Brain Tumor Recognition Using Artificial Intelligence Neural-Networks (BRAIN): A Cost-Effective Clean-Energy Platform
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review
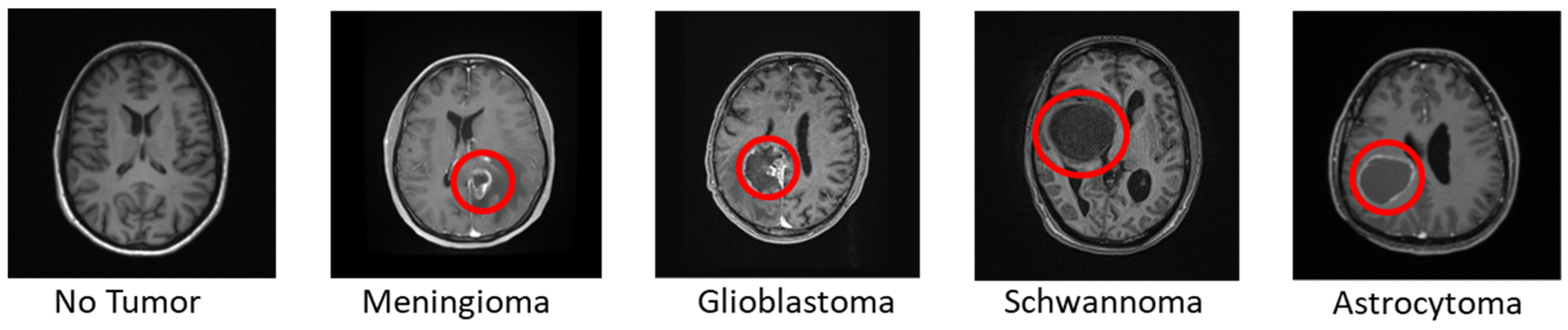
2.2. Data
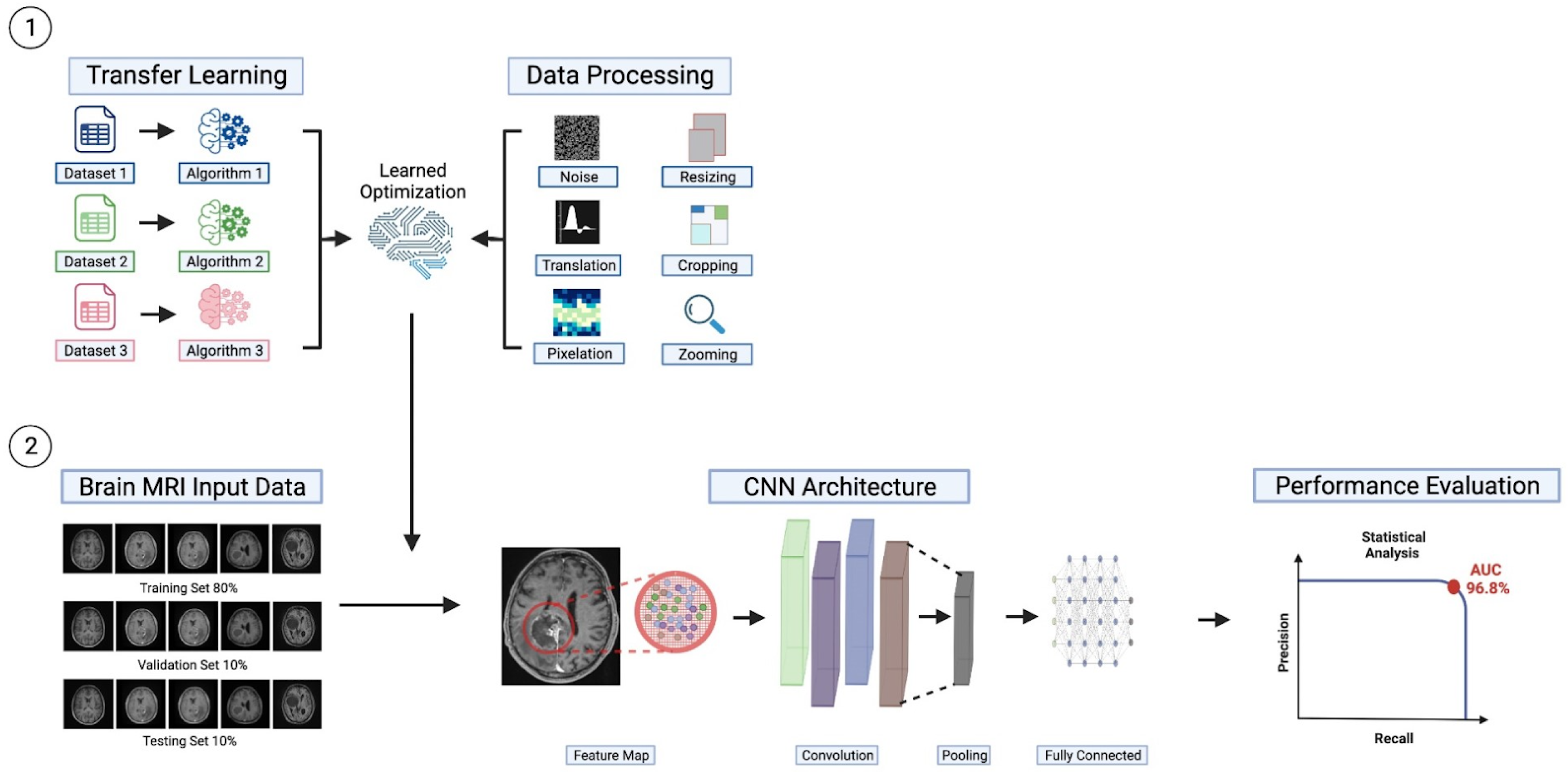
2.3. Workflow
2.4. Improvements to CNN Models for Brain Tumors Classification
3. Results
3.1. Systematic Review
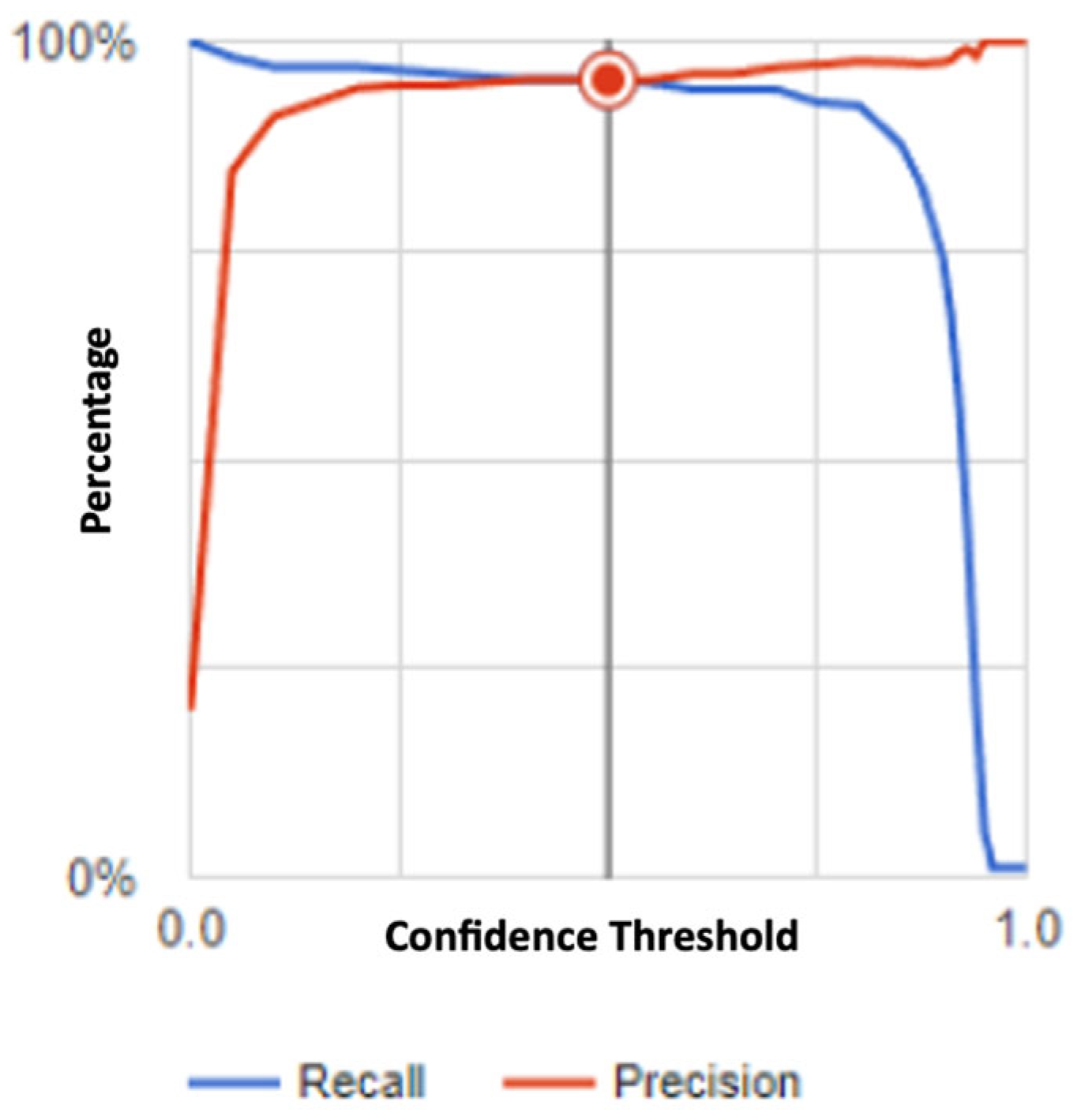
3.2. Evaluation Metrics
3.3. Performance Analysis
3.4. Cost-Effectiveness
4. Discussion
4.1. Our Model Compared to the Current Literature
4.2. Current Limitations of Brain Tumor Classification Models
4.3. Limitations of Our Models
4.4. Future Direction of Brain Tumor Classification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- “Brain Tumor—Statistics”. Cancer.Net. Available online: https://www.cancer.net/cancer-types/brain-tumor/statistics (accessed on 31 May 2023).
