Neurovascular Coupling in Seizures
Abstract
1. Introduction
2. Cerebral Blood Flow
3. Cerebrovascular Autoregulation
4. Physiological Neurovascular Coupling
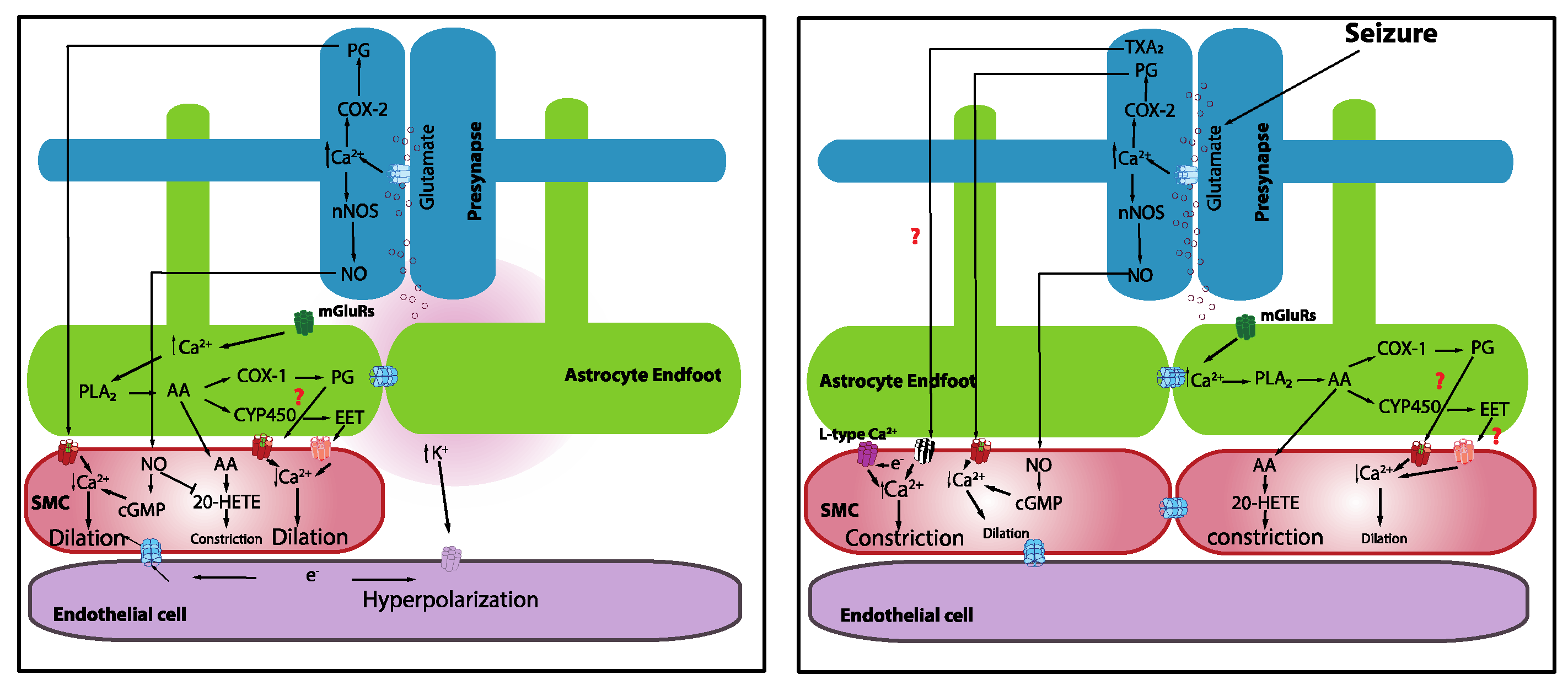
5. Neurovascular Coupling in Seizures
6. Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef]
- Tarantini, S.; Tran, C.H.T.; Gordon, G.R.; Ungvari, Z.; Csiszar, A. Impaired neurovascular coupling in aging and Alzheimer’s disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp. Gerontol. 2017, 94, 52–58. [Google Scholar] [CrossRef]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef]
- McCarron, J.G.; Osol, G.; Halpern, W. Myogenic Responses Are Independent of the Endothelium in Rat Pressurized Posterior Cerebral Arteries. J. Vasc. Res. 1989, 26, 315–319. [Google Scholar] [CrossRef]
- Attwell, D.; Buchan, A.; Charpak, S.; Lauritzen, M.; MacVicar, B.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef]
- Nippert, A.R.; Biesecker, K.R.; Newman, E.A. Mechanisms Mediating Functional Hyperemia in the Brain. Neuroscientist 2017, 24, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Longden, T.; Hill-Eubanks, D.C.; Nelson, M.T. Ion channel networks in the control of cerebral blood flow. Br. J. Pharmacol. 2015, 36, 492–512. [Google Scholar] [CrossRef]
- Stackhouse, T.L.; Mishra, A. Neurovascular Coupling in Development and Disease: Focus on Astrocytes. Front. Cell Dev. Biol. 2021, 9, 702832. [Google Scholar] [CrossRef] [PubMed]
- Baruah, J.; Vasudevan, A.; Köhling, R. Vascular Integrity and Signaling Determining Brain Development, Network Excitability, and Epileptogenesis. Front. Physiol. 2020, 10, 1583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tabatabaei, M.; Bélanger, S.; Girouard, H.; Moeini, M.; Lu, X.; Lesage, F. Astrocytic endfoot Ca2+ correlates with parenchymal vessel responses during 4-AP induced epilepsy: An in vivo two-photon lifetime microscopy study. Br. J. Pharmacol. 2017, 39, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, D.E.; Engel, J.; Phelps, M.E.; Selin, C. Epileptic patterns of local cerebral metabolism and perfusion in humans determined by emission computed tomography of 18FDG and 13NH3. Ann. Neurol. 1980, 8, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, T.; Teaw, S.; Bordey, A. Hypervascularization in mTOR-dependent focal and global cortical malformations displays differential rapamycin sensitivity. Epilepsia 2019, 60, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.Y.; Rühlmann, C.; Blinder, P.; Devor, A.; Drew, P.J.; Friedman, B.; Knutsen, P.M.; Lyden, P.D.; Mateo, C.; Mellander, L.; et al. Robust and fragile aspects of cortical blood flow in relation to the underlying angioarchitecture. Microcirculation 2015, 22, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef] [PubMed]
- Mangold, R.; Sokoloff, L.; Conner, E.; Kleinerman, J.; Therman, P.-O.G.; Kety, S.S. The effects of sleep and lack of sleep on the cerebral circulation and metabolism of normal young men 1. J. Clin. Investig. 1955, 34, 1092–1100. [Google Scholar] [CrossRef]
- Gusnard, D.A.; Raichle, M.E. Searching for a baseline: Functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001, 2, 685–694. [Google Scholar] [CrossRef]
