Abstract
This review synthesizes the emerging understanding of the roles of glial cells and non-coding RNAs (ncRNAs) in the pathogenesis and progression of Alzheimer’s disease and related dementias (ADRDs). ADRDs encompass a spectrum of neurodegenerative disorders characterized by cognitive decline, memory impairment, and functional deterioration. The interplay between the most common types of glial cells—astrocytes, microglia, and oligodendrocytes—and ncRNAs is emerging as a critical factor in the development of ADRDs. Glial cells are essential for maintaining homeostasis within the central nervous system (CNS); however, their dysregulation can lead to neuroinflammation and neuronal dysfunction, exacerbating neurodegeneration. Reactive astrocytes and activated microglia can create neurotoxic environments that further impair neuronal health. Concurrently, ncRNAs, particularly long non-coding RNAs (lncRNAs) and microRNAs (miRNAs), have emerged as significant regulators of glial gene expression, influencing inflammatory responses and glial cell function. Understanding the complex interactions between glial cells and ncRNAs is crucial for developing targeted therapeutic strategies. By elucidating the mechanisms underlying their interactions, this review aims to highlight the critical importance of glial cells and ncRNAs in the context of neurodegenerative diseases, paving the way for innovative approaches to prevent and treat ADRDs. Ultimately, enhancing our understanding of these processes may lead to novel therapies and improved outcomes for individuals affected by these debilitating conditions.
1. Introduction
Alzheimer’s disease and related dementias (ADRDs) encompass a spectrum of neurodegenerative disorders characterized by progressive cognitive decline, memory impairment, and functional deterioration. Among these, Alzheimer’s disease (AD), frontotemporal dementia (FTD), Lewy body dementia (LBD), vascular contributions to cognitive impairment and dementia (VCID), and multiple etiology dementia (MED) represent the most significant and common forms of dementia. Each of these conditions exhibits distinct clinical features, underlying pathophysiology, and implications for diagnosis and management.
1.1. Overview of ADRDs
Alzheimer’s disease, the most prevalent form of dementia, accounts for approximately 60–80% of all dementia cases [1]. AD is primarily characterized by progressive memory loss, language difficulties, and impaired reasoning abilities. Neuropathologically, AD is marked by the accumulation of extracellular amyloid plaques and intracellular neurofibrillary tangles composed of hyperphosphorylated tau protein, leading to neuronal loss and brain atrophy [2,3]. The clinical trajectory typically begins with mild cognitive impairment, which can progress to more severe cognitive deficits, ultimately affecting daily functioning, independence and overall quality of life [4]. The prevalence of AD is projected to rise significantly, with estimates suggesting that by 2060, around 14 million individuals in the United States will be living with the disease [5,6].
Frontotemporal dementia, while less common than AD, constitutes a significant subset of ADRDs, particularly among younger individuals. Unlike AD, FTD typically affects individuals in their 50 s and 60 s, presenting unique challenges for patients and their families [7]. FTD is characterized by progressive changes in personality, behavior, and language, often preceding memory loss [8]. The underlying pathology involves degeneration of the frontal and temporal lobes of the brain, which can manifest in various clinical syndromes, including behavioral variant FTD and primary progressive aphasia [9]. These syndromes highlight the diverse presentations of FTD, complicating diagnosis and management [10].
Lewy body dementia represents another critical form of ADRDs, distinguished by the presence of Lewy bodies—abnormal protein aggregates composed of alpha-synuclein—in the brain. LBD can present with fluctuating cognition, visual hallucinations, and parkinsonism, making it particularly challenging to differentiate from both AD and Parkinson’s disease [11]. The overlap of symptoms can lead to misdiagnosis, impacting treatment decisions and care strategies. Patients with LBD may experience significant fluctuations in attention and alertness, further complicating their clinical management [12]. Recognizing LBD as a distinct entity within the spectrum of ADRDs is essential for appropriate therapeutic approaches and caregiver support [13].
Vascular contributions to cognitive impairment and dementia (VCID) represent a significant but often under-recognized aspect of ADRDs. VCID arises from cerebrovascular disease, leading to reduced blood flow to the brain (hypoperfusion) and subsequent cognitive decline. This form of dementia can result from multiple small strokes or chronic ischemia and is frequently associated with vascular risk factors such as hypertension and diabetes [14]. The clinical presentation of VCID can vary widely, often overlapping with symptoms of AD and other dementias, complicating diagnosis [15]. Effective management of VCID requires addressing the underlying vascular risk factors to prevent further cognitive decline [16].
Multiple-etiology dementia (MED) refers to the coexistence of multiple types of dementia pathology, most commonly AD and vascular dementia [17]. This condition is increasingly recognized as a prevalent form of dementia among older adults, as many individuals exhibit overlapping features of different dementias [18]. The presence of mixed pathology can influence the clinical course and response to treatment, necessitating a comprehensive approach to diagnosis and management [19]. Understanding the interplay between different dementia types is crucial for developing effective therapeutic strategies and optimizing patient outcomes [20].
The growing prevalence of ADRDs poses significant challenges for healthcare systems, caregivers, and society at large. The economic burden associated with these conditions is substantial, encompassing costs related to medical care, long-term care, and lost productivity [21]. Furthermore, the emotional and psychological toll on caregivers is profound, as they navigate the complexities of providing care for individuals with progressive cognitive decline [22]. Addressing the needs of both patients and caregivers is essential for improving quality of life and ensuring effective support systems are in place [23].
1.2. Glial Cells in ADRDs
In recent years, the role of glial cells—specifically astrocytes, microglia, and oligodendrocytes—in the pathophysiology of ADRDs has garnered increasing attention. Astrocytes, the most abundant glial cell type in the CNS, provide structural, metabolic, and trophic support to neurons. They are involved in neurotransmitter uptake, ion homeostasis, and the maintenance of the blood–brain barrier [24,25]. However, in the context of neurodegeneration, astrocytes can undergo reactive changes that contribute to neuronal dysfunction. Reactive astrocytes release pro-inflammatory cytokines and neurotoxic factors, exacerbating neuroinflammation and promoting neuronal death [26,27]. Studies have shown that astrocytic dysfunction is linked to the progression of ADRDs, where astrocytes lose their neuroprotective functions and instead adopt neurotoxic properties [28,29,30,31,32,33]. This duality of astrocytic function underscores the complexity of their role in neurodegenerative processes.
Microglia, the resident immune cells of the central nervous system, play an integral role in both maintaining homeostasis and driving pathological processes in neurodegeneration. These cells continuously monitor the brain environment and are largely responsible for removing cellular debris. However, their activation frequently transitions into a pro-inflammatory state that exacerbates neurodegenerative pathology [34]. Research indicates that microglial activation substantially impacts astrocytic function, establishing a reciprocal interaction that amplifies neuroinflammatory signaling and accelerates neuronal damage [35,36]. For example, activated microglia have been shown to induce a neurotoxic phenotype in astrocytes, further propagating inflammation and neurotoxicity [37,38]. This dynamic underscores the necessity of elucidating glial cell crosstalk within the pathophysiological framework of ADRDs.
Oligodendrocytes, while primarily known for their role in myelination, also contribute to neuronal health and function. They provide metabolic support to axons and are involved in the maintenance of axonal integrity [24,25]. In neurodegenerative diseases, oligodendrocyte dysfunction leads to demyelination, thereby exacerbating the progression of cognitive decline. Emerging evidence suggests that oligodendrocytes may also respond to inflammatory signals from astrocytes and microglia, further complicating the cellular dynamics in ADRDs [24,25].
The role of glial cells in the pathogenesis of ADRDs holds particular significance when examining the mechanisms underlying neuroinflammation and neurodegeneration. For instance, astrocytes are known to secrete factors that regulate neuronal survival and function, including neurotrophic factors and cytokines [24,25]. However, in the context of neurodegeneration, the balance of these secreted factors shifts towards a neurotoxic profile, leading to increased neuronal vulnerability [26,27]. Additionally, astrocytes sustain neuronal function by regulating the metabolic microenvironment through the provision of essential nutrients and energy substrates, while disruptions in this support are implicated in the acceleration of neurodegenerative processes [24,25].
When considering these glial cell contributions to neurodegeneration, it is crucial to understand their temporal dynamics across ADRD progression. Current evidence suggests that glial reactivity, particularly microglial activation, represents one of the earliest detectable cellular responses in ADRDs, often preceding symptom onset and significant neuronal loss [39]. In AD, microglial activation and associated neuroinflammatory changes can be detected in the prodromal and mild cognitive impairment (MCI) stages, suggesting these processes may contribute to disease initiation rather than simply responding to established pathology [40]. Astrocytic reactivity follows a similar pattern, with early morphological and functional changes detectable in preclinical and early symptomatic phases [41]. In contrast, oligodendrocyte dysfunction appears to accumulate gradually throughout disease progression, with more pronounced effects in later stages that correlate with cognitive decline severity [42]. This temporal progression of glial dysfunction provides an important context for understanding both disease mechanisms and potential therapeutic windows, as interventions targeting early glial changes may offer greater neuroprotective benefits before extensive damage occurs [43].
1.3. Non-Coding RNAs in ADRDs
In addition to glial cells, non-coding RNAs, including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and transposable elements, have emerged as significant players in ADRDs. For example, non-coding RNAs are involved in regulating glial gene expression and cellular responses, influencing both neuroinflammation and neurodegeneration. miRNAs can modulate the expression of pro-inflammatory cytokines in glial cells, thereby affecting the inflammatory response in neurodegenerative diseases [44]. Furthermore, lncRNAs have been implicated in the regulation of glial cell function and neuronal health, with dysregulation of these molecules potentially contributing to the pathogenesis of ADRDs [45]. Transposable elements (TEs), which are sequences of DNA capable of moving within the genome, have been implicated in Alzheimer’s disease (AD) through their potential to disrupt genomic stability and contribute to neuroinflammation [46]. Often considered “junk” DNA, TEs have gained attention for their role in neurodegenerative diseases [47]. Recent studies suggest that the activation of transposable elements during aging leads to the production of double-stranded RNA (dsRNA), which may trigger neuroinflammation and contribute to neuronal death [48,49]. Additional mechanisms include the activation of the brain’s immune system, exacerbating neurodegenerative processes, and may also promote the accumulation of toxic proteins such as amyloid-β, further accelerating AD pathology [50].
As the understanding of ADRDs continues to evolve, there is increasing recognition of the intricate roles played by glial cells and non-coding RNAs in the pathogenesis and progression of these neurodegenerative disorders. Glial cells are not merely supportive elements within the central nervous system; they actively participate in neuroinflammatory processes, neuronal health, and the maintenance of homeostasis. Recent research has illuminated the dual nature of glial cells, where reactive changes can lead to neurotoxic environments that exacerbate neuronal dysfunction and contribute to the progression of ADRDs [51]. Understanding the molecular mechanisms underlying these interactions is crucial for developing targeted therapeutic strategies. In parallel, non-coding RNAs, such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and transposable elements, have emerged as significant regulators of gene expression and cellular responses in the context of neurodegeneration. These non-coding RNAs are involved in various processes, including the modulation of inflammatory responses, regulation of glial cell function, and neuronal survival. For instance, specific miRNAs are implicated in the dysregulation of glial cell activation and the propagation of neuroinflammation, while studies demonstrate that lncRNAs influence glial differentiation and function. Additionally, transposable elements can disrupt genomic stability and contribute to the pathophysiological landscape of ADRDs [52]. The interplay between glial cells and non-coding RNAs represents a complex regulatory network that warrants comprehensive exploration.
This review aims to consolidate the current knowledge surrounding the interplay between glial cells and non-coding RNAs in the pathogenesis and progression of ADRDs. By examining the mechanisms by which these cellular and molecular players interact, we hope to elucidate their contributions to neuroinflammation, neuronal health, and disease. Furthermore, we will explore potential therapeutic avenues that target these interactions, with the goal of advancing our understanding and treatment of ADRDs. We aim to provide a comprehensive overview that highlights the critical importance of glial cells and non-coding RNAs in the context of neurodegenerative diseases.
To begin, we will outline the role of astrocytes in the five major ADRDs (AD, FTD, LBD, VCID, and MED). While each of these conditions is associated with distinct pathogenic features, there is a common thread of astrocytic dysfunction. Understanding how astrocytes respond to these pathological insults provides crucial insights into their role in neurodegeneration and highlights the potential for targeting astrocytic pathways to ameliorate disease progression.
Having established the multifaceted roles of glial cells in the pathophysiology of ADRDs, we will first examine astrocytes, the most abundant glial cell type in the CNS. Astrocytes serve as key regulators of neuronal homeostasis through their extensive functions in neurotransmitter recycling, ion balance, and blood–brain barrier maintenance. Understanding how these cells undergo pathological transformations across different ADRDs provides crucial insights into disease progression and potential therapeutic targets.
Having established the complex landscape of ADRDs and the emerging importance of glial cells in their pathogenesis, we will now examine each major glial cell type and its contributions to neurodegenerative processes. We begin with astrocytes, the most abundant glial cells in the CNS, which provide essential support for neuronal function and homeostasis. While each ADRD presents with distinct pathogenic features, astrocytic dysfunction represents a common thread across these conditions, often emerging in early disease stages. Understanding how these cells respond to pathological insults across different ADRDs provides crucial insights into disease mechanisms and reveals potential therapeutic targets for intervention before extensive neurodegeneration occurs.
2. Astrocytes
Astrocytes, a vital component of the central nervous system (CNS), play critical roles in maintaining homeostasis through functions such as regulating the blood–brain barrier (BBB), neurotransmitter recycling, and extracellular ion balance [53,54]. In ADRDs, astrocytes undergo a pathological transformation into reactive states, a process known as reactive astrogliosis [55]. This transition involves significant morphological, functional, and transcriptional changes, which not only impair their protective roles but also exacerbate neuroinflammation, synaptic dysfunction, and neuronal loss [56,57].
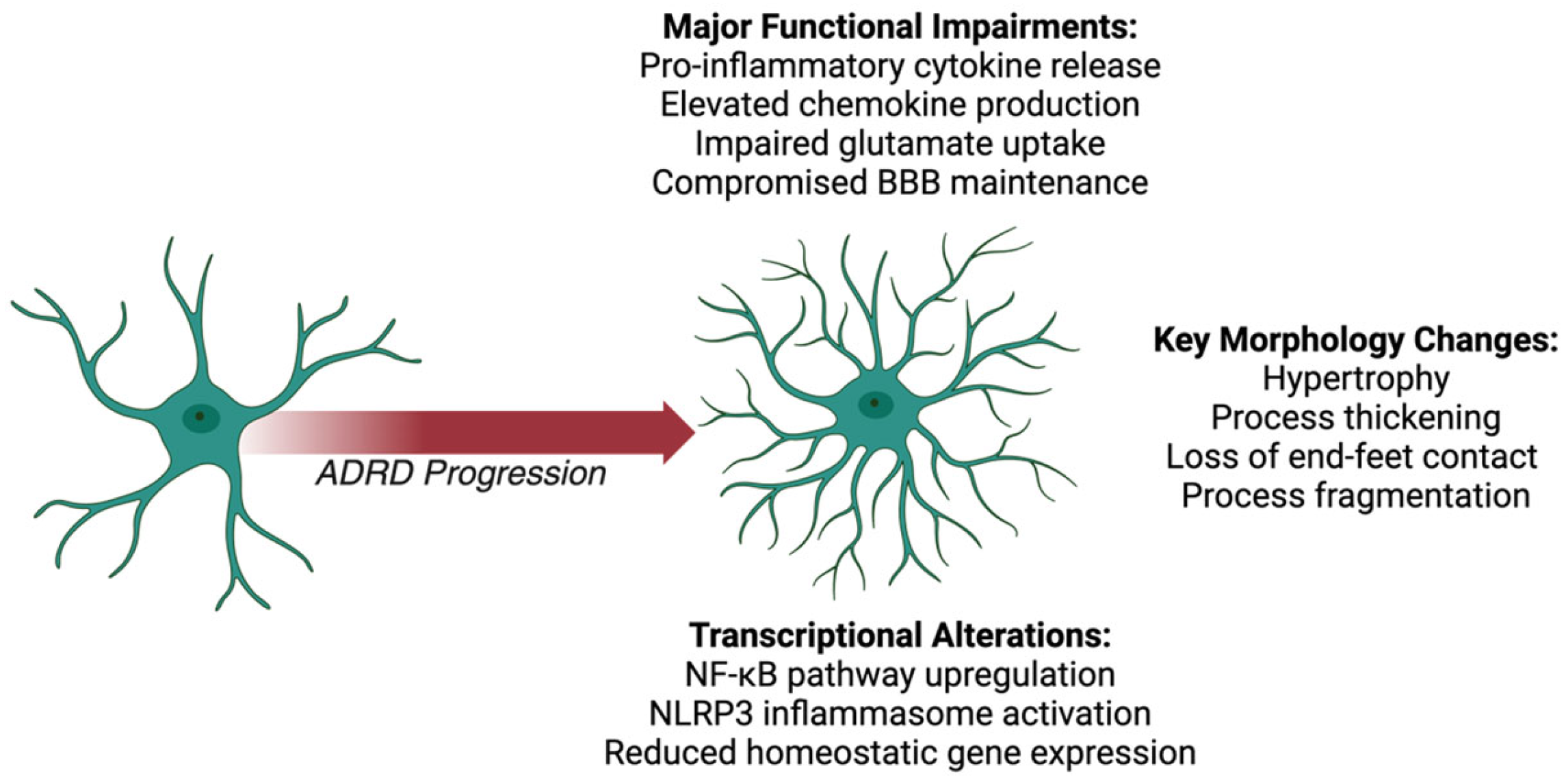
2.1. Astrocyte Morphological Changes
Reactive astrocytes in ADRDs exhibit hypertrophy and increased ramification, reflecting their transition from a homeostatic to a reactive phenotype [58,59]. In AD, astrocytes cluster around amyloid-β (Aβ) plaques, displaying enlarged cell bodies and thickened processes [60,61]. This morphological response is accompanied by the loss of astrocytic end-feet, which disrupts BBB integrity and allows peripheral immune cell infiltration, exacerbating neuroinflammation [62,63].
Similar morphological changes are observed in FTD, where reactive astrocytes are linked to tau pathology [64,65]. These cells exhibit swollen, hypertrophic processes and a fragmented structure, impairing their interactions with neurons and exacerbating tau-mediated damage [66,67]. In LBD, astrocytes respond to α-synuclein aggregates with hypertrophy and abnormal process remodeling, further disrupting synaptic and vascular support6566.
In VCID, chronic hypoperfusion induces widespread astrogliosis, with hypertrophic astrocytes losing their structural association with blood vessels [68,69]. This compromises their role in BBB maintenance and amplifies vascular permeability [27,70]. MEDs, which combine neurodegenerative and vascular pathologies, exhibit more severe astrocytic hypertrophy and process fragmentation. This increased severity results from the combined insults of Aβ deposition and ischemic injury [71,72].
2.2. Astrocyte Functional Impairments
Astrocytic dysfunction in ADRDs significantly disrupts their homeostatic roles, driving Astrocytic dysfunction in ADRDs significantly disrupts their homeostatic roles, driving inflammation and neuronal damage [73,74]. Across these conditions, reactive astrocytes release elevated levels of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) [75,76]. These cytokines propagate neuroinflammation and exacerbate neuronal vulnerability [20,77].
Reactive astrocytes also show dysregulated chemokine production, including elevated levels of CCL2 (MCP-1), CCL5 (RANTES), and CXCL10 (IP-10) [78,79]. These chemokines attract immune cells to sites of injury, further intensifying inflammation [80,81]. Additionally, reactive astrocytes release matrix metalloproteinases (MMPs), which degrade the extracellular matrix and compromise synaptic integrity, particularly in AD and LBD [82,83].
Additionally, impaired Aβ clearance by astrocytes represents a critical functional deficit that contributes to amyloid plaque accumulation in AD. Several molecular mechanisms that underlie this impairment have been proposed. First, reactive astrocytes show decreased expression of Aβ-degrading enzymes such as neprilysin (NEP) and insulin-degrading enzyme (IDE), reducing their capacity to enzymatically process Aβ peptides [84]. Second, the low-density lipoprotein receptor-related protein 1 (LRP1), a key receptor mediating Aβ uptake and clearance, is downregulated in AD astrocytes, further limiting their ability to internalize Aβ [85]. Third, the ATP-binding cassette transporter A1 (ABCA1), which facilitates Aβ clearance through ApoE lipidation, shows reduced functionality in reactive astrocytes [86]. Additionally, AD astrocytes exhibit impaired lysosomal degradation pathways, including defective autophagy and reduced lysosomal acidification, which compromises their ability to degrade internalized Aβ [87]. The ApoE ε4 allele further exacerbates these deficits by reducing astrocytic Aβ clearance efficiency compared to other ApoE isoforms, providing a mechanistic link between this genetic risk factor and impaired Aβ proteostasis [88].