- Mariotto, A.B.; Enewold, L.; Zhao, J.X.; Zeruto, C.A.; Yabroff, K.R. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Akinyelu, A.A.; Zaccagna, F.; Grist, J.T.; Castelli, M.; Rundo, L. Brain Tumor Diagnosis Using Machine Learning, Convolutional Neural Networks, Capsule Neural Networks and Vision Transformers, Applied to MRI: A Survey. J. Imaging 2022, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, X. Progress on the diagnosis and evaluation of brain tumors. Cancer Imaging 2013, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Meyer, J.E.; Mabray, M.C.; Cha, S. Current clinical brain tumor imaging. Neurosurgery 2017, 81, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Havaei, M.; Davy, A.; Warde-Farley, D.; Biard, A.; Courville, A.; Bengio, Y.; Pal, C.; Jodoin, P.M.; Larochelle, H. Brain tumor segmentation with deep neural networks. Med. Image Anal. 2017, 35, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.; Sala, E.; Schönlieb, C.B.; Rundo, L. Focus U-Net: A novel dual attention-gated CNN for polyp segmentation during colonoscopy. Comput. Biol. Med. 2021, 137, 104815. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Sugiyama, O.; Yakami, M.; Ueno, S.; Kubo, T.; Kuroda, T.; Togashi, K. Computer-aided diagnosis of lung nodule classification between benign nodule, primary lung cancer, and metastatic lung cancer at different image size using deep convolutional neural network with transfer learning. PLoS ONE 2018, 13, e0200721. [Google Scholar] [CrossRef]
- Ghauri, M.S.; Reddy, A.J.; Tak, N.; A Tabaie, E.; Ramnot, A.; Esfahani, P.R.; Nawathey, N.; Siddiqi, J. Utilizing Deep Learning for X-ray Imaging: Detecting and Classifying Degenerative Spinal Conditions. Cureus 2023, 15, e41582. [Google Scholar] [CrossRef]
- Arabahmadi, M.; Farahbakhsh, R.; Rezazadeh, J. Deep Learning for Smart Healthcare-A Survey on Brain Tumor Detection from Medical Imaging. Sensors 2022, 22, 1960. [Google Scholar] [CrossRef] [PubMed]
- Biratu, E.S.; Schwenker, F.; Ayano, Y.M.; Debelee, T.G. A Survey of Brain Tumor Segmentation and Classification Algorithms. J. Imaging 2021, 7, 179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hennes, W. Brain Tumor Dataset for 14 Classes. Kaggle.com. Available online: https://www.kaggle.com/datasets/waseemnagahhenes/brain-tumor-for-14-classes (accessed on 28 February 2023).
- Feltrin, F. Brain Tumor MRI Images 17 Classes. Kaggle. Available online: https://www.kaggle.com/datasets/fernando2rad/brain-tumor-mri-images-44c (accessed on 28 February 2023).
- Nickparvar, M. Brain Tumor MRI Dataset. Kaggle.com. Available online: https://www.kaggle.com/datasets/masoudnickparvar/brain-tumor-mri-dataset (accessed on 28 February 2023).
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. NIPS 2012, 60, 84–90. [Google Scholar] [CrossRef]
- Lotlikar, V.S.; Satpute, N.; Gupta, A. Brain Tumor Detection Using Machine Learning and Deep Learning: A Review. Curr. Med. Imaging 2022, 18, 604–622. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, A.; Vilnis, L.; Le, Q.V.; Sutskever, I.; Kaiser, L.; Kurach, K.; Martens, J. Adding Gradient Noise Improves Learning for Very Deep Networks. arXiv 2015, arXiv:1511.06807. [Google Scholar]
- Zaccagna, F.; Grist, J.T.; Quartuccio, N.; Riemer, F.; Fraioli, F.; Caracò, C.; Halsey, R.; Aldalilah, Y.; Cunningham, C.H.; Massoud, T.F.; et al. Imaging and treatment of brain tumors through molecular targeting: Recent clinical advances. Eur. J. Radiol. 2021, 142, 109842. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zaccagna, F.; Rundo, L.; Testa, C.; Agati, R.; Lodi, R.; Manners, D.N.; Tonon, C. Convolutional Neural Network Techniques for Brain Tumor Classification (from 2015 to 2022): Review, Challenges, and Future Perspectives. Diagnostics 2022, 12, 1850. [Google Scholar] [CrossRef] [PubMed]
- Hajiramezanali, E.; Dadaneh, S.Z.; Karbalayghareh, A.; Zhou, M.; Qian, X. Bayesian multi-domain learning for cancer subtype discovery from next-generation sequencing count data. arXiv 2018, arXiv:1810.09433. [Google Scholar]
