Abstract
Müller glia exhibit a remarkable regenerative capacity in zebrafish through spontaneous reprogramming post-injury but remain limited in mammals. This review highlights the key mechanisms underlying Müller glia reprogramming, including gene regulatory networks, cytokine signaling, signal transduction pathways, epigenetic modifications, and transcriptional regulation. Cross-species analyses have uncovered conserved gene networks that suppress neurogenesis in mammals, while injury-induced transcriptional profiles reveal divergent regenerative strategies. Combinatorial approaches may enhance the reprogramming of mammalian Müller glia into functional neurons. Nevertheless, significant challenges remain, such as variability in the efficacy of direct reprogramming methods and the limited regeneration of cone photoreceptors, even in regenerative species. We conclude that targeting epigenetic barriers and species-specific regulatory pathways offers promising avenues for clinical translation in retinal disorders such as glaucoma and retinitis pigmentosa. Moving forward, research efforts should prioritize the functional integration of regenerated neurons and the development of standardized methodologies to accelerate therapeutic advancements.
1. Introduction
Müller glia (MG) are essential retinal glial cells that share functional similarities with astrocytes in the brain [1]. They play a crucial role in maintaining retinal homeostasis under both physiological and pathological conditions [2]. Notably, MG exhibit an intrinsic regenerative capacity, which is highly active in zebrafish, limited in chicks, and significantly restricted in mammals [3]. In zebrafish, retinal injury triggers MG to re-enter the cell cycle, divide asymmetrically, generate multipotent progenitor cells, and regenerate lost neurons [4]. And in chicks, injury-induced MG can produce a small number of inner retinal neurons [5]. Conversely, in mammals, MG primarily undergo reactive gliosis in response to injury—a process that limits neuronal regeneration [6,7]. In zebrafish and chick, the transition from quiescence to reactivity is essential for retinal regeneration, whereas in mice, a dedicated network suppresses neurogenic competence and restores quiescence. Understanding the molecular mechanisms underlying zebrafish MG regeneration offers valuable insights into potential strategies for enhancing the regenerative capacity of mammalian MG.
The structure and function of the vertebrate retina have been remarkably conserved during evolution, reflected in a common developmental program [8]. As in other parts of the CNS, gliogenesis in the retina occurs towards the end of development. Lineage-tracing studies have shown that retinal neurons and MG have a common developmental origin [9]. Interestingly, late-stage retinal progenitor cells that give rise to MG do not completely exit their progenitor state. Instead, they transition into glial cells while retaining certain progenitor-like characteristics [10]. This dual identity suggests an inherent potential for MG to regenerate retinal neurons under appropriate conditions, making them an attractive target for regenerative therapies.
A promising approach to harnessing MG regenerative potential is cell reprogramming, which involves the conversion of one cell type into another through targeted molecular manipulations. This process entails substantial changes in gene expression patterns, allowing cells to acquire new identities and functions. Reprogramming techniques are pivotal in understanding cellular differentiation mechanisms, creating disease models, and developing regenerative therapies [11].
Cell reprogramming can be broadly categorized into indirect and direct approaches. Indirect reprogramming follows a sequential process: induction of adult stem cell proliferation, dedifferentiation into pluripotent stem cells, and subsequent redifferentiation into specific cell types to achieve cellular transformation [5]. In contrast, direct reprogramming bypasses the pluripotent stage, enabling the direct conversion of adult somatic cells into other functional cell types [12]. In zebrafish, MG undergo indirect reprogramming and retain their glial identity. Though both indirect and direct reprogramming approaches have been employed to achieve MG reprogramming in mice, an indirect way seems more conducive to retinal repair as it keeps glial identity similar to regenerative process in zebrafish.
Given MG’s unique characteristics, exploring reprogramming strategies could open new avenues for retinal regeneration and vision restoration therapies. Investigations of a spontaneous reprogramming process in regeneration-competent species (e.g., zebrafish and chicks) and induced reprogramming strategies in mammals both have pinpointed evolutionarily conserved reprogramming molecules (e.g., Ascl1 and Lin28) and signaling pathways (e.g., Hippo/YAP inhibition and Wnt/β-catenin activation) which serve as promising therapeutic targets. These reprogramming mechanisms demonstrate remarkable translational potential for human retinal degenerative disorders like glaucoma and retinitis pigmentosa.
2. Search Strategy
The literature retrieval for this narrative review was conducted using the PubMed database, covering publications from 2000 to 2024. For search terms, the following keyword combinations were employed: “Müller glia” OR “retinal glia” OR “Müller cell”, “Reprogramming” OR “transdifferentiation” OR “cell fate conversion” OR “glia-to-neuron”, and “Retinal regeneration” OR “neuron regeneration” OR “Neurogenesis”. We initially identified a total of 76 records. These papers were meticulously screened to identify the most relevant articles and further evaluated for mechanistic insights into MG reprogramming, including cytokines, signaling pathways, transcriptional regulation, and epigenetic modifications. Additional references were identified through citation tracking of key articles and reviews. Figure 1 highlights milestone events in the field of MG reprogramming research, reflecting the significant advancements and key findings in this area over the past two decades. We especially compare MG reprogramming mechanisms between zebrafish (spontaneous regeneration) and mammals (induced reprogramming) and focus on their implications in retinal regeneration.
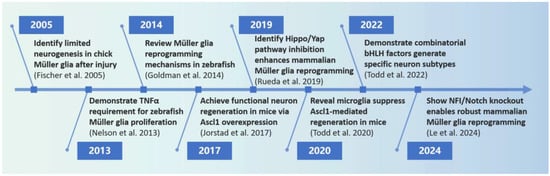
Figure 1.
Timeline showing the history of investigations into MG reprogramming. TNFα: tumor necrosis factor alpha; Ascl1: achaete-scute homolog 1; bHLH factors: basic Helix–Loop–Helix factors; NFI: Nuclear Factor I. References cited in the timeline: [3,4,13,14,15,16,17,18].
3. Müller Glia Responses to Injury
In response to retinal injury, MG undergo reactive gliosis, characterized by cellular hypertrophy, increased expression of intermediate filament proteins, and activation of extracellular regulatory kinases. While this process initially provides neuroprotective effects, chronic reactive gliosis disrupts retinal homeostasis and exacerbates the primary damage [6]. Mammalian MG primarily undergo gliosis fate and rarely divide [7]. However, in teleost fish, gliotic response is temporary after injury, and MG-derived progenitor cells (MGPCs) have the ability to regenerate all types of neurons and restore visual function [4,8]. Although large numbers of proliferating MGPCs are also formed in avian retina, the neurogenic capacity of these cells is relatively limited [13] (Figure 2).
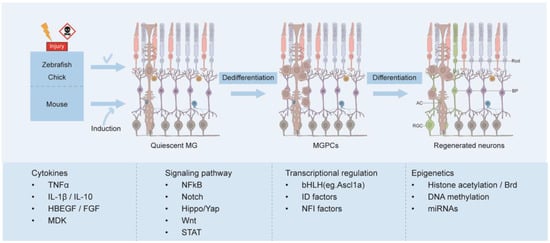
Figure 2.
MG reprogramming process and mechanisms. Following retinal injury, MG can spontaneously reprogram into retinal neurons in zebrafish and chick, whereas in mice, this process occurs only through induction. MG re-enter the cell cycle, dedifferentiate into progenitor cells (MGPCs), and subsequently differentiate into various types of retinal neurons, including rod cell (Rod), amacrine cell (AC), bipolar cell (BP), and retinal ganglion cell (RGC). Multiple mechanisms, including cytokines, signaling pathways, transcriptional regulation, and epigenetic factors, play crucial roles in the MG reprogramming.
Cross-species studies using single-cell RNA sequencing and assay for transposase-accessible chromatin sequencing have uncovered evolutionarily conserved and species-specific regulatory networks that govern the transitions between quiescent, reactive, and proliferative MG states. In mice, these networks restore MG from a reactive to a quiescent state following injury. By contrast, in zebrafish and chicks, genes selectively expressed in reactive MG promote their transition into proliferative and neurogenic progenitor cells [19]. This suggests that targeting gene regulatory networks that suppress neurogenic potential in mammalian MG could facilitate endogenous retinal regeneration.
Different injury models also reveal distinct gene expression and transcriptional regulatory networks. Most studies for MG reprogramming employ acute damage models. Light-induced damage primarily affects photoreceptors, while N-methyl-D-aspartate (NMDA)-induced excitotoxicity damages retinal ganglion cells (RGCs). These two models exhibit significant differences in the transcriptional profiles and regulatory mechanisms governing MG responses [20]. Notably, there are key differences in the MG response to high-intensity acute damage versus chronic neurodegeneration. Age-related retinal neurodegeneration is insufficient to trigger MG reprogramming in zebrafish. However, light damage in the aged zebrafish retina triggers a regenerative response, and numbers of proliferating MGPCs are comparable to younger adult fish. Therefore, aged MG retain the capacity to regenerate neurons, and the lack of compensatory proliferation in response to chronic neuronal loss is likely due to the insufficient levels of the required stimuli for MG reprogramming [21]. Similarly, chronic photoreceptor degeneration in zebrafish did not result in significant apoptosis, MG gliosis, or MG cell-cycle re-entry [22]. Certain signals maybe necessary for MG to reach the injury response threshold to proliferate and regenerate neurons [23]. Inhibition of Notch signaling induced MG of cep290 mutants to re-enter the cell cycle [24].
