Glia Excitation in the CNS Modulates Intact Behaviors and Sensory-CNS-Motor Circuitry
Abstract
1. Introduction
2. Methods and Materials
2.1. Fly Lines and Optogenetic Procedures
2.2. Intact Adult Behavior
2.3. Intact Larval Behavior
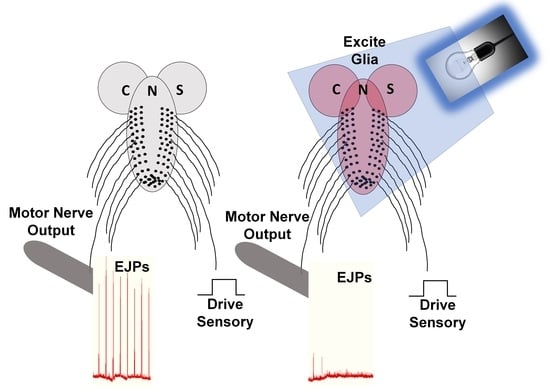
2.4. In Situ Neural Circuit of Larvae
2.5. Study Area
3. Results
3.1. Intact Adult Behavior
3.2. Intact Larval Behavior
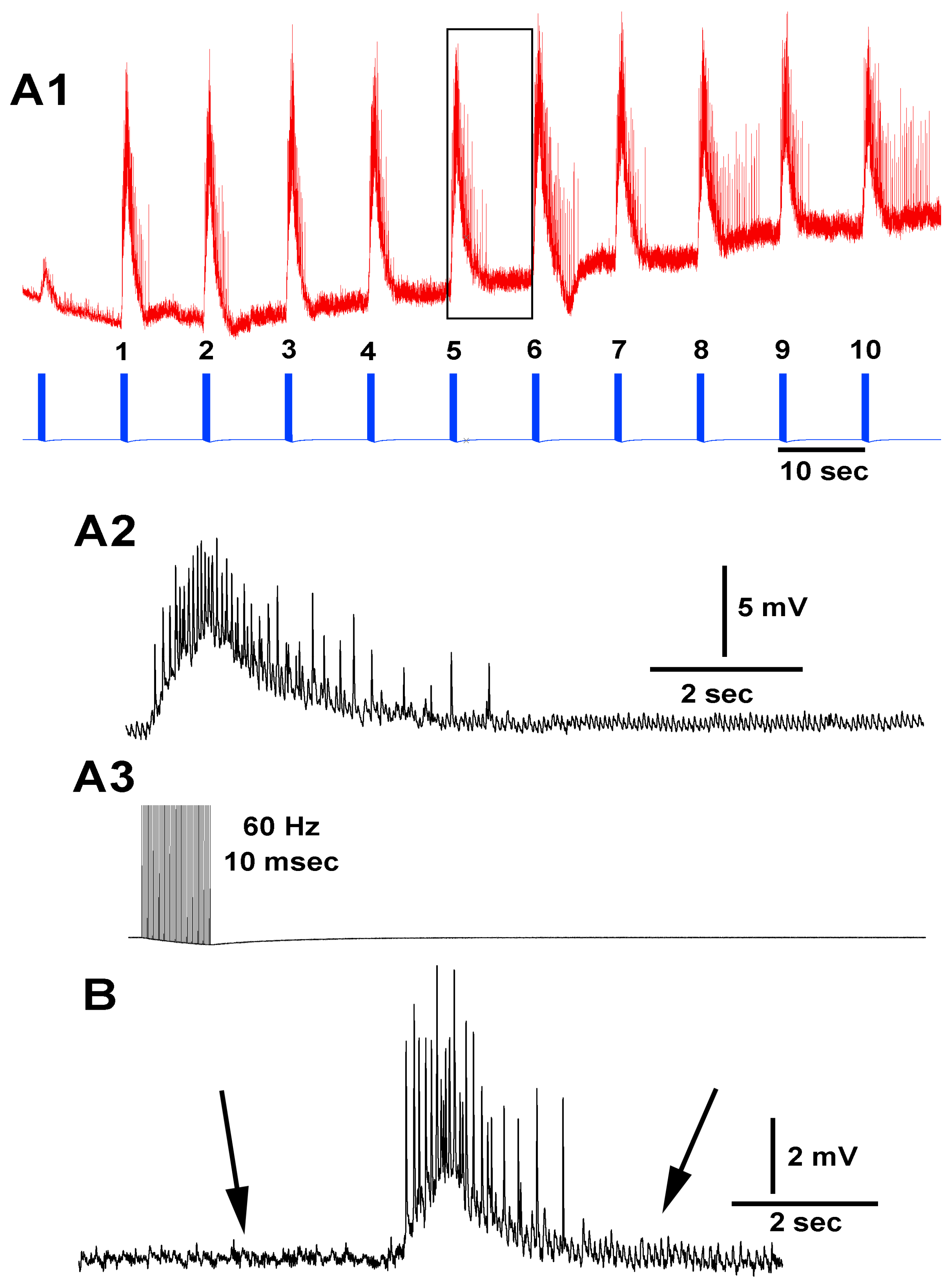
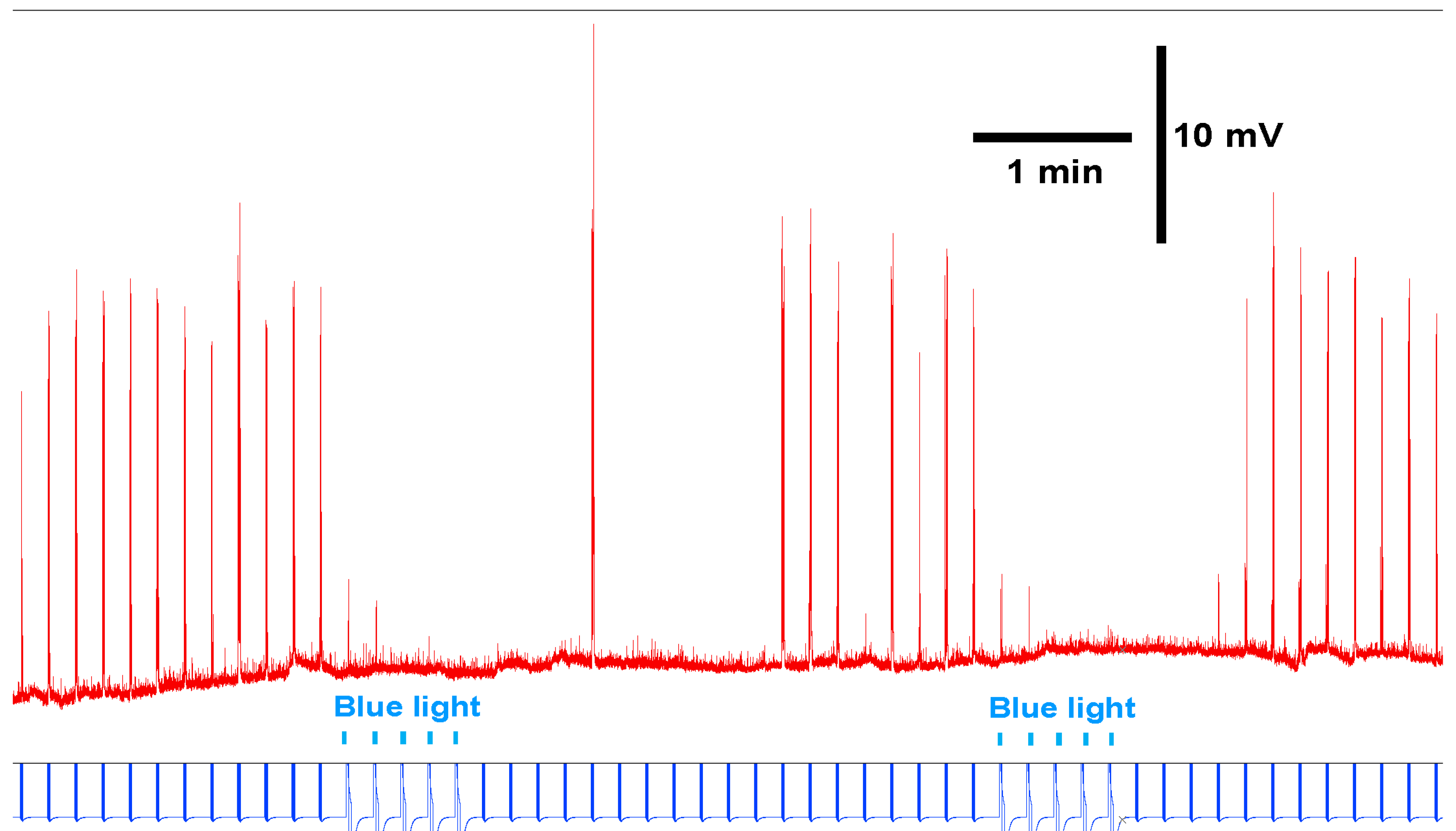
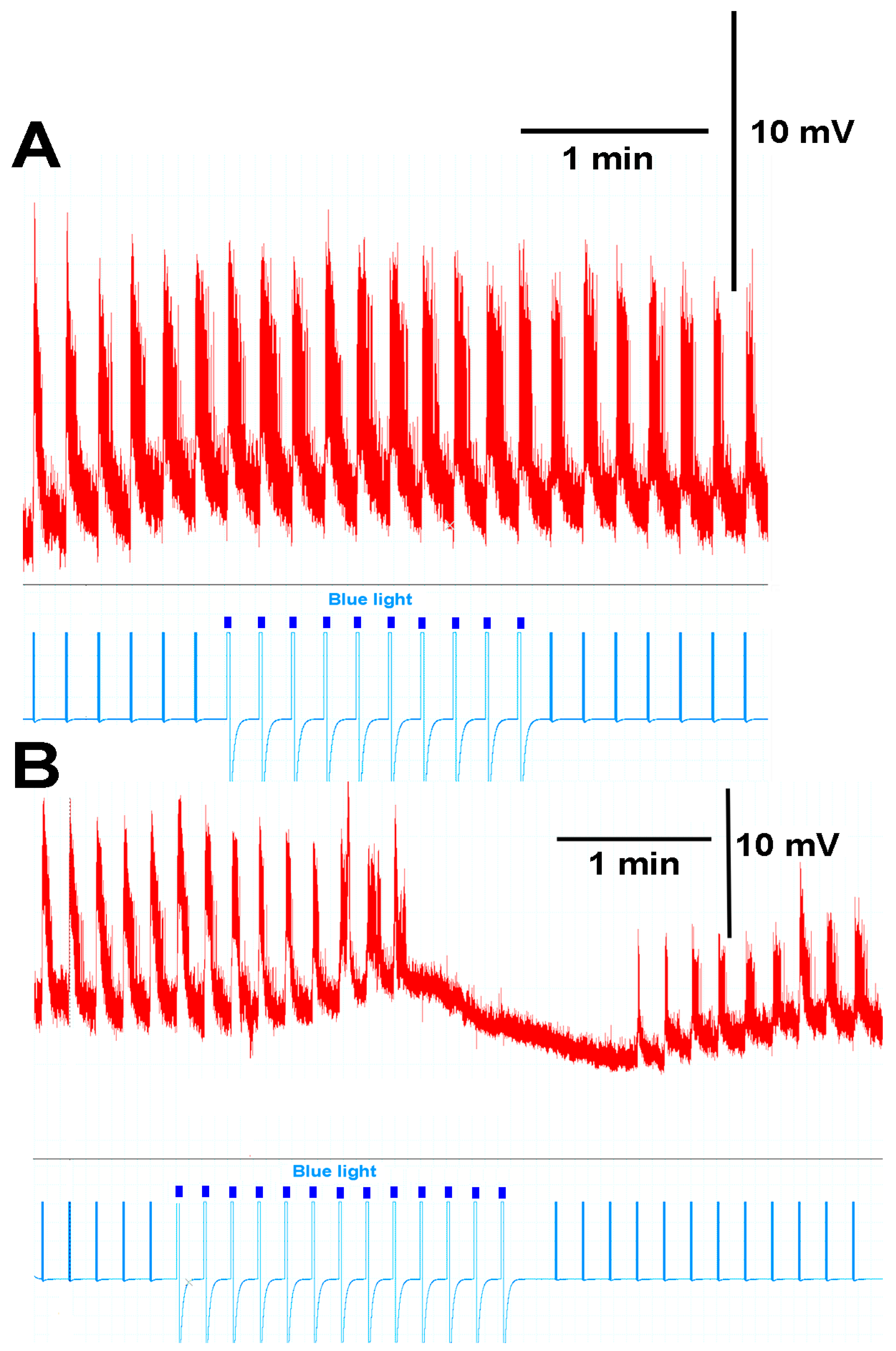
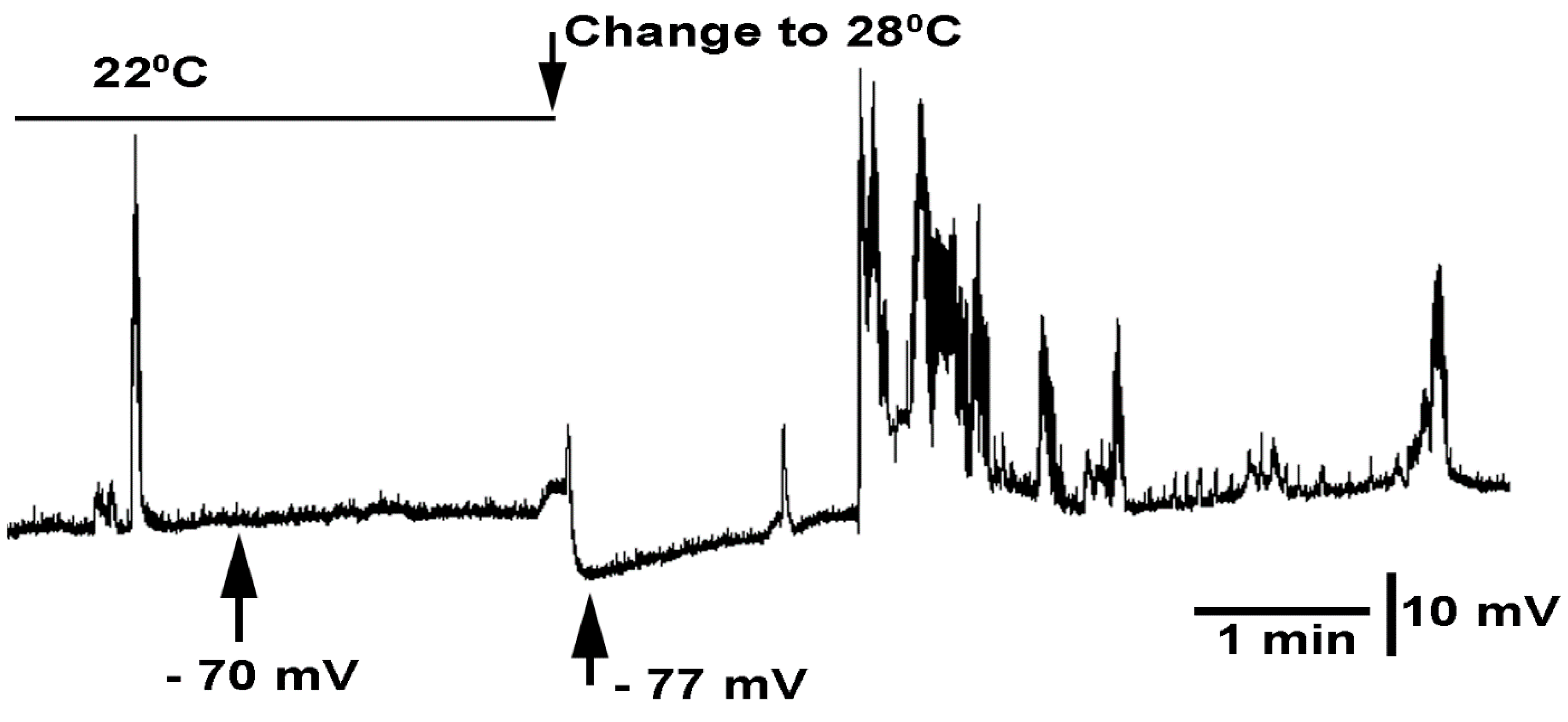
3.3. In Situ Neural Circuit Activity of Larvae
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Ansary, A.; Hassan, W.M.; Qasem, H.; Das, U.N. Identification of biomarkers of impaired sensory profiles among autistic patients. PLoS ONE 2016, 11, e0164153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Yang, H.X.; Cui, X.L.; Shi, L.J.; Gong, J.B.; Lui, S.S.Y.; Cheung, E.F.C.; Watanabe, K.; Chan, R.C.K. Self-reported sensory responsiveness patterns in typically-developing and early-onset schizophrenia adolescents: Its relationship with schizotypal and autistic traits. J. Psychiatr. Res. 2020, 131, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Yang, H.X.; Shi, L.J.; Lui, S.S.Y.; Cheung, E.F.C.; Chan, R.C.K. Correlations between audiovisual temporal processing and sensory responsiveness in adolescents with autistic traits. J. Autism Dev. Disord. 2021, 51, 2450–2460. [Google Scholar] [CrossRef]
- Filippi, M.; Preziosa, P.; Langdon, D.; Lassmann, H.; Paul, F.; Rovira, À.; Schoonheim, M.M.; Solari, A.; Stankoff, B.; Rocca, M.A. Identifying progression in multiple sclerosis: New perspectives. Ann. Neurol. 2020, 88, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Rus, H.; Pardo, C.A.; Hu, L.; Darrah, E.; Cudrici, C.; Niculescu, T.; Niculescu, F.; Mullen, K.M.; Allie, R.; Guo, L.; et al. The voltage-gated potassium channel Kv1.3 is highly expressed on inflammatory infiltrates in multiple sclerosis brain. Proc. Natl. Acad. Sci. USA 2005, 102, 11094–11099. [Google Scholar] [CrossRef] [PubMed]
