J.-Y.; Hong, D.-Y.; Lee, E.-C.; Park, S.-W.; Lee, M.-R.; Oh, J.-S. Neuroinflammation in Post-Traumatic Stress Disorder. Biomedicines 2022, 10, 2518. [Google Scholar] [CrossRef]
- Koizumi, S. Glial Purinergic Signals and Psychiatric Disorders. Front. Cell. Neurosci. 2022, 15, 822614. [Google Scholar] [CrossRef]
- Dickerson, M.R.; Murphy, S.F.; Urban, M.J.; White, Z.; VandeVord, P.J. Chronic Anxiety- and Depression-like Behaviors Are Associated with Glial-Driven Pathology Following Repeated Blast Induced Neurotrauma. Front. Behav. Neurosci. 2021, 15, 787475. [Google Scholar] [CrossRef]
- Tizabi, Y.; Getachew, B.; Hauser, S.R.; Tsytsarev, V.; Manhães, A.C.; da Silva, V.D.A. Role of Glial Cells in Neuronal Function, Mood Disorders, and Drug Addiction. Brain Sci. 2024, 14, 558. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.C.; Ohlsson, H.; Lannoy, S.; Stephenson, M.; Crump, C.; Sundquist, J.; Sundquist, K.; Kendler, K.S. Shared genetic and environmental etiology between substance use disorders and suicidal behavior. Psychol. Med. 2023, 53, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Mendoza, B.; Martínez-Magaña, J.J.; Monroy-Jaramillo, N.; Genis-Mendoza, A.D.; Fresno, C.; Fries, G.R.; Walss-Bass, C.; López Armenta, M.; García-Dolores, F.; Díaz-Otañez, C.E.; et al. Candidate pharmacological treatments for substance use disorder and suicide identified by gene co-expression network-based drug repositioning. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2021, 186, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Martínez Martínez, K.I.; Ojeda Aguilar, Y.L.; Hernández Villafuerte, J.; Contreras-Peréz, M.E. Depression and Suicidal Behavior Comorbidity in Patients Admitted to Substance-Use Residential Treatment in Aguascalientes, Mexico. J. Evid. Based Soc. Work 2023, 20, 508–519. [Google Scholar] [CrossRef]
- Gobbi, G.; Atkin, T.; Zytynski, T.; Wang, S.; Askari, S.; Boruff, J.; Ware, M.; Marmorstein, N.; Cipriani, A.; Dendukuri, N.; et al. Association of Cannabis Use in Adolescence and Risk of Depression, Anxiety, and Suicidality in Young Adulthood: A Systematic Review and Meta-analysis. JAMA Psychiatry 2019, 76, 426–434. [Google Scholar] [CrossRef]
- Moret, R.M.; Sanz-Gómez, S.; Gascón-Santos, S.; Alacreu-Crespo, A. Exploring the Impact of Recreational Drugs on Suicidal Behavior: A Narrative Review. Psychoactives 2024, 3, 337–356. [Google Scholar] [CrossRef]
- Mewton, L.; Andrews, G. Cognitive behavioral therapy for suicidal behaviors: Improving patient outcomes. Psychol. Res. Behav. Manag. 2016, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Stanley, B.; Brown, G.; Brent, D.; Wells, K.; Poling, K.; Curry, J.; Kennard, B.D.; Wagner, A.; Cwik, M.; Klomek, A.B.; et al. Cognitive Behavior Therapy for Suicide Prevention (CBT-SP): Treatment Model, Feasibility and Acceptability. J. Am. Acad. Child. Adolesc. Psychiatry 2009, 48, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, L.; Qian, Y.; Jin, X.-H.; Yu, H.-R.; Du, L.; Fu, X.-L.; Zhu, B.; Chen, H.-L. The significance of cognitive-behavioral therapy on suicide: An umbrella review. J. Affect. Disord. 2022, 317, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.-H.; Xu, J.; Jia, Y.-J.; Ge, M.-W.; Zhang, W.-Q.; Tang, W.; Zhao, D.-Y.; Hu, S.-Q.; Du, W.; Shen, W.-Q.; et al. Non-pharmacological interventions for preventing suicide attempts: A systematic review and network meta-analysis. Asian J. Psychiatry 2024, 93, 103913. [Google Scholar] [CrossRef]
- Zisook, S.; Domingues, I.; Compton, J. Pharmacologic Approaches to Suicide Prevention. Focus 2023, 21, 137–144. [Google Scholar] [CrossRef]
- Xie, W.; Xiang, D.; Li, Y.; Ge, M.; Deng, A. An exploratory study evaluating the 20 medications most commonly associated with suicidal ideation and self-injurious behavior in the FAERS database. BMC Pharmacol. Toxicol. 2025, 26, 24. [Google Scholar] [CrossRef]
- Rajkumar, R.P. Pharmacological Strategies for Suicide Prevention Based on the Social Pain Model: A Scoping Review. Psych 2022, 4, 494–515. [Google Scholar] [CrossRef]
- Zhang, L.; Swaab, D.F. Chapter 22—Neuroglia in suicide. In Handbook of Clinical Neurology; Verkhratsky, A., de Witte, L.D., Aronica, E., Hol, E.M., Eds.; Neuroglia in Neurologic and Psychiatric Disorders, Part II; Elsevier: Amsterdam, The Netherlands, 2025; Volume 210, pp. 371–379. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chahla, M.N.A. The Interplay Between Suicidal Behavior and Mental Disorders: Focusing on the Role of Glial Cells. Neuroglia 2025, 6, 24. https://doi.org/10.3390/neuroglia6030024
Chahla MNA. The Interplay Between Suicidal Behavior and Mental Disorders: Focusing on the Role of Glial Cells. Neuroglia. 2025; 6(3):24. https://doi.org/10.3390/neuroglia6030024
Chicago/Turabian StyleChahla, Maya N. Abou. 2025. "The Interplay Between Suicidal Behavior and Mental Disorders: Focusing on the Role of Glial Cells" Neuroglia 6, no. 3: 24. https://doi.org/10.3390/neuroglia6030024
APA StyleChahla, M. N. A. (2025). The Interplay Between Suicidal Behavior and Mental Disorders: Focusing on the Role of Glial Cells. Neuroglia, 6(3), 24. https://doi.org/10.3390/neuroglia6030024