4. Müller Glia Reprogramming Mechanisms
Taking the spontaneous MG reprogramming process of zebrafish and chick as references, many potential strategies for inducing MG reprogramming in mouse have been developed based on corresponding mechanisms, like cytokines, signal transduction, epigenetic modifications, and transcriptional regulation. These mechanisms have different effects on various phases of MG reprogramming (Table 1).
4.1. Cytokines and Müller Glia Reprogramming
Following retinal injury, various cytokines are released into the extracellular environment, where they bind to receptors on MG, and activate signaling cascades that initiate reprogramming. Dying neurons produce tumor necrosis factor alpha (TNFα), which induces MG reprogramming by upregulating key reprogramming genes such as ascl1a and stat3. Once reprogramming is initiated, MG themselves also synthesize TNFα, regulating the proliferation of MGPCs [14].
Microglia, the resident immune cells of the central nervous system, respond rapidly to retinal neuronal injury by releasing inflammatory signals. In the mouse retina, microglia suppress the neurogenic reprogramming capacity of MG after injury [15]. However, repopulated microglia, which replace ablated microglia, exhibit homeostatic properties, suppress extracellular matrix remodeling, and promote MG transition into a progenitor-like proliferative state [25]. In contrast, in chick and zebrafish retinas, reactive microglia are essential for MG proliferation following injury [26,27].
In zebrafish, microglia release pro-inflammatory cytokine interleukin-1β (IL-1β) and anti-inflammatory cytokine interleukin-10 (IL-10), both of which are required to initiate MG reprogramming in response to photoreceptor damage. And IL-1β alone can drive MG proliferation even in uninjured retinas, whereas IL-10 induces only gliotic response [28]. Photoreceptor-derived TNFα appears to act indirectly by stimulating microglial expression of il-1β and il-10, which then promote MG reprogramming [29].
In chick retinas subjected to NMDA-induced damage, reactive microglia secrete signals that upregulate MG expression of heparin-binding epidermal growth factor (HBEGF) and fibroblast growth factor (FGF). These factors function in an autocrine manner to promote MGPCs formation [30]. Additionally, microglial phagocytosis of dying neurons may modulate the release of neuron-derived cytokines, influencing downstream MG reprogramming [29].
MG also activate signaling networks via autocrine or paracrine mechanisms [4]. Leptin and IL-6 family cytokines synergistically activate Jak/Stat3 signaling to stimulate MG reprogramming and retina regeneration in zebrafish [31]. Various cytokines are thought to work synergistically, amplifying paracrine signals from dying neurons and microglia as well as lowering the activation threshold of intracellular reprogramming cascades [25]. In damaged mouse retinas, MG downregulate midkine (MDK), a factor that promotes MGPCs proliferation and reduces neuronal death, further influencing reprogramming outcomes [32].
4.2. Key Signaling Pathways in Müller Glia Reprogramming
4.2.1. NFkB Signaling
In mouse retinas, NF-κB signaling is activated in MG following NMDA-induced injury. This activation is driven by pro-inflammatory cytokines, such as IL-1β and TNF, released by reactive microglia. Suppressing inflammation with glucocorticoids blocks NF-κB activation in MG. Notably, NF-κB signaling interacts with pathways like Toll-like receptors, ciliary neurotrophic factor/Jak/Stat, osteopontin, and Wnt/GSK3β [33]. NF-κB signaling inhibits Ascl1 overexpression-induced MG reprogramming. Inhibition of its downstream effectors such as TGF-β/Smad and ID transcription factors also improves neurogenic outcomes, highlighting its suppressive role in neuronal regeneration [34].
4.2.2. Notch Signaling
In zebrafish, Notch signaling maintains MG in a quiescent state. Light-induced retinal injury triggers downregulation of Notch3 and upregulation of Notch1a, Notch1b, and Notch2 in zebrafish MG. Notch3 or DeltaB knockdown facilitates MG proliferation even without injury, while silencing Notch1a, Notch1b, or Notch2 inhibits this process [35,36].
The dynamic regulation of Notch signaling plays a distinct role in the regenerative processes of the zebrafish and mammalian retinas [37]. In mammals, mature MG maintain basal Notch signaling activity [19,38]. While Notch signaling may transiently promote MG proliferation after injury, persistent activation suppresses pro-neural gene expression, impairing neuronal regeneration [39]. Notch signaling inhibition, particularly through Rbpj or Notch1/2 deletion, enables efficient reprogramming of MG into retinal neurons, with near-complete conversion achieved when combined with NFI factor loss [16]. These findings indicate Notch signaling as a pivotal suppressor of neurogenesis in mammals.
4.2.3. Hippo/Yap Signaling
The Hippo pathway restricts MG reprogramming by inhibiting Yes-associated protein (Yap), a transcriptional co-activator. Either disruption of Hippo components Lats1/2 or expression of Yap variants Yap5SA resistant to inhibition promotes MG proliferation and dedifferentiation [17]. Conditional Yap deletion in mouse MG prevents cell-cycle gene upregulation upon photoreceptor damage. Yap is also required for Xenopus MG proliferation in response to injury. Yap enables MG to exit from quiescence by interacting with epidermal growth factor receptor signaling, which is a critical step toward regeneration [40]. In zebrafish, Yap activity inhibition attenuates MG proliferation, MGPCs formation, and photoreceptor differentiation. Yap may drive MG reprogramming by regulating the expression of transcription factors like lin28a and ascl1a [41].
4.2.4. Wnt Signaling
Wnt signaling contributes to retinal regeneration in adult mammals. Activation of Wnt signaling by Wnt3a or glycogen synthase kinase-3β inhibitors promotes proliferation of MGPCs and neural regeneration in the retina. Conversely, inhibiting Wnt signaling with Dkk-1 attenuates retinal regeneration [42]. In mouse, NMDA-induced neurotoxic injury activates Wnt signaling, leading to MG proliferation. And Wnt-Lin28-let-7 miRNA axis plays a crucial role in regulating proliferation and neurogenic potential of MG [43]. In addition, Wnt signaling promotes cell fusion induced MG reprogramming. In the photoreceptor degeneration mouse model, transplanted hematopoietic stem and progenitor cells (HSPCs) fuse with MG. Activation of Wnt signaling enhanced survival and proliferation of Müller–HSPC hybrids as well as their reprogramming into photoreceptor precursors [44].
4.2.5. Jak/Stat Signaling
In chick retina, Jak/Stat-signaling promotes the reprogramming of MG into MGPCs and the proliferation of MGPCs but inhibits the neurogenic capacity of MGPCs [45]. Such an effect is also seen in mouse retina. And elevated Stat activity in MGPCs suppresses differentiation may attributed to the induction of ID factor expression, whereas Stat inhibition promotes neurogenesis by enhancing Ascl1 binding to pro-neurogenic genes [46]. Balancing Jak/Stat-signaling is therefore critical for successful neuronal regeneration.
4.2.6. MAPK Signaling
The MAPK/ERK pathway may act as a “pioneer pathway” that initiates MG dedifferentiation by integrating mitogenic signals from ligands such as FGF, IGF, and HB-EGF, which activate their respective receptors, leading to downstream activation of pERK1/2, Egr1, cFos, and pCREB [23,47,48]. This signaling cascade suppresses glial gene expression and promotes MG dedifferentiation and proliferation [49]. In zebrafish, MAPK/ERK functions synergistically with PI3K, Jak/Stat3, and β-catenin pathways to activate pStat3 and β-catenin, which are essential for MG proliferation and multipotency [50]. Conversely, in the chick retina, glucocorticoid receptor (GCR) signaling antagonizes the MAPK pathway by repressing the activation of pERK1/2 and immediate early genes such as Egr1 and cFos, thereby inhibiting the formation of MGPCs [51]. Although MAPK-driven reprogramming mechanism is robust in regenerative species like chick and zebrafish, it is limited in mammals. However, in mice, enhancing MAPK signaling in combination with the removal of key transcriptional repressors (e.g., Nfia/b/x knockout) significantly improves MGPC induction and neurogenic potential [19].
