Abstract
Background/Objectives: The annexin A1 (AnxA1) protein has proven important in ocular disease homeostasis and holds great therapeutic promise. However, its role in the context of the healthy retina remains unknown. Therefore, this study used electroretinography (ERG) to investigate the role of endogenous AnxA1 in the retinal function of wild-type (WT) and AnxA1 knockout mice (AnxA1−/−). Methods: An extensive repertoire of full-field ERG was applied to AnxA1−/− and WT mice to examine retinal physiology. Morphometric analyses of the retina were conducted. Results: Our results revealed significant differences in the implicit time of a-wave and b-wave between the WT and AnxA1−/− groups under scotopic conditions. The negative and positive amplitude components of mesopic ON responses were higher in the AnxA1-/- group than in the WT group. In contrast, the implicit time of mesopic ON responses were significantly higher in the WT group than in the AnxA1-/- WT group. However, in photopic OFF responses, only the implicit time was significantly longer in the WT group than in the AnxA1−/− group. In the histomorphometric analysis, the retina of AnxA1−/− mice shows increased thickness. Conclusions: The absence of AnxA1 alters retinal morphology and physiology.
1. Introduction
Degenerative retinal diseases, such as diabetic retinopathy and age-related macular degeneration, share common characteristics of neuronal loss, gliosis, Müller cell loss, and inflammation [1,2]. When diagnosing and monitoring these diseases, electroretinography (ERG) is essential for assessing the functional status of the retina [3,4].
In addition to advances in using ERG as a biomarker in various conditions of the central nervous system, recent research has investigated the role of annexin A1 (AnxA1) in ophthalmic diseases. AnxA1 is a Ca2+/phospholipid-binding protein that belongs to the annexin family, and its multifunctional role has been explored in the central nervous and visual systems [5]. AnxA1 is known to modulate inflammatory processes, apoptosis, and phagocytosis [6]. AnxA1 is also involved in intracellular signaling processes, cytoskeleton, and membrane dynamics [7], making it essential in regulating and maintaining the visual system.
Proteomic studies of the murine retina have shown that AnxA1 and AnxA2 are the most abundant annexin family members [8]. AnxA1 is more abundant in Müller cells and microglia than in retinal neurons [8]. Due to its crucial anti-inflammatory and pro-resolutive roles, the lack of endogenous AnxA1 was associated with a worse outcome in uveitis and retinal ischemia in animal models [9,10,11]. Furthermore, in an ischemia–reperfusion model, the secretion of AnxA1 by ganglion cells was essential for regulating microglial activation and protecting against the inflammatory damage caused by the disease [12].
While studies on AnxA1 highlight its significant role as a therapeutic target in ocular diseases [5], its normal physiological role in the retina remains unexplored. Therefore, this study used ERG to investigate the role of endogenous AnxA1 in retinal function in WT and AnxA1−/− mice. Our hypothesis is that the lack of Annexin A1 protein can cause functional alterations in the retina of mice. Our hypothesis is that the lack of AnxA1 impairs retinal function, leading to detectable alterations in retinal responses and morphology in mice.
2. Materials and Methods
2.1. Animals
ERG was performed on 14 WT and 15 AnxA1−/− male C57BL/6 mice aged 11–12 weeks, weighing 20–25 g. The mice were obtained from the Center for Development of Experimental Models for Medicine and Biology (CEDEME) at the Federal University of São Paulo (UNIFESP). The animals were maintained with ad libitum access to water and food in a dedicated room with acoustic isolation, controlled temperature (22 °C ± 1 °C), and a 12/12 h light/dark cycle. The experimental procedures were approved by the Ethics Committee on Animal Experimentation at UNIFESP (No.: 858230821; ID: 011028) and IP-USP (No.: 1583180822; ID: 000309) and performed according to the ARRIVE guidelines.
2.2. Animal Preparation
The protocols and procedures were performed as described previously [13,14]. Briefly, the mice were dark-adapted for at least 12 h, and all animal preparation and electrode placement were performed under deep red illumination to maintain the retina in a dark-adapted state. Anesthesia was performed by intramuscular injection with a single dose of 10% ketamine (Dopalen; Ceva, São Paulo, SP, Brazil) and 2% xylazine (Anasedan; Ceva), 1:1—1 µL/g. During the ERG recordings, mice were positioned on a water-heated platform (38 °C) to maintain their body temperature during anesthesia. Subcutaneous injections of 0.9% saline (300 μL before and 100 μL after the recordings) were given to prevent dehydration. The pupils were fully dilated using 1% tropicamide eye drops (Ciclomidrin®; Cristalia, São Paulo, SP, Brazil) followed by anesthetic eye drops (0.4% oxybuprocaine hydrochloride; Oxinest®; Cristalia) to prevent any discomfort on the cornea. Goldring electrodes (Ø 1 mm; Roland Consult, Brandenburg, Germany) were positioned on the corneas of both eyes (active electrodes) with 2% methylcellulose (Ophthalmos, São Paulo, SP, Brazil) to prevent cornea dehydration (methylcellulose was again placed on the cornea after the recordings). Two needle electrodes were placed subcutaneously medial to the two ears (reference electrodes), and one was positioned subcutaneously at the base of the tail (ground electrode; Concentric Subdermal Steel Needle; Roland Consult).
2.3. ERG Setup and Recordings
Binocular recordings of full-field ERGs and stimulus presentations were performed with the RetiPort system (Roland Consult) using a Ganzfeld bowl (Q450SC; Roland Consult). Signals were amplified 100,000 times, filtered with a bandpass filter between 1 and 300 Hz, and digitized at a rate of 512 Hz (flashes) or 1024 Hz (flicker). ERGs were recorded in order of increasing mean luminance for the following stimulus conditions.