- Coggan, J.S.; Bittner, S.; Stiefel, K.M.; Meuth, S.G.; Prescott, S.A. Physiological dynamics in demyelinating diseases: Unraveling complex relationships through computer modeling. Intl. J. Mol. Sci. 2015, 16, 21215–21236. [Google Scholar] [CrossRef] [PubMed]
- Auld, D.S.; Robitaille, R. Glial cells and neurotransmission: An inclusive view of synaptic function. Neuron 2003, 40, 389–400. [Google Scholar] [CrossRef]
- Sancho, L.; Contreras, M.; Allen, N.J. Glia as sculptors of synaptic plasticity. Neurosci. Res. 2021, 167, 17–29. [Google Scholar] [CrossRef]
- Einheber, S.; Bhat, M.; Salzer, J. Disrupted axo-glial junctions result in accumulation of abnormal mitochondria at nodes of Ranvier. Neuron Glia Biol. 2006, 2, 165–174. [Google Scholar] [CrossRef][Green Version]
- Kuffler, S.W.; Potter, D.D. Glia in the leech central nervous system: Physiological properties and neuron-glia relationship. J. Neurophysiol. 1964, 27, 290–320. [Google Scholar] [CrossRef]
- Sasaki, T.; Beppu, K.; Tanaka, K.F.; Fukazawa, Y.; Shigemoto, R.; Matsui, K. Application of an optogenetic byway for perturbing neuronal activity via glial photostimulation. Proc. Natl. Acad. Sci. USA 2012, 109, 20720–20725. [Google Scholar] [CrossRef] [PubMed]
- Rennels, M.L.; Gregory, T.F.; Blaumanis, O.R.; Fujimoto, K.; Grady, P.A. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985, 326, 47–63. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Baylor, D.A.; Nicholls, J.G. Changes in extracellular potassium concentration produced by neuronal activity in the central nervous system of the leech. J. Physiol. 1969, 203, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Kuffler, S.W.; Nicholls, J.G.; Orkand, R.K. Physiological properties of glial cells in the central nervous system of amphibia. J. Neurophysiol. 1966, 29, 768–787. [Google Scholar] [CrossRef] [PubMed]