Furthermore, in ADRDs, astrocytes fail to regulate glutamate homeostasis, contributing to excitotoxicity [89]. Impaired glutamate uptake, due to the reduced expression or dysfunction of transporters such as GLT-1 leads to excessive extracellular glutamate levels [90,91]. This excitotoxicity damages neurons and synapses, particularly in AD, FTD, and LBD [92,93]. The loss of astrocytic end-feet in ADRDs further compromises BBB integrity [94,95]. In AD, FTD, LBD, and VCID, BBB disruption allows peripheral immune cells and neurotoxic substances to infiltrate the CNS, aggravating inflammation and neuronal damage [96,97]. MEDs demonstrate compounded BBB dysfunction due to overlapping pathological mechanisms [98,99].
2.3. Astrocyte Transcriptional Alterations
Astrocytic transcriptional profiles in ADRDs reflect extensive reprogramming associated with inflammation, oxidative stress, and metabolic dysfunction [100,101]. Across ADRDs, upregulation of genes linked to the NF-κB pathway drives cytokine production (e.g., IL-1β and IL-6) and inflammasome activation (e.g., NLRP3) [53,54]. These transcriptional changes perpetuate neuroinflammation and impair glutamate homeostasis [55,102].
In AD and LBD, astrocytes show transcriptional dysregulation of genes involved in Aβ and α-synuclein clearance, respectively [56,57]. Reduced expression of genes critical for lysosomal function further hinders the degradation of these aggregates, exacerbating neurotoxicity [58,59]. In FTD, astrocytes upregulate IL-6-related signaling pathways and genes promoting tau aggregation, linking transcriptional reprogramming to tau pathology [60,61]. VCID astrocytes display increased expression of hypoxia-related genes and pathways such as AIM2 inflammasome signaling, highlighting their role in ischemia-induced damage [62,63].
Genetic factors also play a pivotal role in astrocytic dysfunction. In AD, mutations in APP, PSEN1, and PSEN2 increase Aβ accumulation, triggering astrocyte activation [64,65]. The ApoE ε4 allele enhances astrocytic reactivity, promoting inflammation and impairing lipid transport [66,67]. FTD-associated mutations in MAPT and GRN disrupt tau clearance and exacerbate inflammatory signaling [103,104]. In LBD, GBA mutations impair astrocytic lysosomal function, facilitating α-synuclein aggregation [68,69]. In VCID and MEDs, APOE ε4 and C9orf72 mutations amplify astrocytic reactivity, compounding vascular and neurodegenerative damage [27,70].
In conclusion, astrocytes play a pivotal role in the progression of various dementias, where their dysfunction contributes to neuroinflammation, neuronal damage, and cognitive decline (Figure 1, Table 1). In ADRDs, reactive astrogliosis alters astrocytic functions, including the secretion of pro-inflammatory cytokines, chemokines, and the disruption of the blood-brain barrier. The extensive analysis of astrocytic contributions to ADRD pathogenesis illustrates how these traditionally supportive cells can become key drivers of neuroinflammation and neurodegeneration when their homeostatic functions are compromised. However, astrocytes do not respond to pathological insults in isolation. Their activation states and functional outputs are intimately linked to other glial populations, particularly microglia, with whom they engage in continuous bidirectional communication.

Figure 1.
Alterations in Astrocytes during NMPD Progression.
Microglia, as the resident immune cells of the CNS, represent the next critical population in our examination of glial contributions to ADRDs. Unlike astrocytes, which derive from neural progenitors, microglia originate from yolk sac-derived myeloid precursors and enter the CNS during early development, establishing themselves as the primary mediators of innate immunity within the brain [105,106]. Under physiological conditions, these highly ramified cells continuously survey their microenvironment, rapidly responding to disturbances in CNS homeostasis [107]. The distinctive ontogeny and immune functions of microglia position them as key responders to the protein aggregates, cellular damage, and inflammatory signals characteristic of ADRDs. The following section will explore how microglial activation states evolve throughout disease progression, their disease-specific responses across different ADRDs, and how their interactions with astrocytes create complex inflammatory environments that influence neuronal health and cognitive outcomes.

Table 1.
Summary of the morphological changes, functional impairments, and transcriptional alterations of astrocytes in ADRDs.
Table 1.
Summary of the morphological changes, functional impairments, and transcriptional alterations of astrocytes in ADRDs.
| Morphological Changes | Functional Impairments | Transcriptional Alterations | |
|---|---|---|---|
| All ADRDs |
|
|
|
| AD |
|
|
|
| FTD |
|
|
|
| LBD |
|
|
|
| VCID |
|
|
|
| MEDs |
|
|
|
3. Microglia
Microglia, the resident immune cells of the central nervous system (CNS), are indispensable for maintaining neural homeostasis. These cells continuously surveil their microenvironment, responding dynamically to injury, infection, and disease [97,98]. In ADRDs, microglial activation emerges early and persists as a driver of pathology [99,100]. Upon activation, microglia exhibit profound morphological, molecular, and functional changes, leading to the release of pro-inflammatory cytokines, chemokines, and reactive oxygen species (ROS) [101,102]. While this response is vital for debris clearance and neuronal support under normal conditions, its dysregulation in ADRDs establishes a chronic inflammatory state that accelerates neurodegeneration [103,104].
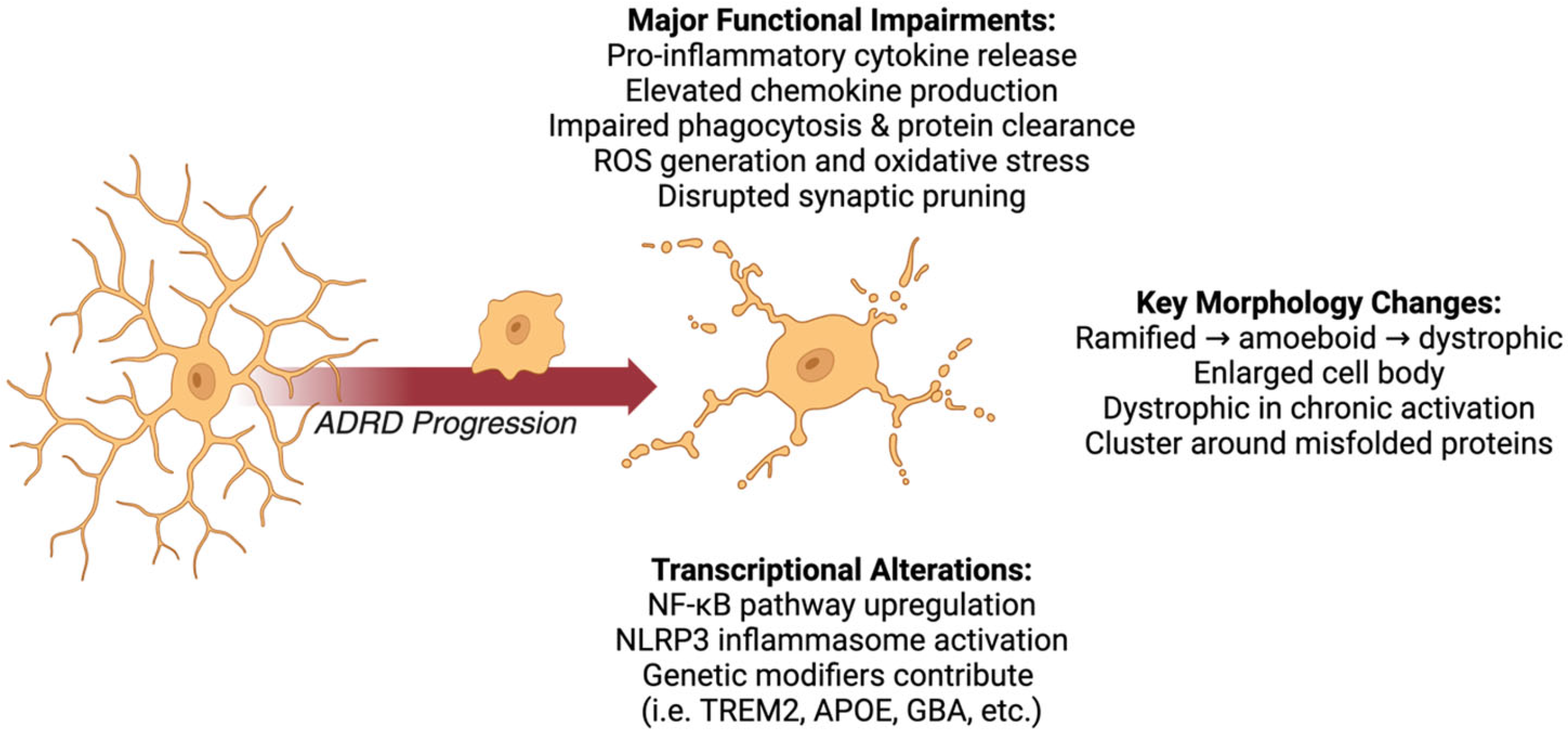
3.1. Microglial Morphological Changes
Activated microglia across ADRDs are characterized by hypertrophy, increased ramification, and process retraction, reflecting their transition from a homeostatic to a reactive state [108,109]. In AD, microglia cluster around amyloid-β (Aβ) plaques, adopting a dystrophic morphology with fragmented processes and rounded cell bodies [110,111]. These dystrophic microglia are less effective at clearing plaques and contribute to chronic inflammation, exacerbated by pro-inflammatory cytokines such as IL-1β and TNF-α [112,113,114]. This persistent activation has been linked to neurodegeneration, highlighting the dual role of microglia as both protectors and contributors to neurodegenerative processes [115].
Similar morphological changes are observed in FTD, where hypertrophic microglia are associated with tau pathology [116,117]. These cells often display disorganized processes, which compromise their interactions with neurons and exacerbate tau-mediated neurotoxicity [118,119]. In LBD, α-synuclein aggregates induce microglial hyper-ramification, impairing synaptic support and promoting oxidative stress, which contributes to dopaminergic neuron loss [120]. This suggests that microglial activation in LBD may play a role in disease progression rather than merely responding to neuronal damage [121].
In VCID, chronic ischemia triggers microglial activation, leading to hypertrophy and the loss of processes critical for maintaining neurovascular unit integrity [122]. MEDs exhibit amplified morphological abnormalities, with microglial hypertrophy and widespread structural dysfunction contributing to blood–brain barrier (BBB) breakdown and neuronal injury [123,124]. Recent studies indicate that microglial activation varies significantly across different neurodegenerative diseases and stages, suggesting that the underlying pathological substrates influence microglial behavior [125]. Understanding these dynamics is crucial for elucidating the role of microglia in ADRDs and their interactions with neurons and other glial cells [126].
3.2. Microglial Functional Impairments
Microglial activation in ADRDs disrupts their homeostatic roles, driving chronic inflammation and neuronal damage [127,128]. Across all ADRDs, microglia release elevated levels of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) [129]. These cytokines exacerbate neuronal injury, impair synaptic function, and perpetuate neurotoxic feedback loops with other glial cells, particularly astrocytes [129,130].
Dysregulated chemokine production is also a hallmark of microglial activation. In AD, FTD, and LBD, elevated levels of CCL2 (MCP-1), CCL5 (RANTES), and CXCL10 (IP-10) are observed, promoting peripheral immune cell recruitment and intensifying neuroinflammation [131,132]. This chemokine dysregulation further amplifies microglial activation, creating a self-sustaining inflammatory cycle [133,134].
Microglia exhibit several molecular mechanisms of impaired Aβ clearance in AD. First, the downregulation of Aβ-binding receptors, including triggering receptor expressed on myeloid cells 2 (TREM2), CD36, and Toll-like receptors (TLRs), reduces the microglial capacity for Aβ recognition and phagocytosis [135]. TREM2 dysfunction is particularly significant, as TREM2 variants are established risk factors for AD, and TREM2 signaling is essential for microglial phagocytic activity and metabolic fitness around amyloid plaques [136]. Second, chronic exposure to Aβ induces microglial immune tolerance and dysfunction of the lysosomal-autophagy system, impairing the degradation of internalized Aβ [137]. Third, pro-inflammatory cytokines such as TNF-α and IL-1β, which are elevated in AD, inhibit microglial phagocytic capacity while promoting a neurotoxic phenotype [138]. Additionally, microglia in AD show decreased expression of Aβ-degrading enzymes, including neprilysin and the insulin-degrading enzyme. The ApoE ε4 allele further suppresses microglial Aβ clearance through altered lipid metabolism and inflammatory signaling, providing another mechanism by which this genetic risk factor contributes to AD pathogenesis [139].
ROS production by microglia is another shared feature across ADRDs, contributing to oxidative stress and mitochondrial dysfunction in neurons [123,140]. In LBD and VCID, oxidative damage is exacerbated by impaired microglial support for synaptic and vascular structures, leading to synaptic disintegration and BBB compromise [119,141].
Microglia also exhibit impaired clearance of pathological proteins in ADRDs. In AD, their capacity to phagocytose Aβ is reduced, while in LBD, lysosomal dysfunction impairs the degradation of α-synuclein aggregates [142]. In FTD, specific tau protein isoforms, particularly hyperphosphorylated tau and aggregated tau species containing the 4R isoform (which predominates in many FTD variants), significantly disrupt microglial function through several mechanisms [143]. First, extracellular tau oligomers activate microglial toll-like receptors (particularly TLR2 and TLR4), triggering pro-inflammatory signaling cascades that shift microglia toward a dysfunctional phenotype with reduced phagocytic capacity [144]. Second, internalized tau impairs the microglial lysosomal function by disrupting acidification and cathepsin activity, compromising their ability to degrade protein aggregates [145]. Third, tau-activated microglia upregulate NLRP3 inflammasome activity, resulting in sustained IL-1β release that further promotes tau phosphorylation and aggregation in neurons, creating a detrimental feedback loop [144]. Additionally, tau pathology induces microglial senescence characterized by telomere shortening and accumulated DNA damage, leading to decreased expression of homeostatic genes and phagocytic receptors such as TREM2, CD33, and Cx3cr1 [146]. These molecular changes collectively impair the microglial clearance of pathological tau species and cellular debris, exacerbating tau propagation and neurotoxicity in FTD [147,148].
3.3. Microglial Transcriptional Alterations
Microglial transcriptional profiles across ADRDs reflect disease-specific and overlapping changes that drive their pathological activation [149,150]. Upregulation of genes associated with the NF-κB signaling pathway is a consistent feature, promoting the production of inflammatory cytokines (e.g., IL-1β) and activating inflammasome components such as NLRP3 [151,152] These transcriptional shifts contribute to chronic inflammation, oxidative stress, and impaired protein clearance mechanisms [152,153].
In FTD and LBD, microglia exhibit additional transcriptional changes, including the upregulation of genes involved in IL-6 signaling and metabolic dysfunction, further driving neuroinflammation and synaptic damage [133,154]. In VCID, ischemia-induced transcriptional reprogramming activates pathways linked to hypoxia and the AIM2 inflammasome, exacerbating neuronal injury and BBB disruption.
In AD, mutations in APP, PSEN1, and PSEN2 drive Aβ accumulation, triggering sustained microglial activation [155,156]. The ApoE ε4 allele amplifies these responses, promoting pro-inflammatory states and metabolic deficits [157]. FTD-associated mutations in MAPT and GRN impair tau clearance and enhance inflammatory signaling [157,158]. In LBD, GBA mutations disrupt lysosomal function, worsening α-synuclein aggregation and neuroinflammation [159,160]. In VCID and MEDs, APOE ε4 and C9orf72 mutations heighten microglial reactivity, accelerating the inflammatory and vascular components of pathology [161,162].
To summarize, microglia play a pivotal role in ADRDs, transitioning from protective to pathological states as the diseases progress. Their activation is marked by profound morphological changes, including hypertrophy, ramification, and process retraction, often leading to the adoption of a dystrophic morphology. Functionally, activated microglia release excessive pro-inflammatory cytokines (e.g., TNF-α, IL-1β, and IL-6) and chemokines (e.g., CCL2, CCL5, and CXCL10), creating a self-perpetuating inflammatory cycle that exacerbates neuronal damage and disrupts synaptic function. Additionally, microglial dysfunction impairs their capacity to clear pathological aggregates such as Aβ, tau, and α-synuclein, further amplifying neurotoxicity. Transcriptional reprogramming, driven by pathways such as NF-κB and influenced by genetic mutations (e.g., APP, GRN, GBA, and APOE ε4), compounds these effects by promoting chronic inflammation, oxidative stress, and metabolic disruption.
Our analysis of microglial contributions to ADRD pathogenesis reveals their central role in orchestrating neuroinflammatory responses and their potential to both protect against and exacerbate neurodegenerative processes (Figure 2, Table 2). As we have seen, the complex interactions between microglia and astrocytes create inflammatory feedback loops that significantly impact disease trajectory. However, this neuroimmune axis represents only part of the glial response to ADRDs. To fully understand the multicellular basis of neurodegeneration, we must also consider oligodendrocytes, which have received comparatively less attention in ADRD research despite growing evidence of their involvement.

Figure 2.
Alteration in Microglia during NMPD Progression.
Oligodendrocytes, the myelinating cells of the CNS, differ fundamentally from astrocytes and microglia in both origin and primary function. Derived from oligodendrocyte precursor cells (OPCs) that persist throughout adulthood, these cells are primarily responsible for producing and maintaining the myelin sheaths that insulate axons, enabling saltatory conduction and providing metabolic support to neurons [163]. Traditionally, oligodendrocyte dysfunction has been associated with demyelinating disorders such as multiple sclerosis rather than ADRDs. However, emerging evidence indicates significant oligodendrocyte and white matter abnormalities across the spectrum of neurodegenerative diseases [164]. The following section will examine how oligodendrocytes respond to and contribute to ADRD pathogenesis, their interactions with other glial populations, and how myelin dysfunction influences cognitive decline in these conditions. This analysis will complete our examination of individual glial cell types before we explore their collective interactions in driving neuroinflammation and neurodegeneration.

Table 2.
Summary of the morphological changes, functional impairments, and transcriptional alterations of microglia in ADRDs.
Table 2.
Summary of the morphological changes, functional impairments, and transcriptional alterations of microglia in ADRDs.
| Morphological Changes | Functional Impairments | Transcriptional Alterations | |
|---|---|---|---|
| All ADRDs |
|
|
|
| AD |
|
|
|
| FTD |
|
|
|
| LBD |
|
|
|
| VCID |
|
|
|
| MEDs |
|
|
|
4. Oligodendrocytes
Oligodendrocytes, the myelinating cells of the central nervous system (CNS), play critical roles in supporting neuronal function by maintaining myelin integrity, providing metabolic support, and regulating extracellular ion balance [170,171]. In ADRDs, oligodendrocytes undergo pathological changes that disrupt these essential functions, contributing to neuroinflammation, demyelination, and neuronal injury [172]. Their involvement in ADRDs has emerged as a significant area of study, offering insights into their roles in disease pathogenesis [173].