Scotopic flashes: Dark-adapted rod and mixed rod-cone mediated ERG responses were recorded to white light flashes of −3.7, −2.7, −1.7, −0.7, and 0.3 log cd.s/m2 intensities on a dark background. The number of sweeps decreased with increasing flash intensity (12, 10, 8, 8, and 4, respectively). The interval between stimuli was progressively increased with increasing flash intensity (1, 2, 5, 10, and 20 s, respectively) to preserve dark adaptation. In addition, the interval between each intensity series increased from 30 to 120 s as the flash intensity increased.
Mesopic ON and OFF responses: After a pre-adaptation period of 2 min to 1 cd/m2 mean luminance on a white background, rapid-on and -off sawtooth stimuli (white light), evoking ON responses (to luminance increments) and OFF responses (to luminance decrements), respectively, were obtained at a mean luminance of 1 cd/m2 (white light). The sawtooth was presented at 4 Hz (period = 250 ms) and 100% luminance (Michelson) contrast. The first 2 s after stimulus onset were discarded to avoid onset artifacts. The averages of at least forty 1 s sweeps were obtained.
Photopic flashes: After a pre-adaptation period of 2 min on a white background of 25 cd/m2, white flashes of 0.3 log cd.s/m2 intensity were presented. Responses to 24 flashes were averaged with an interstimulus interval of 1 s.
Photopic sine-wave: After a pre-adaptation period of 2 min to 60 cd/m2 on a white background, ERGs to sinusoidal luminance modulation (100% Michelson contrast; 60 cd/m2 mean luminance [white light]) were measured at 10 randomly presented temporal frequencies between 3 and 30 Hz. The first 2 s after stimulus onset were discarded to avoid onset artifacts. The averages of at least twenty 1 s sweeps were obtained.
Photopic ON and OFF responses: Rapid-on and -off sawtooth stimuli (white light) evoking ON responses (to luminance increments) and OFF responses (to luminance decrements), respectively, were obtained at a mean luminance of 60 cd/m2. As in the mesopic assessment, the sawtooth was presented at 4 Hz with a temporal profile of 250 ms and 100% luminance (Michelson) contrast. The first 2 s after stimulus onset were discarded to avoid onset artifacts. The averages of at least forty 1 s sweeps were obtained.
2.4. ERG Signal Processing
The ERG components were analyzed offline, including peak/trough detection, baseline measurements, and Fourier analysis, using Matlab® routines (The Mathworks Inc., Natick, MA, USA) written and provided by Professor Jan Kremers’ group (FAU Erlangen-Nürnberg, Erlangen, Germany) and Excel spreadsheets (Microsoft Office 2016; Microsoft Corp., Redmond, WA, USA). The processing and analysis of the ERG signals were performed as previously described [13,14]. The oscillatory potentials (OPs) were isolated using a variable filtering method [15], and ERGs without OPs were used to measure a- and b-wave parameters. Isolated OPs were also analyzed.
The amplitude of the scotopic a-wave was defined as the difference in μV between the baseline (average of recordings 16 ms before the flash) and the minimum within 50 ms after stimulus onset. The amplitude of the scotopic b-wave was the difference in μV between the a-wave trough and the peak of the b-wave. The implicit time corresponds to the interval between the stimulus onset and the peak of the a-wave or b-wave. The OPs were analyzed in the frequency domain (Fourier analysis), and the amplitudes are presented as a function of flash intensity. The b-waves of the light-adapted flash ERG were measured as described for the scotopic b-wave. As shown previously [13,14], when applying the same analysis (Matlab routines), the photopic a-wave components were considerably reduced and were not included in the analyses. For the ON and OFF recordings, the first troughs were taken from the baseline, with subsequent component amplitudes measured from the preceding peak or trough. Steady-state ERGs in response to sine-wave modulation were Fourier analyzed to obtain the amplitudes and phases of the first harmonic (fundamental) components. Noise was defined as the mean of the amplitudes at the stimulus frequencies ±1 Hz. The first harmonic phase values were included in subsequent analyses only if the response signal-to-noise ratio was ≥2, and the first harmonic amplitude was corrected by subtracting the noise.
2.5. Histomorphometric Analysis
The eyes were fixed in 4% paraformaldehyde (#30525-89-4, Sigma-Aldrich, St. Louis, MO, USA) for 24 h, washed in tap water, dehydrated in a decreasing ethanol series, and embedded in paraffin. Sections of 4 μm thickness were obtained using a Leica RM2155 microtome (Leica Microsystems, Nussloch, Germany) and stained with hematoxylin and eosin for morphometric analysis of the retina. Slides were scanned using the Aperio® CS2 slide scanner equipment (Leica Microsystems, Wetzlar, Germany). The total, central and peripheral thickness of the retina was measured Using QuPath software version 0.3.2 (University of Edinburgh, UK), Three perpendicular lines were taken in the peripheral nasal and temporal regions of the retina and the central region, and the average of the three measurements of each region was taken for later analysis. The central retina was considered to be the area located approximately 0.5–1.0 mm from the optic nerve, a region with greater cell and vascular density. The peripheral retina corresponds to the area located more than 2.0 mm from the optic disk towards the ora serrata, characterized by a gradual reduction in the thickness of the neuronal layers and capillary complexity. The software quantified the total number of cells in the outer nuclear layer (ONL) and inner nuclear layer (INL). The thickness values (in μm) and total number of cells (mm2) are presented as the mean ± standard error of the mean (±SEM).