- Abiwinanda, N.; Hanif, M.; Hesaputra, S.T.; Handayani, A.; Mengko, T.R. Brain Tumor Classification Using Convolutional Neural Network. In World Congress on Medical Physics and Biomedical Engineering 2018; Lhotska, L., Sukupova, L., Lacković, I., Ibbott, G.S., Eds.; IFMBE Proceedings; Springer: Singapore, 2019; Volume 68. [Google Scholar] [CrossRef]
- Ge, C.; Gu, I.Y.-H.; Jakola, A.S.; Yang, J. Deep Learning and Multi-Sensor Fusion for Glioma Classification Using Multistream 2D Convolutional Networks. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 5894–5897. [Google Scholar] [CrossRef]
- Deepak, S.; Ameer, P.M. Brain tumor classification using deep CNN features via transfer learning. Comput. Biol. Med. 2019, 111, 103345. [Google Scholar] [CrossRef]
- Hemanth, M.; Janardhan, M.; Sujihelen, L. Design and Implementing Brain Tumor Detection Using. Machine Learning Approach. In Proceedings of the 2019 3rd International Conference on Trends in Electronics and Informatics (ICOEI), Tirunelveli, India, 23–25 April 2019; pp. 1289–1294. [Google Scholar] [CrossRef]
- Kutlu, H.; Avcı, E. A Novel Method for Classifying Liver and Brain Tumors Using Convolutional Neural Networks, Discrete Wavelet Transform and Long Short-Term Memory Networks. Sensors 2019, 19, 1992. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Gu, I.Y.-H.; Jakola, A.S.; Yang, J. Deep semi-supervised learning for brain tumor classification. BMC Med. Imaging 2020, 20, 87. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Jue, W.; Mushtaq, M.; Mushtaq, M.U. Brain tumor classification in MRI image using convolutional neural network. Math. Biosci. Eng. MBE 2020, 17, 6203–6216. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ashraf, I.; Alhaisoni, M.; Damaševičius, R.; Scherer, R.; Rehman, A.; Bukhari, S.A.C. Multimodal Brain Tumor Classification Using Deep Learning and Robust Feature Selection: A Machine Learning Application for Radiologists. Diagnostics 2020, 10, 565. [Google Scholar] [CrossRef] [PubMed]
- Manni, F.; van der Sommen, F.; Fabelo, H.; Zinger, S.; Shan, C.; Edström, E.; Elmi-Terander, A.; Ortega, S.; Callicó, G.M.; de With, P.H.N. Hyperspectral Imaging for Glioblastoma Surgery: Improving Tumor Identification Using a Deep Spectral-Spatial Approach. Sensors 2020, 20, 6955. [Google Scholar] [CrossRef] [PubMed]
- Mzoughi, H.; Njeh, I.; Wali, A.; Ben Slima, M.; BenHamida, A.; Mhiri, C.; Ben Mahfoudhe, K. Deep Multi-Scale 3D Convolutional Neural Network (CNN) for MRI Gliomas Brain Tumor Classification. J. Digit. Imaging 2020, 33, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Vidyaratne, L.; Rahman, M.; Iftekharuddin, K.M. Context aware deep learning for brain tumor segmentation, subtype classification, and survival prediction using radiology images. Sci. Rep. 2020, 10, 19726. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Naz, S.; Razzak, M.I.; Akram, F.; Imran, M. A Deep learning-based framework for automatic brain tumors classification using transfer learning. Circ. Syst. Signal. Process. 2020, 39, 757–775. [Google Scholar] [CrossRef]
- Tandel, G.S.; Balestrieri, A.; Jujaray, T.; Khanna, N.N.; Saba, L.; Suri, J.S. Multiclass magnetic resonance imaging brain tumor classification using artificial intelligence paradigm. Comput. Biol. Med. 2020, 122, 103804. [Google Scholar] [CrossRef]
- Kader, I.A.E.; Xu, G.; Shuai, Z.; Saminu, S.; Javaid, I.; Ahmad, I.S. Differential Deep Convolutional Neural Network Model for Brain Tumor Classification. Brain Sci. 2021, 11, 352. [Google Scholar] [CrossRef]
- El Kader, I.A.; Xu, G.; Shuai, Z.; Saminu, S. Brain Tumor Detection and Classification by Hybrid CNN-DWA Model Using MR Images. Curr. Med. Imaging 2021, 17, 1248–1255. [Google Scholar] [CrossRef]