- Hyder, F.; Rothman, D.L.; Shulman, R.G. Total neuroenergetics support localized brain activity: Implications for the interpretation of fMRI. Proc. Natl. Acad. Sci. USA 2002, 99, 10771–10776. [Google Scholar] [CrossRef]
- Smith, A.J.; Blumenfeld, H.; Behar, K.; Rothman, D.L.; Shulman, R.G.; Hyder, F. Cerebral energetics and spiking frequency: The neurophysiological basis of fMRI. Proc. Natl. Acad. Sci. USA 2002, 99, 10765–10770. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Laughlin, S. An Energy Budget for Signaling in the Grey Matter of the Brain. Br. J. Pharmacol. 2001, 21, 1133–1145. [Google Scholar] [CrossRef]
- Howarth, C.; Gleeson, P.; Attwell, D. Updated Energy Budgets for Neural Computation in the Neocortex and Cerebellum. Br. J. Pharmacol. 2012, 32, 1222–1232. [Google Scholar] [CrossRef]
- Faraci, F.; Heistad, D. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ. Res. 1990, 66, 8–17. [Google Scholar] [CrossRef]
- Cipolla, M.J.; Smith, J.; Kohlmeyer, M.M.; Godfrey, J.A. SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: Effect of ischemia and reperfusion. Stroke 2009, 40, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.; Raichle, M.E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. USA 1986, 83, 1140–1144. [Google Scholar] [CrossRef]
- Lecoq, J.; Tiret, P.; Najac, M.; Shepherd, G.M.; Greer, C.A.; Charpak, S. Odor-evoked oxygen consumption by action potential and synaptic transmission in the olfactory bulb. J. Neurosci. 2009, 29, 1424–1433. [Google Scholar] [CrossRef]
- Parpaleix, A.; Houssen, Y.G.; Charpak, S. Imaging local neuronal activity by monitoring PO2 transients in capillaries. Nat. Med. 2013, 19, 241–246. [Google Scholar] [CrossRef]
- Devor, A.; Sakadžić, S.; Saisan, P.A.; Yaseen, M.A.; Roussakis, E.; Srinivasan, V.J.; Vinogradov, S.A.; Rosen, B.R.; Buxton, R.B.; Dale, A.M.; et al. ‘Overshoot’ of O2 is required to maintain baseline tissue oxygenation at locations distal to blood vessels. J. Neurosci. 2011, 31, 13676–13681. [Google Scholar] [CrossRef]
- Paulson, O.B.; Hasselbalch, S.G.; Rostrup, E.; Knudsen, G.M.; Pelligrino, D. Cerebral Blood Flow Response to Functional Activation. Br. J. Pharmacol. 2009, 30, 2–14. [Google Scholar] [CrossRef]
- Logothetis, N.K.; Pauls, J.; Augath, M.; Trinath, T.; Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001, 412, 150–157. [Google Scholar] [CrossRef]
- Devor, A.; Ulbert, I.; Dunn, A.K.; Narayanan, S.N.; Jones, S.; Andermann, M.L.; Boas, D.A.; Dale, A.M. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proc. Natl. Acad. Sci. USA 2005, 102, 3822–3827. [Google Scholar] [CrossRef] [PubMed]
- Shmuel, A.; Yacoub, E.; Pfeuffer, J.; Van de Moortele, P.-F.; Adriany, G.; Hu, X.; Ugurbil, K. Sustained Negative BOLD, Blood Flow and Oxygen Consumption Response and Its Coupling to the Positive Response in the Human Brain. Neuron 2002, 36, 1195–1210. [Google Scholar] [CrossRef]
- Shmuel, A.; Augath, M.; Oeltermann, A.; Logothetis, N.K. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat. Neurosci. 2006, 9, 569–577. [Google Scholar] [CrossRef]