2.6. Statistical Analyses
All ERG data are expressed as means ± standard deviations. Statistical analyses to verify genotype and group differences were performed with Jamovi software (version 2.3; The Jamovi Project, Sydney, Australia) using one or two-way analysis of variance, depending on the presence of a within-subject repeated measure (intensity, frequency). The histomorphometric analysis data were analyzed using GraphPad Prism software (version 9.1). The data were first descriptively analyzed and assessed for normality (Kolmogorov–Smirnov test). Normally distributed variables were compared between groups using a t-test. For all tests, a p-value of <0.05 was considered statistically significant.
3. Results
3.1. Dark- and Light-Adapted Flash ERGs
The recordings obtained under dark-adapted (scotopic) and light-adapted (photopic) conditions assess the integrity of the photoreceptors (cones and rods) and bipolar cells in the retina and the inner retina circuitry involved in generating Ops. In both WT and AnxA1−/− mice, the dark-adapted flash protocol evoked a negative a-wave followed by a positive b-wave. The a-wave appeared only at intensities equal to or higher than −1.7 log cd.s/m2 (Figure 1a). As expected, increasing the light flash intensity during scotopic recordings led to a progressive increase in the amplitudes of both the a-waves and b-waves (Figure 1b, upper plots).
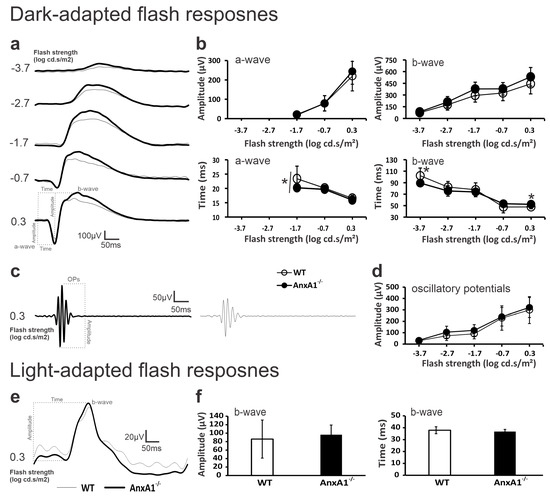
Figure 1.
The scotopic and photopic flash ERGs of WT (thin traces/open symbols) and AnxA1−/− (bold traces/filled symbols) mice. (a) The average dark-adapted waveforms without OPs of WT and AnxA1−/− mice. (b) The mean (±standard deviation) amplitudes (μV; upper plots) and implicit times (ms; lower plots) of scotopic a- and b-waves in WT and AnxA1−/− mice. (c) The average OP traces isolated from the strongest scotopic flash (0.3 log cd.s/m2) in WT AnxA1−/− mice. (d) The mean (±standard deviation) amplitude of OPs as a function of flash intensity in WT and AnxA1−/− mice. (e) The average light-adapted waveforms of WT and AnxA1−/− mice. (f) Histograms showing the mean (±standard deviation) amplitude and implicit time of the photopic b-wave. The dotted lines in a, c, and e show the physiological hallmarks used to measure implicit times and/or amplitudes. Recordings were made in the 18 eyes of 9 WT mice and 20 eyes of 10 AnxA1−/− mice. Asterisks indicate significant differences (p < 0.05). The asterisk in the implicit times of the scotopic a-wave indicates a genotypic effect.
The analysis showed no significant differences in the amplitude of the a-waves (genotype effect: F = 0.848, p = 0.365; genotype × amplitude interaction: F = 2.96, p = 0.060) or b-waves (genotype effect: F = 3.51, p = 0.056; genotype × amplitude interaction: F = 4.00, p = 0.200) between WT and AnxA1−/− mice at any of the tested intensities (Figure 1b, upper plots). However, the implicit time defined as the interval between the stimulus (flash) and the peak of the retinal electrical response significantly differed between the two genotypes for the a-wave (genotype effect: F = 4.82, p = 0.035; no significant genotype × implicit time interaction: F = 2.74, p = 0.072). For the b-wave, significant differences in implicit time appeared at intensities of −3.7 and 0.3 log cd.s/m2 (genotype × implicit time interaction: F = 9.92, p = 0.009 and 0.007, respectively), although the genotype effect remained non-significant (F = 2.95, p = 0.094).
Oscillatory potentials (OPs) did not show significant differences between WT and AnxA1−/− mice at any of the tested intensities (genotype effect: F = 1.31, p = 0.259; genotype × amplitude interaction: F = 0.575, p = 0.681; Figure 1d). Under photopic conditions, a flash of 0.3 log cd.s/m2 elicited similar b-wave implicit times (genotype × implicit time interaction: F = 1.860, p = 0.171) and amplitudes (genotype × amplitude interaction: F = 0.969, p = 0.316; Figure 1f) in both genotypes (Figure 1e,f).
3.2. Sawtooth ON and OFF ERGs
Sawtooth stimuli with a rapid on increment and rapid off decrement allow the integrity of the visual pathways mediated by ON and OFF bipolar cells to be assessed [16]. We recorded the responses under mesopic and photopic conditions. Under mesopic conditions, ON responses exhibited a small initial negative component followed by a larger positive component. In contrast, OFF responses showed a larger initial negative component followed by a smaller positive component (Figure 2a,b).