- Hashemzehi, R.; Mahdavi, S.J.S.; Kheirabadi, M.; Kamel, S.R. Y-net: A reducing gaussian noise convolutional neural network for MRI brain tumor classification with NADE concatenation. Biomed. Phys. Eng. Express 2021, 7, 055006. [Google Scholar] [CrossRef]
- Irmak, E. Multi-Classification of brain tumor MRI images using deep convolutional neural network with fully optimized framework. Iran. J. Sci. Technol. Trans. Electr. Eng. 2021, 45, 1015–1036. [Google Scholar] [CrossRef]
- Kang, J.; Ullah, Z.; Gwak, J. MRI-Based Brain Tumor Classification Using Ensemble of Deep Features and Machine Learning Classifiers. Sensors 2021, 21, 2222. [Google Scholar] [CrossRef]
- Latif, G.; Iskandar, D.A.; Alghazo, J.; Butt, M.M. Brain MR Image Classification for Glioma Tumor detection using Deep Convolutional Neural Network Features. Curr. Med. Imaging 2021, 17, 56–63. [Google Scholar] [CrossRef]
- Murthy, M.Y.B.; Koteswararao, A.; Babu, M.S. Adaptive fuzzy deformable fusion and optimized CNN with ensemble classification for automated brain tumor diagnosis. Biomed. Eng. Lett. 2021, 12, 37–58. [Google Scholar] [CrossRef]
- Amou, M.A.; Xia, K.; Kamhi, S.; Mouhafid, M. A Novel MRI Diagnosis Method for Brain Tumor Classification Based on CNN and Bayesian Optimization. Healthcare 2022, 10, 494. [Google Scholar] [CrossRef]
- Alanazi, M.F.; Ali, M.U.; Hussain, S.J.; Zafar, A.; Mohatram, M.; Irfan, M.; AlRuwaili, R.; Alruwaili, M.; Ali, N.H.; Albarrak, A.M. Brain Tumor/Mass Classification Framework Using Magnetic-Resonance-Imaging-Based Isolated and Developed Transfer Deep-Learning Model. Sensors 2022, 22, 372. [Google Scholar] [CrossRef]
- Almalki, Y.E.; Ali, M.U.; Kallu, K.D.; Masud, M.; Zafar, A.; Alduraibi, S.K.; Irfan, M.; Basha, M.A.A.; Alshamrani, H.A.; Alduraibi, A.K.; et al. Isolated Convolutional-Neural-Network-Based Deep-Feature Extraction for Brain Tumor Classification Using Shallow Classifier. Diagnostics 2022, 12, 1793. [Google Scholar] [CrossRef]
- Aurna, N.F.; Abu Yousuf, M.; Abu Taher, K.; Azad, A.; Moni, M.A. A classification of MRI brain tumor based on two stage feature level ensemble of deep CNN models. Comput. Biol. Med. 2022, 146, 105539. [Google Scholar] [CrossRef]
- Haq, A.U.; Li, J.P.; Khan, S.; Alshara, M.A.; Alotaibi, R.M.; Mawuli, C. DACBT: Deep learning approach for classification of brain tumors using MRI data in IoT healthcare environment. Sci. Rep. 2022, 12, 15331. [Google Scholar] [CrossRef]
- Ker, J.; Bai, Y.; Lee, H.Y.; Rao, J.; Wang, L. Automated brain histology classification using machine learning. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas 2019, 66, 239–245. [Google Scholar] [CrossRef]
- Kibriya, H.; Amin, R.; Alshehri, A.H.; Masood, M.; Alshamrani, S.S.; Alshehri, A. A Novel and Effective Brain Tumor Classification Model Using Deep Feature Fusion and Famous Machine Learning Classifiers. Comput. Intell. Neurosci. 2022, 2022, 7897669. [Google Scholar] [CrossRef]
- Ramya, M.; Kirupa, G.; Rama, A. Brain tumor classification of magnetic resonance images using a novel CNN-based medical image analysis and detection network in comparison with AlexNet. J. Popul. Ther. Clin. Pharmacol. J. Ther. Popul. Pharmacol. Clin. 2022, 29, e97–e108. [Google Scholar] [CrossRef]
- Sekhar, A.; Biswas, S.; Hazra, R.; Sunaniya, A.K.; Mukherjee, A.; Yang, L. Brain Tumor Classification Using Fine-Tuned GoogLeNet Features and Machine Learning Algorithms: IoMT Enabled CAD System. IEEE J. Biomed. Health Inform. 2022, 26, 983–991. [Google Scholar] [CrossRef]
- Srinivas, C.; KS, N.P.K.; Zakariah, M.; Alothaibi, Y.A.; Shaukat, K.; Partibane, B.; Awal, H. Deep Transfer Learning Approaches in Performance Analysis of Brain Tumor Classification Using MRI Images. J. Healthc. Eng. 2022, 2022, 3264367. [Google Scholar] [CrossRef]
- Taher, F.; Shoaib, M.R.; Emara, H.M.; Abdelwahab, K.M.; El-Samie, F.E.A.; Haweel, M.T. Efficient framework for brain tumor detection using different deep learning techniques. Front. Public Healthc. 2022, 10, 959667. [Google Scholar] [CrossRef]