4.3. Transcriptional Regulation in Müller Glia Reprogramming
4.3.1. bHLH Transcription Factors
Proneural basic Helix–Loop–Helix (bHLH) transcription factors, including Ascl1, Neurog2, Atoh1/7, and Neurod1, participate in glia reprogramming in the mammalian central nervous system [52]. Ascl1 plays a critical role in initiating injury-dependent MG reprogramming in zebrafish, whereas Ascl1 is not expressed in mammalian MG after injury [53,54]. Overexpressing Ascl1 combined with using histone deacetylase inhibitors, like Trichostatin A (TSA), has successfully induced neuron regeneration in mouse injury models, mostly bipolar cells [3]. Further, using combinations of transcription factors significantly improves reprogramming efficiency, enabling MG to differentiate into specific neuronal subtypes. Overexpression of Ascl1/Atoh1 promotes the regeneration of ganglion or amacrine-like neurons, even in the absence of injury or TSA treatment [55]. And Islet1/Pou4f2/Ascl1 stimulates the genesis of ganglion-like neurons, while Atoh1 supports the further differentiation and maturation of these neurons [18]. However, in human MG cultures, Ascl1 overexpression results in the generation of RGCs and amacrine-like neurons, rather than the bipolar cells seen in mice, indicating that these transcription factor combinations cannot apply to human MG directly [56].
In addition, ID (inhibitor of DNA binding) factors, which belong to the HLH family but lack the DNA-binding domain, inhibit the activity of Class I and II bHLH factors by heterodimerizing [57]. In chick retinas, ID4 was prominently expressed by resting MG, but following retinal damage ID4 was rapidly upregulated and then downregulated in MGPCs. By contrast, ID1/2/3 were at low levels in resting MG but upregulated in MGPCs. Inhibiting ID factors following retinal damage decreased numbers of proliferating MGPCs by increasing levels of p21Cip1 in MG. Inhibiting ID factors after MGPCs proliferation promotes the differentiation of progenitors into amacrine-like neurons [58]. In zebrafish, ID factor levels significantly decrease during MGPC differentiation into neurons [36]. In NMDA-injured mouse retinas, inhibiting ID factors in Ascl1-overexpressing MG promotes the regeneration of bipolar-like neurons [34]. Thus, ID factors drive the proliferation of MGPCs and suppress the neurogenic potential of MGPCs.
4.3.2. NFI Factors
Nuclear Factor I (NFI), including NFIA, NFIB, NFIC, and NFIX, plays an essential role in the development of glial cells in the central nervous system [59]. NFI expression patterns are conserved across species, including chick, pig, and macaque retinas, where NFIA and NFIB are prominently expressed in MG, possibly contributing to the maintenance of the glial phenotype. NFI levels in MG and MGPCs in chicks show injury-dependent decrease [60]. Conditional knockout of Nfia/b/x promotes MG reprogramming into neurons in mice, suggesting that NFI factors act as negative regulators of injury-induced reprogramming in MG [19].
4.4. Epigenetics in Müller Glia Reprogramming
4.4.1. Histone Modifications
Histone modifications are closely linked to the expression of pluripotency factors during MG reprogramming [61]. Histone acetyltransferases (HATs) and histone deacetylases (HDACs) serve to activate or repress gene transcription, respectively, through the loosening or tightening of chromatin structure [62,63]. Inhibiting HDACs with TSA facilitates MG reprogramming by enhancing the accessibility of key loci involved in neurodevelopment and regeneration [3]. Inhibiting the HDAC SIRT6 via siRNA also promotes MG reprogramming with Sox9 upregulation [64]. These findings suggest that histone acetylation plays a crucial role in driving MG transdifferentiation into neurons. The Bromo domain (Brd) recognizes acetylated lysine residues on histones. Brd inhibitor (+)-JQ1 treatment prevents cell-cycle re-entry of MG and the generation of MGPCs [65]. Furthermore, histone modifications such as methylation and phosphorylation also influence MG reprogramming [66,67].
4.4.2. DNA Methylation
DNA methylation participates in cell fate conversion [68]. Oct4 methylation-mediated silencing prevents MG dedifferentiation in mice [69]. In zebrafish, MG reprogramming into MGPCs relies on dynamic DNA methylation regulation. Demethylation predominates in early stages, while later stages show increased methylation, suggesting that DNA demethylation is crucial for initiating MG reprogramming [70].
DNA methylation also regulates MG redifferentiation. Genes required for phototransduction (e.g., Opn1mw, Opn1sw, Arr3, Pde6c for cones; Gngt1, Gucy2f for rods) are located in highly methylated regions of the MG genome. TET-family demethylases (Tet2/3) are essential for removing these epigenetic barriers and enabling neuronal differentiation [71]. In zebrafish, loss of Tet2/3 disrupts the differentiation of RGCs and photoreceptors, highlighting the role of DNA demethylation in retinal neuron regeneration [72].
4.4.3. MicroRNA-Mediated Gene Silencing
MicroRNAs (miRNAs) mediate post-transcriptional gene silencing by targeting the 3′ untranslated regions of mRNAs, in which way regulate MG reprogramming [73]. The Dicer-dependent miRNA biogenesis pathway is crucial for neuronal regeneration in zebrafish following light-induced damage [74]. Overexpressing miR-124/miR-9, miR-25/miR-124, or inhibiting let-7 enhances Ascl1-induced reprogramming of MG in mice [75,76]. And Lin-28 overexpression, which lowers the let-7 level, also promotes MG dedifferentiation into MGPCs [77].
In zebrafish, miR-203 expression is downregulated during MG regeneration, and maintaining its expression inhibits retinal regeneration [78]. The target of miR-203, Pax6b, is expressed in neural progenitor cells, suggesting that inhibiting miR-203 allows Pax6b expression and supports retinal regeneration [79]. Similarly, miR-216a downregulation after retinal damage in zebrafish increases its mRNA target Dot1l expression, which may promote MG dedifferentiation by influencing chromatin accessibility and activating Wnt target genes [80].
Recent studies have found that miR-18a expression increases after light-induced damage in zebrafish retinas. miR-18a deficiency delays photoreceptor regeneration but increases the number of MGPCs [81]. Understanding the full range of miRNA expression changes and their target genes during MG reprogramming could provide new strategies for retinal regeneration.
In mature MG, the loss of reprogramming ability is associated with decreased chromatin accessibility, suggesting that epigenetic factors limit retinal regeneration [82]. Overcoming epigenetic inhibition may therefore be a more effective approach for inducing neurogenesis in mammalian MG than transferring species-specific genes.


Table 1.
Influence of reprogramming-related factors on Müller glia neurogenesis.
Table 1.
Influence of reprogramming-related factors on Müller glia neurogenesis.
| Mechanism | Influence | Factors | Model | Reference |
|---|---|---|---|---|
| Cytokines | Induce MG proliferation | TNFα, IL-1β and IL-10 | Zebrafish | [14,28] |
| Promote MGPCs formation | HBEGF and FGF, MDK | Chick, Mouse | [30,32] | |
| Signaling pathway | Induce MG proliferation | Yap, Wnt | Mouse, Xenopus | [40,43] |
| Suppress MG proliferation | NFkB, Notch | Zebrafish, Mouse, Rat | [16,34,35,36,39] | |
| Promote MGPCs formation | Yap | Zebrafish | [41] | |
| prevent MGPCs formation | Hippo | Mouse | [17] | |
| Suppress differentiation of MGPCs into neurons | STAT | Mouse | [46] | |
| Transcriptional regulation | Enhance MG neurogenesis | Ascl1, Ascl1 + TSA, Atoh1/Ascl1 | Zebrafish, Mouse, Human MG culture | [3,46,53,55] |
| Influence regenerated neuron types | Atoh1/Ascl1, Ascl1/Pou4f2/Islet1/Atoh1 | Mouse | [18,46] | |
| Promote MGPCs proliferation | ID factors | Chick | [58] | |
| Suppress differentiation of MGPCs into neurons | ID factors, NFI factors | Chick, Zebrafish, Mouse | [19,34,36,58,60] | |
| Epigenetics | Suppress MG proliferation | Brd, miR-216a | Zebrafish | [65,80] |
| Promote MGPCs formation | miR-124/miR-9, miR-25/miR-124 | Mouse, Mouse MG culture | [75,76] | |
| Prevent MGPCs formation | Histone acetylation, Brd, DNA methylation, let 7, miR-216a | Zebrafish, Mouse, Rat | [61,65,69,70,77,80] | |
| Promote MGPCs proliferation | Dicer-dependent miRNA biogenesis pathway | Zebrafish | [78] | |
| Suppress MGPCs proliferation | Histone acetylation, miR-203 | Zebrafish | [61,78] | |
| Promote differentiation of MGPCs into neurons | DNA methylation, miR-18a | Zebrafish, Mouse | [70,81] | |
| Suppress Neuron differentiation | DNA methylation | Zebrafish | [72] | |
| Suppress MG neurogenesis | Histone acetylation, miR-25/miR-124 | Mouse | [3,76] |
4.5. Microenvironment in Müller Glia Reprogramming
The microniche surrounding MG critically influences their stem cell potential and ability to generate progenitors. After neural injury, the extracellular matrix (ECM) undergoes remodeling, and abnormally deposited ECM forms a physical barrier that hinders neural regeneration [83,84]. Mechanical signals from the ECM regulate the reprogramming of somatic cells into stem cells in vitro [85]. For example, degradation of the ECM by gelatinases promotes MG reprogramming in the chick retina by reducing matrix stiffness [86].