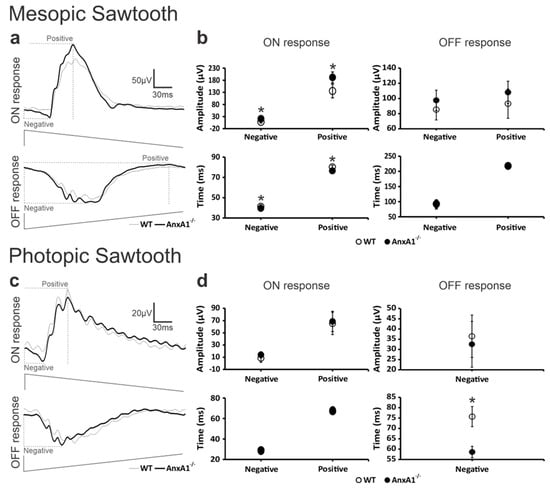
Figure 2.
The ON- and OFF-mediated ERG components. The averaged waveforms of (a) mesopic ON- and OFF-mediated responses and (c) photopic ON- and OFF-mediated responses in WT (thin traces) and AnxA1−/− (bold traces) mice. Representations of the luminance modulation for the rapid-on and the rapid-off protocols are shown below the traces. Definitions of key components for measurements of amplitudes and/or implicit times (dotted lines) for negative and positive components obtained with mesopic and photopic stimulation are indicated. The plots show the mean (± standard deviation) amplitudes (μV) and implicit times (ms) for the (b) mesopic and (d) photopic sawtooth protocols. Recordings were made in the 10 eyes of five animals for both AnxA1−/− and WT mice. Asterisks indicate significant (p < 0.05) genotype differences.
In mesopic ON responses, the WT group exhibited significantly longer implicit times for both negative and positive components compared to the AnxA1−/− group (genotype effect: F = 28.9, p = 0.001; genotype × implicit time [−] interaction: F = 10.9, p = 0.027; genotype × implicit time [+] interaction: p = 0.001). Additionally, the AnxA1−/− group showed significantly higher amplitudes and longer implicit times in both components when compared to the WT group (genotype effect: F = 35.3, p = 0.001; genotype × amplitude [−] interaction: F = 13.6, p = 0.001; genotype × amplitude [+] interaction: p = 0.001).
In mesopic OFF responses, we observed no significant differences between the groups. The implicit times remained similar in both WT and AnxA1−/− mice (genotype effect: F = 0.0753, p = 0.787; genotype × implicit time [−] interaction: F = 0.0035, p = 0.996; genotype × implicit time [+] interaction: p = 0.988), while the AnxA1−/− group presented slightly higher amplitudes that did not reach statistical significance (genotype effect: F = 4.00, p = 0.061; genotype × amplitude [−] interaction: F = 1.27, p = 0.221; genotype × amplitude [+] interaction: p = 0.241).
Under photopic conditions, ON responses mirrored the mesopic ON pattern, showing a small initial negative component followed by a larger positive component. Conversely, photopic OFF responses presented only a negative component (Figure 2c,d). In photopic ON responses, we found no significant differences between the groups for any of the examined parameters. The implicit times were similar between genotypes (genotype effect: F = 0.0019, p = 0.966; genotype × implicit time [−] interaction: F = 3.04, p = 0.900; genotype × implicit time [+] interaction: p = 0.845). Although the AnxA1−/− group showed slightly larger amplitudes, the difference was not statistically significant (genotype effect: F = 0.811, p = 0.380; genotype × amplitude [−] interaction: F = 0.136, p = 0.198; genotype × amplitude [+] interaction: p = 0.972).
Unlike mesopic OFF responses, photopic OFF responses exhibited significantly longer implicit times in the WT group compared to the AnxA1−/− group (genotype × implicit time [−] interaction: F = 93.591, p = 0.001), while the amplitude remained statistically unchanged (genotype × amplitude [−] interaction: F = 0.660, p = 0.427).
These findings demonstrate that the absence of AnxA1 alters retinal ON and OFF responses in mice. Under mesopic conditions, the ON responses exhibited significant changes in both implicit time and amplitude, while the OFF responses remained intact. Under photopic conditions, only the implicit time of the negative component of the OFF response changed, with no alteration in amplitude.
3.3. ERGs to Luminance Sine-Wave Modulations
We assessed the integrity of post-receptoral mechanisms in the cone system—which contribute to the processing of temporal luminance—by applying sinusoidal wave stimuli at ten temporal frequencies ranging from 3 to 30 Hz (Figure 3a). We analyzed both the phase and amplitude of the first harmonic across these frequencies.
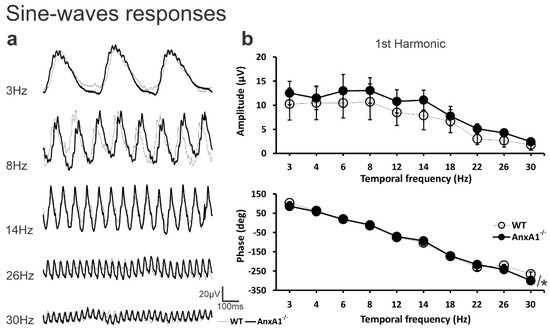
Figure 3.
The responses to sine-wave stimuli. (a) The averaged waveforms of WT (thin traces) and AnxA1−/− (thick traces) mice to sine-wave stimuli at 3, 8, 14, 26, and 30 Hz. (b) The mean (± standard deviation) for first harmonic amplitudes (μV; upper plot) and phases (degrees; lower plot) as a function of temporal frequency between 3 and 30 Hz in WT (open symbols) and AnxA1−/− (filled symbols) mice. Recordings were made in the 10 eyes of five animals from AnxA1−/− and WT mice. Asterisks indicate significant (p < 0.05) genotype differences.