- Mitarai, G.; Svaetichin, G.; Vallecalle, E.; Fatechand, R.; Villegas, J.; Laufer, M. Glia-neuron interactions and adaptational mechanisms of the retina. In The Visual System; Jung, R., Kornhuber, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1961. [Google Scholar]
- Robitaille, R. Purinergic receptors and their activation by endogenous purines at perisynaptic glial cells of the frog neuromuscular junction. J. Neurosci. 1995, 15, 7121–7131. [Google Scholar] [CrossRef]
- Todd, K.J.; Robitaille, R. Purinergic modulation of synaptic signalling at the neuromuscular junction. Pflugers Arch. 2006, 452, 608–614. [Google Scholar] [CrossRef]
- Barik, A.; Li, L.; Sathyamurthy, A.; Xiong, W.C.; Mei, L. Schwann cells in neuromuscular junction formation and maintenance. J. Neurosci. 2016, 36, 9770–9781. [Google Scholar] [CrossRef]
- Dawydow, A.; Gueta, R.; Ljaschenko, D.; Ullrich, S.; Hermann, M.; Ehmann, N.; Gao, S.; Fiala, A.; Langenhan, T.; Nagel, G.; et al. Channelrhodopsin-2-XXL, a powerful optogenetic tool for low-light applications. Proc. Natl. Acad. Sci. USA 2014, 111, 13972–13977. [Google Scholar] [CrossRef]
- Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 2015, 18, 1213–1225. [Google Scholar] [CrossRef]
- Han, X.; Boyden, E.S. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE 2007, 2, e299. [Google Scholar] [CrossRef] [PubMed]
- Pankau, C.; McCubbin, S.; Cooper, R.L. The effect of optogenetically activating glia on neuronal function. Neuroglia 2021, 2, 57–67. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Lee, I.S. Drosophila Glia: Models for Human Neurodevelopmental and Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 4859. [Google Scholar] [CrossRef] [PubMed]