Within the immune microenvironment, microglial activation is essential for modulating the immune landscape to facilitate MG reprogramming in zebrafish following retinal injury [87,88]. Pro-inflammatory microglia exacerbate gliosis and inhibit neurogenesis, whereas anti-inflammatory microglia promote neuronal regeneration [89]. In mammals, microglia remain chronically overactivated during retinal injury or degeneration, leading to persistent inflammation that accelerates photoreceptor apoptosis and MG gliosis [90]. Repopulated microglia (Rep-MiG) can suppress pathological ECM remodeling and reprogram MG, thereby protecting photoreceptors and improving visual function [25]. Additionally, multiple signaling pathways linked to cytokine-mediated immune responses, as discussed in the former part, further shape MG plasticity.
In the metabolic microenvironment, eliminating metabolic barriers enhances direct neuronal reprogramming efficiency [91]. A metabolic shift from low-to-high glycolysis may be necessary for MG fate conversion, as elevated glycolysis supports stemness maintenance [92]. Inhibiting oxidative damage also aids neuronal regeneration [91]. Furthermore, mitochondrial structure and function, along with extracellular vesicles, influence cellular reprogramming by regulating energy metabolism and intercellular communication [93,94].
Emerging evidence indicates that interventions on different reprogramming mechanisms demonstrate variations in the types of regenerated neurons and reprogramming efficiency [95]. High-efficiency strategies universally rely on multifactorial synergy, whereas single-factor approaches yield significantly reduced efficacy. The combined overexpression of β-catenin with Otx2/Crx/Nrl achieves a rod photoreceptor reprogramming efficiency of 97.40%, while Pou4f2/Atoh7 overexpression induces RGCs reprogramming in approximately 30% [96,97]. An epigenetic strategy combining Ascl1 and Atoh1 drives amacrine cell reprogramming to 80% efficiency, and the addition of a STAT inhibitor to Ascl1 elevates bipolar cell reprogramming to 50–60% [46,55]. Furthermore, the completeness of functional validation correlates positively with reprogramming efficiency. Actually, divergent reprogramming mechanisms are not independent in regeneration-competent species; instead, they form a complex gene regulatory network and interfere with each other. Therefore, the collaborative utilization of multiple mechanisms represents a promising direction for MG reprogramming strategies.
5. Contribution of Müller Glia Reprogramming to Retinal Neuron Regeneration
MG reprogramming plays a pivotal role in retinal neuron regeneration, contributing to neural repair through multiple mechanisms. One significant function is neuronal replacement, where reprogrammed MG generate new neurons to compensate for those lost due to retinal injury or degenerative diseases. In zebrafish and early postnatal rodents, MG re-enter the cell cycle upon injury, producing photoreceptors and bipolar cells [42,98]. While adult mammalian MG exhibit limited regenerative capacity, studies with human retinal organoids demonstrate their potential to differentiate into photoreceptor or RGC precursors [99,100].
Beyond direct neuronal replacement, MG reprogramming also exerts a neuroprotective effect [101]. The neuronal progenitor cells generated through this process secrete neurotrophic factors such as brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), and ciliary neurotrophic factor (CNTF), which enhance the survival of damaged neurons [102]. Extracellular vesicles (EVs) released by MG further mediate neuroprotection by delivering miRNAs that inhibit apoptotic pathways [103].
Furthermore, MG reprogramming contributes to the restoration of retinal homeostasis by reconstructing retinal structure and function. MG-derived neurons and supporting cells aids in the re-establishment of the retina laminar organization and functional connectivity. MG emerge late in development, contributing to structural organization and functional maturation of the retina [104,105]. Transplanted MG-derived precursors improve retinal function in animal models by integrating into host circuits or modulating the microenvironment [100]. Given these multifaceted contributions, MG reprogramming holds great potential for clinical applications in the treatment of retinal neurodegenerative diseases.
6. Potential Implications of Müller Glia Reprogramming for Retinal Diseases
Retinal neurodegenerative diseases, including retinitis pigmentosa (RP), diabetic retinopathy (DR), age-related macular degeneration (AMD), and glaucoma, represent major causes of vision loss worldwide. Current treatments are largely limited to slowing disease progression rather than restoring lost vision, underscoring the urgent need for regenerative strategies. In RP, an inherited disorder characterized by progressive photoreceptor loss, MG reprogramming offers a promising avenue for neuronal replacement [106]. Studies have demonstrated that Yap activity inhibition or Wnt signaling activation can induce MG to generate photoreceptor progenitor cells, which integrate into the retina and restore visual function [41,44].
Similarly, in DR, a complication of diabetes involving retinal vascular damage and neuronal degeneration, MG reprogramming not only generates new neurons but also enhances neuroprotection through the secretion of neurotrophic factors, mitigating neuronal loss [107]. Additionally, it plays a role in vascular repair, improving retinal blood supply and stabilizing the retinal microenvironment [108]. In AMD, a leading cause of vision impairment among the elderly that primarily affects macular photoreceptors, MG reprogramming holds potential for photoreceptor regeneration, restoring macular function [109]. Moreover, by secreting anti-inflammatory factors, MG can reduce inflammation in the macula, thereby slowing disease progression [110].
In glaucoma, which is marked by the degeneration of RGCs and results in irreversible vision loss, MG reprogramming offers a potential solution by generating new RGCs to replace damaged ones [111]. Research indicates that overexpression of transcription factors such as Islet1, Pou4f2, and Ascl1 can drive MG differentiation into RGCs, which subsequently integrate into the retinal circuitry as functional neurons [18]. Collectively, these findings highlight the vast regenerative potential of MG reprogramming, positioning it as a promising therapeutic strategy for a range of retinal disorders.
7. Discussion
Retinal neuron loss caused by genetic and age-related diseases such as retinitis pigmentosa, diabetic retinopathy, macular degeneration, and glaucoma leads to severe vision impairment. Despite advancements in understanding retinal neurodegeneration, effective therapeutic interventions remain elusive. Harnessing the neurogenic potential of MG offers a promising avenue for promoting endogenous neuronal regeneration and restoring vision [101].
Recent studies have made considerable progress in understanding spontaneous regeneration mechanisms in zebrafish as well as developing induced reprogramming strategies in mammals. MG reprogramming mechanisms including cytokines, signaling pathways, transcriptional factors, and epigenetic modifications operate together to regulate MG gene expression. The future of MG-mediated neuronal regeneration depends on combining multiple strategies to improve reprogramming efficiency and achieve precise targeting.
Despite notable progress, mammalian MG reprogramming faces significant challenges, and some direct reprogramming techniques remain controversial. For example, while the knockdown of Ptbp1 has been reported to promote the transdifferentiation of MG into ganglion- or cone-like neurons [112,113], other studies using advanced AAV tools and MG-lineage tracing mouse models have failed to confirm NeuroD1- or Ptbp1-mediated MG reprogramming. This discrepancy has been attributed to issues such as AAV vector leakage induced by the GFAP minipromoter, which leads to the mislabeling of endogenous neurons [114,115,116]. Moreover, lineage tracing experiments used to test in vivo glia-to-neuron conversion underscore the limitations of Cre-loxP recombination system, which potentially building a higher barrier for cell transformation [117]. Thus, a more comprehensive understanding of inherent effects and limitations of techniques like AAV vectors and Cre-loxP recombination systems is crucial before employing them to manipulate and assess cell identity.
The success of reprogramming ultimately depends on the functional integration of newly generated neurons into existing retinal circuits. This process requires precise migration to the appropriate retinal layers, the extension of dendrites and axons, and the establishment of functional synapses with host neurons [118]. To achieve functional vision recovery through MG-derived neurogenesis, research efforts must address the critical challenges of cellular integration and neural circuit reformation [95].
The therapeutic potential of MG reprogramming relies on its accuracy to regenerate specific retinal neuron types affected by different retinal diseases. In zebrafish, the gene regulatory networks involved in MG reprogramming vary significantly depending on the types of retinal injury [20]. However, cone regeneration presents a notable challenge, even in species capable of spontaneous MG reprogramming, such as zebrafish and Xenopus. For instance, chronic cone degeneration caused by cep290/bbs2 mutations in zebrafish fails to induce cone regeneration, despite transcriptional changes resembling light-induced injury responses [119]. Similarly, while Xenopus MG can regenerate several neuronal types, they do not regenerate cones, which are the primary cells affected in CoCl2-induced retinal degeneration [120]. The lack of a clearly defined mechanism for cone regeneration poses a significant obstacle to achieving this outcome in mammals. Understanding and developing such mechanisms are crucial steps toward facilitating the restoration of functional vision through MG-derived neurogenesis in future.