Although the AnxA1−/− group consistently showed higher amplitudes than the WT group at all tested frequencies, this difference did not reach statistical significance for the first harmonic amplitude (genotype effect: F = 32.48, p = 0.080; genotype × amplitude interaction: F = 1.22, p = 0.285). Despite the similarity in overall amplitude, we detected a significant difference in the phase of the first harmonic between the two groups (genotype effect: F = 6.67, p = 0.019). Notably, the phases at 26 and 30 Hz differed significantly between WT and AnxA1−/− mice (genotype × frequency interaction: F = 17.5 [p = 0.002] and p < 0.001, respectively; Figure 3b).
3.4. Histomorphometric Analysis
We conducted a histomorphometric evaluation of retinal tissue (Figure 4A–I). The assessment included both central and peripheral retinal regions from WT and AnxA1−/− mice (Figure 4A–D). By analyzing hematoxylin-stained sections, we quantified the total number of nuclei in the outer nuclear layer (ONL) and inner nuclear layer (INL). This analysis revealed no significant differences between the two genotypes (Figure 4E,F). Further examination of retinal thickness showed distinct patterns. When we measured the overall thickness across central and peripheral areas (Figure 4G), as well as when we evaluated the central (Figure 4H) and peripheral (Figure 4I) regions individually, we detected a consistent increase in retinal thickness in AnxA1−/− mice in comparison to WT animals, with statistical significance indicated at * p < 0.05 and ** p < 0.01.
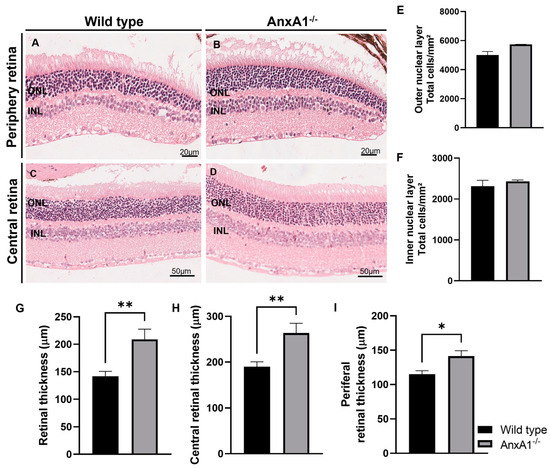
Figure 4.
Histomorphometric analysis. (A–D) The morphological analysis of the retina of wild-type and AnxA1−/− mice was carried out using microphotographs of the peripheral (20 μm) and central (50 μm) regions of the retinas of both groups (n = 6). (E) The graphs show the quantification of the total number of cells in the outer nuclear layer (ONL), counted using hematoxylin staining of the cell nuclei. (F) Quantification of the total number of cells in the inner nuclear layer (INL) was also carried out using cell nuclei staining. (G) In addition, the total thickness of the retina was analyzed, considering the central and peripheral regions. (H,I) Thickness of the peripheral and central retina. Cell number and thickness results are presented as mean ± SEM. Significant differences between groups were observed, with * p < 0.05 and ** p < 0.01, according to statistical analysis using the t-test.
4. Discussion
This study examined the electrophysiological response of AnxA1−/− mice, describing their visual phenotype. Under scotopic conditions, no significant changes were observed in the amplitudes of a- and b-waves in AnxA1−/− mice compared to WT mice at all tested intensities, indicating no changes in the photoreceptors (the primary contributor to the a-wave) and inner retina/bipolar cells (b-wave; OPs) under these conditions.
The AnxA1−/− mice used in the autoimmune uveitis model showed a reduction in the amplitude of both the a-waves and b-waves under scotopic and photopic conditions [11], which was significantly greater in AnxA1−/− mice (90%) than in WT mice (50%). This finding reveals a marked deterioration in the visual function of mice lacking AnxA1, consistent with the photoreceptor lesions identified in histological analysis [10]. These results suggest a direct correlation between the absence of AnxA1 and compromised visual capacity, highlighting its crucial importance in maintaining the integrity of the visual system in mice [10]. No changes were observed on day 0 after uveitis induction. The result found in the induction time on day 0 is similar to our findings [11]. In retinal ischemia, the amplitudes of the a- and b-waves were smaller in AnxA1−/− mice at higher intensities (2.5, 7.96, and 79.06 cd.s/m2) than in WT mice [11]. However, it is important to note that these studies did not compare control mice of both genotypes or evaluate the response time.
Regarding the implicit time of scotopic responses, the lack of AnxA1 modulated the response time of the b-wave at the tested intensities (−3.7 and 0.3 log cd.s/m2). The implicit time of the a-wave also differed significantly by genotype (WT vs. AnxA1−/−). We can conclude that the lack of AnxA1 alters the response time of the photoreceptors responsible for the a-wave, which respond slightly faster in AnxA1−/− mice than in WT mice. It also alters the response time of the ON bipolar cells responsible for the b-wave, which responds faster at both lower and higher flash intensities in AnxA1−/− than in WT mice. A functional analysis of visual performance, addressing aspects such as acuity and contrast, could be beneficial in validating these findings and determining whether AnxA1 plays a more evident role in visual function [17].
While the role of AnxA1 in photoreceptors remains unknown, other annexins, such as AnxA2 and AnxA5, are important for photoreceptor renewal, with roles in the phagocytosis of the outer segments of photoreceptors [18,19]. The genetic ablation of AnxA2 in human retinal pigment epithelial cells (ARPE-19) and mice reduced the efficiency of the phagocytosis of outer segments. In AnxA2−/− mice, these segments accumulated in the apical processes of the retinal pigment epithelium (RPE), indicating a delay in phagosome transport [18].
