Unique Astrocyte Cytoskeletal and Nuclear Morphology in a Three-Dimensional Tissue-Engineered Rostral Migratory Stream
Abstract
:1. Introduction
2. Materials and Methods
2.1. Astrocyte Cell Culture
2.2. Fabrication of Hydrogel Micro-Columns
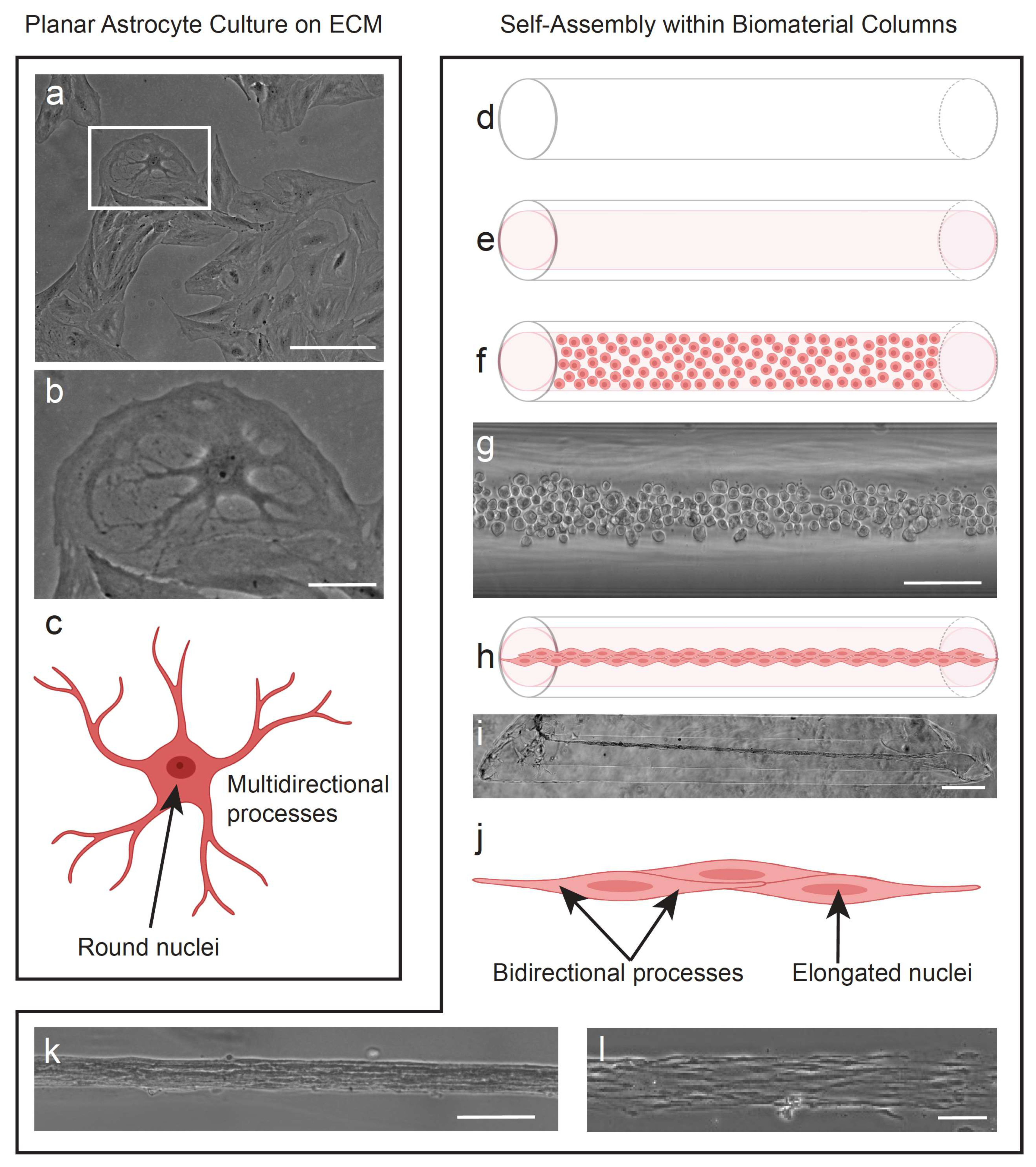
2.3. Fabrication of Tissue-Engineered Rostral Migratory Streams and Planar Astrocyte Cultures
2.4. Immunocytochemistry
2.5. Immunohistochemistry
2.6. Imaging
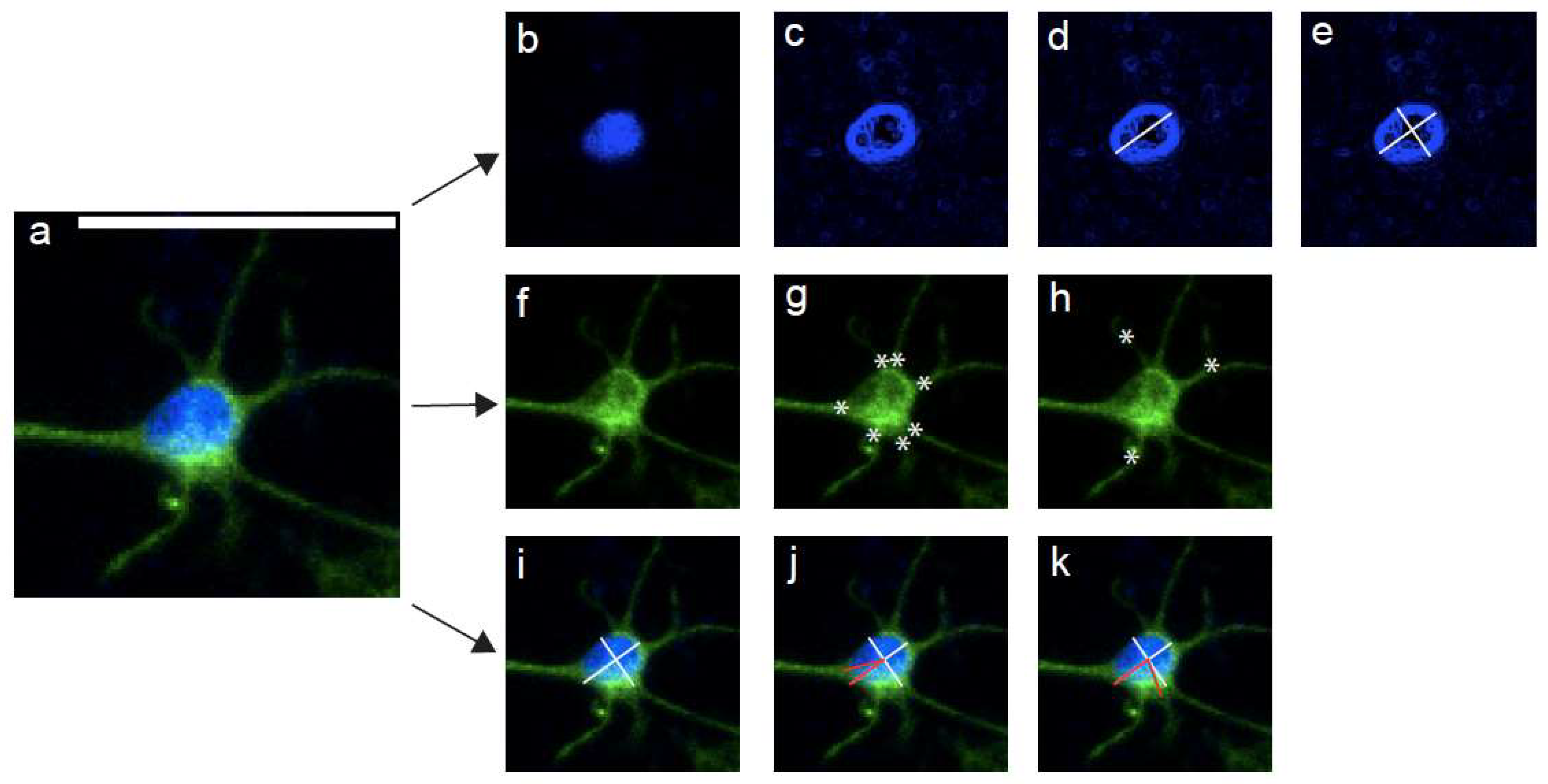
2.7. Imaging Analyses, Statistics, and Reproducibility
3. Results
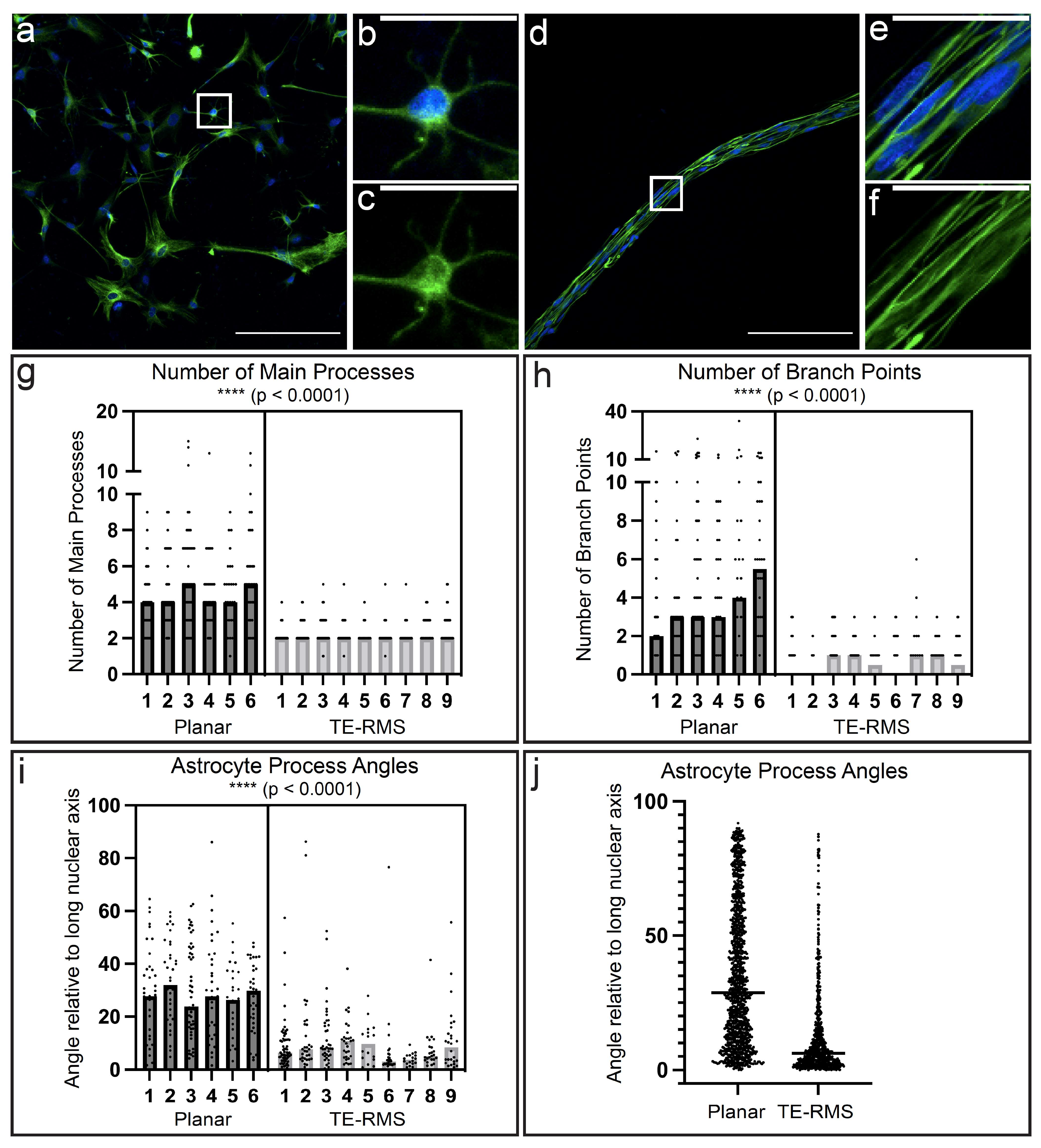
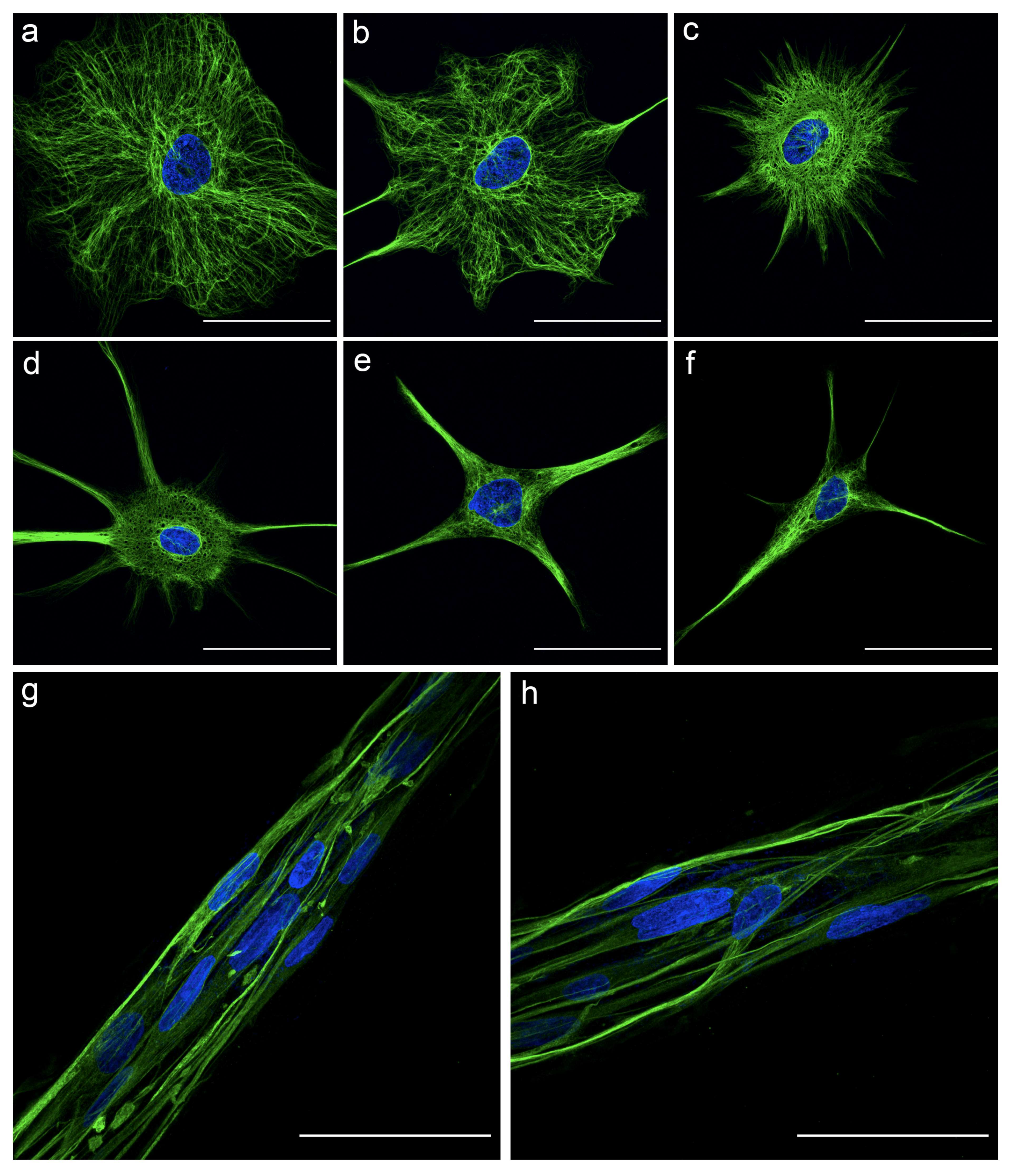
3.1. Planar Astrocytes Have a More Complex and Varied Cytoskeletal Arrangement Compared to TE-RMS Astrocytes
3.2. Endogenous Protoplasmic Astrocytes Have a More Complex and Varied Cytoskeletal Arrangement Compared to Endogenous RMS Astrocytes in Rat Brain
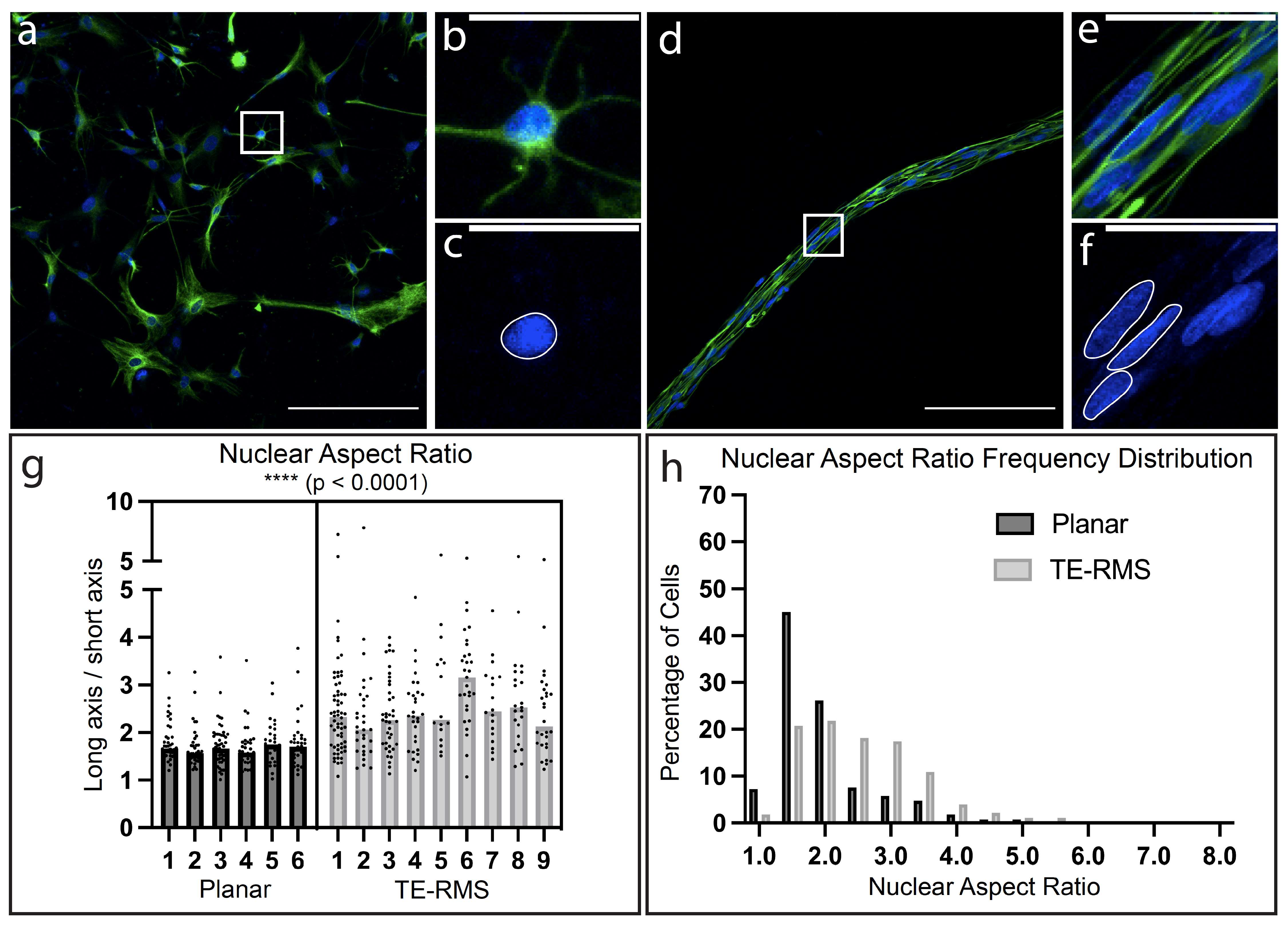
3.3. TE-RMS Astrocytes Possess Elongated Nuclei Compared to Planar Astrocyte Sister Cultures
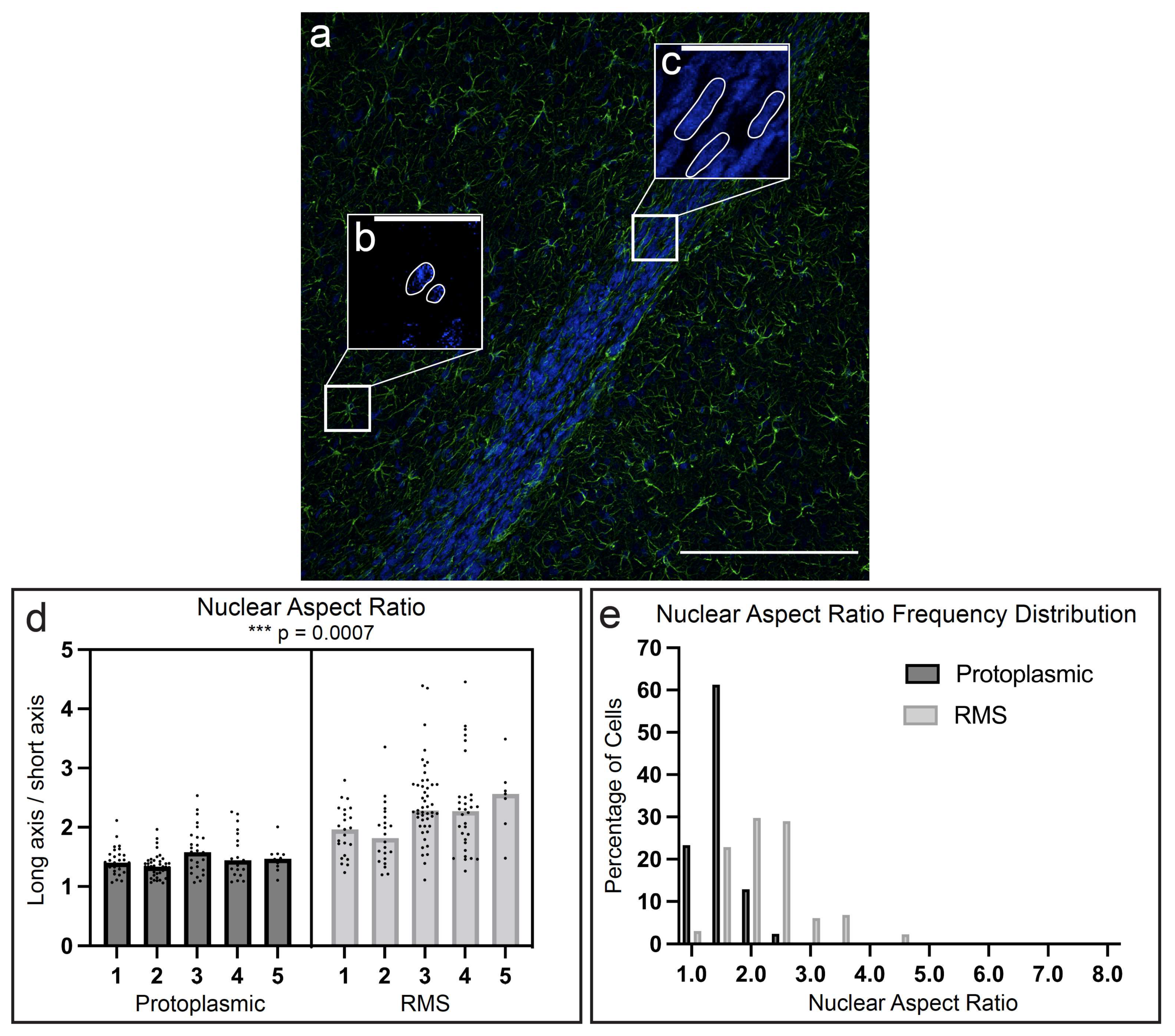
3.4. RMS Astrocytes Possess Elongated Nuclei Compared to Protoplasmic Astrocytes in the Endogenous Rat Brain
3.5. High Magnification Fluorescent Imaging Highlights Profound Differences in Nuclear Morphology and Cytoskeletal Arrangement between Planar and TE-RMS Astrocytes
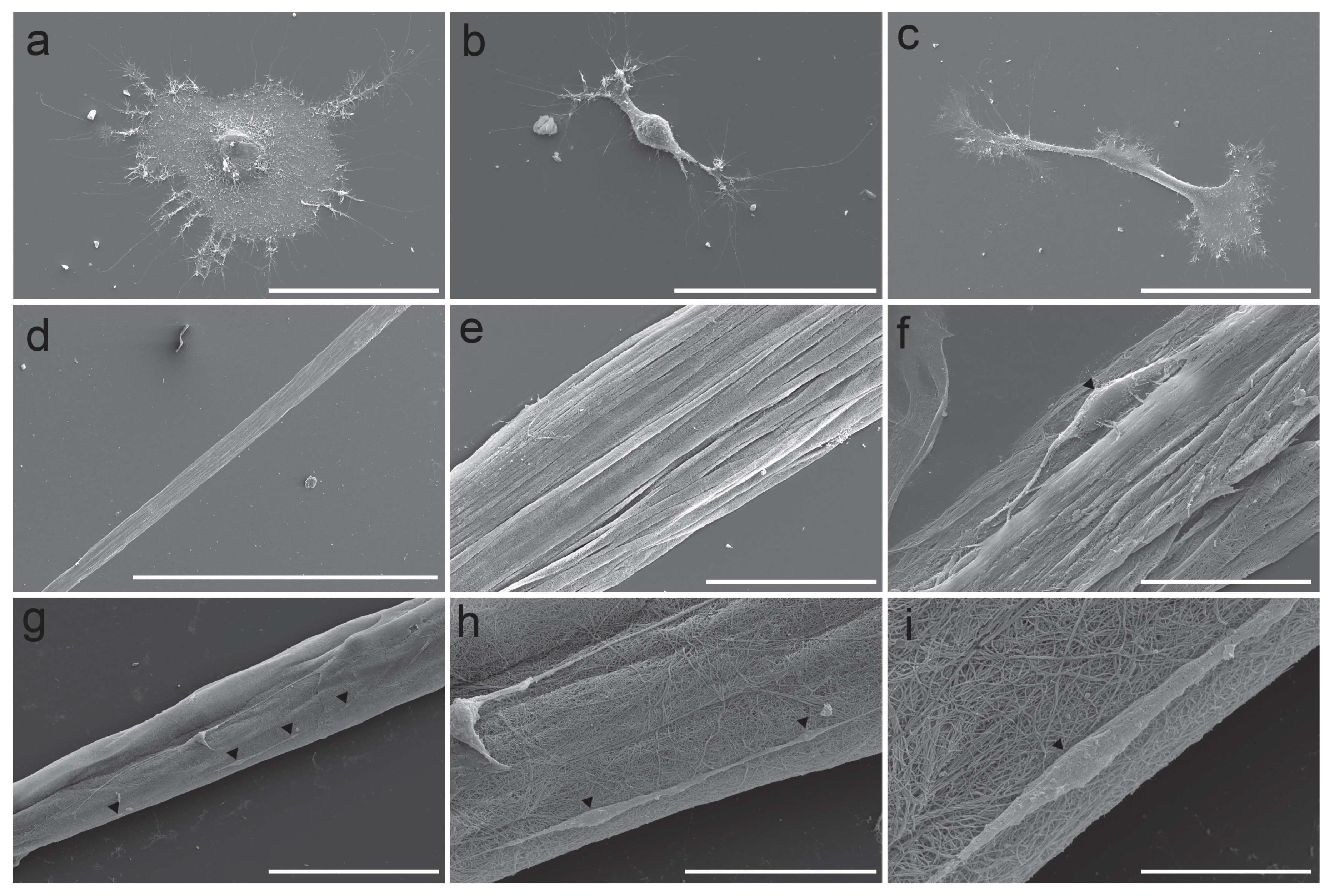
3.6. Scanning Electron Microscopy Imaging Confirms Novel Astrocytic Morphology of the TE-RMS
3.7. Comparison of TE-RMS and Endogenous Rat RMS Astrocytes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ming, G.-L.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and Functional Implications of Adult Neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lledo, P.-M.; Merkle, F.T.; Alvarez-Buylla, A. Origin and Function of Olfactory Bulb Interneuron Diversity. Trends Neurosci. 2008, 31, 392–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, D.A.; Alvarez-Buylla, A. The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018820. [Google Scholar] [CrossRef]