- Tiwari, P.; Pant, B.; Elarabawy, M.M.; Abd-Elnaby, M.; Mohd, N.; Dhiman, G.; Sharma, S. CNN Based Multiclass Brain Tumor Detection Using Medical Imaging. Comput. Intell. Neurosci. 2022, 2022, 1830010. [Google Scholar] [CrossRef]
- Yazdan, S.A.; Ahmad, R.; Iqbal, N.; Rizwan, A.; Khan, A.N.; Kim, D.-H. An Efficient Multi-Scale Convolutional Neural Network Based Multi-Class Brain MRI Classification for SaMD. Tomography 2022, 8, 1905–1927. [Google Scholar] [CrossRef]
- Zahoor, M.M.; Qureshi, S.A.; Bibi, S.; Khan, S.H.; Khan, A.; Ghafoor, U.; Bhutta, M.R. A New Deep Hybrid Boosted and Ensemble Learning-Based Brain Tumor Analysis Using MRI. Sensors 2022, 22, 2726. [Google Scholar] [CrossRef]
- El-Wahab, B.S.A.; Nasr, M.E.; Khamis, S.; Ashour, A.S. BTC-fCNN: Fast Convolution Neural Network for Multi-class Brain Tumor Classification. Healthc. Inf. Sci. Syst. 2023, 11, 3. [Google Scholar] [CrossRef]
- Al-Azzwi, Z.H.N.; Nazarov, A. Brain Tumor Classification based on Improved Stacked Ensemble Deep Learning Methods. Asian Pac. J. Cancer Prev. 2023, 24, 2141–2148. [Google Scholar] [CrossRef]
- AlTahhan, F.E.; Khouqeer, G.A.; Saadi, S.; Elgarayhi, A.; Sallah, M. Refined Automatic Brain Tumor Classification Using Hybrid Convolutional Neural Networks for MRI Scans. Diagnostics 2023, 13, 864. [Google Scholar] [CrossRef]
- Alturki, N.; Umer, M.; Ishaq, A.; Abuzinadah, N.; Alnowaiser, K.; Mohamed, A.; Saidani, O.; Ashraf, I. Combining CNN Features with Voting Classifiers for Optimizing Performance of Brain Tumor Classification. Cancers 2023, 15, 1767. [Google Scholar] [CrossRef]
- Khan, F.; Ayoub, S.; Gulzar, Y.; Majid, M.; Reegu, F.A.; Mir, M.S.; Soomro, A.B.; Elwasila, O. MRI-Based Effective Ensemble Frameworks for Predicting Human Brain Tumor. J. Imaging 2023, 9, 163. [Google Scholar] [CrossRef]
- Kumar, S.; Choudhary, S.; Jain, A.; Singh, K.; Ahmadian, A.; Bajuri, M.Y. Brain Tumor Classification Using Deep Neural Network and Transfer Learning. Brain Topogr. 2023, 36, 305–318. [Google Scholar] [CrossRef]
- Kurdi, S.Z.; Ali, M.H.; Jaber, M.M.; Saba, T.; Rehman, A.; Damaševičius, R. Brain Tumor Classification Using Meta-Heuristic Optimized Convolutional Neural Networks. J. Pers. Med. 2023, 13, 181. [Google Scholar] [CrossRef]
- Muezzinoglu, T.; Baygin, N.; Tuncer, I.; Barua, P.D.; Baygin, M.; Dogan, S.; Tuncer, T.; Palmer, E.E.; Cheong, K.H.; Acharya, U.R. PatchResNet: Multiple Patch Division–Based Deep Feature Fusion Framework for Brain Tumor Classification Using MRI Images. J. Digit. Imaging 2023, 36, 973–987. [Google Scholar] [CrossRef]
- Özkaraca, O.; Bağrıaçık, O.; Gürüler, H.; Khan, F.; Hussain, J.; Khan, J.; e Laila, U. Multiple Brain Tumor Classification with Dense CNN Architecture Using Brain MRI Images. Life 2023, 13, 349. [Google Scholar] [CrossRef]
- Rasheed, Z.; Ma, Y.-K.; Ullah, I.; Al Shloul, T.; Bin Tufail, A.; Ghadi, Y.Y.; Khan, M.Z.; Mohamed, H.G. Automated Classification of Brain Tumors from Magnetic Resonance Imaging Using Deep Learning. Brain Sci. 2023, 13, 602. [Google Scholar] [CrossRef]
- Ravinder, M.; Saluja, G.; Allabun, S.; Alqahtani, M.S.; Abbas, M.; Othman, M.; Soufiene, B.O. Enhanced brain tumor classification using graph convolutional neural network architecture. Sci. Rep. 2023, 13, 14938. [Google Scholar] [CrossRef]
- ZainEldin, H.; Gamel, S.A.; El-Kenawy, E.-S.M.; Alharbi, A.H.; Khafaga, D.S.; Ibrahim, A.; Talaat, F.M. Brain Tumor Detection and Classification Using Deep Learning and Sine-Cosine Fitness Grey Wolf Optimization. Bioengineering 2022, 10, 18. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, X.; Chen, W.; Du, G.; Fu, Q.; Zhang, H.; Jiang, H. EFF_D_SVM: A robust multi-type brain tumor classification system. Front. Neurosci. 2023, 17, 1269100. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very Deep CNN for Large-Scale Image Recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Huang, G.; Liu, Z.; Van Der Maaten, L.; Weinberger, K.Q. Densely connected convolutional networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 4700–4708. [Google Scholar]