The same pattern is observed for AnxA5, where the lack of AnxA5 affects retinal clearance by reducing the levels of αvβ5 receptors on the apical phagocytic surface of RPE cells [19]. While this study did not address the mechanisms underlying the reduced response time in the a-wave and b-wave under scotopic conditions, it is plausible to hypothesize that AnxA1 may function analogously to AnxA2 and AnxA5. The absence of AnxA1 could impede the renewal process of outer segments in photoreceptors. Indeed, AnxA1 plays a pivotal role in phagocytosis by modulating phagocytic receptors [20]. In Alzheimer’s disease, treatment with recombinant human AnxA1 has been shown to reduce β-amyloid levels by enhancing degradation in neuroblastoma cells and promoting phagocytosis through microglia [21].
AnxA1 is the most abundant annexin family member in the retina [8]. However, it is notable that the primary sources of AnxA1 are Müller glial cells and microglia, not retinal neurons [8]. To date, only Grosche et al. have examined AnxA1 expression in indistinguishable retinal neurons, leaving a significant gap in understanding its expression in isolated neuronal cells. This lack of detailed investigation may limit a comprehensive understanding of the potential role of AnxA1 in ophthalmic diseases.
AnxA1 is an essential regulatory protein, playing important roles in forming the blood–retinal barrier and mediating fundamental cellular responses, such as apoptosis in response to damage, and resolving inflammation [22]. Moreover, the absence of AnxA1 in the retina renders the retinal microenvironment more susceptible to inflammatory processes [5].
Distinguishing between ON and OFF responses using the full-field ERG is valuable for characterizing post-receptoral responses [23]. Analyzing the integrity of pathways involving ON and OFF bipolar cells allows for a more detailed understanding of the response profile of photoreceptors, rods, cones, and post-receptor cells, including ON and OFF bipolar cells [23]. No differences were observed in the responses of ON/OFF bipolar cells under photopic conditions, while the absence of AnxA1 reduced the onset and offset times of the responses of ON bipolar cells or the cone pathway of bipolar cells under mesopic conditions, suggesting potential changes in the signal conduction speed between them. Furthermore, AnxA1−/− mice had greater amplitudes in both the negative and positive ON components than WT mice.
Regarding time under mesopic conditions, AnxA1−/− mice had shorter response times in both positive and negative ON components than WT mice. These findings suggest that the absence of AnxA1 affects the function of photoreceptors, rods, cones, and bipolar cells related to the mesopic ON response. This observation may have significant implications for understanding how AnxA1 influences the responses of retinal cells and visual processing in the retina under mesopic conditions. Regarding the mesopic OFF response, response times and amplitudes did not differ significantly between the WT and AnxA1−/− mice, suggesting that the absence of AnxA1 does not affect the time or amplitude of the responses of OFF bipolar cells under mesopic conditions.
Dysfunctions in cellular excitability can lead to the onset of neurodegenerative processes since the exchange of chemical mediators in retinal synapses is essential for the proper functioning of visual activity. A reduction in the functionality of this system or its complete loss may result in neurodegenerative conditions [24,25]. In glaucoma, reduced AnxA1 secretion is associated with the apoptosis of ganglion cells. The translocation of AnxA1 to the nucleus has been linked to the apoptosis of these cells [26]. Moreover, treatment with a mimetic peptide of AnxA1 reduced the apoptosis of retinal ganglion cells [12], demonstrating its importance in neurodegenerative conditions of the retina. AnxA1 has also been proven crucial in neurons beyond the retina, such as dorsal root neurons, where homozygous AnxA1 deletion sensitized transient receptor potential cation channel subfamily V member 1 (TRPV1) receptors related to nociception [27]. AnxA1 deletion also caused a reduction in mature dendritic spines without a deficit in dendritic complexity [28]. Therefore, the presence or absence of AnxA1 can have significant implications for the function and health of the retina and neurodegenerative processes in various neuronal contexts.
Regarding the amplitude of the first harmonic, AnxA1−/− mice had slightly greater amplitudes than WT mice in the testing conditions and frequencies, although the differences were nonsignificant. However, while the phases were similar between the AnxA1−/− and WT mice, a significant genotype effect was observed, meaning the phase response behaves differently in each genotype. The fact that AnxA1−/− mice exhibited slightly greater phase synchronization suggests that the absence of AnxA1 influences the synchronization of photoreceptors. This phase change indicates a possible alteration in the synchronization or timing of observed sinusoidal luminance modulation. The precise synchronization of electrical activities in the retina is crucial in effectively transmitting visual information to the brain [29].
To assess whether the changes observed with ERG could impact morphology, we conducted histomorphometric analyses. We observed increased thickness of the total, peripheral, and central retina in AnxA1−/− mice, which may be associated with some form of retinal edema or inflammation, commonly observed in diabetic retinopathy or age-related macular edema. A previous study reported Müller cell hypertrophy and increased retinal and INL volumes in non-proliferative diabetic retinopathy [30]. Therefore, the greater thickness of the retina may be associated with compensation for the lack of AnxA1 to maintain retinal functionality, which appears to be altered based on the ERG recordings.
Our findings represent a milestone since they demonstrate, for the first time, that AnxA1 is crucial in efficiently processing response times originating from photoreceptors and bipolar cells. This knowledge is pivotal for assessing the implications of AnxA1 deficiency and may pave the way for targeted interventions and treatments aimed at restoring retinal function in conditions associated with its absence. Our results highlight the crucial role of AnxA1 in retinal physiology.
A limitation of this study is the exclusive use of male mice. This choice was made in order to minimize the variability associated with hormonal fluctuations present in females. However, we recognize that sex is an important biological variable, capable of influencing the progression and molecular mechanisms of retinal diseases. Future studies that include both sexes are needed to investigate possible sex-specific responses and broaden the translational relevance of the findings.