Author Contributions
S.Q. and Y.Z.: conceptualization, methodology; Y.Z.: literature search, writing the original draft, and illustration preparation; S.Q. and H.W.: editing, reviewing, and finalizing the draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (No.31871477; 32170971) awarded to Song Qin, and the Natural Science Foundation of Shanghai (20ZR1409700) awarded to Haixiang Wu.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank members of the Qin laboratory for suggestions and comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller Cells in the Healthy and Diseased Retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-Neuron Interactions in the Mammalian Retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef]
- Jorstad, N.L.; Wilken, M.S.; Grimes, W.N.; Wohl, S.G.; VandenBosch, L.S.; Yoshimatsu, T.; Wong, R.O.; Rieke, F.; Reh, T.A. Stimulation of Functional Neuronal Regeneration from Müller Glia in Adult Mice. Nature 2017, 548, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D. Müller Glial Cell Reprogramming and Retina Regeneration. Nat. Rev. Neurosci. 2014, 15, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Lahne, M.; Nagashima, M.; Hyde, D.R.; Hitchcock, P.F. Reprogramming Müller Glia to Regenerate Retinal Neurons. Annu. Rev. Vis. Sci. 2020, 6, 171–193. [Google Scholar] [CrossRef]
- Bringmann, A.; Iandiev, I.; Pannicke, T.; Wurm, A.; Hollborn, M.; Wiedemann, P.; Osborne, N.N.; Reichenbach, A. Cellular Signaling and Factors Involved in Müller Cell Gliosis: Neuroprotective and Detrimental Effects. Prog. Retin. Eye Res. 2009, 28, 423–451. [Google Scholar] [CrossRef]
- Nishino, R.; Nomura-Komoike, K.; Iida, T.; Fujieda, H. Cell Cycle-Dependent Activation of Proneural Transcription Factor Expression and Reactive Gliosis in Rat Müller Glia. Sci. Rep. 2023, 13, 22712. [Google Scholar] [CrossRef]
- Lenkowski, J.R.; Raymond, P.A. Müller Glia: Stem Cells for Generation and Regeneration of Retinal Neurons in Teleost Fish. Prog. Retin. Eye Res. 2014, 40, 94–123. [Google Scholar] [CrossRef]
- Turner, D.L.; Snyder, E.Y.; Cepko, C.L. Lineage-Independent Determination of Cell Type in the Embryonic Mouse Retina. Neuron 1990, 4, 833–845. [Google Scholar] [CrossRef]
- Jadhav, A.P.; Roesch, K.; Cepko, C.L. Development and Neurogenic Potential of Müller Glial Cells in the Vertebrate Retina. Prog. Retin. Eye Res. 2009, 28, 249–262. [Google Scholar] [CrossRef]
- Graf, T.; Enver, T. Forcing Cells to Change Lineages. Nature 2009, 462, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Y.; Liu, J.; Qian, L. Direct Cell Reprogramming: Approaches, Mechanisms and Progress. Nat. Rev. Mol. Cell Biol. 2021, 22, 410–424. [Google Scholar] [CrossRef]
- Fischer, A.J. Neural Regeneration in the Chick Retina. Progress. Retin. Eye Res. 2005, 24, 161–182. [Google Scholar] [CrossRef]
- Nelson, C.M.; Ackerman, K.M.; O’Hayer, P.; Bailey, T.J.; Gorsuch, R.A.; Hyde, D.R. Tumor Necrosis Factor-Alpha Is Produced by Dying Retinal Neurons and Is Required for Muller Glia Proliferation during Zebrafish Retinal Regeneration. J. Neurosci. 2013, 33, 6524–6539. [Google Scholar] [CrossRef] [PubMed]
- Todd, L.; Finkbeiner, C.; Wong, C.K.; Hooper, M.J.; Reh, T.A. Microglia Suppress Ascl1-Induced Retinal Regeneration in Mice. Cell Rep. 2020, 33, 108507. [Google Scholar] [CrossRef]
- Le, N.; Vu, T.-D.; Palazzo, I.; Pulya, R.; Kim, Y.; Blackshaw, S.; Hoang, T. Robust Reprogramming of Glia into Neurons by Inhibition of Notch Signaling and Nuclear Factor I (NFI) Factors in Adult Mammalian Retina. Sci. Adv. 2024, 10, eadn2091. [Google Scholar] [CrossRef]
- Rueda, E.M.; Hall, B.M.; Hill, M.C.; Swinton, P.G.; Tong, X.; Martin, J.F.; Poché, R.A. The Hippo Pathway Blocks Mammalian Retinal Müller Glial Cell Reprogramming. Cell Rep. 2019, 27, 1637–1649.e6. [Google Scholar] [CrossRef] [PubMed]
- Todd, L.; Jenkins, W.; Finkbeiner, C.; Hooper, M.J.; Donaldson, P.C.; Pavlou, M.; Wohlschlegel, J.; Ingram, N.; Rieke, F.; Reh, T.A.; et al. Reprogramming Müller Glia to Regenerate Ganglion-like Cells in Adult Mouse Retina with Developmental Transcription Factors. Sci. Adv. 2022, 8, eabq7219. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.; Wang, J.; Boyd, P.; Wang, F.; Santiago, C.; Jiang, L.; Yoo, S.; Lahne, M.; Todd, L.J.; Jia, M.; et al. Gene Regulatory Networks Controlling Vertebrate Retinal Regeneration. Science 2020, 370, eabb8598. [Google Scholar] [CrossRef]
- Lyu, P.; Iribarne, M.; Serjanov, D.; Zhai, Y.; Hoang, T.; Campbell, L.J.; Boyd, P.; Palazzo, I.; Nagashima, M.; Silva, N.J.; et al. Common and Divergent Gene Regulatory Networks Control Injury-Induced and Developmental Neurogenesis in Zebrafish Retina. Nat. Commun. 2023, 14, 8477. [Google Scholar] [CrossRef]
- Martins, R.R.; Zamzam, M.; Tracey-White, D.; Moosajee, M.; Thummel, R.; Henriques, C.M.; MacDonald, R.B. Müller Glia Maintain Their Regenerative Potential despite Degeneration in the Aged Zebrafish Retina. Aging Cell 2022, 21, e13597. [Google Scholar] [CrossRef] [PubMed]
- Turkalj, B.; Quallich, D.; Bessert, D.A.; Kramer, A.C.; Cook, T.A.; Thummel, R. Development and Characterization of a Chronic Photoreceptor Degeneration Model in Adult Zebrafish That Does Not Trigger a Regenerative Response. Exp. Eye Res. 2021, 209, 108630. [Google Scholar] [CrossRef] [PubMed]
- Taylor, O.B.; El-Hodiri, H.M.; Palazzo, I.; Todd, L.; Fischer, A.J. Regulating the Formation of Müller Glia-Derived Progenitor Cells in the Retina. Glia 2025, 73, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Fogerty, J.; Song, P.; Boyd, P.; Grabinski, S.E.; Hoang, T.; Reich, A.; Cianciolo, L.T.; Blackshaw, S.; Mumm, J.S.; Hyde, D.R.; et al. Notch Inhibition Promotes Regeneration and Immunosuppression Supports Cone Survival in a Zebrafish Model of Inherited Retinal Dystrophy. J. Neurosci. 2022, 42, 5144–5158. [Google Scholar] [CrossRef]
- Cheng, X.; Gao, H.; Tao, Z.; Yin, Z.; Cha, Z.; Huang, X.; Zhang, Y.; Zeng, Y.; He, J.; Ge, L.; et al. Repopulated Retinal Microglia Promote Müller Glia Reprogramming and Preserve Visual Function in Retinal Degenerative Mice. Theranostics 2023, 13, 1698–1715. [Google Scholar] [CrossRef]
- Fischer, A.J.; Zelinka, C.; Gallina, D.; Scott, M.A.; Todd, L. Reactive Microglia and Macrophage Facilitate the Formation of Müller Glia-Derived Retinal Progenitors. Glia 2014, 62, 1608–1628. [Google Scholar] [CrossRef]
- Conedera, F.M.; Pousa, A.M.Q.; Mercader, N.; Tschopp, M.; Enzmann, V. Retinal Microglia Signaling Affects Müller Cell Behavior in the Zebrafish Following Laser Injury Induction. Glia 2019, 67, 1150–1166. [Google Scholar] [CrossRef]
- Lu, C.; Hyde, D.R. Cytokines IL-1β and IL-10 Are Required for Müller Glia Proliferation Following Light Damage in the Adult Zebrafish Retina. Front. Cell Dev. Biol. 2024, 12, 1406330. [Google Scholar] [CrossRef]
- Yin, M.; Chen, Z.; Ouyang, Y.; Zhang, H.; Wan, Z.; Wang, H.; Wu, W.; Yin, X. Thrombin-Induced, TNFR-Dependent miR-181c Downregulation Promotes MLL1 and NF-κB Target Gene Expression in Human Microglia. J. Neuroinflamm. 2017, 14, 132. [Google Scholar] [CrossRef]