- Lazarini, F.; Lledo, P.-M. Is Adult Neurogenesis Essential for Olfaction? Trends Neurosci. 2011, 34, 20–30. [Google Scholar] [CrossRef]
- Brill, M.S.; Ninkovic, J.; Winpenny, E.; Hodge, R.D.; Ozen, I.; Yang, R.; Lepier, A.; Gascón, S.; Erdelyi, F.; Szabo, G.; et al. Adult Generation of Glutamatergic Olfactory Bulb Interneurons. Nat. Neurosci. 2009, 12, 1524–1533. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Manaenko, A.; Hu, Q. Targeting Adult Neurogenesis for Poststroke Therapy. Stem Cells Int. 2017, 2017, 5868632. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Wang, Y.; Xie, L.; Mao, X.; Won, S.J.; Galvan, V.; Jin, K. Effect of Neural Precursor Proliferation Level on Neurogenesis in Rat Brain during Aging and after Focal Ischemia. Neurobiol. Aging 2009, 30, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Chang, E.H.; Adorjan, I.; Mundim, M.V.; Sun, B.; Dizon, M.L.V.; Szele, F.G. Traumatic Brain Injury Activation of the Adult Subventricular Zone Neurogenic Niche. Front. Neurosci. 2016, 10, 332. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, N.; Herranz-Pérez, V.; Otsuka, T.; Sano, H.; Ohno, N.; Omata, T.; Nguyen, H.B.; Thai, T.Q.; Nambu, A.; Kawaguchi, Y.; et al. New Neurons Use Slit-Robo Signaling to Migrate through the Glial Meshwork and Approach a Lesion for Functional Regeneration. Sci. Adv. 2018, 4, eaav0618. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.-W.; Wang, Y.-Q.; Xu, M.; Shen, D.-H.; Wang, J.-J.; Huang, F.; Yu, Z.; Sun, F.