Future studies examining the expression and localization of AnxA1 in the retina, as well as its specific functions across distinct retinal cell types, would be highly valuable. A more detailed approach, analyzing each retinal cell type individually in AnxA1−/− mice, could provide significant insights into the molecular mechanisms underlying the reduced synchronization of retinal electrical activity observed in these animals. Moreover, exploring the involvement of key inflammatory mediators (e.g., cytokines), phagocytosis-related proteins (such as Mertk), and markers of Müller cell activation (e.g., GFAP) will be essential to elucidate AnxA1’s contribution to retinal homeostasis. Investigating the regulation of oxidative stress and its potential interplay with AnxA1 may also provide further mechanistic support for our hypothesis. Collectively, these investigations would enable a more comprehensive understanding of the cell-specific functions of AnxA1 in the retina.
5. Conclusions
This study highlights the effects of the absence of AnxA1 under both scotopic and mesopic conditions in mice. While the amplitudes of a- and b-waves did not change under scotopic conditions, the changes in time responses indicate that AnxA1 influences photoreceptors and bipolar cells. Moreover, the response of bipolar cells was enhanced in AnxA1−/− mice under mesopic conditions, emphasizing the crucial role of AnxA1 in regulating their function. The changes in the temporal phase in AnxA1−/− mice suggest a potential negative effect on photoreceptor synchronization, with implications for sine wave luminance modulation. In addition, the greater thickness of the retina may be associated with compensation for the lack of AnxA1 to maintain retinal functionality.
Author Contributions
Conceptualization, formal analysis, investigation, data curation, and writing—original draft preparation, R.A.d.S. and A.M.P.L.; methodology, D.D.d.S., F.M.M.-C., L.P.d.S.F., M.S., and M.F.C.; funding acquisition and writing—review and editing, D.F.V.; supervision, funding acquisition, project administration, and writing—review and editing, C.D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by São Paulo Research Foundation (FAPESP, grant number 22/12027-0 to R.A.d.S., 19/00777-1 to A.M.P.L., 22/00191-0 to D.F.V.) and Brazilian National Council for Scientific and Technological Development (CNPq) (Grant Nos. 314630/2020-1 to D.F.V., 308524/2022-5 to C.D.G.).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee on Animal Experimentation at UNIFESP (No.: 858230821; ID: 011028) and IP-USP (No.: 1583180822; ID: 000309) and performed according to the ARRIVE guidelines.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Youngblood, H.; Robinson, R.; Sharma, A.; Sharma, S. Proteomic Biomarkers of Retinal Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2019, 20, 4755. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef]
- Forshaw, T.R.J.; Subhi, Y.; Andréasson, S.; Sørensen, T.L. Full-Field Electroretinography Changes Associated with Age-Related Macular Degeneration: A Systematic Review with Meta-Analyses. Ophthalmologica 2022, 245, 195–203. [Google Scholar] [CrossRef]
- McAnany, J.J.; Persidina, O.S.; Park, J.C. Clinical electroretinography in diabetic retinopathy: A review. Surv. Ophthalmol. 2022, 67, 712–722. [Google Scholar] [CrossRef]
- André da Silva, R.; Moraes de Paiva Roda, V.; Philipe de Souza Ferreira, L.; Oliani, S.M.; Paula Girol, A.; Gil, C.D. Annexins as potential targets in ocular diseases. Drug Discov. Today 2022, 27, 103367. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Vago, J.P.; Teixeira, M.M.; Sousa, L.P. Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance. J. Immunol. Res. 2016, 2016, 8239258. [Google Scholar] [CrossRef] [PubMed]
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461. [Google Scholar] [CrossRef]
- Grosche, A.; Hauser, A.; Lepper, M.F.; Mayo, R.; von Toerne, C.; Merl-Pham, J.; Hauck, S.M. The Proteome of Native Adult Müller Glial Cells From Murine Retina. Mol. Cell Proteom. 2016, 15, 462–480. [Google Scholar] [CrossRef]
- Girol, A.P.; Mimura, K.K.; Drewes, C.C.; Bolonheis, S.M.; Solito, E.; Farsky, S.H.; Gil, C.D.; Oliani, S.M. Anti-inflammatory mechanisms of the annexin A1 protein and its mimetic peptide Ac2-26 in models of ocular inflammation in vivo and in vitro. J. Immunol. 2013, 190, 5689–5701. [Google Scholar] [CrossRef]
- Yazid, S.; Gardner, P.J.; Carvalho, L.; Chu, C.J.; Flower, R.J.; Solito, E.; Lee, R.W.; Ali, R.R.; Dick, A.D. Annexin-A1 restricts Th17 cells and attenuates the severity of autoimmune disease. J. Autoimmun. 2015, 58, 1–11. [Google Scholar] [CrossRef]
- Hui, Q.; Zheng, F.; Qin, L.; Pei, C. Annexin A1 Promotes Reparative Angiogenesis and Ameliorates Neuronal Injury in Ischemic Retinopathy. Curr. Eye Res. 2022, 47, 791–801. [Google Scholar] [CrossRef]