- El-Hodiri, H.M.; Bentley, J.R.; Reske, A.G.; Taylor, O.B.; Palazzo, I.; Campbell, W.A.; Halloy, N.R.; Fischer, A.J. Heparin-Binding Epidermal Growth Factor and Fibroblast Growth Factor 2 Rescue Müller Glia-Derived Progenitor Cell Formation in Microglia- and Macrophage-Ablated Chick Retinas. Development 2023, 150, dev202070. [Google Scholar] [CrossRef]
- Zhao, X.-F.; Wan, J.; Powell, C.; Ramachandran, R.; Myers, M.G.; Goldman, D. Leptin and IL-6 Family Cytokines Synergize to Stimulate Müller Glia Reprogramming and Retina Regeneration. Cell Rep. 2014, 9, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.A.; Fritsch-Kelleher, A.; Palazzo, I.; Hoang, T.; Blackshaw, S.; Fischer, A.J. Midkine Is Neuroprotective and Influences Glial Reactivity and the Formation of Müller Glia-Derived Progenitor Cells in Chick and Mouse Retinas. Glia 2021, 69, 1515–1539. [Google Scholar] [CrossRef]
- Palazzo, I.; Kelly, L.; Koenig, L.; Fischer, A.J. Patterns of NFkB Activation Resulting from Damage, Reactive Microglia, Cytokines, and Growth Factors in the Mouse Retina. Exp. Neurol. 2023, 359, 114233. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, I.; Todd, L.J.; Hoang, T.V.; Reh, T.A.; Blackshaw, S.; Fischer, A.J. NFkB-Signaling Promotes Glial Reactivity and Suppresses Müller Glia-Mediated Neuron Regeneration in the Mammalian Retina. Glia 2022, 70, 1380–1401. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.J.; Hobgood, J.S.; Jia, M.; Boyd, P.; Hipp, R.I.; Hyde, D.R. Notch3 and DeltaB Maintain Müller Glia Quiescence and Act as Negative Regulators of Regeneration in the Light-Damaged Zebrafish Retina. Glia 2021, 69, 546–566. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Devi, S.; Jui, J.; Goldman, D. Notch Signaling via Hey1 and Id2b Regulates Müller Glia’s Regenerative Response to Retinal Injury. Glia 2021, 69, 2882–2898. [Google Scholar] [CrossRef]
- Campbell, L.J.; Levendusky, J.L.; Steines, S.A.; Hyde, D.R. Retinal Regeneration Requires Dynamic Notch Signaling. Neural Regen. Res. 2022, 17, 1199–1209. [Google Scholar] [CrossRef]
- Elsaeidi, F.; Macpherson, P.; Mills, E.A.; Jui, J.; Flannery, J.G.; Goldman, D. Notch Suppression Collaborates with Ascl1 and Lin28 to Unleash a Regenerative Response in Fish Retina, But Not in Mice. J. Neurosci. 2018, 38, 2246–2261. [Google Scholar] [CrossRef]
- Del Debbio, C.B.; Mir, Q.; Parameswaran, S.; Mathews, S.; Xia, X.; Zheng, L.; Neville, A.J.; Ahmad, I. Notch Signaling Activates Stem Cell Properties of Müller Glia through Transcriptional Regulation and Skp2-Mediated Degradation of p27Kip1. PLoS ONE 2016, 11, e0152025. [Google Scholar] [CrossRef]
- Hamon, A.; García-García, D.; Ail, D.; Bitard, J.; Chesneau, A.; Dalkara, D.; Locker, M.; Roger, J.E.; Perron, M. Linking YAP to Müller Glia Quiescence Exit in the Degenerative Retina. Cell Rep. 2019, 27, 1712–1725.e6. [Google Scholar] [CrossRef]
- Lourenço, R.; Brandão, A.S.; Borbinha, J.; Gorgulho, R.; Jacinto, A. Yap Regulates Müller Glia Reprogramming in Damaged Zebrafish Retinas. Front. Cell Dev. Biol. 2021, 9, 667796. [Google Scholar] [CrossRef] [PubMed]
- Osakada, F.; Ooto, S.; Akagi, T.; Mandai, M.; Akaike, A.; Takahashi, M. Wnt Signaling Promotes Regeneration in the Retina of Adult Mammals. J. Neurosci. 2007, 27, 4210–4219. [Google Scholar] [CrossRef]
- Yao, K.; Qiu, S.; Tian, L.; Snider, W.D.; Flannery, J.G.; Schaffer, D.V.; Chen, B. Wnt Regulates Proliferation and Neurogenic Potential of Müller Glial Cells via a Lin28/Let-7 miRNA-Dependent Pathway in Adult Mammalian Retinas. Cell Rep. 2016, 17, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Sanges, D.; Simonte, G.; Di Vicino, U.; Romo, N.; Pinilla, I.; Nicolás, M.; Cosma, M.P. Reprogramming Müller Glia via in Vivo Cell Fusion Regenerates Murine Photoreceptors. J. Clin. Investig. 2016, 126, 3104–3116. [Google Scholar] [CrossRef]
- Todd, L.; Squires, N.; Suarez, L.; Fischer, A.J. Jak/Stat Signaling Regulates the Proliferation and Neurogenic Potential of Müller Glia-Derived Progenitor Cells in the Avian Retina. Sci. Rep. 2016, 6, 35703. [Google Scholar] [CrossRef]
- Jorstad, N.L.; Wilken, M.S.; Todd, L.; Finkbeiner, C.; Nakamura, P.; Radulovich, N.; Hooper, M.J.; Chitsazan, A.; Wilkerson, B.A.; Rieke, F.; et al. STAT Signaling Modifies Ascl1 Chromatin Binding and Limits Neural Regeneration from Muller Glia in Adult Mouse Retina. Cell Rep. 2020, 30, 2195–2208.e5. [Google Scholar] [CrossRef]
- Fischer, A.J.; Scott, M.A.; Ritchey, E.R.; Sherwood, P. Mitogen-Activated Protein Kinase-Signaling Regulates the Ability of Müller Glia to Proliferate and Protect Retinal Neurons against Excitotoxicity. Glia 2009, 57, 1538–1552. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor Signaling Pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef]
- Kelly, L.E.; El-Hodiri, H.M.; Crider, A.; Fischer, A.J. Protein Phosphatases Regulate the Formation of Müller Glia-Derived Progenitor Cells in the Chick Retina. Mol. Cell. Neurosci. 2024, 129, 103932. [Google Scholar] [CrossRef]
- Wan, J.; Zhao, X.-F.; Vojtek, A.; Goldman, D. Retinal Injury, Growth Factors, and Cytokines Converge on β-Catenin and pStat3 Signaling to Stimulate Retina Regeneration. Cell Rep. 2014, 9, 285–297. [Google Scholar] [CrossRef]
- Gallina, D.; Zelinka, C.; Fischer, A.J. Glucocorticoid Receptors in the Retina, Müller Glia and the Formation of Müller Glia-Derived Progenitors. Development 2014, 141, 3340–3351. [Google Scholar] [CrossRef] [PubMed]
- Todd, L. Inducing Neural Regeneration from Glia Using Proneural bHLH Transcription Factors. Adv. Exp. Med. Biol. 2023, 1415, 577–582. [Google Scholar] [CrossRef]
- Fausett, B.V.; Gumerson, J.D.; Goldman, D. The Proneural Basic Helix-Loop-Helix Gene Ascl1a Is Required for Retina Regeneration. J. Neurosci. 2008, 28, 1109–1117. [Google Scholar] [CrossRef]
- Karl, M.O.; Reh, T.A. Regenerative Medicine for Retinal Diseases: Activating Endogenous Repair Mechanisms. Trends Mol. Med. 2010, 16, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Todd, L.; Hooper, M.J.; Haugan, A.K.; Finkbeiner, C.; Jorstad, N.; Radulovich, N.; Wong, C.K.; Donaldson, P.C.; Jenkins, W.; Chen, Q.; et al. Efficient Stimulation of Retinal Regeneration from Müller Glia in Adult Mice Using Combinations of Proneural bHLH Transcription Factors. Cell Rep. 2021, 37, 109857. [Google Scholar] [CrossRef]
- Wohlschlegel, J.; Finkbeiner, C.; Hoffer, D.; Kierney, F.; Prieve, A.; Murry, A.D.; Haugan, A.K.; Ortuño-Lizarán, I.; Rieke, F.; Golden, S.A.; et al. ASCL1 Induces Neurogenesis in Human Müller Glia. Stem Cell Rep. 2023, 18, 2400–2417. [Google Scholar] [CrossRef] [PubMed]
- Torres-Machorro, A.L. Homodimeric and Heterodimeric Interactions among Vertebrate Basic Helix-Loop-Helix Transcription Factors. Int. J. Mol. Sci. 2021, 22, 12855. [Google Scholar] [CrossRef]
- Taylor, O.B.; Patel, S.P.; Hawthorn, E.C.; El-Hodiri, H.M.; Fischer, A.J. ID Factors Regulate the Ability of Müller Glia to Become Proliferating Neurogenic Progenitor-like Cells. Glia 2024, 72, 1236–1258. [Google Scholar] [CrossRef]
- Wilczynska, K.M.; Singh, S.K.; Adams, B.; Bryan, L.; Rao, R.R.; Valerie, K.; Wright, S.; Griswold-Prenner, I.; Kordula, T. Nuclear Factor I Isoforms Regulate Gene Expression during the Differentiation of Human Neural Progenitors to Astrocytes. Stem Cells 2009, 27, 1173–1181. [Google Scholar] [CrossRef]