-Y. Functional Integration of Newly Generated Neurons into Striatum after Cerebral Ischemia in the Adult Rat Brain. Stroke 2008, 39, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, Y.; Zheng, M.; Zhong, J.; Ma, F.; Zhou, B.; Zhu, J. Neurogenic Niche Conversion Strategy Induces Migration and Functional Neuronal Differentiation of Neural Precursor Cells Following Brain Injury. Stem Cells Dev. 2020, 29, 235–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohab, J.J.; Fleming, S.; Blesch, A.; Carmichael, S.T. A Neurovascular Niche for Neurogenesis after Stroke. J. Neurosci. 2006, 26, 13007–13016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, B.; Morshead, C.; Gonzalez, C.; Kim, M.; Gregg, C.; Shingo, T.; Weiss, S. Growth Factor-Stimulated Generation of New Cortical Tissue and Functional Recovery after Stroke Damage to the Motor Cortex of Rats. J. Cereb. Blood Flow Metab. 2007, 27, 983–997. [Google Scholar] [CrossRef] [Green Version]
- Schäbitz, W.-R.; Steigleder, T.; Cooper-Kuhn, C.M.; Schwab, S.; Sommer, C.; Schneider, A.; Kuhn, H.G. Intravenous Brain-Derived Neurotrophic Factor Enhances Poststroke Sensorimotor Recovery and Stimulates Neurogenesis. Stroke 2007, 38, 2165–2172. [Google Scholar] [CrossRef] [Green Version]
- Jinnou, H.; Sawada, M.; Kawase, K.; Kaneko, N.; Herranz-Pérez, V.; Miyamoto, T.; Kawaue, T.; Miyata, T.; Tabata, Y.; Akaike, T.; et al. Radial Glial Fibers Promote Neuronal Migration and Functional Recovery after Neonatal Brain Injury. Cell Stem Cell 2018, 22, 128–137.e9. [Google Scholar] [CrossRef] [Green Version]
- Gundelach, J.; Koch, M. Redirection of Neuroblast Migration from the Rostral Migratory Stream into a Lesion in the Prefrontal Cortex of Adult Rats. Exp. Brain Res. 2018, 236, 1181–1191. [Google Scholar] [CrossRef]
- Clark, A.R.; Carter, A.B.; Hager, L.E.; Price, E.M. In Vivo Neural Tissue Engineering: Cylindrical Biocompatible Hydrogels That Create New Neural Tracts in the Adult Mammalian Brain. Stem Cells Dev. 2016, 25, 1109–1118. [Google Scholar] [CrossRef]