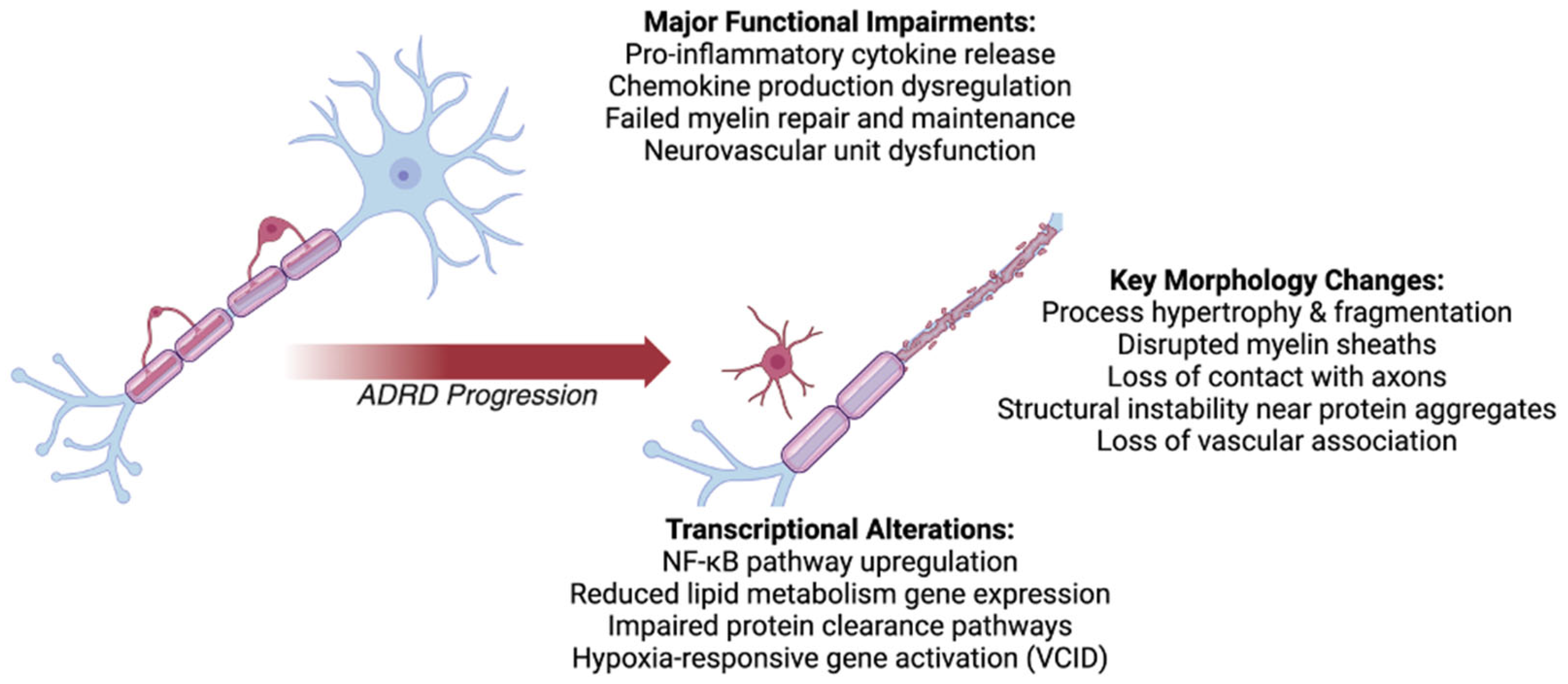
4.1. Oligodendrocyte Morphological Changes
Oligodendrocytes in ADRDs exhibit distinct morphological changes indicative of their reactive state [174]. Across all dementia types, hypertrophy and increased ramification are commonly observed, reflecting an attempt to respond to pathological conditions [175]. In AD, oligodendrocytes exhibit swelling, process fragmentation, and reduced structural stability, often clustering near Aβ plaques [176]. This morphological disruption compromises their ability to maintain myelin integrity, contributing to neuronal vulnerability and exacerbating cognitive decline [177].
In FTD, oligodendrocytes exhibit fragmented and disorganized processes associated with tau protein accumulation [178,179]. These changes impair their capacity to support neuronal health and amplify tau-mediated neurotoxicity. Similarly, LBD oligodendrocytes display hypertrophy and process instability in response to α-synuclein aggregates, further contributing to demyelination and myelin degradation [180].
In VCID, chronic cerebral hypoperfusion induces reactive oligodendrocytes with hypertrophied and dysfunctional processes [178]. These cells lose their structural associations with blood vessels, exacerbating BBB breakdown and facilitating peripheral immune cell infiltration [181]. MEDs present compounded structural abnormalities, including extensive process fragmentation and severe demyelination, reflecting the dual burden of vascular and neurodegenerative insults [182].
4.2. Oligodendrocyte Functional Impairments
Reactive oligodendrocytes in ADRDs display significant functional impairments that disrupt their homeostatic roles and contribute to disease progression [183,184,185]. Across dementia types, oligodendrocytes release elevated levels of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6 [183,184]. These cytokines foster a neuroinflammatory environment, exacerbating neuronal damage and impairing synaptic integrity [186].
Dysregulated chemokine production is another hallmark of oligodendrocytic dysfunction. Elevated levels of CCL2 (MCP-1), CCL5 (RANTES), and CXCL10 (IP-10) are commonly observed in ADRDs, facilitating the recruitment of peripheral immune cells to the CNS [187,188]. This chemokine-mediated inflammation creates a self-perpetuating cycle, amplifying microglial and astrocytic reactivity [189].
Oligodendrocytes also fail to effectively repair myelin in ADRDs. The loss of myelin integrity increases neuronal susceptibility to oxidative stress and excitotoxicity, further impairing neural networks critical for cognitive function [190,191]. This failure is particularly pronounced in VCID, where ischemic damage exacerbates oligodendrocytic dysfunction and contributes to neuronal and vascular instability [192]. Mixed dementias further highlight the compounded effects of these deficits, with oligodendrocytes struggling to manage the dual demands of vascular and neurodegenerative stressors [193].
4.3. Oligodendrocyte Transcriptional Alterations
Oligodendrocytes in ADRDs undergo significant transcriptional reprogramming, driven by inflammatory, oxidative, and metabolic stress pathways [173,194]. Upregulation of NF-κB signaling is a consistent feature across dementia types, promoting the production of inflammatory cytokines such as IL-1β and IL-6, which exacerbate neuroinflammation and impair oligodendrocytic functions [171,195]. In AD, this is accompanied by the reduced expression of genes involved in lipid metabolism and Aβ clearance, further amplifying toxicity [196,197].
Similar patterns are observed in frontotemporal dementia, where IL-6 signaling and tau-related genes are upregulated, impairing myelin maintenance and promoting tau aggregation [177,198]. LBD oligodendrocytes show transcriptional shifts that reduce lysosomal function, hindering α-synuclein degradation and exacerbating inflammation [199]. In VCID, transcriptional changes reflect ischemia-induced activation of hypoxia-responsive genes and AIM2 inflammasome signaling, contributing to vascular and neuronal instability [200]. MEDs combine these transcriptional disruptions, with heightened inflammatory and metabolic stress pathways exacerbating oligodendrocytic dysfunction [201].
Genetic mutations further compound these transcriptional changes. In Alzheimer’s disease, mutations in APP, PSEN1, and PSEN2 drive Aβ accumulation and inflammatory activation, while ApoE ε4 amplifies metabolic deficits and reactivity [202]. FTD mutations in MAPT and GRN impair tau clearance and promote inflammatory signaling [203]. LBD-associated GBA mutations reduce lysosomal efficiency, worsening α-synuclein pathology [204]. APOE ε4 and C9orf72 mutations in VCID and mixed dementias exacerbate inflammatory activation and BBB disruption, linking genetic and transcriptional dysfunction across ADRDs [205].
As discussed, oligodendrocytes—critical for maintaining myelin integrity and providing both metabolic and structural support to neurons—undergo significant pathological changes in ADRDs (Figure 3, Table 3). Reactive oligodendrocytes present in ADRDs display hypertrophy, process fragmentation, and structural destabilization, contributing to demyelination, blood-brain barrier breakdown, and increased neuronal vulnerability. By releasing pro-inflammatory cytokines (e.g., TNF-α and IL-1β) and chemokines (e.g., CCL2 and CXCL10), they foster a neuroinflammatory environment that amplifies glial and neuronal dysfunction. Transcriptional disruptions, driven by NF-κB activation and disease-related mutations (e.g., APP, GRN and GBA), further exacerbate oxidative stress and impair critical homeostatic functions. Despite these detrimental changes, oligodendrocytes remain a compelling therapeutic target, with potential interventions focusing on reducing inflammation and restoring their capacity for myelin repair.

Figure 3.
Alteration in Oligodendrocytes during NMPD Progression.
The preceding sections have individually characterized how astrocytes, microglia, and oligodendrocytes respond to and contribute to ADRD pathogenesis. Each glial cell type exhibits unique patterns of reactivity and dysfunction that reflect their distinct physiological roles within the CNS. Astrocytes show compromised homeostatic functions and adopt neurotoxic secretory profiles, microglia develop persistent pro-inflammatory activation states with impaired phagocytic capacity, and oligodendrocytes exhibit myelin abnormalities and metabolic dysfunction that compromise axonal integrity. While examining these cell-specific responses provides valuable insights, it presents an inherently compartmentalized view of glial contributions to neurodegeneration. In reality, the CNS represents a highly integrated cellular network where no glial cell functions in isolation. Astrocytes, microglia, and oligodendrocytes engage in continuous, multidirectional communication through direct contact, secreted factors, and extracellular vesicles [206]. These intercellular dialogues create complex signaling networks that collectively determine the neuroinflammatory environment and neuronal fate in ADRDs. The following section will explore these critical glial interactions, focusing on how communication between different glial populations amplifies or mitigates neurodegenerative processes. Understanding these cellular conversations is essential for developing therapeutic strategies that target not just individual cell types but the dysfunctional multicellular networks characteristic of ADRDs.

Table 3.
Summary of the morphological changes, functional impairments, and transcriptional alterations of oligodendrocytes in ADRDs.
Table 3.
Summary of the morphological changes, functional impairments, and transcriptional alterations of oligodendrocytes in ADRDs.
| Morphological Changes | Functional Impairments | Transcriptional Alterations | |
|---|---|---|---|
| All ADRDs |
|
|
|
| AD |
|
|
|
| FTD |
|
|
|
| LBD |
|
|
|
| VCID |
|
|
|
| MEDs |
|
|
|
5. Interactions Among Glial Cells
In the pathogenesis and progression of ADRDs. These interactions are complex and involve many signaling pathways, inflammatory responses, and metabolic processes that can protect or harm neuronal health. In ADRDs, microglial activation plays a central role in initiating and sustaining neuroinflammation. As previously discussed, microglia release a variety of pro-inflammatory cytokines upon activation, such as IL-1α, TNF-α, and C1q, which propagate a neurotoxic environment. These cytokines induce a phenotypic transformation in astrocytes, inducing a reactive state. Reactive astrocytes are characterized by their neurotoxic properties, contributing to the death of neurons and oligodendrocytes. In the context of AD, the resultant neuroinflammation further exacerbates the accumulation of Aβ plaques, triggering the activation of additional microglia. The ensuing cascade initiates a chronic, low-grade inflammation that perpetuates neuronal degeneration and cognitive decline [208]. Microglial activation is not limited to cytokine release but also involves the secretion of extracellular vesicles that carry neurotoxic factors, further enhancing neuronal damage. These vesicles can contain a variety of harmful molecules, including inflammatory mediators and neurotoxic proteins, thus amplifying the inflammatory cascade, and contributing to the neurodegenerative process [209]. The interplay between microglia, astrocytes, and neurons underscores the complexity of neuroinflammation in AD, among other ADRDs, suggesting that targeting microglial activation and its downstream effects may provide potential therapeutic avenues for mitigating disease progression.
Astrocytes, traditionally viewed as supportive cells, play a dual role in ADRDs. While they are essential for maintaining homeostasis and supporting neuronal function, reactive astrocytes can adopt a neurotoxic phenotype under the influence of activated microglia. This phenotypic transformation is especially pronounced in AD, where reactive astrocytes not only exacerbate neuroinflammation but directly contribute to synaptic dysfunction and neuronal loss [210]. The interaction between astrocytes and microglia is integral to disease progression; activated microglia amplify the neurotoxic effects of astrocytes, thereby establishing a detrimental feedback loop that perpetuates neurodegeneration and cognitive decline [95,211]. Beyond their role in neuroinflammation, astrocytes are also critical for the regulation of oligodendrocyte survival and myelination, processes that are essential for the maintenance of neuronal function and overall CNS integrity [210]. Thus, while astrocytes are pivotal in the preservation of neuronal homeostasis, their dysregulation in ADRDs highlights their dual role as both protectors and contributors to neurodegeneration, further complicating the pathophysiology of these diseases.
Oligodendrocytes, the myelinating cells of the CNS, are significantly affected by the interactions between microglia and astrocytes. Under the inflammatory conditions stimulated by activated microglia and reactive astrocytes in ADRDs, oligodendrocytes become increasingly susceptible to apoptosis [212,213]. The loss of oligodendrocytes due to these inflammatory processes leads to demyelination, a critical event that disrupts neuronal conduction and contributes to the cognitive deficits observed in ADRDs [213]. In addition to mature oligodendrocytes, oligodendrocyte precursor cells (OPCs) are also affected by the inflammatory microenvironment shaped by microglia and astrocytes. Depending on the nature of these signals, OPCs can either be driven toward differentiation into mature oligodendrocytes or subjected to dysfunction, impairing their ability to regenerate and maintain myelin [214,215]. The balance between promoting OPC differentiation and preventing their dysfunction is crucial for preserving white matter integrity and overall brain health. Disruptions in this balance, particularly in the inflammatory environment of neurodegenerative diseases, exacerbate myelin loss and contribute significantly to the progressive cognitive decline seen in ADRDs.
As the primary functional units of the CNS, neurons are central to these interactive processes. Neurons not only communicate with glial cells but also influence their behavior through the release of signaling molecules. In ADRDs, neuronal dysfunction can lead to altered glial responses, creating a vicious cycle of neurodegeneration. For instance, in AD, the loss of synaptic integrity and neuronal death can further activate microglia and astrocytes, exacerbating the inflammatory response and promoting the progression of the disease [205]. Additionally, the metabolic interactions between neurons, astrocytes, and oligodendrocytes are critical for maintaining energy homeostasis in the brain, and disruptions in these interactions can lead to neuronal damage [215,216].
The interplay between these cell types is also influenced by various signaling pathways and molecular mechanisms. For example, the complement system, particularly the activation of complement component C1q, has been implicated in microglial-mediated synaptic pruning, which can be detrimental in neurodegenerative context [211]. Furthermore, microglia and astrocytes release extracellular vesicles carrying neurotoxic factors that affect neuronal health. This highlights the importance of intercellular communication in ADRD progression [209].
The interactions among glial cells and neurons manifest differently across specific ADRDs. In AD, the accumulation of amyloid plaques and tau tangles leads to a pronounced inflammatory response characterized by activated microglia and reactive astrocytes. These activated cells contribute to synaptic loss and neuronal death [208]. In FTD, the presence of tau pathology can similarly activate glial cells, resulting in neuroinflammation and neurodegeneration [217]. In LBD, the aggregation of alpha-synuclein in neurons can also provoke a neuroinflammatory response, with microglia and astrocytes playing significant roles in the disease’s progression [218]. VCID and MED present unique challenges as well, as they often involve vascular factors that can influence glial cell behavior. In VCID, ischemic events can lead to the activation of microglia and astrocytes, resulting in a cascade of inflammatory responses that can exacerbate neuronal injury [210,219]. The interplay between these cell types in the context of vascular pathology is critical, as it can determine the extent of neuronal damage and cognitive decline. Moreover, the role of oligodendrocytes in these diseases cannot be overlooked. In conditions such as multiple sclerosis, which shares features with VCID, oligodendrocyte loss and demyelination are key pathological features that can be influenced by the inflammatory milieu created by activated microglia and astrocytes [212,213]. The interactions among these cell types can significantly impact the regenerative capacity of the CNS, as oligodendrocyte dysfunction can hinder remyelination and recovery following injury [215,216].
The intricate interactions among glial cells play a pivotal role in the pathogenesis of age-related neurodegenerative diseases (ADRDs). These intercellular communications establish neuroinflammatory cascades that can either exacerbate or mitigate neurodegenerative processes, depending on their activation states [220]. This dynamic interplay is crucial for maintaining the neurovascular unit and overall brain health, yet its dysregulation can lead to detrimental outcomes, including increased microgliosis and the release of neurotoxic factors that perpetuate neuronal damage and cognitive decline. Understanding the mechanisms regulating these glial interactions requires examining the molecular control systems governing their phenotypes and activation states [221]. Among these regulatory mechanisms, non-coding RNAs have emerged as particularly important modulators of glial function. Non-coding RNAs, particularly microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), represent sophisticated molecular switches that fine-tune gene expression without being translated into proteins. These RNA species regulate numerous aspects of glial biology, from differentiation and morphological plasticity to inflammatory signaling and phagocytic capacity. In the context of ADRDs, specific patterns of ncRNA dysregulation are associated with pathological glial phenotypes, suggesting their potential involvement in disease mechanisms [222]. The following section will explore how non-coding RNAs influence glial cell morphology and function, their dysregulation in ADRDs, and their potential as both biomarkers and therapeutic targets. This examination will bridge our understanding of cellular interactions with the molecular mechanisms that control them, providing a more comprehensive picture of the complex regulatory networks underlying neuroinflammation and neurodegeneration.
6. Non-Coding RNAs in Glial Cell Ramification, Morphology, and Neuroinflammation
Non-coding RNAs (ncRNAs) represent a diverse class of RNA molecules that, despite lacking protein-coding potential, exert profound regulatory control over gene expression and cellular processes. Among the various ncRNAs, microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) have garnered particular attention for their pivotal roles in the modulation of glial cell behavior, including their differentiation, morphological changes, and response to neuroinflammatory stimuli. As described in more detail below, both miRNAs and lncRNAs are regulators of gene expression, but miRNAs primarily function through post-transcriptional regulation, whereas lncRNAs have more diverse mechanisms that influence transcriptional regulation, chromatin remodeling, and protein-protein interactions [223].
These ncRNAs are synthesized in the nucleus from their respective genomic loci in glial cells and can be transported to the cytoplasm, where they can elicit a response (e.g., an immune response during injury or disease [222]. ncRNAs can also be secreted into the extracellular space via transport mechanisms, including via small-membrane vesicles called extracellular vesicles (EVs) [224]. These processes may also involve specialized RNA-binding proteins and intracellular vesicles to facilitate intercellular communication (described in more detail below). Once in the cytoplasm, ncRNAs can bind to target mRNAs, regulating gene expression either by repressing translation or promoting mRNA degradation [225].
miRNAs, such as miR-124 and miR-9, govern the balance between the homeostatic and reactive states of glial cells, impacting their structure and function in neurodegenerative conditions such as Alzheimer’s disease. Similarly, lncRNAs, including Bdnf-AS and NEAT1, regulate cytoskeletal dynamics and inflammatory pathways, further influencing glial cell activation and neuroinflammatory responses. Given their central roles in glial biology, these ncRNAs present promising therapeutic targets for neurodegenerative diseases, offering potential strategies to mitigate neuroinflammation and restore cellular homeostasis. The continued exploration of their mechanisms will be critical for developing ncRNA-based treatments for diseases such as Alzheimer’s.
6.1. miRNAs
miRNAs are classified based on their primary sequences and evolutionary conservation across species. Their nomenclature follows a system based on the sequence of their precursor stem-loop structures and their discovery as specific regulatory RNAs in the nervous system [226]. These miRNAs are approximately 22 nucleotides in length and regulate gene expression post-transcriptionally, typically resulting in the downregulation of target genes [227].
In the context of glial cells, miRNAs may elicit their responses as endogenous miRNAs, which are produced within the glial cells themselves, or exogenous miRNAs, which are secreted by other cells. Endogenous miRNAs can regulate glial cells’ internal processes, including differentiation, response to injury, and inflammation [228]. Exogenous RNAs, on the other hand, are secreted from cells, including neurons and other glial cells. They can be transferred to neighboring cells via the mechanisms described above. This form of intercellular communication allows miRNAs to influence the behavior of distant or neighboring glial cells, even across the blood–brain barrier in some cases. In this scenario, miRNAs secreted by neurons can affect glial cells, modulating their activity and potentially influencing synaptic function, neuroinflammation, or myelination [229].
Emerging evidence suggests that miRNAs are centrally involved in the regulation of astrocyte and microglial gene expression and function [96]. For example, miRNAs such as miRNA-124 and miRNA-9 are involved in regulating glial cell morphology, with alterations in this morphology linked to neuroinflammation and ADRDs [47,230,231]. For example, miRNA-9, which is expressed in all glial cells, can regulate neuroinflammation, neurogenesis, and neuronal differentiation [232]. miRNA-124 is also a brain-specific miRNA and is highly enriched in neurons, although it is also expressed in glial cells, particularly astrocytes [96]. One mechanism by which miR-124 may be involved in neuronal health and differentiation is through its regulation of the mTOR (mechanistic target of rapamycin) signaling pathway, which is crucial for cell growth, protein synthesis, and metabolism [233]. miRNA-9, on the other hand, has been implicated in the NF-κB pathway, a critical signaling pathway involved in inflammation and immune responses. miR-9 can target genes in the NF-κB pathway, modulating its activation and thus regulating neuroinflammation [234]. Given the roles of these miRNAs in acting on these proteins, evidence suggests that these miRNAs play an important role in neuronal differentiation, regulating neuroinflammation, synaptic plasticity, and glial cell differentiation. In addition to affecting cellular morphology, miRNAs may also directly modulate neuroinflammatory responses—an intrinsic feature of ADRDs—by influencing microglial activation states, transitioning from homeostatic to reactive phenotypes [235].
Homeostatic microglia exhibit a highly branched, neuroprotective morphology, which is associated with the release of anti-inflammatory cytokines [236]. In contrast, reactive microglia adopt an amoeboid, rounded morphology with shortened processes, accompanied by an enhanced capacity for phagocytosis and the production of pro-inflammatory cytokines [235]. Disruptions in miRNA expression are strongly associated with the induction of the reactive microglial phenotype, mediating morphological alterations through the activation of critical transcription factors and signaling pathways, including NF-κB, a central mediator of neuroinflammation [235]. Specific miRNAs, such as miRNA-146, miRNA-223, and miRNA-155, have been implicated in these processes, with links to changes in microglial morphology and activation, as well as astrogliosis and broader neuroinflammatory responses [237].