- El-Hodiri, H.M.; Campbell, W.A.; Kelly, L.E.; Hawthorn, E.C.; Schwartz, M.; Jalligampala, A.; McCall, M.A.; Meyer, K.; Fischer, A.J. Nuclear Factor I in Neurons, Glia and during the Formation of Müller Glia-Derived Progenitor Cells in Avian, Porcine and Primate Retinas. J. Comp. Neurol. 2022, 530, 1213–1230. [Google Scholar] [CrossRef]
- Mitra, S.; Sharma, P.; Kaur, S.; Khursheed, M.A.; Gupta, S.; Ahuja, R.; Kurup, A.J.; Chaudhary, M.; Ramachandran, R. Histone Deacetylase-Mediated Müller Glia Reprogramming through Her4.1-Lin28a Axis Is Essential for Retina Regeneration in Zebrafish. iScience 2018, 7, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Strahl, B.D.; Allis, C.D. The Language of Covalent Histone Modifications. Nature 2000, 403, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zang, C.; Cui, K.; Schones, D.E.; Barski, A.; Peng, W.; Zhao, K. Genome-Wide Mapping of HATs and HDACs Reveals Distinct Functions in Active and Inactive Genes. Cell 2009, 138, 1019–1031. [Google Scholar] [CrossRef]
- Sanhueza Salas, L.F.; García-Venzor, A.; Beltramone, N.; Capurro, C.; Toiber, D.; Silberman, D.M. Metabolic Imbalance Effect on Retinal Müller Glial Cells Reprogramming Capacity: Involvement of Histone Deacetylase SIRT6. Front. Genet. 2021, 12, 769723. [Google Scholar] [CrossRef]
- Lee, J.; Lee, B.-K.; Gross, J.M. Brd Activity Regulates Müller Glia-Dependent Retinal Regeneration in Zebrafish. Glia 2023, 71, 2866–2883. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Iwagawa, T.; Ochiai, G.; Koso, H.; Nakauchi, H.; Nagasaki, M.; Suzuki, Y.; Watanabe, S. Analysis of Müller Glia Specific Genes and Their Histone Modification Using Hes1-Promoter Driven EGFP Expressing Mouse. Sci. Rep. 2017, 7, 3578. [Google Scholar] [CrossRef]
- Sawicka, A.; Seiser, C. Histone H3 Phosphorylation—A Versatile Chromatin Modification for Different Occasions. Biochimie 2012, 94, 2193–2201. [Google Scholar] [CrossRef]
- Coppedè, F. Mitochondrial DNA Methylation and Mitochondria-Related Epigenetics in Neurodegeneration. Neural Regen. Res. 2024, 19, 405–406. [Google Scholar] [CrossRef]
- Reyes-Aguirre, L.I.; Lamas, M. Oct4 Methylation-Mediated Silencing As an Epigenetic Barrier Preventing Müller Glia Dedifferentiation in a Murine Model of Retinal Injury. Front. Neurosci. 2016, 10, 523. [Google Scholar] [CrossRef]
- Powell, C.; Grant, A.R.; Cornblath, E.; Goldman, D. Analysis of DNA Methylation Reveals a Partial Reprogramming of the Müller Glia Genome during Retina Regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 19814–19819. [Google Scholar] [CrossRef]
- Dvoriantchikova, G.; Seemungal, R.J.; Ivanov, D. Development and Epigenetic Plasticity of Murine Müller Glia. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Seritrakul, P.; Gross, J.M. Tet-Mediated DNA Hydroxymethylation Regulates Retinal Neurogenesis by Modulating Cell-Extrinsic Signaling Pathways. PLoS Genet. 2017, 13, e1006987. [Google Scholar] [CrossRef]
- Konar, G.J.; Ferguson, C.; Flickinger, Z.; Kent, M.R.; Patton, J.G. miRNAs and Müller Glia Reprogramming During Retina Regeneration. Front. Cell Dev. Biol. 2020, 8, 632632. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, K.; Harding, R.L.; Bailey, T.; Patton, J.G.; Hyde, D.R. Dynamic miRNA Expression Patterns during Retinal Regeneration in Zebrafish: Reduced Dicer or miRNA Expression Suppresses Proliferation of Müller Glia-Derived Neuronal Progenitor Cells. Dev. Dyn. 2014, 243, 1591–1605. [Google Scholar] [CrossRef]
- Wohl, S.G.; Reh, T.A. miR-124-9-9* Potentiates Ascl1-Induced Reprogramming of Cultured Müller Glia. Glia 2016, 64, 743–762. [Google Scholar] [CrossRef]
- Wohl, S.G.; Hooper, M.J.; Reh, T.A. MicroRNAs miR-25, Let-7 and miR-124 Regulate the Neurogenic Potential of Müller Glia in Mice. Development 2019, 146, dev179556. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Tao, Z.; Xue, L.; Zeng, Y.; Wang, Y.; Xu, H.; Yin, Z.Q. Lin28b Stimulates the Reprogramming of Rat Müller Glia to Retinal Progenitors. Exp. Cell Res. 2017, 352, 164–174. [Google Scholar] [CrossRef]
- Rajaram, K.; Harding, R.L.; Hyde, D.R.; Patton, J.G. miR-203 Regulates Progenitor Cell Proliferation during Adult Zebrafish Retina Regeneration. Dev. Biol. 2014, 392, 393–403. [Google Scholar] [CrossRef]
- Thummel, R.; Kassen, S.C.; Enright, J.M.; Nelson, C.M.; Montgomery, J.E.; Hyde, D.R. Characterization of Müller Glia and Neuronal Progenitors during Adult Zebrafish Retinal Regeneration. Exp. Eye Res. 2008, 87, 433–444. [Google Scholar] [CrossRef]
- Kara, N.; Kent, M.R.; Didiano, D.; Rajaram, K.; Zhao, A.; Summerbell, E.R.; Patton, J.G. The miR-216a-Dot1l Regulatory Axis Is Necessary and Sufficient for Müller Glia Reprogramming during Retina Regeneration. Cell Rep. 2019, 28, 2037–2047.e4. [Google Scholar] [CrossRef]
- Magner, E.; Sandoval-Sanchez, P.; Kramer, A.C.; Thummel, R.; Hitchcock, P.F.; Taylor, S.M. Disruption of miR-18a Alters Proliferation, Photoreceptor Replacement Kinetics, Inflammatory Signaling, and Microglia/Macrophage Numbers During Retinal Regeneration in Zebrafish. Mol. Neurobiol. 2022, 59, 2910–2931. [Google Scholar] [CrossRef]
- Si, T.-E.; Li, Z.; Zhang, J.; Su, S.; Liu, Y.; Chen, S.; Peng, G.-H.; Cao, J.; Zang, W. Epigenetic Mechanisms of Müller Glial Reprogramming Mediating Retinal Regeneration. Front. Cell Dev. Biol. 2023, 11, 1157893. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, S.; Yong, V.W. The Extracellular Matrix as Modifier of Neuroinflammation and Remyelination in Multiple Sclerosis. Brain 2021, 144, 1958–1973. [Google Scholar] [CrossRef]
- Melrose, J.; Hayes, A.J.; Bix, G. The CNS/PNS Extracellular Matrix Provides Instructive Guidance Cues to Neural Cells and Neuroregulatory Proteins in Neural Development and Repair. Int. J. Mol. Sci. 2021, 22, 5583. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, M.; Xie, D.; Liu, X.; Yan, H. Role of the Extracellular Matrix and YAP/TAZ in Cell Reprogramming. Differ. Res. Biol. Divers. 2021, 122, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.A., IV; Deshmukh, A.; Blum, S.; Todd, L.; Mendonca, N.; Weist, J.; Zent, J.; Hoang, T.V.; Blackshaw, S.; Leight, J.; et al. Matrix-Metalloproteinase Expression and Gelatinase Activity in the Avian Retina and Their Influence on Müller Glia Proliferation. Exp. Neurol. 2019, 320, 112984. [Google Scholar] [CrossRef]
- Silva, N.J.; Nagashima, M.; Li, J.; Kakuk-Atkins, L.; Ashrafzadeh, M.; Hyde, D.R.; Hitchcock, P.F. Inflammation and Matrix Metalloproteinase 9 (Mmp-9) Regulate Photoreceptor Regeneration in Adult Zebrafish. Glia 2020, 68, 1445–1465. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, H.; Yu, S.; Zhou, C.; Zhang, X.; Li, N.; Zhang, S.; Song, K.; Lu, Y.; Liu, D.; et al. Inflammation-Induced Mammalian Target of Rapamycin Signaling Is Essential for Retina Regeneration. Glia 2020, 68, 111–127. [Google Scholar] [CrossRef]
- Vay, S.U.; Flitsch, L.J.; Rabenstein, M.; Rogall, R.; Blaschke, S.; Kleinhaus, J.; Reinert, N.; Bach, A.; Fink, G.R.; Schroeter, M.; et al. The Plasticity of Primary Microglia and Their Multifaceted Effects on Endogenous Neural Stem Cells in Vitro and in Vivo. J. Neuroinflamm. 2018, 15, 226. [Google Scholar] [CrossRef]