- Klingner, C.M.; Ebenau, K.; Hasler, C.; Brodoehl, S.; Görlich, Y.; Witte, O.W. Influences of negative BOLD responses on positive BOLD responses. NeuroImage 2011, 55, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Harel, N.; Lee, S.-P.; Nagaoka, T.; Kim, D.-S.; Kim, S.-G. Origin of negative blood oxygenation lev-el-dependent fMRI signals. J. Cereb. Blood Flow Metab. 2002, 22, 908–917. [Google Scholar] [CrossRef]
- Blinder, P.; Tsai, P.S.; Kaufhold, J.; Knutsen, P.M.; Suhl, H.; Kleinfeld, D. The cortical angiome: An interconnected vascular network with noncolumnar patterns of blood flow. Nat. Neurosci. 2013, 16, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.A.; Berthiaume, A.-A.; Grant, R.I.; Harrill, S.A.; Koski, T.; Tieu, T.; McDowell, K.P.; Faino, A.V.; Kelly, A.L.; Shih, A.Y. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat. Neurosci. 2021, 24, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Hill, M. Signaling Mechanisms Underlying the Vascular Myogenic Response. Physiol. Rev. 1999, 79, 387–423. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, W.M.; Hill, L.; Gulland, G.L. On Intra-Cranial Pressure and the Cerebral Circulation: Part I. Physiological; Part II. Histological. J. Physiol. 1895, 18, 334–362. [Google Scholar] [CrossRef] [PubMed]
- Lassen, N.A. Cerebral Blood Flow and Oxygen Consumption in Man. Physiol. Rev. 1959, 39, 183–238. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.O.; Hamner, J.W.; Taylor, J.A. The role of myogenic mechanisms in human cerebrovascular regulation. J. Physiol. 2013, 591, 5095–5105. [Google Scholar] [CrossRef]
- Brayden, J.E.; Earley, S.; Nelson, M.T.; Reading, S. Transient receptor potential (trp) channels, vascular tone and autoregulation of cerebral blood flow. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1116–1120. [Google Scholar] [CrossRef]
- Meininger, G.A.; Davis, M.J. Cellular mechanisms involved in the vascular myogenic response. Am. J. Physiol. Circ. Physiol. 1992, 263, H647–H659. [Google Scholar] [CrossRef]
- VanBavel, E.; Wesselman, J.P.M.; Spaan, J.A.E. Myogenic Activation and Calcium Sensitivity of Cannulated Rat Mesenteric Small Arteries. Circ. Res. 1998, 82, 210–220. [Google Scholar] [CrossRef]
- Brayden, J.; Nelson, M. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 1992, 256, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Harder, D.R.; Lange, A.R.; Gebremedhin, D.; Birks, E.K.; Roman, R.J. Cytochrome P450 metabolites of arachidonic acid as intracellular signaling molecules in vascular tissue. J. Vasc. Res. 1997, 34, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.H.; Gordon, G.R. Acute two-photon imaging of the neurovascular unit in the cortex of active mice. Front. Cell. Neurosci. 2015, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Busija, D.W.; Bari, F.; Domoki, F.; Louis, T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res. Rev. 2007, 56, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Domoki, F.; Perciaccante, J.V.; Shimizu, K.; Puskar, M.; Busija, D.W.; Bari, F. N-methyl-d-aspartate-induced vasodilation is mediated by endothelium-independent nitric oxide release in piglets. Am. J. Physiol. Circ. Physiol. 2002, 282, H1404–H1409. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Meng, W.; Ayata, C.; Huang, P.L.; Fishman, M.C.; Moskowitz, M.A. L-NNA-sensitive regional cerebral blood flow augmentation during hypercapnia in type III NOS mutant mice. Am. J. Physiol. Circ. Physiol. 1996, 271, H1717–H1719. [Google Scholar] [CrossRef]