- Rasool, M.; Ismail, N.A.; Boulila, W.; Ammar, A.; Samma, H.; Yafooz, W.M.S.; Emara, A.-H.M. A Hybrid Deep Learning Model for Brain Tumour Classification. Entropy 2022, 24, 799. [Google Scholar] [CrossRef]
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.; Anguelov, D.; Erhan, D.; Vanhoucke, V.; Rabinovich, A. Going deeper with convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 1–9. [Google Scholar]
- Yasaka, K.; Akai, H.; Kunimatsu, A.; Kiryu, S.; Abe, O. Deep learning with convolutional neural network in radiology. Jpn. J. Radiol. 2018, 36, 257–272. [Google Scholar] [CrossRef]
- Zeineldin, R.A.; Karar, M.E.; Elshaer, Z.; Coburger, J.; Wirtz, C.R.; Burgert, O.; Mathis-Ullrich, F. Explainability of deep neural networks for MRI analysis of brain tumors. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1673–1683. [Google Scholar] [CrossRef]
- Haque, R.; Hassan, M.; Bairagi, A.K.; Islam, S.M.S. NeuroNet19: An explainable deep neural network model for the classification of brain tumors using magnetic resonance imaging data. Sci. Rep. 2024, 14, 1524. [Google Scholar] [CrossRef]


| Author (Year) | Brain Tumor Subtypes | Model Algorithm | Average Accuracy (%) | Dataset Size | Dataset Used |
|---|---|---|---|---|---|
| Abiwinanda (2018) [23] | Glioma Pituitary Adenoma Meningioma | 13-layer CNN | 84.19 | 3064 | Kaggle |
| Ge C (2018) [24] | IDH Mutation IDH Wild-type Gliomas with/without 1p19q codeletion | Multistream CNN and Fusion Network | 90.13 | 444 | MICCAI BraTS 2017 Mayo Clinic |
| Deepak (2019) [25] | Meningioma Pituitary Glioma | GoogleNet | 91.8 | 3064 | Kaggle |
| Hemanth (2019) [26] | Normal Tumor | CNN | 94.5 | 220 | NS |
| Kutlu (2019) [27] | Meningioma Pituitary Glioma | CNN–DWT–LSTM hybrid | 98.6 | 3064 | NS |
| Ge C (2020) [28] | IDH Mutation IDH Wild-type Glioma (high vs. low grade) | GAN-augmented Multi-stream 2D CNN | 88.62 | 485 | TCGA MICCAI |
| Khan HA (2020) [29] | Benign tumor Malignant tumor | CNN | 100 | 253 | Kaggle |
| Khan MA (2020) [30] | T1, T2, T1CE, and Flair | VGG16 and VGG19 feature extraction and fusion, ELM classification | 95.73 | BraTs2015/17/18 | |
| Manni (2020) [31] | Normal Glioblastoma Multiforme | 3D–2D hybrid | 80 | 26 | In vivo HS human-brain image database |
| Mzoughi (2020) [32] | Glioma (high v low grade) | 3Ddeep CNN | 96.49 | 284 | BraTS-2018 |
| Pei (2020) [33] | Glioma (high v low grade) | 3DCNN | 48.4 | 335 | BraTS-2019–2020 TCIA |
| Rehman (2020) [34] | Glioma Pituitary Adenoma Meningioma | AlexNet GoogleNet VGGNet | 95.63 | 8934 | Kaggle Nanfang Hospital |
| Tandel (2020) [35] | Normal Tumor Astrocytoma Oligodendroglioma Glioblastoma Multiforme | AlexNet | 99 | NS | REMBRANDT |
| Kader (2021) [36] | Tumor, Normal | Differential Deep-CNN | 99.25 | 25,000 | Tianjin Universal Center of Medical Imaging and Diagnostic (TUCMD) |
| El Kader (2021) [37] | Normal Tumor | Hybrid CNN-DWA | 98 | 3650 | BRATS2012–2015 ISLES-SISS 2015 |
| Hashemzehi (2021) [38] | Normal Meningioma Pituitary Glioma | Y-Net with NADE | 82.91 | 6328 | Figshare Kaggle |
| Irmak (2021) [39] | Glioma Pituitary Adenoma Meningioma Metastatic | 25-layer CNN | 92.66 | 424,486 | RIDER Repository of Molecular Brain Neoplasia Data (REMBRANDT) KAGGLE TCGA-LGG |
| Kang (2021) [40] | Glioma Pituitary Adenoma Meningioma | CNN models with machine-learning classifiers | 93.72 | 3064 | Kaggle |
| Latif (2021) [41] | Glioma (high v low grade) | CNN | 98.77 | 40,300 | BRATS-2015 |
| Murthy (2021) [42] | Normal Tumor | Optimized Convolutional Neural Network with Ensemble Classification (OCNN-EC) | 95.3 | 253 | Kaggle website (figshare, SARTAJ dataset, Br35H) |
| Amou (2022) [43] | Meningioma Pituitary Glioma | optimized CNN | 98.7 | 3064 | Figshare Tianjin Medical University |
| Alanazi (2022) [44] | Glioma Pituitary Adenoma Meningioma | Developed transfer-learned CNN | 96.33 | 3000 | Kaggle |