Another well-studied miRNA in the context of glial cell differentiation, morphology, and activation is miR-124. For instance, during the morphological transition of microglia from a ramified to an amoeboid state, several immune cell surface markers, including CD45 and major histocompatibility complexes (MHCs), are upregulated, coinciding with the downregulation of miR-124 [238]. Furthermore, the expression of both miR-124 and miR-9 has been implicated in the regulation of genes that govern cytoskeletal dynamics, which in turn contributes to the development of the characteristic star-shaped, branched morphology of astrocytes [96]. Additionally, miR-9 has been shown to modulate astrocyte morphology by influencing the extension and branching of processes through the regulation of actin- and tubulin-related genes [239]. Beyond these specific examples, miRNAs in general are broadly differentially expressed in AD and other dementias, driving alterations in glial morphology and promoting reactive phenotypes [240]. For example, the upregulation of miRNA-146a has been linked to reactive astrocytes—through synaptic dysfunction and impaired repair mechanisms—promoting neuroinflammation and neuronal damage [237]. Similarly, miR-223 and miR-155 have been associated with changes in astrocyte morphology, reactive astrogliosis, and downstream neurotoxicity [241,242]. However, the precise mechanisms by which other miRNAs contribute to disease pathogenesis remain incompletely understood, representing a critical area for future research.
The growing body of evidence highlighting the critical role of miRNAs in regulating glial cell function, morphology, and activation suggests their potential as therapeutic targets in ADRDs. In this context, both the use of miRNA inhibitors, which aim to mitigate the adverse effects of dysregulated miRNAs, and miRNA mimetics, designed to downregulate specific target genes, represent promising avenues of research. Strategies that modulate miRNA expression, such as targeting specific miRNAs with synthetic oligonucleotide inhibitors, have the potential to restore normal glial cell morphology and function, potentially preventing gliosis and neuroinflammation [243,244,245,246,247]. Additionally, the overexpression of artificial miRNAs is emerging as another promising strategy to improve glial health and counteract neurodegenerative processes [248,249,250].
6.2. lncRNAs
lncRNAs, typically >200 nucleotides in length, are also emerging as RNA regulators of glial cell ramification and morphology, influencing processes such as differentiation, synaptic interactions and neuroinflammation [251]. Growing evidence demonstrates that multiple lncRNAs can regulate the cytoskeleton, gene expression, and cellular signaling, thus shaping the structure and function of glial cells [252]. Much of this research suggests that lncRNAs contribute to glial changes by modulating cytoskeletal dynamics (including actin and tubulin levels), branching, and morphology (e.g., by binding with and interacting with actin filaments and microtubules) [253]. As such, they may also represent novel targets for therapeutic interventions in neurological disorders that involve glial cell dysfunction/reactivity, again in a bidirectional fashion. For example, increasing select lncRNAs has been reported to improve cognitive function and reduce glial cell inflammation [254], whereas decreasing other lncRNAs may reduce microglia-associated inflammation [255].
In addition to regulating cytoskeletal dynamics, lncRNAs have also been shown to interact with a variety of proteins that directly influence glial cytoskeletal dynamics. For example, the lncRNA brain-derived neurotrophic factor antisense (Bdnf-AS) can influence astrocyte morphology and branching by interacting with the mTOR signaling pathway, a key regulator of cytoskeletal dynamics (e.g., dendrite branching) [256]. Additional lncRNAs, including nuclear rich abundant transcript 1 (NEAT1) can modulate glial activation (reducing NEAT1 seems to be neuroprotective) in part by modulating transcriptional activity of NF-kB [257,258]. As described above, NEAT1 may also be involved in MTOR signaling, highlighting its role in neuron homeostasis and survival [259]. Another lncRNA, H19, can induce the activation of astrocytes, microglia, and the release of pro-inflammatory cytokines (interleukin-1β, interleukin-6, and tumor necrosis factor-α) [260].
In neurodegenerative diseases such as AD, which is characterized by heightened levels of neuroinflammation, glial cells undergo significant changes in morphology. As described above, lncRNAs can modulate glial cell activation, process extension, and the inflammatory response (e.g., by shifting microglia from an M2 state to an M1 activated state), which is associated with pathological progression [235]. Recent evidence suggests that lncRNAs can contribute to glial cell responses during pathological conditions, including neuroinflammation and reactive gliosis.
To summarize, the roles of miRNAs and lncRNAs in regulating glial cell morphology and function are pivotal in understanding neuroinflammatory processes and neurodegenerative diseases such as Alzheimer’s disease Table 4. miRNAs, including miR-124 and miR-9, significantly influence the transition between the reactive and homeostatic states of microglia, thereby affecting neuroinflammation and neuronal health (Table 4). Concurrently, lncRNAs modulate cytoskeletal dynamics and inflammatory responses, further contributing to glial cell behavior during pathological conditions. The therapeutic potential of targeting these non-coding RNAs is promising, as restoring normal expression levels may mitigate neuroinflammation and promote glial health, highlighting the need for further research to elucidate their specific mechanisms and implications in neurodegenerative pathology.

Table 4.
Summary of miRNAs and lncRNAs that regulate glial cell morphology and function in ADRDs.
7. Therapeutic Outlooks
In ADRDs, the complex interactions between glial cells are central to disease progression. These cells do not function in isolation; rather, they engage in dynamic interactions that regulate neuroinflammation, synaptic function, and neuronal health. Astrocytes provide metabolic support to neurons and modulate synaptic transmission, while microglia act as the primary immune cells of the CNS, responding to pathological changes by altering their activation states [261,262]. Oligodendrocytes, responsible for myelinating axons, also play a role in maintaining neuronal integrity and function. The intricate interplay among these glial populations, combined with their regulation by ncRNAs, presents a promising therapeutic target for mitigating the pathophysiological processes underlying ADRDs [263,264].
Therapeutic strategies that modulate the activation and function of glial cells, while also targeting ncRNAs, may restore the delicate balance required for proper neuroinflammatory responses and neuronal protection. For example, restoring astrocytic function through the inhibition of pro-inflammatory cytokines such as IL-1β and TNF-α, or by enhancing astrocytic glutamate uptake, could help mitigate the neurotoxic environment that promotes neurodegeneration [265,266].
Similarly, microglial activation is tightly regulated; strategies aimed at restoring their neuroprotective functions while dampening excessive pro-inflammatory activation show promise for slowing disease progression. Targeting chemokines, such as CCL2 and CXCL10, or modulating the activation of NF-κB and inflammasome signaling pathways, could prevent inflammatory escalation and restore glial homeostasis [267,268]. Moreover, the interactions between these glial cells and their ability to influence each other’s states further highlight the importance of a coordinated therapeutic approach. Enhancing astrocytic functions could, in turn, modulate microglial responses, offering a comprehensive strategy to restore the balance between pro-inflammatory and neuroprotective states in the brain [269,270].
ncRNAs, particularly miRNAs, have emerged as critical regulators of glial cell behavior and activation. miRNAs regulate key processes such as glial morphology, activation, and the inflammatory response. By modulating miRNA expression—through either miRNA inhibitors to prevent their adverse effects or mimetics to enhance their activity—it is possible to restore normal glial cell function. For instance, miRNAs such as miRNA-124 and miRNA-9, which influence the morphology of both astrocytes and microglia, could be targeted to promote protective glial phenotypes and counteract reactive transformations [271,272]. Additionally, modulating miRNAs involved in microglial activation could prevent neuroinflammation and mitigate neuronal damage. Targeting these pathways could be particularly effective in ADRDs, where dysregulated glial responses contribute to neuronal dysfunction and disease progression [273].
lncRNAs add another layer of complexity to glial regulation. Acting as competing endogenous RNAs (ceRNAs), lncRNAs can sequester miRNAs and modulate the expression of target mRNAs, many of which are involved in inflammatory processes [262,274]. Dysregulated lncRNA expression has been associated with increased neuroinflammation, and targeting these molecules could help restore glial homeostasis and reduce the pathological activation of glial cells. For example, lncRNAs such as NEAT1 have been implicated in the regulation of inflammatory responses in microglia, suggesting that their modulation could be beneficial in the context of ADRDs [121,275]. By modulating both miRNAs and lncRNAs, therapeutic strategies may be able to restore a balanced inflammatory response, mitigate neurodegeneration, and improve neuronal survival [276,277]. Several techniques might be used to inhibit ncRNAs, including antisense oligonucleotides, RNA interference (RNAi), and the development of other small molecules to target these RNAs [278]. The therapeutic potential of targeting glial cells and their interactions with ncRNAs offers a multifaceted approach to treating ADRDs. By addressing the dysregulation of both glial function and the molecular pathways that govern their activation, these strategies offer the potential to reprogram glial cells and halt or reverse the neurodegenerative processes that characterize ADRDs.
The temporal progression of glial activation and ncRNA dysregulation in ADRDs has significant implications for therapeutic development. Since these changes emerge in prodromal and early disease stages, they represent promising targets for early intervention before extensive neurodegeneration and cognitive decline occur [279]. Stage-specific therapeutic approaches that consider the evolving nature of glial phenotypes throughout disease progression may be particularly effective. For instance, interventions targeting microglial activation might be most beneficial in preclinical and early symptomatic phases, while treatments addressing oligodendrocyte dysfunction and myelin integrity might have a greater impact in later disease stages [280,281]. Similarly, ncRNA-based therapies may need to be tailored to the temporal expression patterns of specific ncRNAs throughout disease progression. This highlights the need for reliable biomarkers of glial activation and ncRNA dysregulation to identify appropriate therapeutic windows and monitor treatment efficacy in clinical trials [282].
Several challenges must be addressed in these therapeutic areas. For example, the blood–brain barrier (BBB) poses a challenge for delivering therapeutic agents (including miRNAs and lncRNAs) to the brain. Because the molecules are large and negatively charged, it is difficult to effectively target delivery in glial cells or neurons. Other challenges, including specificity and off-target effects, are also a concern. Various strategies, including lipid nanoparticles, exosomes, and viral vectors, are being explored to improve ncRNA delivery. Ongoing trials are focusing on these techniques, as well as the development of more efficient delivery systems [283]. As research into the role of glial cells and ncRNAs continues to evolve, the integration of ncRNA-based therapies with traditional cell-based approaches may provide a more comprehensive strategy to combat these complex diseases [284,285].
8. Concluding Remarks
The interplay between glial cells and non-coding RNAs (ncRNAs) is increasingly recognized as a critical factor in the pathogenesis and progression of ADRDs. Glial cells, including astrocytes, microglia, and oligodendrocytes, play essential roles in maintaining homeostasis within the central nervous system (CNS). However, their dysregulation can lead to neuroinflammation and neuronal dysfunction, contributing to the progression of neurodegenerative diseases. Recent studies have highlighted the dual nature of glial cells, where reactive changes can result in neurotoxic environments that exacerbate neuronal damage [285,286]. Furthermore, the identification of specific long non-coding RNAs (lncRNAs) that modulate glial cell behavior and fate determination has opened new avenues for understanding how these cells contribute to ADRDs [287].
ncRNAs, particularly lncRNAs and miRNAs, have emerged as significant regulators of gene expression and cellular responses in the context of neurodegeneration. These molecules are involved in various processes, including the modulation of inflammatory responses, regulation of glial cell function, and neuronal survival. For instance, lncRNAs have been shown to interact with transcription factors and other regulatory proteins, influencing the expression of genes associated with neuroinflammation and neurodegeneration [288,289]. Additionally, miRNAs can act as post-transcriptional regulators, fine-tuning the expression of genes involved in glial activation and neuronal health [290,291]. The complex regulatory networks involving glial cells and ncRNAs underscore the need for a comprehensive understanding of their roles in ADRDs.
Another important aspect of glial-mediated neuroinflammation in ADRDs involves the complement system, which serves as a critical bridge between glial reactivity and synaptic pathology. Reactive astrocytes and microglia upregulate the expression and secretion of various complement components, particularly C1q, C3, and C4, which tag vulnerable synapses for elimination [292]. For example, in AD, Aβ deposits activate the classical complement pathway, while in FTD, pathological tau induces similar complement activation [293]. Oligodendrocytes also contribute to this process by releasing complement factors in response to inflammatory stimuli [294]. This complement cascade creates a feed-forward mechanism where complement-mediated microglial phagocytosis of synapses drives network dysfunction before substantial neuronal loss occurs. Therapeutic strategies targeting this pathway, such as C1q or C3 inhibitors, have shown promise in preclinical models by preserving synaptic integrity while modulating glial reactivity [295]. Regarding ncRNA-based interventions, several innovative approaches are emerging. Beyond conventional antisense oligonucleotides (ASOs) targeting specific miRNAs, novel techniques include miRNA sponges that act as competitive inhibitors, CRISPR-Cas9-based approaches for modifying ncRNA expression, and small molecule inhibitors of ncRNA-protein interactions [296]. Extracellular vesicle-based delivery systems show particular promise for CNS targeting, as they can cross the blood-brain barrier and achieve cell-specific delivery [297]. Additionally, circular RNAs and aptamer-conjugated nanoparticles offer enhanced stability and specificity for ncRNA modulation in glial cells [298]. These complementary approaches—targeting both the complement system and ncRNA dysregulation—may provide synergistic benefits in restoring glial homeostasis and mitigating neuroinflammation in ADRDs [299].
Given the interplay between oligodendrocytes, astrocytes, and microglia, combinatory therapies addressing multiple glial dysfunctions may provide the most comprehensive approach to treating ADRDs [121]. Looking ahead, future research should focus on elucidating the specific mechanisms by which glial cells and ncRNAs interact. Advanced techniques such as single-cell RNA sequencing and spatial transcriptomics will be invaluable in mapping the expression profiles of glial cells and ncRNAs in both healthy and diseased states [300,301]. Understanding the temporal dynamics of these interactions will provide insights into how glial cells transition from protective to detrimental roles during the progression of neurodegenerative diseases. Moreover, the therapeutic potential of targeting glial cells and ncRNAs in ADRDs should be explored. For instance, modulating the expression of specific lncRNAs or miRNAs could restore normal glial function and mitigate neuroinflammation. Additionally, the development of ncRNA-based biomarkers for early diagnosis and the monitoring of disease progression could significantly enhance the clinical management of ADRDs [287]. As our understanding of the roles of glial cells and ncRNAs deepens, it may lead to innovative therapeutic strategies that target these pathways, improving outcomes for individuals affected by ADRDs.
In summary, the interaction between glial cells and non-coding RNAs represents a complex and dynamic regulatory network that plays a crucial role in the pathogenesis and progression of Alzheimer’s disease and related dementia. The dual roles of glial cells, coupled with the regulatory functions of ncRNAs, highlight the importance of these cellular and molecular players in maintaining CNS homeostasis and their potential contributions to neurodegeneration. As research continues to uncover the intricate mechanisms underlying these interactions, there is a promising opportunity to develop novel therapeutic strategies that target glial cells and ncRNAs. By advancing our understanding of these processes, we can pave the way for innovative approaches to prevent and treat ADRDs, enhancing the quality of life for affected individuals and their families.