- Tran, C.H.; Peringod, G.; Gordon, G.R. Astrocytes Integrate Behavioral State and Vascular Signals during Functional Hyperemia. Neuron 2018, 100, 1133–1148.e3. [Google Scholar] [CrossRef] [PubMed]
- Lindauer, U.; Megow, D.; Matsuda, H.; Dirnagl, U. Nitric oxide: A modulator, but not a mediator, of neuro-vascular coupling in rat somatosensory cortex. Am. J. Physiol. 1999, 277, H799–H811. [Google Scholar] [CrossRef]
- Uhlirova, H.; Kılıç, K.; Tian, P.; Thunemann, M.; Desjardins, M.; Saisan, P.A.; Sakadžić, S.; Ness, T.V.; Mateo, C.; Cheng, Q.; et al. Cell type specificity of neurovascular coupling in cerebral cortex. eLife 2016, 5, e14315. [Google Scholar] [CrossRef] [PubMed]
- Zonta, M.; Angulo, M.C.; Gobbo, S.; Rosengarten, B.; Hossmann, K.-A.; Pozzan, T.; Carmignoto, P. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 2002, 6, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, S.J.; MacVicar, B. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 2004, 431, 195–199. [Google Scholar] [CrossRef]
- Girouard, H.; Bonev, A.D.; Hannah, R.M.; Meredith, A.; Aldrich, R.W.; Nelson, M.T. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc. Natl. Acad. Sci. USA 2010, 107, 3811–3816. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.R.J.; Choi, H.B.; Rungta, R.; Ellis-Davies, G.C.R.; MacVicar, B.A. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 2008, 456, 745–749. [Google Scholar] [CrossRef]
- Nett, W.J.; Oloff, S.H.; McCarthy, K.D. Hippocampal Astrocytes In Situ Exhibit Calcium Oscillations That Occur Independent of Neuronal Activity. J. Neurophysiol. 2002, 87, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Lind, B.L.; Brazhe, A.R.; Jessen, S.B.; Tan, F.C.C.; Lauritzen, M. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, E4678–E4687. [Google Scholar] [CrossRef] [PubMed]
- Lind, B.L.; Jessen, S.B.; Lønstrup, M.; Joséphine, C.; Bonvento, G.; Lauritzen, M. Fast Ca2+ responses in astrocyte end-feet and neurovascular coupling in mice. Glia 2017, 66, 348–358. [Google Scholar] [CrossRef]
- Winship, I.R.; Plaa, N.; Murphy, T.H. Rapid Astrocyte Calcium Signals Correlate with Neuronal Activity and Onset of the Hemodynamic Response In Vivo. J. Neurosci. 2007, 27, 6268–6272. [Google Scholar] [CrossRef]
- Nizar, K.; Uhlirova, H.; Tian, P.; Saisan, P.A.; Cheng, Q.; Reznichenko, L.; Weldy, K.L.; Steed, T.; Sridhar, V.B.; Macdonald, C.L.; et al. In vivo Stimulus-Induced Vasodilation Occurs without IP3 Receptor Activation and May Precede Astrocytic Calcium Increase. J. Neurosci. 2013, 33, 8411–8422. [Google Scholar] [CrossRef]