- Stork, T.; Engelen, D.; Krudewig, A.; Silies, M.; Bainton, R.J.; Klämbt, C. Organization and function of the blood-brain barrier in Drosophila. J. Neurosci. 2008, 28, 587–597. [Google Scholar] [CrossRef] [PubMed]
- MacNamee, S.E.; Liu, K.E.; Gerhard, S.; Tran, C.T.; Fetter, R.D.; Cardona, A.; Tolbert, L.P.; Oland, L.A. Astrocytic glutamate transport regulates a Drosophila CNS synapse that lacks astrocyte ensheathment. J. Comp. Neurol. 2016, 524, 1979–1998. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Urban, J.; Technau, G.M. Distribution, classification, and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Rouxs Arch. Dev. Biol. 1995, 204, 284–307. [Google Scholar] [CrossRef]
- Beckervordersandforth, R.M.; Rickert, C.; Altenhein, B.; Technau, G.M. Postembryonic development of the midline glia in the CNS of Drosophila: Proliferation, programmed cell death, and endocrine regulation. Mech. Dev. 2008, 125, 542–557. [Google Scholar] [CrossRef]
- Awasaki, T.; Lai, S.-L.; Ito, K.; Lee, T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J. Neurosci. 2008, 28, 13742–13753. [Google Scholar] [CrossRef]
- Muthukumar, A.K.; Stork, T.; Freeman, M.R. Activity-dependent regulation of astrocyte GAT levels during synaptogenesis. Nat. Neurosci. 2014, 17, 1340–1350. [Google Scholar] [CrossRef]
- Peco, E.; Davla, S.; Camp, D.; Stacey, S.M.; Landgraf, M.; van Meyel, D.J. Drosophila astrocytes cover specific territories of the CNS neuropil and are instructed to differentiate by Prospero, a key effector of Notch. Development 2016, 143, 1170–1181. [Google Scholar] [CrossRef]
- Higgins, J.; Hermanns, C.; Malloy, C.; Cooper, R.L. Considerations in repetitive activation of light sensitive ion channels for long term studies: Channel rhodopsin in the Drosophila model. Neurosci. Res. 2017, 125, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; Ocorr, K. Drug discovery through functional screening in the Drosophila heart. Methods Mol. Biol. 2009, 577, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Bellen, H.J.; Tong, C.; Tsuda, H. 100 years of Drosophila research and its impact on vertebrate neuroscience: A history lesson for the future. Nat. Rev. Neurosci. 