Author Contributions
Conceptualization, S.J.R., D.W., T.J.L. and J.A.M.; writing—original draft preparation, S.J.R. and D.W.; writing—review and editing, S.J.R., D.W., T.J.L. and J.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liang, C.-S.; Li, D.-J.; Yang, F.-C.; Tseng, P.-T.; Carvalho, A.F.; Stubbs, B.; Thompson, T.; Mueller, C.; Shin, J.I.; Radua, J.; et al. Mortality Rates in Alzheimer’s Disease and Non-Alzheimer’s Dementias: A Systematic Review and Meta-Analysis. Lancet Healthy Longev. 2021, 2, e479–e488. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, E.; Lin, P.S.; Kamdar, N.; Gonzales, G.; Norcott, A.; Peterson, M.D. Risk of Early- and Late-onset Alzheimer Disease and Related Dementia in Adults with Cerebral Palsy. Dev. Med. Child Neurol. 2021, 64, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, K.D.; Girling, L.; Berlinger, N. Inclusion of People Living with Alzheimer’s Disease or Related Dementias Who Lack a Study Partner in Social Research: Ethical Considerations from a Qualitative Evidence Synthesis. Dementia 2022, 21, 1200–1218. [Google Scholar] [CrossRef] [PubMed]
- Jicha, G.; Abner, E.L.; Arnold, S.E.; Carrillo, M.C.; Dodge, H.H.; Edland, S.D.; Fargo, K.N.; Feldman, H.; Goldstein, L.B.; Hendrix, J.A.; et al. Committee on High-quality Alzheimer’s Disease Studies (CHADS) Consensus Report. Alzheimer’s Dement. 2021, 18, 1109–1118. [Google Scholar] [CrossRef]
- Jutkowitz, E.; Halladay, C.; Tsai, J.; Hooshyar, D.; Quach, L.; O’Toole, T.; Rudolph, J.L. Prevalence of Alzheimer’s Disease and Related Dementias Among Veterans Experiencing Housing Insecurity. Alzheimer’s Dement. 2021, 18, 1306–1313. [Google Scholar] [CrossRef]
- Keohane, L.M.; Nikpay, S.; Braun, K.; Cheng, A.; Stevenson, D.G.; Buntin, M.B.; Yu, D.; Blot, W.J.; Lipworth, L. Association of Race and Income with Incident Diagnosis of Alzheimer’s Disease and Related Dementias Among Black and White Older Adults. J. Appl. Gerontol. 2022, 42, 898–908. [Google Scholar] [CrossRef]
- Orth, J.; Flowers, D.; Betz, G.; Cagle, J.G. A Systematic Review of Specialty Dementia Care Units in Long-term Care Settings. Int. J. Geriatr. Psychiatry 2023, 38, e5907. [Google Scholar] [CrossRef]
- Trammell, A.R. Characterization of African-American Super-Agers in the National Alzheimer’s Coordinating Center Cohort. J. Am. Geriatr. Soc. 2024, 72, 1995–2005. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Borson, S.; Khang, P.; Langer-Gould, A.; Wang, S.E.; Carrol, J.A.; Lee, J. Dementia Diagnosis and Utilization Patterns in a Racially Diverse Population Within an Integrated Health Care Delivery System. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12279. [Google Scholar] [CrossRef]
- Torossian, M. The Dignity of Older Individuals with Alzheimer’s Disease and Related Dementias: A Scoping Review. Dementia 2021, 20, 2891–2915. [Google Scholar] [CrossRef]
- Gianattasio, K.Z.; Moghtaderi, A.; Lupu, D.; Prather, C.; Power, M.C. Evaluation of Federal Policy Changes to the Hospice Benefit and Use of Hospice for Persons With ADRDS. Jama Health Forum 2022, 3, e220900. [Google Scholar] [CrossRef] [PubMed]
- Peacock, S.; Bayly, M.; Duggleby, W.; Ploeg, J.; Pollard, L.; Swindle, J.; Lee, H.J.; Williams, A.; Markle-Reid, M.; McAiney, C. Women’s Caregiving Experience of Older Persons Living with Alzheimer Disease and Related Dementias and Multiple Chronic Conditions: Using Wuest’s Theory. Sage Open Nurs. 2020, 6, 2377960820974816. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Yelton, B.; Ezeanya, V.; Kannaley, K.; Friedman, D.B. Review of the Content and Quality of Mobile Applications About Alzheimer’s Disease and Related Dementias. J. Appl. Gerontol. 2018, 39, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Pooley, J.; Lee, M.; Shankle, W.R. Understanding Memory Impairment with Memory Models and Hierarchical Bayesian Analysis. J. Math. Psychol. 2011, 55, 47–56. [Google Scholar] [CrossRef]
- Couret, A.; Lapeyre-Mestre, M.; Gombault-Datzenko, E.; Renoux, A.; Villars, H.; Gardette, V. Healthcare Use Patterns Before Alzheimer’s Disease and Related Diseases Identification and Future Healthcare Trajectory. Int. J. Geriatr. Psychiatry 2022, 38, e5849. [Google Scholar] [CrossRef]
- Brown, C.S. Trends in Cause-Specific Mortality Among Persons With Alzheimer’s Disease in South Carolina: 2014 to 2019. Front. Aging Neurosci. 2024, 16, e1387082. [Google Scholar] [CrossRef]
- Langa, K.M.; Foster, N.L.; Larson, E.B. Mixed Dementia: Emerging Concepts and Therapeutic Implications. JAMA 2004, 292, 2901. [Google Scholar] [CrossRef]
- Cross, D.A. Access to Preferred Skilled Nursing Facilities: Transitional Care Pathways for Patients WithAlzheimer’s Disease and Related Dementias. Health Serv. Res. 2023, 59, e14263. [Google Scholar] [CrossRef]
- Shepard, V.; Snih, S.A.; Burke, R.; Downer, B.; Kuo, Y.; Malagaris, I.; Raji, M. Characteristics Associated with Mexican-American Hospice Use: Retrospective Cohort Study Using the Hispanic Established Population for the Epidemiologic Study of the Elderly (H-Epese). Am. J. Hosp. Palliat. Med. 2022, 40, 480–491. [Google Scholar] [CrossRef]
- Lott, S.A. Digital Health Technologies for Alzheimer’s Disease and Related Dementias: Initial Results from a Landscape Analysis and Community Collaborative Effort. J. Prev. Alzheimer’s Dis. 2024, 11, 1480–1489. [Google Scholar] [CrossRef]
- Tosun, D.; Rosen, H.J.; Miller, B.L.; Weiner, M.W.; Schuff, N. MRI Patterns of Atrophy and Hypoperfusion Associations Across Brain Regions in Frontotemporal Dementia. Neuroimage 2012, 59, 2098–2109. [Google Scholar] [CrossRef] [PubMed]
- Mina, C.B.M.; Ana-Marija, V.T.; Olga, Ž.V.; Milana, O.S.; Sanja, P.S.; Sanja, B.S. Kleptomania, a Symptom of Depression or Frontotemporal Dementia? World J. Biol. Pharm. Health Sci. 2021, 6, 29–33. [Google Scholar] [CrossRef]
- Ting, H.S.; Yashodhara, B.M.; Yousuf, U.A.M.; Dar, B.A.; Abas, A.L. A Case of Frontotemporal Dementia. Int. J. Case Rep. Images 2013, 4, 179. [Google Scholar] [CrossRef]
- Bessing, Y.F. Mixed Dementia Alzheimer and Lewy Body with Congenital Diseases: Case Study. World J. Adv. Res. Rev. 2023, 20, 137–140. [Google Scholar] [CrossRef]
- Foster, E.R.; Campbell, M.C.; Burack, M.A.; Hartlein, J.; Flores, H.; Cairns, N.J.; Hershey, T.; Perlmutter, J.S. Amyloid Imaging of Lewy Body-associated Disorders. Mov. Disord. 2010, 25, 2516–2523. [Google Scholar] [CrossRef]
- Al-faham, Z.; Zein, R.; Wong, C.-Y.O. 18f-FDG PET Assessment of Lewy Body Dementia with Cerebellar Diaschisis. J. Nucl. Med. Technol. 2014, 42, 306–307. [Google Scholar] [CrossRef]
- Huang, J.; Li, C.; Shang, H. Astrocytes in Neurodegeneration: Inspiration from Genetics. Front. Neurosci. 2022, 16, e882316. [Google Scholar] [CrossRef]
- Mavroudis, I.; Petridis, F.; Chatzikonstantinou, S.; Mckenn, J.; Karantali, E.; Kazis, D. The Role of Astrocytes in Astrocytes Alzheimer’s Disease. Ann. Acad. Rom. Sci. Ser. Biol. Sci. 2020, 9, 65–79. [Google Scholar] [CrossRef]
- Vicente-Acosta, A.; Giménez-Cassina, A.; Díaz-Nido, J.; Loría, F. The Smoothened Agonist SAG Reduces Mitochondrial Dysfunction and Neurotoxicity of Frataxin-Deficient Astrocytes. J. Neuroinflamm. 2022, 19, 93. [Google Scholar] [CrossRef]
- Peteri, U.-K.; Niukkanen, M.; Castrén, M. Astrocytes in Neuropathologies Affecting the Frontal Cortex. Front. Cell. Neurosci. 2019, 13, 44. [Google Scholar] [CrossRef]
- Early, A.N.; Weekman, E.M.; Lee, T.; Wilcock, D.M. Contribution of Astrocytic MMP9 toward Progression of VCID Pathology. Alzheimer’s Dement. 2022, 18 (Suppl. S3), e065982. [Google Scholar] [CrossRef]
- Sompol, P. Targeting Reactive Astrocytes in Vascular Dementia: Investigation of Neuronal-Astrocyte-Vascular Interactions. J. Exp. Neurosci. 2024, 19, 26331055241255332. [Google Scholar] [CrossRef] [PubMed]
- Altay, M.F.; Liu, A.K.L.; Holton, J.L.; Parkkinen, L.; Lashuel, H.A. Prominent Astrocytic Alpha-Synuclein Pathology with Unique Post-Translational Modification Signatures Unveiled across Lewy Body Disorders. Acta Neuropathol. Commun. 2022, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Meares, G.P.; Benveniste, E.N. Inflammation and the Pathophysiology of Astrocytes in Neurodegenerative Diseases. In Neuroinflammation and Neurodegeneration; Springer: New York, NY, USA, 2014; pp. 61–80. [Google Scholar] [CrossRef]
- Monterey, M.; Wei, H.; Wu, X.; Wu, J.Q. The Many Faces of Astrocytes in Alzheimer’s Disease. Front. Neurol. 2021, 12, 619626. [Google Scholar] [CrossRef]
- Bi, F.; Huang, C.; Tong, J.; Qiu, G.; Huang, B.; Wu, Q.; Li, F.; Xu, Z.; Bowser, R.; Xia, X.-G.; et al. Reactive astrocytes secrete lcn2 to promote neuron death. Proc. Natl. Acad. Sci. USA 2013, 110, 4069–4074. [Google Scholar] [CrossRef]
- Pérez-Sala, D.; Pajares, M.A. Appraising the Role of Astrocytes as Suppliers of Neuronal Glutathione Precursors. Int. J. Mol. Sci. 2023, 24, 8059. [Google Scholar] [CrossRef]
- Barbierato, M.; Borri, M.; Facci, L.; Zusso, M.; Skaper, S.D.; Giusti, P. Expression and Differential Responsiveness of Central Nervous System Glial Cell Populations to the Acute Phase Protein Serum Amyloid A. Sci. Rep. 2017, 7, 12158. [Google Scholar] [CrossRef]
- Yasuno, F.; Kosaka, J.; Ota, M.; Higuchi, M.; Ito, H.; Fujimura, Y.; Nozaki, S.; Takahashi, S.; Mizukami, K.; Asada, T.; et al. Increased binding of peripheral benzodiazepine receptor in mild cognitive impairment–dementia converters measured by positron emission tomography with [11C]DAA1106. Psychiatry Res. 2012, 203, 67–74. [Google Scholar] [CrossRef]
- Hamelin, L.; Lagarde, J.; Dorothée, G.; Leroy, C.; Labit, M.; Comley, R.A.; de Souza, L.C.; Corne, H.; Dauphinot, L.; Bertoux, M.; et al. Early and protective microglial activation in Alzheimer’s disease: A prospective study using18F-DPA-714 PET imaging. Brain 2016, 139 Pt 4, 1252–1264. [Google Scholar] [CrossRef]
- Carter, S.F.; Herholz, K.; Rosa-Neto, P.; Pellerin, L.; Nordberg, A.; Zimmer, E.R. Astrocyte Biomarkers in Alzheimer’s Disease. Trends Mol. Med. 2019, 25, 77–95. [Google Scholar] [CrossRef]
- Dean, D.C.; Sojkova, J.; Hurley, S.; Kecskemeti, S.; Okonkwo, O.; Bendlin, B.B.; Theisen, F.; Johnson, S.C.; Alexander, A.L.; Gallagher, C.L. Alterations of Myelin Content in Parkinson’s Disease: A Cross-Sectional Neuroimaging Study. PLoS ONE 2016, 11, e0163774. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Rogers, J. Inflammation in Alzheimer Disease—A Brief Review of the Basic Science and Clinical Literature. Cold Spring Harb. Perspect. Med. 2011, 2, a006346. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Kim, W.-K.; Wellman, L.L.; Sanford, L.D.; Guo, M.-L. Short-Term Sleep Fragmentation Dysregulates Autophagy in a Brain Region-Specific Manner. Life 2021, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Krupp, S.; Hubbard, I.; Tam, O.; Hammell, G.M.; Dubnau, J. TDP-43 pathology in Drosophila induces glial-cell type specific toxicity that can be ameliorated by knock-down of SF2/SRSF1. PLoS Genet. 2023, 19, e1010973. [Google Scholar] [CrossRef]
- Wahl, D.; Grant, R.A.; LaRocca, T.J. The reverse transcriptase inhibitor 3TC modulates hippocampal transcriptome signatures of inflammation in tauopathy model mice. Exp. Gerontol. 2024, 192, 112458. [Google Scholar] [CrossRef]
- Wahl, D.; Smith, M.E.; McEntee, C.M.; Cavalier, A.N.; Osburn, S.C.; Burke, S.D.; Grant, R.A.; Nerguizian, D.; Lark, D.S.; Link, C.D.; et al. The reverse transcriptase inhibitor 3TC protects against age-related cognitive dysfunction. Aging Cell 2023, 22, e13798. [Google Scholar] [CrossRef]
- Ochoa, E.; Ramirez, P.; Gonzalez, E.; De Mange, J.; Ray, W.J.; Bieniek, K.F.; Frost, B. Pathogenic tau–induced transposable element–derived dsRNA drives neuroinflammation. Sci. Adv. 2023, 9, eabq5423. [Google Scholar] [CrossRef]
- Kempuraj, D.; Khan, M.M.; Thangavel, R.; Xiong, Z.; Yang, E.; Zaheer, A. Glia Maturation Factor Induces Interleukin-33 Release from Astrocytes: Implications for Neurodegenerative Diseases. J. Neuroimmune Pharmacol. 2013, 8, 643–650. [Google Scholar] [CrossRef]
- Thomas, E.O.; Zuniga, G.; Sun, W.; Frost, B. Awakening the dark side: Retrotransposon activation in neurodegenerative disorders. Curr. Opin. Neurobiol. 2020, 61, 65–72. [Google Scholar] [CrossRef]
- Torre, C.D.; Zambello, R.; Cacciavillani, M.; Campagnolo, M.; Berno, T.; Salvalaggio, A.; De March, E.; Barilà, G.; Lico, A.; Lucchetta, M.; et al. Lenalidomide long-term neurotoxicity: Clinical and neurophysiologic prospective study. Neurology 2016, 87, 1161–1166. [Google Scholar] [CrossRef]
- de Medicina, I.P.N.M.E.; Vespero, F.H. Epigenetic Regulation of Gene Expression in the Development of Neurodegenerative Diseases: A Narrative Review. Int. J. Med. Sci. Clin. Res. Stud. 2023, 3, 2385–2393. [Google Scholar] [CrossRef]
- Neal, M.; Richardson, J.R. Epigenetic regulation of astrocyte function in neuroinflammation and neurodegeneration. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, N.; Delekate, A.; Plescher, M.; Schmitt, F.; Krauss, S.; Blank, N.; Halle, A.; Petzold, G.C. Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer’s disease model. EMBO Mol. Med. 2019, 11, e9665. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.L.; Pasini, S.; Lambert, W.S.; D’Alessandro, K.B.; Yao, V.; Risner, M.L.; Calkins, D.J. Redistribution of metabolic resources through astrocyte networks mitigates neurodegenerative stress. Proc. Natl. Acad. Sci. USA 2020, 117, 18810–18821. [Google Scholar] [CrossRef]
- Martorana, F.; Foti, M.; Virtuoso, A.; Gaglio, D.; Aprea, F.; Latronico, T.; Rossano, R.; Riccio, P.; Papa, M.; Alberghina, L.; et al. Differential Modulation of NF-κB in Neurons and Astrocytes Underlies Neuroprotection and Antigliosis Activity of Natural Antioxidant Molecules. Oxidative Med. Cell. Longev. 2019, 2019, 8056904. [Google Scholar] [CrossRef]
- Duran, R.C.-D.; Wang, C.-Y.; Zheng, H.; Deneen, B.; Wu, J.Q. Brain Region-Specific Gene Signatures Revealed by Distinct Astrocyte Subpopulations Unveil Links to Glioma and Neurodegenerative Diseases. ENEURO 2019, 6, ENEURO.0288-18.2019. [Google Scholar] [CrossRef]
- Diaz-Castro, B.; Gangwani, M.R.; Yu, X.; Coppola, G.; Khakh, B.S. Astrocyte molecular signatures in Huntington’s disease. Sci. Transl. Med. 2019, 11, eaaw8546. [Google Scholar] [CrossRef]
- Reid, J.K.; Kuipers, H.F. She Doesn’t Even Go Here: The Role of Inflammatory Astrocytes in CNS Disorders. Front. Cell. Neurosci. 2021, 15, 704884. [Google Scholar] [CrossRef]
- Smith, H.L.; Freeman, O.J.; Butcher, A.J.; Holmqvist, S.; Humoud, I.; Schätzl, T.; Hughes, D.T.; Verity, N.C.; Swinden, D.P.; Hayes, J.; et al. Astrocyte Unfolded Protein Response Induces a Specific Reactivity State that Causes Non-Cell-Autonomous Neuronal Degeneration. Neuron 2020, 105, 855–866.e5. [Google Scholar] [CrossRef]
- Hartmann, K.; Sepulveda-Falla, D.; Rose, I.V.L.; Madore, C.; Muth, C.; Matschke, J.; Butovsky, O.; Liddelow, S.; Glatzel, M.; Krasemann, S. Complement 3+-astrocytes are highly abundant in prion diseases, but their abolishment led to an accelerated disease course and early dysregulation of microglia. Acta Neuropathol. Commun. 2019, 7, 83. [Google Scholar] [CrossRef]
- Vallee, K.-A.J.; Fields, J.A. Caloric Restriction Mimetic 2-Deoxyglucose Reduces Inflammatory Signaling in Human Astrocytes: Implications for Therapeutic Strategies Targeting Neurodegenerative Diseases. Brain Sci. 2022, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Sibon, O.C.M.; Dijkers, P.F. Inhibition of NF-κB in astrocytes is sufficient to delay neurodegeneration induced by proteotoxicity in neurons. J. Neuroinflamm. 2018, 15, 261. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.P.; Koledova, V.V.; Schneider, K.; Sambandan, T.G.; Grayson, A.; Zeidman, G.; Artamonova, A.; Sambanthamurthi, R.; Fairus, S.; Sinskey, A.J.; et al. Palm Fruit Bioactives modulate human astrocyte activity in vitro altering the cytokine secretome reducing levels of TNFα, RANTES and IP-10. Sci. Rep. 2018, 8, 16423. [Google Scholar] [CrossRef] [PubMed]
- Alami, N.O.; Schurr, C.; Heuvel, F.O.; Tang, L.; Li, Q.; Tasdogan, A.; Kimbara, A.; Nettekoven, M.; Ottaviani, G.; Raposo, C.; et al. NF-κB activation in astrocytes drives a stage-specific beneficial neuroimmunological response in ALS. EMBO J. 2018, 37, e98697. [Google Scholar] [CrossRef]
- Oksanen, M.; Lehtonen, S.; Jaronen, M.; Goldsteins, G.; Hämäläinen, R.H.; Koistinaho, J. Astrocyte alterations in neurodegenerative pathologies and their modeling in human induced pluripotent stem cell platforms. Cell. Mol. Life Sci. 2019, 76, 2739–2760. [Google Scholar] [CrossRef]
- Leng, K.; Rooney, B.; McCarthy, F.; Xia, W.; Rose, I.V.L.; Bax, S.; Chin, M.; Fathi, S.; Herrington, K.A.; Leonetti, M.; et al. mTOR activation induces endolysosomal remodeling and nonclassical secretion of IL-32 via exosomes in inflammatory reactive astrocytes. J. Neuroinflamm. 2024, 21, 198. [Google Scholar] [CrossRef]
- Miyazaki, I.; Asanuma, M. Neuron-Astrocyte Interactions in Parkinson’s Disease. Cells 2020, 9, 2623. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef]
- Yin, S.; Ma, X.-Y.; Sun, Y.-F.; Yin, Y.-Q.; Long, Y.; Zhao, C.-L.; Ma, J.-W.; Li, S.; Hu, Y.; Li, M.-T.; et al. RGS5 augments astrocyte activation and facilitates neuroinflammation via TNF signaling. J. Neuroinflamm. 2023, 20, 203. [Google Scholar] [CrossRef]
- Limbad, C.; Oron, T.R.; Alimirah, F.; Davalos, A.R.; Tracy, T.E.; Gan, L.; Desprez, P.-Y.; Campisi, J. Astrocyte senescence promotes glutamate toxicity in cortical neurons. PLoS ONE 2020, 15, e0227887. [Google Scholar] [CrossRef]
- Xingi, E.; Koutsoudaki, P.N.; Thanou, I.; Phan, M.-S.; Margariti, M.; Scheller, A.; Tinevez, J.-Y.; Kirchhoff, F.; Thomaidou, D. LPS-Induced Systemic Inflammation Affects the Dynamic Interactions of Astrocytes and Microglia with the Vasculature of the Mouse Brain Cortex. Cells 2023, 12, 1418. [Google Scholar] [CrossRef] [PubMed]
- Perriot, S.; Mathias, A.; Perriard, G.; Canales, M.; Jonkmans, N.; Merienne, N.; Meunier, C.; El Kassar, L.; Perrier, A.L.; Laplaud, D.-A.; et al. Human Induced Pluripotent Stem Cell-Derived Astrocytes Are Differentially Activated by Multiple Sclerosis-Associated Cytokines. Stem Cell Rep. 2018, 11, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Taha, D.M.; Clarke, B.E.; Hall, C.E.; Tyzack, G.E.; Ziff, O.J.; Greensmith, L.; Kalmar, B.; Ahmed, M.; Alam, A.; Thelin, E.P.; et al. Astrocytes display cell autonomous and diverse early reactive states in familial amyotrophic lateral sclerosis. Brain 2022, 145, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.E.; Taha, D.M.; Tyzack, G.E.; Patani, R. Regionally encoded functional heterogeneity of astrocytes in health and disease: A perspective. Glia 2020, 69, 20–27. [Google Scholar] [CrossRef]
- Michal, I.; Guy, S.S.; Michel, R. Astrocytes in Pathogenesis of Multiple Sclerosis and Potential Translation Into Clinic. Glia Health Dis. 2020, 131. Available online: https://www.intechopen.com/chapters/68613 (accessed on 3 April 2025).