- Bonder, D.E.; McCarthy, K.D. Astrocytic Gq-GPCR-Linked IP3R-Dependent Ca2+ Signaling Does Not Mediate Neurovascular Coupling in Mouse Visual Cortex In Vivo. J. Neurosci. 2014, 34, 13139–13150. [Google Scholar] [CrossRef]
- Stobart, J.; Ferrari, K.D.; Barrett, M.J.; Glück, C.; Stobart, M.J.; Zuend, M.; Weber, B. Cortical Circuit Activity Evokes Rapid Astrocyte Calcium Signals on a Similar Timescale to Neurons. Neuron 2018, 98, 726–735.e4. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Nedergaard, M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007, 10, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Gordon, G.R.J.; Tran, C.H.T. Heterogeneity of Sensory-Induced Astrocytic Ca2+ Dynamics During Functional Hyperemia. Front. Physiol. 2020, 11, 587-10. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.H. Neurovascular Coupling and Epilepsy: Hemodynamic Markers for Localizing and Predicting Seizure Onset. Epilepsy Curr. 2007, 7, 91–94. [Google Scholar] [CrossRef]
- Farrell, J.S.; Wolff, M.D.; Teskey, G.C. Neurodegeneration and Pathology in Epilepsy: Clinical and Basic Perspectives. Neurodegener. Dis. 2017, 15, 317–334. [Google Scholar] [CrossRef]
- Leonhardt, G.; De Greiff, A.; Weber, J.; Ludwig, T.; Wiedemayer, H.; Forsting, M.; Hufnagel, A. Brain Perfusion Following Single Seizures. Epilepsia 2005, 46, 1943–1949. [Google Scholar] [CrossRef]
- Fong, G.C.Y.; Fong, K.Y.; Mak, W.; Tsang, K.L.; Chan, K.H.; Cheung, R.T.F.; Ho, S.L. Postictal psychosis related regional cerebral hyperfusion. J. Neurol. Neurosurg. Psychiatr. 2000, 68, 100–101. [Google Scholar] [CrossRef]
- Tatlidil, R. Persistent postictal hyperperfusion demonstrated with PET. Epilepsy Res. 2000, 42, 83–88. [Google Scholar] [CrossRef]
- Gotman, J.; Kobayashi, E.; Bagshaw, A.; Bénar, C.-G.; Dubeau, F. Combining EEG and fMRI: A multimodal tool for epilepsy research. J. Magn. Reson. Imaging 2006, 23, 906–920. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, H.; Suh, M.; Schwartz, T.H. Spatiotemporal Dynamics of Perfusion and Oximetry during Ictal Discharges in the Rat Neocortex. J. Neurosci. 2009, 29, 2814–2823. [Google Scholar] [CrossRef]
- Penfield, W.; Santha von, K.; Cipriani, A. Cerebral Blood Flow During Induced Epileptiform Seizures in Animals and Man. J. Neurophysiol. 1939, 2, 257–267. [Google Scholar] [CrossRef]
- Penfield, W. Epilepsy and the cerebral lesions of birth and infancy. Can. Med. Assoc. J. 1939, 41, 527–534. [Google Scholar]
- Bahar, S.; Suh, M.; Zhao, M.; Schwartz, T.H. Intrinsic optical signal imaging of neocortical seizures: The ‘epileptic dip’. NeuroReport 2006, 17, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.; Ma, H.; Zhao, M.; Sharif, S.; Schwartz, T.H. Neurovascular Coupling and Oximetry During Epileptic Events. Mol. Neurobiol. 2006, 33, 181–198. [Google Scholar] [CrossRef]
- Tran, C.H.T.; George, A.G.; Teskey, G.C.; Gordon, G.R. Seizures elevate gliovascular unit Ca2+ and cause sustained vasoconstriction. JCI Insight 2020, 5, e136469. [Google Scholar] [CrossRef]
- Gómez-Gonzalo, M.; Losi, G.; Chiavegato, A.; Zonta, M.; Cammarota, M.; Brondi, M.; Vetri, F.; Uva, L.; Pozzan, T.; de Curtis, M.; et al. An excitatory loop with astrocytes contributes to drive neurons to seizure thresh-old. PLoS Biol. 2010, 8, e1000352. [Google Scholar] [CrossRef]