- Li, L.; Xu, L.; Chen, W.; Li, X.; Xia, Q.; Zheng, L.; Duan, Q.; Zhang, H.; Zhao, Y. Reduced Annexin A1 Secretion by ABCA1 Causes Retinal Inflammation and Ganglion Cell Apoptosis in a Murine Glaucoma Model. Front. Cell Neurosci. 2018, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.I.; Barboni, M.T.; Nagy, B.V.; Roux, M.J.; Rendon, A.; Ventura, D.F.; Kremers, J. Asymmetrical Functional Deficits of ON and OFF Retinal Processing in the mdx3Cv Mouse Model of Duchenne Muscular Dystrophy. Invest. Ophthalmol. Vis. Sci. 2016, 57, 5788–5798. [Google Scholar] [CrossRef]
- Barboni, M.T.S.; Vaillend, C.; Joachimsthaler, A.; Liber, A.M.P.; Khabou, H.; Roux, M.J.; Vacca, O.; Vignaud, L.; Dalkara, D.; Guillonneau, X.; et al. Rescue of Defective Electroretinographic Responses in Dp71-Null Mice with AAV-Mediated Reexpression of Dp71. Investig. Ophthalmol. Vis. Sci. 2020, 61, 11. [Google Scholar] [CrossRef]
- Harazny, J.; Scholz, M.; Buder, T.; Lausen, B.; Kremers, J. Electrophysiological deficits in the retina of the DBA/2J mouse. Doc. Ophthalmol. 2009, 119, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Sustar, M.; Holder, G.E.; Kremers, J.; Barnes, C.S.; Lei, B.; Khan, N.W.; Robson, A.G. ISCEV extended protocol for the photopic On-Off ERG. Doc. Ophthalmol. 2018, 136, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.J.; Lee, J.I.; Jang, Y.J.; An, S.; Kim, J.H.; Fried, S.I.; Im, M. Retinal Degeneration Reduces Consistency of Network-Mediated Responses Arising in Ganglion Cells to Electric Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1921–1930. [Google Scholar] [CrossRef]
- Law, A.L.; Ling, Q.; Hajjar, K.A.; Futter, C.E.; Greenwood, J.; Adamson, P.; Wavre-Shapton, S.T.; Moss, S.E.; Hayes, M.J. Annexin A2 regulates phagocytosis of photoreceptor outer segments in the mouse retina. Mol. Biol. Cell 2009, 20, 3896–3904. [Google Scholar] [CrossRef]
- Yu, C.; Muñoz, L.E.; Mallavarapu, M.; Herrmann, M.; Finnemann, S.C. Annexin A5 regulates surface αvβ5 integrin for retinal clearance phagocytosis. J. Cell Sci. 2019, 132, jcs232439. [Google Scholar] [CrossRef]
- Patel, D.M.; Ahmad, S.F.; Weiss, D.G.; Gerke, V.; Kuznetsov, S.A. Annexin A1 is a new functional linker between actin filaments and phagosomes during phagocytosis. J. Cell Sci. 2011, 124, 578–588. [Google Scholar] [CrossRef]
- Ries, M.; Watts, H.; Mota, B.C.; Lopez, M.Y.; Donat, C.K.; Baxan, N.; Pickering, J.A.; Chau, T.W.; Semmler, A.; Gurung, B.; et al. Annexin A1 restores cerebrovascular integrity concomitant with reduced amyloid-β and tau pathology. Brain 2021, 144, 1526–1541. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Huang, J.; Lou, X.; Yao, K.; Ye, M.; Mou, Q.; Wen, Z.; Duan, Q.; Zhang, H.; Zhao, Y. Endothelial CYP2J2 overexpression restores the BRB via METTL3-mediated ANXA1 upregulation. FASEB J. 2022, 36, e22619. [Google Scholar] [CrossRef] [PubMed]
- Pasmanter, N.; Petersen-Jones, S.M. Characterization of scotopic and mesopic rod signaling pathways in dogs using the On-Off electroretinogram. BMC Vet. Res. 2022, 18, 422. [Google Scholar] [CrossRef]
- Wu, S.M. Synaptic organization of the vertebrate retina: General principles and species-specific variations: The Friedenwald lecture. Invest. Ophthalmol. Vis. Sci. 2010, 51, 1263–1274. [Google Scholar] [CrossRef]
- Euler, T.; Haverkamp, S.; Schubert, T.; Baden, T. Retinal bipolar cells: Elementary building blocks of vision. Nat. Rev. Neurosci. 2014, 15, 507–519. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, L.; Li, X.; Chen, W.; Lu, Z. Tat-NTS Suppresses the Proliferation, Migration and Invasion of Glioblastoma Cells by Inhibiting Annexin-A1 Nuclear Translocation. Cell Mol. Neurobiol. 2021, 42, 2715–2725. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, S.; Ke, X.; Yi, Y.; Yu, H.; Yu, D.; Li, Q.; Shang, Y.; Lu, Y.; Pei, L. The mechanism of Annexin A1 to modulate TRPV1 and nociception in dorsal root ganglion neurons. Cell Biosci. 2021, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yi, W.; Sun, C.; Ma, S.; Wang, J.; Liu, S.; Chen, Y. EphB2 activates CREB-dependent expression of Annexin A1 to regulate dendritic spine morphogenesis. Biochem. Biophys. Res. Commun. 2021, 584, 107–115. [Google Scholar] [CrossRef]
- Rider, A.T.; Henning, G.B.; Eskew, R.T.; Stockman, A. Harmonics added to a flickering light can upset the balance between ON and OFF pathways to produce illusory colors. Proc. Natl. Acad. Sci. USA 2018, 115, E4081–E4090. [Google Scholar] [CrossRef]
- Carpi-Santos, R.; de Melo Reis, R.A.; Gomes, F.C.A.; Calaza, K.C. Contribution of Müller Cells in the Diabetic Retinopathy Development: Focus on Oxidative Stress and Inflammation. Antioxidants 2022, 11, 617. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).