- Yu, S.-J.; Tseng, K.-Y.; Shen, H.; Harvey, B.K.; Airavaara, M.; Wang, Y. Local Administration of AAV-BDNF to Subventricular Zone Induces Functional Recovery in Stroke Rats. PLoS ONE 2013, 8, e81750. [Google Scholar] [CrossRef]
- Erlandsson, A.; Lin, C.-H.A.; Yu, F.; Morshead, C.M. Immunosuppression Promotes Endogenous Neural Stem and Progenitor Cell Migration and Tissue Regeneration after Ischemic Injury. Exp. Neurol. 2011, 230, 48–57. [Google Scholar] [CrossRef]
- Petraglia, A.L.; Marky, A.H.; Walker, C.; Thiyagarajan, M.; Zlokovic, B.V. Activated Protein C Is Neuroprotective and Mediates New Blood Vessel Formation and Neurogenesis after Controlled Cortical Impact. Neurosurgery 2010, 66, 165–171, discussion 171-2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popa-Wagner, A.; Stöcker, K.; Balseanu, A.T.; Rogalewski, A.; Diederich, K.; Minnerup, J.; Margaritescu, C.; Schäbitz, W.-R. Effects of Granulocyte-Colony Stimulating Factor After Stroke in Aged Rats. Stroke 2010, 41, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Purvis, E.M.; O’Donnell, J.C.; Chen, H.I.; Cullen, D.K. Tissue Engineering and Biomaterial Strategies to Elicit Endogenous Neuronal Replacement in the Brain. Front. Neurol. 2020, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.P.; Silva-Correia, J.; Reis, R.L.; Oliveira, J.M. Biomaterials Developments for Brain Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1078, 323–346. [Google Scholar] [CrossRef]
- Pettikiriarachchi, J.T.S.; Parish, C.L.; Shoichet, M.S.; Forsythe, J.S.; Nisbet, D.R. Biomaterials for Brain Tissue Engineering. Aust. J. Chem. 2010, 63, 1143. [Google Scholar] [CrossRef]
- Winter, C.C.; Katiyar, K.S.; Hernandez, N.S.; Song, Y.J.; Struzyna, L.A.; Harris, J.P.; Cullen, D.K. Transplantable Living Scaffolds Comprised of Micro-Tissue Engineered Aligned Astrocyte Networks to Facilitate Central Nervous System Regeneration. Acta Biomater. 2016, 38, 44–58. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, K.S.; Winter, C.C.; Gordián-Vélez, W.J.; O’Donnell, J.C.; Song, Y.J.; Hernandez, N.S.; Struzyna, L.A.; Cullen, D.K. Three-Dimensional Tissue Engineered Aligned Astrocyte Networks to Recapitulate Developmental Mechanisms and Facilitate Nervous System Regeneration. J. Vis. Exp. 2018, 55848. [Google Scholar] [CrossRef]
- O’Donnell, J.; Katiyar, K.; Panzer, K.; Cullen, D.K. A Tissue-Engineered Rostral Migratory Stream for Directed Neuronal Replacement. Neural Regen. Res. 2018, 13, 1327. [Google Scholar] [CrossRef]
- O’Donnell, J.C.; Purvis, E.M.; Helm, K.V.T.; Adewole, D.O.; Zhang, Q.; Le, A.D.; Cullen, D.K. An Implantable Human Stem Cell-Derived Tissue-Engineered Rostral Migratory Stream for Directed Neuronal Replacement. Commun. Biol. 2021, 4, 879. [Google Scholar] [CrossRef]
- Struzyna, L.A.; Adewole, D.O.; Gordián-Vélez, W.J.; Grovola, M.R.; Burrell, J.C.; Katiyar, K.S.; Petrov, D.; Harris, J.P.; Cullen, D.K. Anatomically Inspired Three-Dimensional Micro-Tissue Engineered Neural Networks for Nervous System Reconstruction, Modulation, and Modeling. J. Vis. Exp. 2017, 123, e55609. [Google Scholar] [CrossRef]
- Struzyna, L.A.; Browne, K.D.; Brodnik, Z.D.; Burrell, J.C.; Harris, J.P.; Chen, H.I.; Wolf, J.A.; Panzer, K.V.; Lim, J.; Duda, J.E.; et al. Tissue Engineered Nigrostriatal Pathway for Treatment of Parkinson’s Disease. J. Tissue Eng. Regen. Med. 2018, 12, 1702–1716. [Google Scholar] [CrossRef] [PubMed]
- Braet, F.; De Zanger, R.; Wisse, E. Drying Cells for SEM, AFM and TEM by Hexamethyldisilazane: A Study on Hepatic Endothelial Cells. J. Microsc. 1997, 186, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peretto, P.; Merighi, A.; Fasolo, A.; Bonfanti, L. Glial Tubes in the Rostral Migratory Stream of the Adult Rat. Brain Res. Bull. 1997, 42, 9–21. [Google Scholar] [CrossRef]