2010, 11, 514–522. [Google Scholar] [CrossRef]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef]
- Lewis, E.B. A new standard food medium. Drosoph. Inf. Ser. 1960, 34, 117–118. [Google Scholar]
- Zhao, S.; Cunha, C.; Zhang, F.; Liu, Q.; Gloss, B.; Deisseroth, K.; Augustine, G.J.; Feng, G. Improved expression of halorhodopsin for light-induced silencing of neuronal activity. Brain Cell Biol. 2008, 36, 141–154. [Google Scholar] [CrossRef]
- Govorunova, E.G.; Sineshchekov, O.A.; Janz, R.; Liu, X.; Spudich, J.L. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science 2015, 349, 647–650. [Google Scholar] [CrossRef]
- Potter, S.; Sifers, J.; Yocom, E.; Blümich, S.L.E.; Potter, R.; Nadolski, J.; Harrison, D.A.; Cooper, R.L. Acute and chronic effects of inhibiting dTOR by rapamycin on development, behavior, and physiology in Drosophila. Biol. Open 2019, 8, bio046508. [Google Scholar] [CrossRef]
- Majeed, Z.R.; Abdeljaber, E.; Soveland, R.; Cornwell, K.; Bankemper, A.; Koch, F.; Cooper, R.L. Modulatory action by the serotonergic system: Behavior and neurophysiology in Drosophila melanogaster. Neural Plast. 2016, 2016, 7291438. [Google Scholar] [CrossRef]
- Titlow, J.S.; Rice, J.; Majeed, Z.R.; Holsopple, E.; Biecker, S.; Cooper, R.L. Anatomical and genotype-specific mechanosensory responses in Drosophila melanogaster larvae. Neurosci. Res. 2014, 83, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Cooper, R.L. Modulation of sensory to motor circuits by serotonin, octopamine, and dopamine in semi-intact Drosophila larva. Neurosci. Res. 2004, 48, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Malloy, C.; Somasundaram, E.; Omar, A.; Bhutto, U.; Medley, M.; Dzubuk, N.; Cooper, R.L. Pharmacological identification of cholinergic receptor subtypes: Modulation of locomotion and neural circuit excitability in Drosophila larvae. Neurosci. IBRO 2019, 411, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Frankenhauser, B.; Hodgkin, A.L. The after-effects of impulses in the giant nerve fibers of Loligo. J. Physiol. 1956, 131, 341–376. [Google Scholar] [CrossRef]
- Katz, B.; Schmitt, O.H. Electric interaction between two adjacent nerve fibres. J Physiol. 1940, 97, 471–488. [Google Scholar] [CrossRef]
- Freiha, J.; Riachi, N.; Chalah, M.A.; Zoghaib, R.; Ayache, S.S.; Ahdab, R. Paroxysmal symptoms in multiple sclerosis-A review of the literature. J. Clin. Med. 2020, 9, 3100. [Google Scholar] [CrossRef]
- Friede, R. Der Kohlenhydratgehalt der Glia von Hirudo bei verschiedenen Funktionszustanden. Z. Zellforsch. 1954, 41, 509–520. [Google Scholar] [CrossRef]
- Angstadt, J.D.; Friesen, W.O. Modulation of swimming behavior in the medicinal leech. II. Ionic conductances underlying serotonergic modulation of swim-gating cell 204. J. Comp. Physiol. A 1993, 172, 235–248. [Google Scholar] [CrossRef]
- Akaishi, T.; Takahashi, T.; Himori, N.; Takeshita, T.; Nakazawa, T.; Aoki, M.; Nakashima, I. Chloride imbalance is involved in the pathogenesis of optic neuritis in neuromyelitis optica. J. Neuroimmunol. 2018, 320, 98–100. [Google Scholar] [CrossRef]
- Bondoli, A.; Magalini, S.I.; de Angelis, C.; Foti, A.; Rodolà, F.; Mascaro, A.; Ranieri, R. Changes in plasma and cerebrospinal fluid electrolytes in hypercapnia. Resuscitation 1981, 9, 99–102. [Google Scholar] [CrossRef]
- Honjo, K.; Hwang, R.Y.; Tracey, W.D., Jr. Optogenetic manipulation of neural circuits and behavior in Drosophila larvae. Nat. Protoc. 2012, 7, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Armache, J.-P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nat. Cell Biol. 2015, 520, 511–517. [Google Scholar] [CrossRef]