- Tremblay, M.-E.; Cookson, M.R.; Civiero, L. Glial phagocytic clearance in Parkinson’s disease. Mol. Neurodegener. 2019, 14, 16. [Google Scholar] [CrossRef]
- Shigetomi, E.; Saito, K.; Sano, F.; Koizumi, S. Aberrant Calcium Signals in Reactive Astrocytes: A Key Process in Neurological Disorders. Int. J. Mol. Sci. 2019, 20, 996. [Google Scholar] [CrossRef]
- Pathak, D.; Sriram, K. Neuron-astrocyte omnidirectional signaling in neurological health and disease. Front. Mol. Neurosci. 2023, 16, 1169320. [Google Scholar] [CrossRef]
- Huang, X.; Su, Y.; Wang, N.; Li, H.; Li, Z.; Yin, G.; Chen, H.; Niu, J.; Yi, C. Astroglial Connexins in Neurodegenerative Diseases. Front. Mol. Neurosci. 2021, 14, 657514. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.W.; Kim, K.-T. Region-Specific Characteristics of Astrocytes and Microglia: A Possible Involvement in Aging and Diseases. Cells 2022, 11, 1902. [Google Scholar] [CrossRef]
- Santiago-Balmaseda, A.; Aguirre-Orozco, A.; Valenzuela-Arzeta, I.E.; Villegas-Rojas, M.M.; Pérez-Segura, I.; Jiménez-Barrios, N.; Hurtado-Robles, E.; Rodríguez-Hernández, L.D.; Rivera-German, E.R.; Guerra-Crespo, M.; et al. Neurodegenerative Diseases: Unraveling the Heterogeneity of Astrocytes. Cells 2024, 13, 921. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, J.; Zheng, J.; Qin, S. Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis. 2019, 10, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Ries, M.; Sastre, M. Mechanisms of Aβ Clearance and Degradation by Glial Cells. Front. Aging Neurosci. 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Hu, J.; Zhao, N.; Wang, J.; Wang, N.; Cirrito, J.R.; Kanekiyo, T.; Holtzman, D.M.; Bu, G. Astrocytic LRP1 Mediates Brain Aβ Clearance and Impacts Amyloid Deposition. J. Neurosci. 2017, 37, 4023–4031. [Google Scholar] [CrossRef]
- Wahrle, S.E.; Jiang, H.; Parsadanian, M.; Legleiter, J.; Han, X.; Fryer, J.D.; Kowalewski, T.; Holtzman, D.M. ABCA1 Is Required for Normal Central Nervous System ApoE Levels and for Lipidation of Astrocyte-secreted apoE. J. Biol. Chem. 2004, 279, 40987–40993. [Google Scholar] [CrossRef]
- Pomilio, C.; Pavia, P.; Gorojod, R.M.; Vinuesa, A.; Alaimo, A.; Galvan, V.; Kotler, M.L.; Beauquis, J.; Saravia, F. Glial alterations from early to late stages in a model of Alzheimer’s disease: Evidence of autophagy involvement in Aβ internalization. Hippocampus 2015, 26, 194–210. [Google Scholar] [CrossRef]
- Prasad, H.; Rao, R. Amyloid clearance defect in ApoE4 astrocytes is reversed by epigenetic correction of endosomal pH. Proc. Natl. Acad. Sci. USA 2018, 115, E6640–E6649. [Google Scholar] [CrossRef]
- Gotoh, M.; Miyamoto, Y.; Ikeshima-Kataoka, H. Astrocytic Neuroimmunological Roles Interacting with Microglial Cells in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 1599. [Google Scholar] [CrossRef]
- Mulica, P.; Grünewald, A.; Pereira, S.L. Astrocyte-Neuron Metabolic Crosstalk in Neurodegeneration: A Mitochondrial Perspective. Front. Endocrinol. 2021, 12, 668517. [Google Scholar] [CrossRef]
- Suga, M.; Kondo, T.; Inoue, H. Modeling Neurological Disorders with Human Pluripotent Stem Cell-Derived Astrocytes. Int. J. Mol. Sci. 2019, 20, 3862. [Google Scholar] [CrossRef]
- Leventoux, N.; Morimoto, S.; Imaizumi, K.; Sato, Y.; Takahashi, S.; Mashima, K.; Ishikawa, M.; Sonn, I.; Kondo, T.; Watanabe, H.; et al. Human Astrocytes Model Derived from Induced Pluripotent Stem Cells. Cells 2020, 9, 2680. [Google Scholar] [CrossRef] [PubMed]
- Ceyzériat, K.; Ben Haim, L.; Denizot, A.; Pommier, D.; Matos, M.; Guillemaud, O.; Palomares, M.-A.; Abjean, L.; Petit, F.; Gipchtein, P.; et al. Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwon, S.-H.; Kam, T.-I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e7. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Kam, T.-I.; Lee, S.; Park, H.; Oh, Y.; Kwon, S.-H.; Song, J.-J.; Kim, D.; Kim, H.; Jhaldiyal, A.; et al. Blocking microglial activation of reactive astrocytes is neuroprotective in models of Alzheimer’s disease. Acta Neuropathol. Commun. 2021, 9, 78. [Google Scholar] [CrossRef]
- Bai, Y.; Su, X.; Piao, L.; Jin, Z.; Jin, R. Involvement of Astrocytes and microRNA Dysregulation in Neurodegenerative Diseases: From Pathogenesis to Therapeutic Potential. Front. Mol. Neurosci. 2021, 14, 556215. [Google Scholar] [CrossRef]
- Boal, A.M.; Risner, M.L.; Cooper, M.L.; Wareham, L.K.; Calkins, D.J. Astrocyte Networks as Therapeutic Targets in Glaucomatous Neurodegeneration. Cells 2021, 10, 1368. [Google Scholar] [CrossRef]
- Matias, I.; Morgado, J.; Gomes, F.C.A. Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front. Aging Neurosci. 2019, 11, 59. [Google Scholar] [CrossRef]
- Edison, P. Astroglial activation: Current concepts and future directions. Alzheimer’s Dement. 2024, 20, 3034–3053. [Google Scholar] [CrossRef]
- Jacquet, A.d.R. Preparation and Co-Culture of iPSC-Derived Dopaminergic Neurons and Astrocytes. Curr. Protoc. Cell Biol. 2019, 85, e98. [Google Scholar] [CrossRef]
- Tassoni, A.; Farkhondeh, V.; Itoh, Y.; Itoh, N.; Sofroniew, M.V.; Voskuhl, R.R. The astrocyte transcriptome in EAE optic neuritis shows complement activation and reveals a sex difference in astrocytic C3 expression. Sci. Rep. 2019, 9, 10010. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Wang, E.; Wang, M.; Sin, W.C.; Brennand, K.J.; Schadt, E.; Naus, C.C.; Buxbaum, J.; Zhang, B. GJA1 (connexin43) is a key regulator of Alzheimer’s disease pathogenesis. Acta Neuropathol. Commun. 2018, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Chen, F.; Song, N.; Xie, J. In vivo Direct Conversion of Astrocytes to Neurons Maybe a Potential Alternative Strategy for Neurodegenerative Diseases. Front. Aging Neurosci. 2021, 13, 689276. [Google Scholar] [CrossRef] [PubMed]
- Leng, K.; Rose, I.V.; Kim, H.; Xia, W.; Romero-Fernández, W.; Rooney, B.; Koontz, M.; Li, E.; Ao, Y.; Wang, S.; et al. CRISPRi Screens in Human Astrocytes Elucidate Regulators of Distinct Inflammatory Reactive States. Nat. Neurosci. 2021, 25, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Prinz, M.; Priller, J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014, 15, 300–312. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Wickstead, E.; Karim, H.A.; Manuel, R.E.; Biggs, C.S.; Getting, S.J.; McArthur, S. Reversal of Β-Amyloid Induced Microglial Toxicityin Vitroby Activation of Fpr2/3. Oxidative Med. Cell. Longev. 2020, 2020, 2139192. [Google Scholar] [CrossRef]
- Xu, J.-J.; Guo, S.; Xue, R.; Xiao, L.; Kou, J.-N.; Liu, Y.-Q.; Han, J.-Y.; Fu, J.-J.; Wei, N. Adalimumab ameliorates memory impairments and neuroinflammation in chronic cerebral hypoperfusion rats. Aging 2021, 13, 14001–14014. [Google Scholar] [CrossRef]
- Chmielarz, M.; Sobieszczańska, B.; Teisseyre, A.; Wawrzyńska, M.; Bożemska, E.; Środa-Pomianek, K. Palmitic Acid Modulates Microglial Cell Response to Metabolic Endotoxemia in an In Vitro Study. Nutrients 2023, 15, 3463. [Google Scholar] [CrossRef]
- Lee, S.-H.; Meilandt, W.J.; Xie, L.; Gandham, V.D.; Ngu, H.; Barck, K.H.; Rezzonico, M.G.; Imperio, J.; Lalehzadeh, G.; Huntley, M.A.; et al. Trem2 restrains the enhancement of tau accumulation and neurodegeneration by β-amyloid pathology. Neuron 2021, 109, 1283–1301.e6. [Google Scholar] [CrossRef]
- Hsieh, C.-F.; Liu, C.-K.; Lee, C.-T.; Yu, L.-E.; Wang, J.-Y. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci. Rep. 2019, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Hara, H.; Haniu, H.; Matsuda, Y. The Combined Effects of Lysophospholipids against Lipopolysaccharide-induced Inflammation and Oxidative Stress in Microglial Cells. J. Oleo Sci. 2021, 70, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Kloske, C.M.; Dugan, A.J.; Weekman, E.M.; Winder, Z.; Patel, E.; Nelson, P.T.; Fardo, D.W.; Wilcock, D.M. Inflammatory Pathways Are Impaired in Alzheimer Disease and Differentially Associated With Apolipoprotein E Status. J. Neuropathol. Exp. Neurol. 2021, 80, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Palavicini, J.P.; Wang, J.; Gonzalez, N.S.; He, S.; Dustin, E.; Zou, C.; Ding, L.; Bhattacharjee, A.; Van Skike, C.E.; et al. Adult-onset CNS myelin sulfatide deficiency is sufficient to cause Alzheimer’s disease-like neuroinflammation and cognitive impairment. Mol. Neurodegener. 2021, 16, 64. [Google Scholar] [CrossRef]
- Mondal, M.; Solomon, S.; Sun, J.; Sampathkumar, N.K.; Carre, I.; Cotel, M.C.; Mehta, P.R.; Rajendran, L.; Vernon, A.C.; Fang, F.; et al. Olanzapine, Risperidone and Clozapine Prescribing Is Associated With Increased Risk for Alzheimer’s Disease Reflecting Antipsychotic-Specific Effects on Microglial Phagocytosis. medRxiv 2023. [Google Scholar] [CrossRef]
- Lokesh, M.; Bandaru, L.J.M.; Rajanna, A.; Dhayal, V.S.; Challa, S. M1 polarization induction by lead and amyloid peptides in microglial cells: Implications for neurodegeneration process. Environ. Toxicol. 2024, 39, 4267–4277. [Google Scholar] [CrossRef]
- Wu, H.; Bao, H.; Liu, C.; Zhang, Q.; Huang, A.; Quan, M.; Li, C.; Xiong, Y.; Chen, G.; Hou, L. Extracellular Nucleosomes Accelerate Microglial Inflammation via C-Type Lectin Receptor 2D and Toll-Like Receptor 9 in mPFC of Mice With Chronic Stress. Front. Immunol. 2022, 13, 854202. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, Y.; Wang, H.; Alam, A.; Kang, S.S.; Ahn, E.H.; Liu, X.; Jia, J.; Ye, K. Gut inflammation triggers C/EBPβ/δ-secretase-dependent gut-to-brain propagation of Aβ and Tau fibrils in Alzheimer’s disease. EMBO J. 2021, 40, e106320. [Google Scholar] [CrossRef]
- Kohli, M.A.; John-Williams, K.; Rajbhandary, R.; Naj, A.; Whitehead, P.; Hamilton, K.; Carney, R.M.; Wright, C.; Crocco, E.; Gwirtzman, H.E.; et al. Repeat expansions in the C9ORF72 gene contribute to Alzheimer’s disease in Caucasians. Neurobiol. Aging 2013, 34, 1519.e5–1519.e12. [Google Scholar] [CrossRef]
- Shi, Y.; Holtzman, D.M. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat. Rev. Immunol. 2018, 18, 759–772. [Google Scholar] [CrossRef]
- Piers, T.M.; East, E.; Villegas-Llerena, C.; Sevastou, I.G.; Matarin, M.; Hardy, J.; Pocock, J.M. Soluble Fibrinogen Triggers Non-cell Autonomous ER Stress-Mediated Microglial-Induced Neurotoxicity. Front. Cell. Neurosci. 2018, 12, 404. [Google Scholar] [CrossRef] [PubMed]
- Essadek, S.; Bouchab, H.; El Kebbaj, R.; Gondcaille, C.; El Kamouni, S.; Savary, S.; Vamecq, J.; Essamadi, A.; Cherkaoui-Malki, M.; Nasser, B.; et al. Effects of a Short-Term Lipopolysaccharides Challenge on Mouse Brain and Liver Peroxisomal Antioxidant and β-oxidative Functions: Protective Action of Argan Oil. Pharmaceuticals 2022, 15, 465. [Google Scholar] [CrossRef] [PubMed]
- Figarella, K.; Uzcategui, N.L.; Mogk, S.; Wild, K.; Fallier-Becker, P.; Neher, J.J.; Duszenko, M. Morphological changes, nitric oxide production, and phagocytosis are triggered in vitro in microglia by bloodstream forms of Trypanosoma brucei. Sci. Rep. 2018, 8, 15002. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, Y.; Kassai, M.; Yoneyama, H. The Impact of Intestinal Microbiota and Toll-like Receptor 2 Signaling on α-Synuclein Pathology in Nontransgenic Mice Injected with α-Synuclein Preformed Fibrils. Microorganisms 2024, 12, 106. [Google Scholar] [CrossRef]
- Li, Y.; Niu, M.; Zhao, A.; Kang, W.; Chen, Z.; Luo, N.; Zhou, L.; Zhu, X.; Lu, L.; Liu, J. CXCL12 is involved in α-synuclein-triggered neuroinflammation of Parkinson’s disease. J. Neuroinflamm. 2019, 16, 263. [Google Scholar] [CrossRef]
- Raas, Q.; Saih, F.-E.; Gondcaille, C.; Trompier, D.; Hamon, Y.; Leoni, V.; Caccia, C.; Nasser, B.; Jadot, M.; Ménétrier, F.; et al. A microglial cell model for acyl-CoA oxidase 1 deficiency. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 567–576. [Google Scholar] [CrossRef]
- Abubaker, A.A.; Vara, D.; Visconte, C.; Eggleston, I.; Torti, M.; Canobbio, I.; Pula, G. Amyloid Peptide β1-42 Induces Integrin αIIbβ3 Activation, Platelet Adhesion, and Thrombus Formation in a NADPH Oxidase-Dependent Manner. Oxidative Med. Cell. Longev. 2019, 2019, 1050476. [Google Scholar] [CrossRef]
- Valcarcel-Ares, M.N.; Tucsek, Z.; Kiss, T.; Giles, C.B.; Tarantini, S.; Yabluchanskiy, A.; Balasubramanian, P.; Gautam, T.; Galvan, V.; Ballabh, P.; et al. Obesity in Aging Exacerbates Neuroinflammation, Dysregulating Synaptic Function-Related Genes and Altering Eicosanoid Synthesis in the Mouse Hippocampus: Potential Role in Impaired Synaptic Plasticity and Cognitive Decline. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 74, 290–298. [Google Scholar] [CrossRef]
- Yang, L.; Wang, W.; Chen, J.; Wang, N.; Zheng, G. A comparative study of resveratrol and resveratrol-functional selenium nanoparticles: Inhibiting amyloid β aggregation and reactive oxygen species formation properties. J. Biomed. Mater. Res. A 2018, 106, 3034–3041. [Google Scholar] [CrossRef]
- Kaneshwaran, K.; Olah, M.; Tasaki, S.; Yu, L.; Bradshaw, E.M.; Schneider, J.A.; Buchman, A.S.; Bennett, D.A.; De Jager, P.L.; Lim, A.S.P. Sleep fragmentation, microglial aging, and cognitive impairment in adults with and without Alzheimer’s dementia. Sci. Adv. 2019, 5, eaax7331. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Cai, X.; Weng, Y.; Wang, Y.; Shen, Q.; Shi, X. Milk Fat Globule-Epidermal Growth Factor-Factor 8 Reverses Lipopolysaccharide-Induced Microglial Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, 2601394. [Google Scholar] [CrossRef] [PubMed]
- Piers, T.M.; Cosker, K.; Mallach, A.; Johnson, G.T.; Guerreiro, R.; Hardy, J.; Pocock, J.M. A locked immunometabolic switch underlies TREM2 R47H loss of function in human iPSC-derived microglia. FASEB J. 2019, 34, 2436–2450. [Google Scholar] [CrossRef] [PubMed]
- Asthana, S.; Pandey, S.K.; Gautam, A.S.; Singh, R.K. MK2 inhibitor PF-3644022 shows protective effect in mouse microglial N9 cell line induced with cigarette smoke extract. Chem. Biol. Drug Des. 2024, 104, e14592. [Google Scholar] [CrossRef] [PubMed]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef]
- Ulland, T.K.; Song, W.M.; Huang, S.C.-C.; Ulrich, J.D.; Sergushichev, A.; Beatty, W.L.; Loboda, A.A.; Zhou, Y.; Cairns, N.J.; Kambal, A.; et al. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell 2017, 170, 649–663.e13. [Google Scholar] [CrossRef]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial Dysfunction and Defective β-Amyloid Clearance Pathways in Aging Alzheimer’s Disease Mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581.E9. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Enomoto, S.; Ohgidani, M.; Sagata, N.; Inamine, S.; Kato, T.A. Preliminary analysis of hippocampus synaptic apoptosis and microglial phagocytosis induced by severe restraint stress. Neuropsychopharmacol. Rep. 2022, 43, 120–125. [Google Scholar] [CrossRef]
- Howe, A.-M.; Burke, S.; O’reilly, M.E.; McGillicuddy, F.C.; Costello, D.A. Palmitic Acid and Oleic Acid Differently Modulate TLR2-Mediated Inflammatory Responses in Microglia and Macrophages. Mol. Neurobiol. 2022, 59, 2348–2362. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J.; Cairns, N.J.; Grossman, M.; McMillan, C.T.; Lee, E.B.; Van Deerlin, V.M.; Lee, V.M.-Y.; Trojanowski, J.Q. Frontotemporal lobar degeneration: Defining phenotypic diversity through personalized medicine. Acta Neuropathol. 2014, 129, 469–491. [Google Scholar] [CrossRef] [PubMed]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Perea, J.R.; Llorens-Martín, M.; Ávila, J.; Bolós, M. The Role of Microglia in the Spread of Tau: Relevance for Tauopathies. Front. Cell. Neurosci. 2018, 12, 172. [Google Scholar] [CrossRef]
- Striet, W.J.; Xue, Q.-S.; Tischer, J.; Bechmann, I. Microglial pathology. Acta Neuropathol. Commun. 2014, 2, 142. [Google Scholar] [CrossRef]
- Kurioka, T.; Mogi, S.; Yamashita, T. Decreasing auditory input induces neurogenesis impairment in the hippocampus. Sci. Rep. 2021, 11, 423. [Google Scholar] [CrossRef]
- Malko, P.; Mortadza, S.A.S.; McWilliam, J.; Jiang, L.-H. TRPM2 Channel in Microglia as a New Player in Neuroinflammation Associated With a Spectrum of Central Nervous System Pathologies. Front. Pharmacol. 2019, 10, 239. [Google Scholar] [CrossRef]
- Minami, Y.; Sonoda, N.; Hayashida, E.; Makimura, H.; Ide, M.; Ikeda, N.; Ohgidani, M.; Kato, T.A.; Seki, Y.; Maeda, Y.; et al. p66Shc Signaling Mediates Diabetes-Related Cognitive Decline. Sci. Rep. 2018, 8, 3213. [Google Scholar] [CrossRef]
- Ide, M.; Sonoda, N.; Inoue, T.; Kimura, S.; Minami, Y.; Makimura, H.; Hayashida, E.; Hyodo, F.; Yamato, M.; Takayanagi, R.; et al. The dipeptidyl peptidase-4 inhibitor, linagliptin, improves cognitive impairment in streptozotocin-induced diabetic mice by inhibiting oxidative stress and microglial activation. PLoS ONE 2020, 15, e0228750. [Google Scholar] [CrossRef]
- Trares, K.; Gào, X.; Perna, L.; Rujescu, D.; Stocker, H.; Möllers, T.; Beyreuther, K.; Brenner, H.; Schöttker, B. Associations of urinary 8-iso-prostaglandin F2α levels with all-cause dementia, Alzheimer’s disease, and vascular dementia incidence: Results from a prospective cohort study. Alzheimer’s Dement. 2020, 16, 804–813. [Google Scholar] [CrossRef]
- Hu, B.; Duan, S.; Wang, Z.; Li, X.; Zhou, Y.; Zhang, X.; Zhang, Y.-W.; Xu, H.; Zheng, H. Insights Into the Role of CSF1R in the Central Nervous System and Neurological Disorders. Front. Aging Neurosci. 2021, 13, 789834. [Google Scholar] [CrossRef]