| Almalki (2022) [45] | Normal Meningioma Pituitary Glioma | 22-layer deep feature trained SVM | 98 | 2970 | Kaggle |
| Aurna (2022) [46] | NS | PCA + CNN | 99.13 | NS | NS |
| Haq (2022) [47] | Normal Meningioma Pituitary Glioma | ResNet50-CNN | 99.9 | 253 | Nanfang Hospital Tianjing Medical University Kaggle |
| Ker (2022) [48] | Normal Glioma (high vs. low grade) | Google Inception V3 CNN | 6154 | Tan Tock Seng Hospital | |
| Kibriya (2022) [49] | Meningioma Pituitary Glioma | AlexNet, GoogLeNet, ResNet18, feature extraction and fusion, SVM and KNN classification | 99.7 | 15,320 | Figshare |
| Ramya (2022) [50] | Normal Tumor | MIDNet18 14-layer CNN | 98.54 | 4588 | Kaggle |
| Sekhar (2022) [51] | Glioma Meningioma Pituitary | GoogLeNet | NS | NS | NS |
| Srinivas (2022) [52] | Normal Tumor | VGG-16, ResNet-50, and Inception-v3 models | 88.2 | 233 | Kaggle |
| Taher (2022) [53] | NS | BRAIN-TUMOR-net | 93.67 | NS | NS |
| Tiwari (2022) [54] | Normal Glioma Meningioma Pituitary | CNN | 99 | 3264 | Kaggle |
| Yazdan (2022) [55] | Glioma Meningioma Pituitary Non-tumor | CNN | 91.2 | 3264 | Kaggle |
| Zahoor (2022) [56] | Normal Tumor | CNN | 99.2 | 5058 | CE-MRI |
| Abd El-Wahab (2023) [57] | Meningioma Pituitary Glioma | BTC-fCNN | 98.86 | 3064 | Figshare |
| Al-Azzwi (2023) [58] | Normal Tumor | VGG-119, stacked ensemble DL | 96.6 | 50 | Kaggle |
| AlTahhan (2023) [59] | Normal Meningioma Pituitary Glioma | AlexNet-KNN | 98.6 | 2880 | Figshare SARTAJ Br35h |
| Alturki (2023) [60] | Tumor, Normal | CNN features + voting classifier | 99.9 | 3762 | Kaggle |
| Khan (2023) [61] | Normal Tumor | XG-Ada-RF (Ensemble of Extreme Gradient Boosting, Ada-Boost, and Random Forest) | 95.9 | 3762 | Figshare |
| Kumar (2023) [62] | Benign tumor Malignant tumor | Improved Res-Net | 96.8 | 1572 | ACRIN-DSC-MR-Brain (ACRIN 6677/RTOG 0625) CPTAC-GBM ACRIN-FMISO-Brain (ACRIN 6684) |
| Kurdi (2023) [63] | Normal Tumor | Harris Hawks optimized CNN (HHOCNN) | 98 | 253 | Kaggle |
| Muezzinoglu (2023) [64] | Normal Meningioma Pituitary Glioblastoma Multiforme | PatchResNet | 98.1 | 3264 | Kaggle website (figshare, SARTAJ dataset, Br35H) |
| Özkaraca (2023) [65] | Normal Meningioma Pituitary Glioma | VGG16, ResNet | 92 | 7021 | Kaggle Figshare SARTAJ Br35H |
| Rasheed (2023) [66] | Glioma Meningioma Pituitary | CNN (16-layer) | 98.04 | 3064 | Kaggle |
| Ravinder (2023) [67] | Normal Meningioma Pituitary Glioma | Graph Neural Network (GNN) | 95.01 | 3264 | Kaggle |
| ZainEldin (2023) [68] | Normal Tumor | BCM-CNN | 99.98 | 3064 | Figshare |
| Zhang (2023) [69] | Glioma Meningioma Pituitary Normal | EFF_D_SVM | 98.59 | 3264 | Kaggle |
| Proposed BRAIN model | Normal Meningioma Glioblastoma Schwannoma Astrocytoma | BRAIN | 96.8 | 2611 | Kaggle Hospital Dataset |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghauri, M.S.; Wang, J.-Y.; Reddy, A.J.; Shabbir, T.; Tabaie, E.; Siddiqi, J. Brain Tumor Recognition Using Artificial Intelligence Neural-Networks (BRAIN): A Cost-Effective Clean-Energy Platform. Neuroglia 2024, 5, 105-118. https://doi.org/10.3390/neuroglia5020008
Ghauri MS, Wang J-Y, Reddy AJ, Shabbir T, Tabaie E, Siddiqi J. Brain Tumor Recognition Using Artificial Intelligence Neural-Networks (BRAIN): A Cost-Effective Clean-Energy Platform. Neuroglia. 2024; 5(2):105-118. https://doi.org/10.3390/neuroglia5020008
Chicago/Turabian StyleGhauri, Muhammad S., Jen-Yeu Wang, Akshay J. Reddy, Talha Shabbir, Ethan Tabaie, and Javed Siddiqi. 2024. "Brain Tumor Recognition Using Artificial Intelligence Neural-Networks (BRAIN): A Cost-Effective Clean-Energy Platform" Neuroglia 5, no. 2: 105-118. https://doi.org/10.3390/neuroglia5020008
APA StyleGhauri, M. S., Wang, J.-Y., Reddy, A. J., Shabbir, T., Tabaie, E., & Siddiqi, J. (2024). Brain Tumor Recognition Using Artificial Intelligence Neural-Networks (BRAIN): A Cost-Effective Clean-Energy Platform. Neuroglia, 5(2), 105-118. https://doi.org/10.3390/neuroglia5020008