- Moshayedi, P.; da F Costa, L.; Christ, A.; Lacour, S.P.; Fawcett, J.; Guck, J.; Franze, K. Mechanosensitivity of Astrocytes on Optimized Polyacrylamide Gels Analyzed by Quantitative Morphometry. J. Phys. Condens. Matter 2010, 22, 194114. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Braga, A.; Yu, Y.; Esteras, N.; Korsak, A.; Theparambil, S.M.; Hadjihambi, A.; Hosford, P.S.; Teschemacher, A.G.; Marina, N.; et al. Mechanosensory Signaling in Astrocytes. J. Neurosci. 2020, 40, 9364–9371. [Google Scholar] [CrossRef]
- Marina, N.; Christie, I.N.; Korsak, A.; Doronin, M.; Brazhe, A.; Hosford, P.S.; Wells, J.A.; Sheikhbahaei, S.; Humoud, I.; Paton, J.F.R.; et al. Astrocytes Monitor Cerebral Perfusion and Control Systemic Circulation to Maintain Brain Blood Flow. Nat. Commun. 2020, 11, 131. [Google Scholar] [CrossRef] [Green Version]
- Kirby, T.J.; Lammerding, J. Emerging Views of the Nucleus as a Cellular Mechanosensor. Nat. Cell Biol. 2018, 20, 373–381. [Google Scholar] [CrossRef]
- Graham, D.M.; Burridge, K. Mechanotransduction and Nuclear Function. Curr. Opin. Cell Biol. 2016, 40, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Zimmerli, C.E.; Allegretti, M.; Rantos, V.; Goetz, S.K.; Obarska-Kosinska, A.; Zagoriy, I.; Halavatyi, A.; Hummer, G.; Mahamid, J.; Kosinski, J.; et al. Nuclear Pores Dilate and Constrict in Cellulo. Science 2021, 374, eabd9776. [Google Scholar] [CrossRef]
- dos Santos, Á.; Toseland, C.P. Regulation of Nuclear Mechanics and the Impact on DNA Damage. Int. J. Mol. Sci. 2021, 22, 3178. [Google Scholar] [CrossRef] [PubMed]
- Versaevel, M.; Grevesse, T.; Gabriele, S. Spatial Coordination between Cell and Nuclear Shape within Micropatterned Endothelial Cells. Nat. Commun. 2012, 3, 671. [Google Scholar] [CrossRef] [Green Version]