- Afridi, R.; Lee, W.-H.; Suk, K. Microglia Gone Awry: Linking Immunometabolism to Neurodegeneration. Front. Cell. Neurosci. 2020, 14, 246. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Rosenzweig, N.; Kleemann, K.L.; Zhang, X.; Brandão, W.; Margeta, M.A.; Schroeder, C.; Sivanathan, K.N.; Silveira, S.; Gauthier, C.; et al. APOE4 impairs the microglial response in Alzheimer’s disease by inducing TGFβ-mediated checkpoints. Nat. Immunol. 2023, 24, 1839–1853. [Google Scholar] [CrossRef] [PubMed]
- Malpetti, M.; Jones, P.S.; Tsvetanov, K.A.; Rittman, T.; van Swieten, J.C.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R.; Graff, C.; et al. Apathy in presymptomatic genetic frontotemporal dementia predicts cognitive decline and is driven by structural brain changes. Alzheimer’s Dement. 2020, 17, 969–983. [Google Scholar] [CrossRef]
- Ferrari-Souza, J.P.; Lussier, F.Z.; Leffa, D.T.; Therriault, J.; Tissot, C.; Bellaver, B.; Ferreira, P.C.L.; Malpetti, M.; Wang, Y.-T.; Povala, G.; et al. APOE ε4 associates with microglial activation independently of Aβ plaques and tau tangles. Sci. Adv. 2023, 9, eade1474. [Google Scholar] [CrossRef]
- Weigand, A.J.; Thomas, K.R.; Bangen, K.J.; Eglit, G.M.; Delano-Wood, L.; Gilbert, P.E.; Brickman, A.M.; Bondi, M.W.; Alzheimer’s Disease Neuroimaging Initiative. APOE interacts with tau PET to influence memory independently of amyloid PET in older adults without dementia. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2021, 17, 61–69. [Google Scholar] [CrossRef]
- Giacomucci, G.; Mazzeo, S.; Bagnoli, S.; Casini, M.; Padiglioni, S.; Polito, C.; Berti, V.; Balestrini, J.; Ferrari, C.; Lombardi, G.; et al. Matching Clinical Diagnosis and Amyloid Biomarkers in Alzheimer’s Disease and Frontotemporal Dementia. J. Pers. Med. 2021, 11, 47. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, G.; Kou, L.; Yin, S.; Han, C.; Hu, J.; Wan, F.; Sun, Y.; Wu, J.; Li, Y.; et al. Reactive microglia enhance the transmission of exosomal α-synuclein via toll-like receptor 2. Brain 2021, 144, 2024–2037. [Google Scholar] [CrossRef]
- Iannucci, J.; Sen, A.; Grammas, P. Isoform-Specific Effects of Apolipoprotein E on Markers of Inflammation and Toxicity in Brain Glia and Neuronal Cells In Vitro. Curr. Issues Mol. Biol. 2021, 43, 215–225. [Google Scholar] [CrossRef]
- Panitch, R.; Hu, J.; Chung, J.; Zhu, C.; Meng, G.; Xia, W.; Bennett, D.A.; Lunetta, K.L.; Ikezu, T.; Au, R.; et al. Integrative brain transcriptome analysis links complement component 4 and HSPA2 to the APOE ε2 protective effect in Alzheimer disease. Mol. Psychiatry 2021, 26, 6054–6064. [Google Scholar] [CrossRef] [PubMed]
- Saab, A.S.; Nave, K.-A. Myelin dynamics: Protecting and shaping neuronal functions. Curr. Opin. Neurobiol. 2017, 47, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Ettle, B.; Schlachetzki, J.C.M.; Winkler, J. Oligodendroglia and Myelin in Neurodegenerative Diseases: More Than Just Bystanders? Mol. Neurobiol. 2016, 53, 3046–3062. [Google Scholar] [CrossRef] [PubMed]
- Rauchmann, B.S.; Brendel, M.; Franzmeier, N.; Trappmann, L.; Zaganjori, M.; Morenas-Rodriguez, E.; Guersel, S.; Burow, L.; Kurz, C.; Haeckert, J.; et al. Microglial Activation and Connectivity in Alzheimer Disease and Aging. Ann. Neurol. 2022, 92, 768–781. [Google Scholar] [CrossRef]
- Taha, M.; Eldemerdash, O.M.; Elshaffei, I.M.; Yousef, E.M.; Soliman, A.S.; Senousy, M.A. Apigenin Attenuates Hippocampal Microglial Activation and Restores Cognitive Function in Methotrexate-Treated Rats: Targeting the miR-15a/ROCK-1/ERK1/2 Pathway. Mol. Neurobiol. 2023, 60, 3770–3787. [Google Scholar] [CrossRef]
- Raas, Q.; Gondcaille, C.; Hamon, Y.; Leoni, V.; Caccia, C.; Ménétrier, F.; Lizard, G.; Trompier, D.; Savary, S. CRISPR/Cas9-mediated knockout of Abcd1 and Abcd2 genes in BV-2 cells: Novel microglial models for X-linked Adrenoleukodystrophy. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 704–714. [Google Scholar] [CrossRef]
- Essadek, S.; Gondcaille, C.; Savary, S.; Samadi, M.; Vamecq, J.; Lizard, G.; El Kebbaj, R.; Latruffe, N.; Benani, A.; Nasser, B.; et al. Two Argan Oil Phytosterols, Schottenol and Spinasterol, Attenuate Oxidative Stress and Restore LPS-Dysregulated Peroxisomal Functions in Acox1−/− and Wild-Type BV-2 Microglial Cells. Antioxidants 2023, 12, 168. [Google Scholar] [CrossRef]
- Wan, Y.; Gao, W.; Zhou, K.; Liu, X.; Jiang, W.; Xue, R.; Wu, W. Role of IGF-1 in neuroinflammation and cognition deficits induced by sleep deprivation. Neurosci. Lett. 2022, 776, 136575. [Google Scholar] [CrossRef]
- Xin, W.; Mironova, Y.A.; Shen, H.; Marino, R.A.; Waisman, A.; Lamers, W.H.; Bergles, D.E.; Bonci, A. Oligodendrocytes Support Neuronal Glutamatergic Transmission via Expression of Glutamine Synthetase. Cell Rep. 2019, 27, 2262–2271.e5. [Google Scholar] [CrossRef]
- Meyer, N.; Richter, N.; Fan, Z.; Siemonsmeier, G.; Pivneva, T.; Jordan, P.; Steinhäuser, C.; Semtner, M.; Nolte, C.; Kettenmann, H. Oligodendrocytes in the Mouse Corpus Callosum Maintain Axonal Function by Delivery of Glucose. Cell Rep. 2018, 22, 2383–2394. [Google Scholar] [CrossRef]
- Saito, E.; Miller, J.; Harari, O.; Cruchaga, C.; Mihindukulasuriya, K.; Kauwe, J.; Bikman, B. Alzheimer’s Disease Alters Oligodendrocytic Glycolytic and Ketolytic Gene Expression. FASEB J. 2021, 35, 1474–1486. [Google Scholar] [CrossRef]
- Hughes, A.N.; Appel, B. Oligodendrocytes express synaptic proteins that modulate myelin sheath formation. Nature communications 2019, 10, 4125. [Google Scholar] [CrossRef] [PubMed]
- Panlilio, J.M.; Hammar, K.M.; Aluru, N.; Hahn, M.E. Developmental exposure to domoic acid targets reticulospinal neurons and leads to aberrant myelination in the spinal cord. Sci. Rep. 2023, 13, 2587. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Macrì, R.; Bava, I.; Gliozzi, M.; Musolino, V.; Nucera, S.; Carresi, C.; Scicchitano, M.; Bosco, F.; Scarano, F.; et al. Myelin Disturbances Produced by Sub-Toxic Concentration of Heavy Metals: The Role of Oligodendrocyte Dysfunction. Int. J. Mol. Sci. 2019, 20, 4554. [Google Scholar] [CrossRef]
- Moore, S.; Meschkat, M.; Ruhwedel, T.; Trevisiol, A.; Tzvetanova, I.D.; Battefeld, A.; Kusch, K.; Kole, M.H.P.; Strenzke, N.; Möbius, W.; et al. A role of oligodendrocytes in information processing. Nat. Commun. 2020, 11, 5497. [Google Scholar] [CrossRef]
- Depp, C.M.; Nave, K.; Lab, K.N. Ageing-Associated Myelin Dysfunction Drives Amyloid Deposition in Mouse Models of Alzheimer’s Disease. Alzheimer’s Dement. 2022, 18, e061183. [Google Scholar] [CrossRef]
- Green, L.A.; Gallant, R.M.; Brandt, J.P.; Nichols, E.L.; Smith, C.J. A Subset of Oligodendrocyte Lineage Cells Interact With the Developing Dorsal Root Entry Zone During Its Genesis. Front. Cell. Neurosci. 2022, 16, 893629. [Google Scholar] [CrossRef]
- Miedema, S.S.M.; Mol, M.O.; Koopmans, F.T.W.; Hondius, D.C.; van Nierop, P.; Menden, K.; Mestdagh, C.F.d.V.; van Rooij, J.; Ganz, A.B.; Paliukhovich, I.; et al. Distinct cell type-specific protein signatures in GRN and MAPT genetic subtypes of frontotemporal dementia. Acta Neuropathol. Commun. 2022, 10, 100. [Google Scholar] [CrossRef]
- Yoon, Y.-S.; Ahn, W.J.; Ricarte, D.; Ortiz, D.; Shin, C.Y.; Lee, S.-J.; Lee, H.-J. Alpha-Synuclein Inclusion Formation in Human Oligodendrocytes. Biomol. Ther. 2021, 29, 83–89. [Google Scholar] [CrossRef]
- Xin, W.; Kaneko, M.; Roth, R.H.; Zhang, A.; Nocera, S.; Ding, J.B.; Stryker, M.P.; Chan, J.R. Oligodendrocytes and myelin limit neuronal plasticity in visual cortex. Nature 2024, 633, 856–863. [Google Scholar] [CrossRef]
- Zhao, C.; Dong, C.; Frah, M.; Deng, Y.; Marie, C.; Zhang, F.; Xu, L.; Ma, Z.; Dong, X.; Lin, Y.; et al. Dual Requirement of CHD8 for Chromatin Landscape Establishment and Histone Methyltransferase Recruitment to Promote CNS Myelination and Repair. Dev. Cell 2018, 45, 753–768.e8. [Google Scholar] [CrossRef] [PubMed]
- Frühbeis, C.; Kuo-Elsner, W.P.; Müller, C.; Barth, K.; Peris, L.; Tenzer, S.; Möbius, W.; Werner, H.B.; Nave, K.-A.; Fröhlich, D.; et al. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol. 2020, 18, e3000621. [Google Scholar] [CrossRef] [PubMed]
- Mitew, S.; Gobius, I.; Fenlon, L.R.; McDougall, S.J.; Hawkes, D.; Xing, Y.L.; Bujalka, H.; Gundlach, A.L.; Richards, L.J.; Kilpatrick, T.J.; et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun. 2018, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Hinman, J.D.; Ngo, K.J.; Kim, D.; Chen, C.; Abraham, C.R.; Ghanbari, M.; Ikram, M.A.; Kushner, S.A.; Kawaguchi, R.; Coppola, G.; et al. miR-142-3p regulates cortical oligodendrocyte gene co-expression networks associated with tauopathy. Hum. Mol. Genet. 2021, 30, 103–118. [Google Scholar] [CrossRef]
- Müller, K.; Schnatz, A.; Schillner, M.; Woertge, S.; Müller, C.; von Graevenitz, I.; Waisman, A.; van Minnen, J.; Vogelaar, C.F. A predominantly glial origin of axonal ribosomes after nerve injury. Glia 2018, 66, 1591–1610. [Google Scholar] [CrossRef]
- Chen, K.; Cambi, F.; Kozai, T.D. Pro-myelinating clemastine administration improves recording performance of chronically implanted microelectrodes and nearby neuronal health. Biomaterials 2023, 301, 122210. [Google Scholar] [CrossRef]
- Eykens, C.; Rossaert, E.; Duqué, S.; Rué, L.; Bento-Abreu, A.; Hersmus, N.; Lenaerts, A.; Kerstens, A.; Corthout, N.; Munck, S.; et al. AAV9-mediated gene delivery of MCT1 to oligodendrocytes does not provide a therapeutic benefit in a mouse model of ALS. Mol. Ther.-Methods Clin. Dev. 2021, 20, 508–519. [Google Scholar] [CrossRef]
- Banerjee, S.; Ghoshal, S.; Girardet, C.; DeMars, K.M.; Yang, C.; Niehoff, M.L.; Nguyen, A.D.; Jayanth, P.; Mersman, B.A.; Xu, F.; et al. Adropin Expression Correlates with Age-Related Neuropathologies in the Human Brain and Improves Neuroinflammation and Cognitive Function in Aging Mice. 2021. Available online: https://pdfs.semanticscholar.org/5a6a/99a4e3bba9a697186be8582c7eeed7fc9dfa.pdf (accessed on 20 December 2024). [CrossRef]
- Forbes, T.A.; Goldstein, E.Z.; Dupree, J.L.; Jablonska, B.; Scafidi, J.; Adams, K.L.; Imamura, Y.; Hashimoto-Torii, K.; Gallo, V. Environmental enrichment ameliorates perinatal brain injury and promotes functional white matter recovery. Nat. Commun. 2020, 11, 964. [Google Scholar] [CrossRef]
- Mukherjee, C.; Kling, T.; Russo, B.; Miebach, K.; Kess, E.; Schifferer, M.; Pedro, L.D.; Weikert, U.; Fard, M.K.; Kannaiyan, N.; et al. Oligodendrocytes Provide Antioxidant Defense Function for Neurons by Secreting Ferritin Heavy Chain. Cell Metab. 2020, 32, 259–272.e10. [Google Scholar] [CrossRef]
- Marton, R.M.; Miura, Y.; Sloan, S.A.; Li, Q.; Revah, O.; Levy, R.J.; Huguenard, J.R.; Pașca, S.P. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat. Neurosci. 2019, 22, 484–491. [Google Scholar] [CrossRef]
- Butt, T.H.; Tobiume, M.; Re, D.B.; Kariya, S. Physical Exercise Counteracts Aging-Associated White Matter Demyelination Causing Cognitive Decline. Aging Dis. 2024, 15, 2136–2148. [Google Scholar] [CrossRef] [PubMed]
- Tiane, A.; Schepers, M.; Rombaut, B.; Hupperts, R.; Prickaerts, J.; Hellings, N.; van den Hove, D.; Vanmierlo, T. From OPC to Oligodendrocyte: An Epigenetic Journey. Cells 2019, 8, 1236. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Dobrowolski, M.; Sockanathan, S. GDE2 expression in oligodendroglia regulates the pace of oligodendrocyte maturation. Dev. Dyn. 2020, 250, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Moloney, R.A.; Pavy, C.L.; Kahl, R.G.S.; Palliser, H.K.; Hirst, J.J.; Shaw, J.C. Dual isolation of primary neurons and oligodendrocytes from guinea pig frontal cortex. Front. Cell. Neurosci. 2024, 17, 1298685. [Google Scholar] [CrossRef]
- Psenicka, M.W.; Smith, B.C.; Tinkey, R.A.; Williams, J.L. Connecting Neuroinflammation and Neurodegeneration in Multiple Sclerosis: Are Oligodendrocyte Precursor Cells a Nexus of Disease? Front. Cell. Neurosci. 2021, 15, 654284. [Google Scholar] [CrossRef]
- Chia, R.; Sabir, M.S.; Bandres-Ciga, S.; Saez-Atienzar, S.; Reynolds, R.H.; Gustavsson, E.; Walton, R.L.; Ahmed, S.; Viollet, C.; Ding, J.; et al. Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture. Nat. Genet. 2021, 53, 294–303. [Google Scholar] [CrossRef]
- Jiang, C.; Hopfner, F.; Katsikoudi, A.; Hein, R.; Catli, C.; Evetts, S.; Huang, Y.; Wang, H.; Ryder, J.W.; Kuhlenbaeumer, G.; et al. Serum neuronal exosomes predict and differentiate Parkinson’s disease from atypical parkinsonism. J. Neurol. Neurosurg. Psychiatry 2020, 91, 720–729. [Google Scholar] [CrossRef]
- Do, J.; McKinney, C.; Sharma, P.; Sidransky, E. Glucocerebrosidase and its relevance to Parkinson disease. Mol. Neurodegener. 2019, 14, 36. [Google Scholar] [CrossRef]
- Kuo, S.-H.; Tasset, I.; Cheng, M.M.; Diaz, A.; Pan, M.-K.; Lieberman, O.J.; Hutten, S.J.; Alcalay, R.N.; Kim, S.; Ximénez-Embún, P.; et al. Mutant glucocerebrosidase impairs α-synuclein degradation by blockade of chaperone-mediated autophagy. Sci. Adv. 2022, 8, eabm6393. [Google Scholar] [CrossRef]
- Johnson, M.E.; Stecher, B.; Labrie, V.; Brundin, L.; Brundin, P. Triggers, Facilitators, and Aggravators: Redefining Parkinson’s Disease Pathogenesis. Trends Neurosci. 2019, 42, 4–13. [Google Scholar] [CrossRef]
- Madureira, M.; Connor-Robson, N.; Wade-Martins, R. “LRRK2: Autophagy and Lysosomal Activity”. Front. Neurosci. 2020, 14, 498. [Google Scholar] [CrossRef] [PubMed]
- Farfel-Becker, T.; Roney, J.C.; Cheng, X.-T.; Li, S.; Cuddy, S.R.; Sheng, Z.-H. Neuronal Soma-Derived Degradative Lysosomes Are Continuously Delivered to Distal Axons to Maintain Local Degradation Capacity. Cell Rep. 2019, 28, 51–64.e4. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Romero, A.; Montpeyó, M.; Martinez-Vicente, M. The Emerging Role of the Lysosome in Parkinson’s Disease. Cells 2020, 9, 2399. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef]
- Zhang, Y.; Chao, F.-L.; Zhou, C.-N.; Jiang, L.; Zhang, L.; Liang, X.; Tang, J.; Tang, Y. Atrophy of lacunosum moleculare layer is important for learning and memory in APP/PS1 transgenic mice. NeuroReport 2021, 32, 596–602. [Google Scholar] [CrossRef]
- Chen, S.; Chang, Y.; Li, L.; Acosta, D.; Li, Y.; Guo, Q.; Wang, C.; Turkes, E.; Morrison, C.; Julian, D.; et al. Spatially resolved transcriptomics reveals genes associated with the vulnerability of middle temporal gyrus in Alzheimer’s disease. Acta Neuropathol Commun. 2022, 10, 188. [Google Scholar] [CrossRef]
- Muraoka, S.; Jedrychowski, M.P.; Iwahara, N.; Abdullah, M.; Onos, K.D.; Keezer, K.J.; Hu, J.; Ikezu, S.; Howell, G.R.; Gygi, S.P.; et al. Enrichment of Neurodegenerative Microglia Signature in Brain-Derived Extracellular Vesicles Isolated from Alzheimer’s Disease Mouse Models. J. Proteome Res. 2021, 20, 1733–1743. [Google Scholar] [CrossRef]
- Gushchina, S.; Pryce, G.; Yip, P.K.; Wu, D.; Pallier, P.; Giovannoni, G.; Baker, D.; Bo, X. Increased expression of colony-stimulating factor-1 in mouse spinal cord with experimental autoimmune encephalomyelitis correlates with microglial activation and neuronal loss. Glia 2018, 66, 2108–2125. [Google Scholar] [CrossRef]
- Wang, G.; Jin, S.; Liu, J.; Li, X.; Dai, P.; Wang, Y.; Hou, S.X. A neuron-immune circuit regulates neurodegeneration in the hindbrain and spinal cord of Arf1-ablated mice. Natl. Sci. Rev. 2023, 12, 222. [Google Scholar] [CrossRef]
- Liu, Y.; Given, K.S.; Harlow, D.E.; Matschulat, A.M.; Macklin, W.B.; Bennett, J.L.; Owens, G.P. Myelin-specific multiple sclerosis antibodies cause complement-dependent oligodendrocyte loss and demyelination. Acta Neuropathol. Commun. 2017, 5, 25. [Google Scholar] [CrossRef]
- Linden, J.R.; Ma, Y.; Zhao, B.; Harris, J.M.; Rumah, K.R.; Schaeren-Wiemers, N.; Vartanian, T. Clostridium perfringens Epsilon Toxin Causes Selective Death of Mature Oligodendrocytes and Central Nervous System Demyelination. mBio 2015, 6, e02513-14. [Google Scholar] [CrossRef] [PubMed]
- Gingele, S.; Merkel, L.; Prajeeth, C.K.; Kronenberg, J.; von Hoevel, F.F.; Skripuletz, T.; Gudi, V.; Stangel, M. Polarized microglia do not influence oligodendrocyte lineage cells via astrocytes. Int. J. Dev. Neurosci. 2019, 77, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.I.; Meisingset, T.W.; Kotter, M.R.; Sonnewald, U. Metabolic Aspects of Neuron-Oligodendrocyte-Astrocyte Interactions. Front. Endocrinol. 2013, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Verrier, J.D.; Jackson, T.C.; Gillespie, D.G.; Janesko-Feldman, K.; Bansal, R.; Goebbels, S.; Nave, K.-A.; Kochanek, P.M.; Jackson, E.K. Role of CNPase in the oligodendrocytic extracellular 2′,3′-cAMP-adenosine pathway. Glia 2013, 61, 1595–1606. [Google Scholar] [CrossRef]
- Jansen, A.H.; van Hal, M.; Kelder, I.C.O.D.; Meier, R.T.; de Ruiter, A.; Schut, M.H.; Smith, D.L.; Grit, C.; Brouwer, N.; Kamphuis, W.; et al. Frequency of nuclear mutant huntingtin inclusion formation in neurons and glia is cell-type-specific. Glia 2016, 65, 50–61. [Google Scholar] [CrossRef]
- Stevenson, T.J.; Murray, H.C.; Turner, C.; Faull, R.L.M.; Dieriks, B.V.; Curtis, M.A. α-synuclein inclusions are abundant in non-neuronal cells in the anterior olfactory nucleus of the Parkinson’s disease olfactory bulb. Sci. Rep. 2020, 10, 6682. [Google Scholar] [CrossRef]
- Zhan, Z.; Wu, Y.; Liu, Z.; Quan, Y.; Li, D.; Huang, Y.; Yang, S.; Wu, K.; Huang, L.; Yu, M. Reduced Dendritic Spines in the Visual Cortex Contralateral to the Optic Nerve Crush Eye in Adult Mice. Investig. Opthalmol. Vis. Sci. 2020, 61, 55. [Google Scholar] [CrossRef]