- Wang, H.; Schupp, M.; Zurborg, S.; Heppenstall, P.A. Residues in the pore region of Drosophila transient receptor potential A1 dictate sensitivity to thermal stimuli. J. Physiol. 2013, 591, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Hamada, F.N.; Rosenzweig, M.; Kang, K.; Pulver, S.R.; Ghezzi, A.; Jegla, T.J.; Garrity, P.A. An internal thermal sensor controlling temperature preference in Drosophila. Nat. Cell Biol. 2008, 454, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.-S.; Zhong, L.; Hsieh, T.-H.; Abooj, M.; Bishnoi, M.; Hughes, L.; Premkumar, L.S. Expression of Transient Receptor Potential Ankyrin 1 (TRPA1) and Its Role in Insulin Release from Rat Pancreatic Beta Cells. PLoS ONE 2012, 7, e38005. [Google Scholar] [CrossRef] [PubMed]
- Nadim, F.; Olsen, O.H.; De Schutter, E.; Calabrese, R.L. Modeling the leech heartbeat elemental oscillator. I. Interactions of intrinsic and synaptic currents. J. Comput. Neurosci. 1995, 3, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Banaclocha, M. Astroglial isopotentiality and calcium-associated biomagnetic field effects on cortical neuronal coupling. Cells 2020, 9, 439. [Google Scholar] [CrossRef]
- Peracchia, C. Increase in gap junction resistance with acidification in crayfish septate axons is closely related to changes in intracellular calcium but not hydrogen ion concentration. J. Membr. Biol. 1990, 113, 75–92. [Google Scholar] [CrossRef]
- Malchow, R.P.; Tchernookova, B.K.; Choi, J.V.; Smith, P.J.S.; Kramer, R.H.; Kreitzer, M.A. Review and hypothesis: A potential common link between glial cells, calcium changes, modulation of synaptic transmission, spreading depression, migraine, and epilepsy-H. Front. Cell Neurosci. 2021, 15, 693095. [Google Scholar] [CrossRef]