- Rougerie, P.; Pieuchot, L.; dos Santos, R.S.; Marteau, J.; Bigerelle, M.; Chauvy, P.-F.; Farina, M.; Anselme, K. Topographical Curvature Is Sufficient to Control Epithelium Elongation. Sci. Rep. 2020, 10, 14784. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.T.T.; Pease, M.E.; Jefferys, J.L.; Kimball, E.C.; Quigley, H.A.; Nguyen, T.D. Pressure-Induced Changes in Astrocyte GFAP, Actin, and Nuclear Morphology in Mouse Optic Nerve. Investig. Opthalmol. Vis. Sci. 2020, 61, 14. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, A.A.; Hou, X.; Ade, A.S.; Fon, G.-V.; Meixner, W.; Higgins, G.A.; Sexton, J.Z.; Wan, X.; Dinov, I.D.; O’Meara, M.J.; et al. Valproic Acid-Induced Changes of 4D Nuclear Morphology in Astrocyte Cells. Mol. Biol. Cell 2021, 32, mbc.E20-08-0502. [Google Scholar] [CrossRef]
- USHIKI, T. Collagen Fibers, Reticular Fibers and Elastic Fibers. A Comprehensive Understanding from a Morphological Viewpoint. Arch. Histol. Cytol. 2002, 65, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Orellana, J.A.; Martinez, A.D.; Retamal, M.A. Gap Junction Channels and Hemichannels in the CNS: Regulation by Signaling Molecules. Neuropharmacology 2013, 75, 567–582. [Google Scholar] [CrossRef]
- Finkbeiner, S. Calcium Waves in Astrocytes-Filling in the Gaps. Neuron 1992, 8, 1101–1108. [Google Scholar] [CrossRef]
- Petrany, M.J.; Millay, D.P. Cell Fusion: Merging Membranes and Making Muscle. Trends Cell Biol. 2019, 29, 964–973. [Google Scholar] [CrossRef]
- Haftbaradaran Esfahani, P.; Knöll, R. Cell Shape: Effects on Gene Expression and Signaling. Biophys. Rev. 2020, 12, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Seelbinder, B.; Ghosh, S.; Schneider, S.E.; Scott, A.K.; Berman, A.G.; Goergen, C.J.; Margulies, K.B.; Bedi, K.C.; Casas, E.; Swearingen, A.R.; et al. Nuclear Deformation Guides Chromatin Reorganization in Cardiac Development and Disease. Nat. Biomed. Eng. 2021, 5, 1500–1516. [Google Scholar] [CrossRef] [PubMed]