- Yu, C.; Roubeix, C.; Sennlaub, F.; Saban, D.R. Microglia versus Monocytes: Distinct Roles in Degenerative Diseases of the Retina. Trends Neurosci. 2020, 43, 433–449. [Google Scholar] [CrossRef]
- Gascón, S.; Murenu, E.; Masserdotti, G.; Ortega, F.; Russo, G.L.; Petrik, D.; Deshpande, A.; Heinrich, C.; Karow, M.; Robertson, S.P.; et al. Identification and Successful Negotiation of a Metabolic Checkpoint in Direct Neuronal Reprogramming. Cell Stem Cell 2016, 18, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Tsogtbaatar, E.; Landin, C.; Minter-Dykhouse, K.; Folmes, C.D.L. Energy Metabolism Regulates Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 87. [Google Scholar] [CrossRef]
- Polyzos, A.A.; Lee, D.Y.; Datta, R.; Hauser, M.; Budworth, H.; Holt, A.; Mihalik, S.; Goldschmidt, P.; Frankel, K.; Trego, K.; et al. Metabolic Reprogramming in Astrocytes Distinguishes Region-Specific Neuronal Susceptibility in Huntington Mice. Cell Metab. 2019, 29, 1258–1273.e11. [Google Scholar] [CrossRef]
- Fridman, E.S.; Ginini, L.; Gil, Z. The Role of Extracellular Vesicles in Metabolic Reprogramming of the Tumor Microenvironment. Cells 2022, 11, 1433. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Kang, J.; Cheng, X.; Gao, H.; Huo, S.; Xu, H. Investigating Müller Glia Reprogramming in Mice: A Retrospective of the Last Decade, and a Look to the Future. Neural Regen. Res. 2025, 20, 946–959. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Qiu, S.; Wang, Y.V.; Park, S.J.H.; Mohns, E.J.; Mehta, B.; Liu, X.; Chang, B.; Zenisek, D.; Crair, M.C.; et al. Restoration of Vision after de Novo Genesis of Rod Photoreceptors in Mammalian Retinas. Nature 2018, 560, 484–488. [Google Scholar] [CrossRef]
- Xiao, D.; Jin, K.; Qiu, S.; Lei, Q.; Huang, W.; Chen, H.; Su, J.; Xu, Q.; Xu, Z.; Gou, B.; et al. In Vivo Regeneration of Ganglion Cells for Vision Restoration in Mammalian Retinas. Front. Cell Dev. Biol. 2021, 9, 755544. [Google Scholar] [CrossRef]
- Bernardos, R.L.; Barthel, L.K.; Meyers, J.R.; Raymond, P.A. Late-Stage Neuronal Progenitors in the Retina Are Radial Müller Glia That Function as Retinal Stem Cells. J. Neurosci. 2007, 27, 7028–7040. [Google Scholar] [CrossRef]
- Jayaram, H.; Jones, M.F.; Eastlake, K.; Cottrill, P.B.; Becker, S.; Wiseman, J.; Khaw, P.T.; Limb, G.A. Transplantation of Photoreceptors Derived from Human Muller Glia Restore Rod Function in the P23H Rat. Stem Cells Transl. Med. 2014, 3, 323–333. [Google Scholar] [CrossRef]
- Eastlake, K.; Wang, W.; Jayaram, H.; Murray-Dunning, C.; Carr, A.J.F.; Ramsden, C.M.; Vugler, A.; Gore, K.; Clemo, N.; Stewart, M.; et al. Phenotypic and Functional Characterization of Müller Glia Isolated from Induced Pluripotent Stem Cell-Derived Retinal Organoids: Improvement of Retinal Ganglion Cell Function upon Transplantation. Stem Cells Transl. Med. 2019, 8, 775–784. [Google Scholar] [CrossRef]
- Eastlake, K.; Lamb, W.D.B.; Luis, J.; Khaw, P.T.; Jayaram, H.; Limb, G.A. Prospects for the Application of Müller Glia and Their Derivatives in Retinal Regenerative Therapies. Prog. Retin. Eye Res. 2021, 85, 100970. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.V.; Bull, N.D.; Martin, K.R. Neurotrophic Factor Delivery as a Protective Treatment for Glaucoma. Exp. Eye Res. 2011, 93, 196–203. [Google Scholar] [CrossRef]
- Mead, B.; Amaral, J.; Tomarev, S. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in Rodent Models of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 702–714. [Google Scholar] [CrossRef]
- Clark, B.S.; Stein-O’Brien, G.L.; Shiau, F.; Cannon, G.H.; Davis-Marcisak, E.; Sherman, T.; Santiago, C.P.; Hoang, T.V.; Rajaii, F.; James-Esposito, R.E.; et al. Single-Cell RNA-Seq Analysis of Retinal Development Identifies NFI Factors as Regulating Mitotic Exit and Late-Born Cell Specification. Neuron 2019, 102, 1111–1126.e5. [Google Scholar] [CrossRef]
- Collin, J.; Queen, R.; Zerti, D.; Dorgau, B.; Hussain, R.; Coxhead, J.; Cockell, S.; Lako, M. Deconstructing Retinal Organoids: Single Cell RNA-Seq Reveals the Cellular Components of Human Pluripotent Stem Cell-Derived Retina. Stem Cells 2019, 37, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis Pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current Understanding of the Molecular and Cellular Pathology of Diabetic Retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef]
- Coughlin, B.A.; Feenstra, D.J.; Mohr, S. Müller Cells and Diabetic Retinopathy. Vis. Res. 2017, 139, 93–100. [Google Scholar] [CrossRef]
- Curcio, C.A.; Medeiros, N.E.; Millican, C.L. Photoreceptor Loss in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1236–1249. [Google Scholar]
- Zhao, N.; Hao, X.-N.; Huang, J.-M.; Song, Z.-M.; Tao, Y. Crosstalk between Microglia and Müller Glia in the Age-Related Macular Degeneration: Role and Therapeutic Value of Neuroinflammation. Aging Dis. 2024, 15, 1132–1154. [Google Scholar] [CrossRef]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The Molecular Basis of Retinal Ganglion Cell Death in Glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Su, J.; Hu, X.; Zhou, C.; Li, H.; Chen, Z.; Xiao, Q.; Wang, B.; Wu, W.; Sun, Y.; et al. Glia-to-Neuron Conversion by CRISPR-CasRx Alleviates Symptoms of Neurological Disease in Mice. Cell 2020, 181, 590–603.e16. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhu, J.; Duan, Y.; Li, G.; Cai, H.; Zheng, L.; Qian, H.; Zhang, C.; Jin, Z.; Fu, X.-D.; et al. Visual Function Restoration in Genetically Blind Mice via Endogenous Cellular Reprogramming. bioRxiv 2020. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, J.; Chen, B. Critical Examination of Ptbp1-Mediated Glia-to-Neuron Conversion in the Mouse Retina. Cell Rep. 2022, 39, 110960. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, J.; Wang, L.-L.; Zhang, C.-L.; Chen, B. New AAV Tools Fail to Detect Neurod1-Mediated Neuronal Conversion of Müller Glia and Astrocytes in Vivo. EBioMedicine 2023, 90, 104531. [Google Scholar] [CrossRef]
- Hoang, T.; Kim, D.W.; Appel, H.; Pannullo, N.A.; Leavey, P.; Ozawa, M.; Zheng, S.; Yu, M.; Peachey, N.S.; Blackshaw, S. Genetic Loss of Function of Ptbp1 Does Not Induce Glia-to-Neuron Conversion in Retina. Cell Rep. 2022, 39, 110849. [Google Scholar] [CrossRef]
- Chen, G. In Vivo Confusion over in Vivo Conversion. Mol. Ther. 2021, 29, 3097–3098. [Google Scholar] [CrossRef]
- Blackshaw, S.; Sanes, J.R. Turning Lead into Gold: Reprogramming Retinal Cells to Cure Blindness. J. Clin. Investig. 2021, 131, e146134. [Google Scholar] [CrossRef]
- Grabinski, S.E.; Parsana, D.; Perkins, B.D. Comparative Analysis of Transcriptional Changes in Zebrafish Cep290 and Bbs2 Mutants by RNA-Seq Reveals Upregulation of Inflammatory and Stress-Related Pathways. Front. Mol. Neurosci. 2023, 16, 1148840. [Google Scholar] [CrossRef]
- Parain, K.; Chesneau, A.; Locker, M.; Borday, C.; Perron, M. Regeneration from Three Cellular Sources and Ectopic Mini-Retina Formation upon Neurotoxic Retinal Degeneration in Xenopus. Glia 2024, 72, 759–776. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).