- Gómez-Gonzalo, M.; Losi, G.; Brondi, M.; Uva, L.; Sato, S.S.; de Curtis, M.; Ratto, G.M.; Carmignoto, G. Ictal but Not Interictal Epileptic Discharges Activate Astrocyte Endfeet and Elicit Cerebral Arteriole Responses. Front. Cell. Neurosci. 2011, 5, 8. [Google Scholar] [CrossRef]
- Zhao, M.; Nguyen, J.; Ma, H.; Nishimura, N.; Schaffer, C.B.; Schwartz, T.H. Preictal and Ictal Neurovascular and Metabolic Coupling Surrounding a Seizure Focus. J. Neurosci. 2011, 31, 13292–13300. [Google Scholar] [CrossRef]
- Devor, A.; Hillman, E.M.C.; Tian, P.; Waeber, C.; Teng, I.C.; Ruvinskaya, L.; Shalinsky, M.H.; Zhu, H.; Haslinger, R.H.; Narayanan, S.N.; et al. Stimulus-Induced Changes in Blood Flow and 2-Deoxyglucose Uptake Dissociate in Ipsilateral Somatosensory Cortex. J. Neurosci. 2008, 28, 14347–14357. [Google Scholar] [CrossRef]
- Rowe, C.C.; Berkovic, S.; Austin, M.C.; Saling, M.; Kalnins, R.M.; McKay, W.J.; Bladin, P.F. Visual and quantitative analysis of interictal SPECT with technetium-99m-HMPAO in temporal lobe epilepsy. J. Nucl. Med. 1991, 32, 1688–1694. [Google Scholar] [PubMed]
- Newton, M.R.; Berkovic, S.F.; Austin, M.C.; Rowe, C.C.; McKay, W.J.; Bladin, P.F. Postictal switch in blood flow distribution and temporal lobe seizures. J. Neurol. Neurosurg. Psychiatry 1992, 55, 891–894. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Farrell, J.S.; Gaxiola-Valdez, I.; Wolff, M.D.; David, L.S.; Dika, H.I.; Geeraert, B.L.; Wang, X.R.; Singh, S.; Spanswick, S.C.; Dunn, J.F.; et al. Postictal behavioural impairments are due to a severe prolonged hypoperfusion/hypoxia event that is COX-2 dependent. Elife 2016, 5, e19352. [Google Scholar] [CrossRef] [PubMed]
- Gokina, N.I.; Osol, G. Temperature and protein kinase C modulate myofilament Ca2+ sensitivity in pressurized rat cerebral arteries. Am. J. Physiol. 1998, 274, H1920–H1927. [Google Scholar] [CrossRef]
- Farrell, J.S.; Colangeli, R.; Dong, A.; George, A.G.; Addo-Osafo, K.; Kingsley, P.J.; Morena, M.; Wolff, M.D.; Dudok, B.; He, K.; et al. In vivo endocannabinoid dynamics at the timescale of physiological and pathological neural activity. Neuron 2021, 109, 2398–2403.e4. [Google Scholar] [CrossRef]
- Devor, A.; Tian, P.; Nishimura, N.; Teng, I.C.; Hillman, E.M.; Narayanan, S.N.; Ulbert, I.; Boas, D.A.; Kleinfeld, D.; Dale, A.M. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J. Neurosci. 2007, 27, 4452–4459. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teskey, G.C.; Tran, C.H.T. Neurovascular Coupling in Seizures. Neuroglia 2021, 2, 36-47. https://doi.org/10.3390/neuroglia2010005
Teskey GC, Tran CHT. Neurovascular Coupling in Seizures. Neuroglia. 2021; 2(1):36-47. https://doi.org/10.3390/neuroglia2010005
Chicago/Turabian StyleTeskey, G. Campbell, and Cam Ha T. Tran. 2021. "Neurovascular Coupling in Seizures" Neuroglia 2, no. 1: 36-47. https://doi.org/10.3390/neuroglia2010005
APA StyleTeskey, G. C., & Tran, C. H. T. (2021). Neurovascular Coupling in Seizures. Neuroglia, 2(1), 36-47. https://doi.org/10.3390/neuroglia2010005