- Ramdas, N.M.; Shivashankar, G.V. Cytoskeletal Control of Nuclear Morphology and Chromatin Organization. J. Mol. Biol. 2015, 427, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Skinner, B.M.; Johnson, E.E.P. Nuclear Morphologies: Their Diversity and Functional Relevance. Chromosoma 2017, 126, 195–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Nuclear and Cytoskeletal Comparison of TE-RMS and Endogenous Rat RMS Astrocytes | |||||
|---|---|---|---|---|---|
| TE-RMS Mean ± SD | RMS Mean ± SD | DF | T Value | p Value | |
| Number of main processes | 2.35 ± 0.69 | 2.31 ± 0.98 | 12 | 0.6326 | 0.5388 |
| Number of branch points | 0.75 ± 0.98 | 0.78 ± 0.93 | 12 | 0.1509 | 0.8825 |
| Angle of main processes | 12.42 ± 16.42 | 18.63 ± 19.39 | 12 | 2.861 | 0.0143 |
| Nuclear aspect ratio | 2.56 ± 0.98 | 2.23 ± 0.67 | 12 | 2.490 | 0.0284 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purvis, E.M.; O’Donnell, J.C.; Cullen, D.K. Unique Astrocyte Cytoskeletal and Nuclear Morphology in a Three-Dimensional Tissue-Engineered Rostral Migratory Stream. Neuroglia 2022, 3, 41-60. https://doi.org/10.3390/neuroglia3010003
Purvis EM, O’Donnell JC, Cullen DK. Unique Astrocyte Cytoskeletal and Nuclear Morphology in a Three-Dimensional Tissue-Engineered Rostral Migratory Stream. Neuroglia. 2022; 3(1):41-60. https://doi.org/10.3390/neuroglia3010003
Chicago/Turabian StylePurvis, Erin M., John C. O’Donnell, and D. Kacy Cullen. 2022. "Unique Astrocyte Cytoskeletal and Nuclear Morphology in a Three-Dimensional Tissue-Engineered Rostral Migratory Stream" Neuroglia 3, no. 1: 41-60. https://doi.org/10.3390/neuroglia3010003
APA StylePurvis, E. M., O’Donnell, J. C., & Cullen, D. K. (2022). Unique Astrocyte Cytoskeletal and Nuclear Morphology in a Three-Dimensional Tissue-Engineered Rostral Migratory Stream. Neuroglia, 3(1), 41-60. https://doi.org/10.3390/neuroglia3010003