- Joshi, A.U.; Minhas, P.S.; Liddelow, S.A.; Haileselassie, B.; Andreasson, K.I.; Dorn, G.W., II; Mochly-Rosen, D. Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat. Neurosci. 2019, 22, 1635–1648. [Google Scholar] [CrossRef]
- Alshaebi, F.; Sciortino, A.; Kayed, R. The Role of Glial Cell Senescence in Alzheimer’s Disease. J. Neurochem. 2025, 169, e70051. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell biology 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Bai, J.; Fu, H.; Qiu, H.; Guo, R. The functions and potential roles of extracellular vesicle noncoding RNAs in gynecological malignancies. Cell Death Discov. 2021, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, T.; Doss C, G.P. Non-coding RNAs in human health and disease: Potential function as biomarkers and therapeutic targets. Funct. Integr. Genom. 2023, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V.; Bartel, B.; Bartel, D.P.; Burge, C.B.; Carrington, J.C.; Chen, X.; Dreyfuss, G.; Eddy, S.R.; Griffiths-Jones, S.; Marshall, M.; et al. A uniform system for microRNA annotation. RNA 2003, 9, 277–279. [Google Scholar] [CrossRef]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef]
- Gil-Jaramillo, N.; Aristizábal-Pachón, A.F.; Aleman, M.A.L.; Gómez, V.G.; Hurtado, H.D.E.; Pinto, L.C.G.; Camacho, J.S.J.; Rojas-Cruz, A.F.; González-Giraldo, Y.; Pinzón, A.; et al. Competing endogenous RNAs in human astrocytes: Crosstalk and interacting networks in response to lipotoxicity. Front. Neurosci. 2023, 17, 1195840. [Google Scholar] [CrossRef]
- Prada, I.; Gabrielli, M.; Turola, E.; Iorio, A.; D’arrigo, G.; Parolisi, R.; De Luca, M.; Pacifici, M.; Bastoni, M.; Lombardi, M.; et al. Glia-to-neuron transfer of miRNAs via extracellular vesicles: A new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol. 2018, 135, 529–550. [Google Scholar] [CrossRef]
- Phatnani, H.; Maniatis, T. Astrocytes in neurodegenerative disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a020628. [Google Scholar] [CrossRef]
- Meraz, R. Medication Nonadherence or Self-care? Understanding the Medication Decision-Making Process and Experiences of Older Adults With Heart Failure. J. Cardiovasc. Nurs. 2019, 35, 26–34. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Z.; Fan, Y.; Zhang, J.; Chen, K.; Gao, L.; Zeng, H.; Cao, J.; Wang, C. MicroRNA-9 Inhibits NLRP3 Inflammasome Activation in Human Atherosclerosis Inflammation Cell Models through the JAK1/STAT Signaling Pathway. Cell. Physiol. Biochem. 2017, 41, 1555–1571. [Google Scholar] [CrossRef]
- Wongrakpanich, A.; Mudunkotuwa, I.A.; Geary, S.M.; Morris, A.S.; Mapuskar, K.A.; Spitz, D.R.; Grassian, V.H.; Salem, A.K. Size-dependent cytotoxicity of copper oxide nanoparticles in lung epithelial cells. Environ. Sci. Nano 2016, 3, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ding, J.; Yang, J.; Guo, X.; Zheng, Y. MicroRNA Roles in the Nuclear Factor Kappa B Signaling Pathway in Cancer. Front. Immunol. 2018, 9, 546. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Reed, K.M.; Carter, A.; Cheng, Y.; Roodsari, S.K.; Pineda, D.M.; Wellman, L.L.; Sanford, L.D.; Guo, M.-L. Sleep-Disturbance-Induced Microglial Activation Involves CRH-Mediated Galectin 3 and Autophagy Dysregulation. Cells 2022, 12, 160. [Google Scholar] [CrossRef]
- Fan, X.-M.; Luo, Y.; Cao, Y.-M.; Xiong, T.-W.; Song, S.; Liu, J.; Fan, Q.-Y. Chronic Manganese Administration with Longer Intervals Between Injections Produced Neurotoxicity and Hepatotoxicity in Rats. Neurochem. Res. 2020, 45, 1941–1952. [Google Scholar] [CrossRef]
- Conrad, A.T.; Dittel, B.N. Taming of macrophage and microglial cell activation by microRNA-124. Cell Res. 2011, 21, 213–216. [Google Scholar] [CrossRef]
- Coolen, M.; Katz, S.; Bally-Cuif, L. miR-9: A versatile regulator of neurogenesis. Front. Cell. Neurosci. 2013, 7, 220. [Google Scholar] [CrossRef]
- Delgado-García, L.M.; Ojalvo-Sanz, A.C.; Nakamura, T.K.E.; Martín-López, E.; Porcionatto, M.; Lopez-Mascaraque, L. Dissecting reactive astrocyte responses: Lineage tracing and morphology-based clustering. Biol. Res. 2024, 57, 54. [Google Scholar] [CrossRef]
- Fonken, L.K.; Frank, M.G.; Gaudet, A.D.; Maier, S.F. Stress and aging act through common mechanisms to elicit neuroinflammatory priming. Brain, Behav. Immun. 2018, 73, 133–148. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K.; Watkins, L.R.; Nelson, R.J.; Popovich, P.G. MicroRNAs: Roles in Regulating Neuroinflammation. Neurosci. 2017, 24, 221–245. [Google Scholar] [CrossRef]
- Pogue, A.; Cui, J.; Li, Y.; Zhao, Y.; Culicchia, F.; Lukiw, W. Micro RNA-125b (miRNA-125b) function in astrogliosis and glial cell proliferation. Neurosci. Lett. 2010, 476, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Song, S.; Pan, X. Knockdown of miR-155 protects microglia against LPS-induced inflammatory injury via targeting RACK1: A novel research for intracranial infection. J. Inflamm. 2017, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Sun, J.; Dai, T.; Xu, Q.; Ding, Y. miR-29c-5p knockdown reduces inflammation and blood–brain barrier disruption by upregulating LRP6. Open Med. 2022, 17, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Zurolo, E.; Prabowo, A.; Fluiter, K.; Spliet, W.G.M.; van Rijen, P.C.; Gorter, J.A.; Aronica, E. MicroRNA-146a: A Key Regulator of Astrocyte-Mediated Inflammatory Response. PLoS ONE 2012, 7, e44789. [Google Scholar] [CrossRef]
- Chipman, L.B.; Pasquinelli, A.E. miRNA Targeting: Growing beyond the Seed. Trends Genet. 2019, 35, 215–222. [Google Scholar] [CrossRef]
- Iyer, M.; Kantarci, H.; Cooper, M.H.; Ambiel, N.; Novak, S.W.; Andrade, L.R.; Lam, M.; Jones, G.; Münch, A.E.; Yu, X.; et al. Oligodendrocyte calcium signaling promotes actin-dependent myelin sheath extension. Nat. Commun. 2024, 15, 265. [Google Scholar] [CrossRef]
- Kieran, N.W.; Suresh, R.; Dorion, M.-F.; MacDonald, A.; Blain, M.; Wen, D.; Fuh, S.-C.; Ryan, F.; Diaz, R.J.; Stratton, J.A.; et al. MicroRNA-210 regulates the metabolic and inflammatory status of primary human astrocytes. J. Neuroinflamm. 2022, 19, 10. [Google Scholar] [CrossRef]
- Chen, Y.; Schlotterer, A.; Kurowski, L.; Li, L.; Dannehl, M.; Hammes, H.-P.; Lin, J. miRNA-124 Prevents Rat Diabetic Retinopathy by Inhibiting the Microglial Inflammatory Response. Int. J. Mol. Sci. 2023, 24, 2291. [Google Scholar] [CrossRef]
- Yang, R.; Yang, B.; Liu, W.; Tan, C.; Chen, H.; Wang, X. Emerging role of non-coding RNAs in neuroinflammation mediated by microglia and astrocytes. J. Neuroinflamm. 2023, 20, 173. [Google Scholar] [CrossRef]
- Zeng, M.; Zhang, T.; Lin, Y.; Lin, Y.; Wu, Z. The Common LncRNAs of Neuroinflammation-Related Diseases. Mol. Pharmacol. 2022, 103, 113–131. [Google Scholar] [CrossRef]
- Sharma, S.; Houfani, A.A.; Foster, L.J. Pivotal functions and impact of long con-coding RNAs on cellular processes and genome integrity. J. Biomed. Sci. 2024, 31, 52. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Chen, B.; Yao, X.; Lei, Y.; Ou, F.; Huang, F. Upregulation of the lncRNA MEG3 improves cognitive impairment, alleviates neuronal damage, and inhibits activation of astrocytes in hippocampus tissues in Alzheimer’s disease through inactivating the PI3K/Akt signaling pathway. J. Cell. Biochem. 2019, 120, 18053–18065. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Yu, Z.; Fang, X.; Liu, M.; Pu, Y.; Shao, Q.; Wang, D.; Zhao, X.; Huang, A.; Xiang, Z.; et al. Lnc RNA GAS 5 inhibits microglial M2 polarization and exacerbates demyelination. Embo Rep. 2017, 18, 1801–1816. [Google Scholar] [CrossRef] [PubMed]
- Takei, N.; Inamura, N.; Kawamura, M.; Namba, H.; Hara, K.; Yonezawa, K.; Nawa, H. Brain-Derived Neurotrophic Factor Induces Mammalian Target of Rapamycin-Dependent Local Activation of Translation Machinery and Protein Synthesis in Neuronal Dendrites. J. Neurosci. 2004, 24, 9760–9769. [Google Scholar] [CrossRef]
- Ni, X.; Su, Q.; Xia, W.; Zhang, Y.; Jia, K.; Su, Z.; Li, G. Knockdown lncRNA NEAT1 regulates the activation of microglia and reduces AKT signaling and neuronal apoptosis after cerebral ischemic reperfusion. Sci. Rep. 2020, 10, 19658. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, T.; Zhao, Z.; Wei, W.; Yang, X.; Wang, X.; Xin, W. Novel Insights into the Emerging Role of Neat1 and Its Effects Downstream in the Regulation of Inflammation. J. Inflamm. Res. 2022, 15, 557–571. [Google Scholar] [CrossRef]
- Zeng, C.; Hu, J.; Chen, F.; Huang, T.; Zhang, L. The Coordination of mTOR Signaling and Non-Coding RNA in Regulating Epileptic Neuroinflammation. Front. Immunol. 2022, 13, 924642. [Google Scholar] [CrossRef]
- Han, C.-L.; Ge, M.; Liu, Y.-P.; Zhao, X.-M.; Wang, K.-L.; Chen, N.; Meng, W.-J.; Hu, W.; Zhang, J.-G.; Li, L.; et al. LncRNA H19 contributes to hippocampal glial cell activation via JAK/STAT signaling in a rat model of temporal lobe epilepsy. J. Neuroinflamm. 2018, 15, 103. [Google Scholar] [CrossRef]
- Passamonti, L.; Tsvetanov, K.A.; Jones, P.S.; Bevan-Jones, W.R.; Arnold, R.; Borchert, R.J.; Mak, E.; Su, L.; O’brien, J.T.; Rowe, J.B. Neuroinflammation and Functional Connectivity in Alzheimer’s Disease: Interactive Influences on Cognitive Performance. J. Neurosci. 2019, 39, 7218–7226. [Google Scholar] [CrossRef]
- Griciuc, A.; Tanzi, R.E. The role of innate immune genes in Alzheimer’s disease. Curr. Opin. Neurol. 2021, 34, 228–236. [Google Scholar] [CrossRef]
- Tejera, D.; Mercan, D.; Sanchez-Caro, J.M.; Hanan, M.; Greenberg, D.; Soreq, H.; Latz, E.; Golenbock, D.; Heneka, M.T. Systemic inflammation impairs microglial Aβ clearance through NLRP 3 inflammasome. EMBO J. 2019, 38, e101064. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gong, J.; Li, S.; Wang, P.; Han, X.; Xu, C.; Luan, H.; Li, R.; Wen, B.; Wei, C. Bibliometric analysis of neuroinflammation and Alzheimer’s disease. Front. Aging Neurosci. 2024, 16, 1423139. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Jung, I.; Na, J.Y.; Lee, Y.; Lee, J.; Lee, J.S.; Lee, J.S. Pseudane-VII Regulates LPS-Induced Neuroinflammation in Brain Microglia Cells through the Inhibition of iNOS Expression. Molecules 2018, 23, 3196. [Google Scholar] [CrossRef] [PubMed]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.-E.; Vernoux, N.; Tremblay, M.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef]
- Tarczyluk-Wells, M.A.; Salzlechner, C.; Najafi, A.R.; Lim, M.J.; Smith, D.; Platt, F.M.; Williams, B.P.; Cooper, J.D. Combined Anti-inflammatory and Neuroprotective Treatments Have the Potential to Impact Disease Phenotypes in Cln3−/− Mice. Front. Neurol. 2019, 10, 963. [Google Scholar] [CrossRef]
- Frigerio, C.S.; Wolfs, L.; Fattorelli, N.; Thrupp, N.; Voytyuk, I.; Schmidt, I.; Mancuso, R.; Chen, W.-T.; Woodbury, M.E.; Srivastava, G.; et al. The Major Risk Factors for Alzheimer’s Disease: Age, Sex, and Genes Modulate the Microglia Response to Aβ Plaques. Cell Rep. 2019, 27, 1293–1306.e6. [Google Scholar] [CrossRef]
- Barroeta-Espar, I.; Weinstock, L.D.; Perez-Nievas, B.G.; Meltzer, A.C.; Chong, M.S.T.; Amaral, A.C.; Murray, M.E.; Moulder, K.L.; Morris, J.C.; Cairns, N.J.; et al. Distinct cytokine profiles in human brains resilient to Alzheimer’s pathology. Neurobiol. Dis. 2019, 121, 327–337. [Google Scholar] [CrossRef]
- Bradburn, S.; Murgatroyd, C.; Ray, N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res. Rev. 2019, 50, 1–8. [Google Scholar] [CrossRef]
- Cheng, Y.; Saville, L.; Gollen, B.; Veronesi, A.A.; Mohajerani, M.; Joseph, J.T.; Zovoilis, A. Increased Alu RNA processing in Alzheimer brains is linked to gene expression changes. Embo Rep. 2021, 22, e52255. [Google Scholar] [CrossRef]
- Muthukumarasamy, I.; Buel, S.M.; Hurley, J.M.; Dordick, J.S. NOX2 inhibition enables retention of the circadian clock in BV2 microglia and primary macrophages. Front. Immunol. 2023, 14, 1106515. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Gong, Z. The Beneficial Roles of SIRT1 in Neuroinflammation-Related Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 6782872. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Wang, Y.; Zhang, X.; Sun, X. Recent Studies on Protective Effects of Walnuts against Neuroinflammation. Nutrients 2022, 14, 4360. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Lee, S.J.; Pyo, J.-S. Effect of acetylcholinesterase inhibitors on post-stroke cognitive impairment and vascular dementia: A meta-analysis. PLoS ONE 2020, 15, e0227820. [Google Scholar] [CrossRef]
- Tan, Q.; Zhang, C.; Rao, X.; Wan, W.; Lin, W.; Huang, S.; Ying, J.; Lin, Y.; Hua, F. The interaction of lipocalin-2 and astrocytes in neuroinflammation: Mechanisms and therapeutic application. Front. Immunol. 2024, 15, 1358719. [Google Scholar] [CrossRef]
- Angelbello, A.J.; Chen, J.L.; Disney, M.D. Small molecule targeting of RNA structures in neurological disorders. Ann. N. Y. Acad. Sci. 2019, 1471, 57–71. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Spangenberg, E.; Severson, P.L.; Hohsfield, L.A.; Crapser, J.; Zhang, J.; Burton, E.A.; Zhang, Y.; Spevak, W.; Lin, J.; Phan, N.Y.; et al. Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat. Commun. 2019, 10, 3758. [Google Scholar] [CrossRef]
- Nasrabady, S.E.; Rizvi, B.; Goldman, J.E.; Brickman, A.M. White matter changes in Alzheimer’s disease: A focus on myelin and oligodendrocytes. Acta Neuropathol. Commun. 2018, 6, 22. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Wang, J.; Tian, T.; Li, X.; Zhang, Y. Noncoding RNAs Emerging as Drugs or Drug Targets: Their Chemical Modification, Bio-Conjugation and Intracellular Regulation. Molecules 2022, 27, 6717. [Google Scholar] [CrossRef] [PubMed]
- El Idrissi, F.; Gressier, B.; Devos, D.; Belarbi, K. A Computational Exploration of the Molecular Network Associated to Neuroinflammation in Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 630003. [Google Scholar] [CrossRef] [PubMed]
- Bruffaerts, R.; Gors, D.; Gallardo, A.B.; Vandenbulcke, M.; Van Damme, P.; Suetens, P.; van Swieten, J.C.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; et al. Hierarchical spectral clustering reveals brain size and shape changes in asymptomatic carriers of C9orf72. Brain Commun. 2022, 4, fcac182. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Deng, Y.; Duan, D.; Sun, S.; Ge, L.; Zhuo, Y.; Yuan, T.; Wu, P.; Wang, H.; Lu, M.; et al. Hyperthermia influences fate determination of neural stem cells with lncRNAs alterations in the early differentiation. PLoS ONE 2017, 12, e0171359. [Google Scholar] [CrossRef]
- Policarpo, R.; Sierksma, A.; De Strooper, B.; D’ydewalle, C. From Junk to Function: LncRNAs in CNS Health and Disease. Front. Mol. Neurosci. 2021, 14, 714768. [Google Scholar] [CrossRef]
- Ye, Z.; Yuan, T. Role and Therapeutic Potential of Non-Coding RNAs in Astrocytes During Neonatal Brain Injury. 2023. Available online: https://www.preprints.org/manuscript/202312.0927/download/final_file (accessed on 20 December 2024). [CrossRef]
- Qureshi, I.A.; Mehler, M.F. Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat. Rev. Neurosci. 2012, 13, 528–541. [Google Scholar] [CrossRef]
- Schirmer, L.; Velmeshev, D.; Holmqvist, S.; Kaufmann, M.; Werneburg, S.; Jung, D.; Vistnes, S.; Stockley, J.H.; Young, A.; Steindel, M.; et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 2019, 573, 75–82. [Google Scholar] [CrossRef]
- Taylor, D.H.; Chu, E.T.; Spektor, R.; Soloway, P.D. Long non-coding RNA regulation of reproduction and development. Mol. Reprod. Dev. 2015, 82, 932–956. [Google Scholar] [CrossRef]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef]
- Shi, Q.; Colodner, K.J.; Matousek, S.B.; Merry, K.; Hong, S.; Kenison, J.E.; Frost, J.L.; Le, K.X.; Li, S.; Dodart, J.-C.; et al. Complement C3-Deficient Mice Fail to Display Age-Related Hippocampal Decline. J. Neurosci. 2015, 35, 13029–13042. [Google Scholar] [CrossRef]
- Farina, C.; Aloisi, F.; Meinl, E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007, 28, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Lansita, J.A.; Mease, K.M.; Qiu, H.; Yednock, T.; Sankaranarayanan, S.; Kramer, S. Nonclinical Development of ANX005: A Humanized Anti-C1q Antibody for Treatment of Autoimmune and Neurodegenerative Diseases. Int. J. Toxicol. 2017, 36, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Costales, M.G.; Aikawa, H.; Li, Y.; Childs-Disney, J.L.; Abegg, D.; Hoch, D.G.; Velagapudi, S.P.; Nakai, Y.; Khan, T.; Wang, K.W.; et al. Small-molecule targeted recruitment of a nuclease to cleave an oncogenic RNA in a mouse model of metastatic cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 2406–2411. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered Anticancer Drugs Across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Zheng, X.; Song, L.; Cao, C.; Sun, S. Multiple roles of circular RNAs in prostate cancer: From the biological basis to potential clinical applications. Eur. J. Med. Res. 2025, 30, 140. [Google Scholar] [CrossRef]
- Heneka, M.T.; McManus, R.M.; Latz, E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018, 19, 610–621. [Google Scholar] [CrossRef]
- (Stămat), L.-R.B.; Dinescu, S.; Costache, M. Regulation of Inflammasome by microRNAs in Triple-Negative Breast Cancer: New Opportunities for Therapy. Int. J. Mol. Sci. 2023, 24, 3245. [Google Scholar] [CrossRef]
- Antoniou, D.; Stergiopoulos, A.; Politis, P.K. Recent advances in the involvement of long non-coding RNAs in neural stem cell biology and brain pathophysiology. Front. Physiol. 2014, 5, 155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).