- Kiyoshi, C.M.; Du, Y.; Zhong, S.; Wang, W.; Taylor, A.T.; Xiong, B.; Ma, B.; Terman, D.; Zhou, M. Syncytial isopotentiality: A system-wide electrical feature of astrocytic networks in the brain. Glia 2018, 66, 2756–2769. [Google Scholar] [CrossRef]
- Shivers, R.R.; Brightman, M.W. Trans-glial channels in ventral nerve roots of crayfish. J. Comp. Neurol. 1976, 167, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Krill, J.L.; Dawson-Scully, K. Characterization of a novel stimulus-induced glial calcium wave in Drosophila larval peripheral segmental nerves and its role in PKG-modulated thermoprotection. J Neurogenet. 2021, 35, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Badre, N.H.; Cooper, R.L. Reduced calcium channel function in Drosophila disrupts associative learning in larva, and behavior in adults. Int. J. Zool. Res. 2008, 4, 152–164. [Google Scholar] [CrossRef]
- O’Neil, A.S.; Kim, C.; Cooper, R.L. Learning and Memory retention in larval Drosophila. J. Entomol. 2020, 17, 36–47. [Google Scholar] [CrossRef][Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCubbin, S.; Harrison, D.A.; Cooper, R.L. Glia Excitation in the CNS Modulates Intact Behaviors and Sensory-CNS-Motor Circuitry. Neuroglia 2022, 3, 23-40. https://doi.org/10.3390/neuroglia3010002
McCubbin S, Harrison DA, Cooper RL. Glia Excitation in the CNS Modulates Intact Behaviors and Sensory-CNS-Motor Circuitry. Neuroglia. 2022; 3(1):23-40. https://doi.org/10.3390/neuroglia3010002
Chicago/Turabian StyleMcCubbin, Shelby, Douglas A. Harrison, and Robin L. Cooper. 2022. "Glia Excitation in the CNS Modulates Intact Behaviors and Sensory-CNS-Motor Circuitry" Neuroglia 3, no. 1: 23-40. https://doi.org/10.3390/neuroglia3010002
APA StyleMcCubbin, S., Harrison, D. A., & Cooper, R. L. (2022). Glia Excitation in the CNS Modulates Intact Behaviors and Sensory-CNS-Motor Circuitry. Neuroglia, 3(1), 23-40. https://doi.org/10.3390/neuroglia3010002