NG2 Glia: Novel Roles beyond Re-/Myelination
Abstract
:1. Introduction
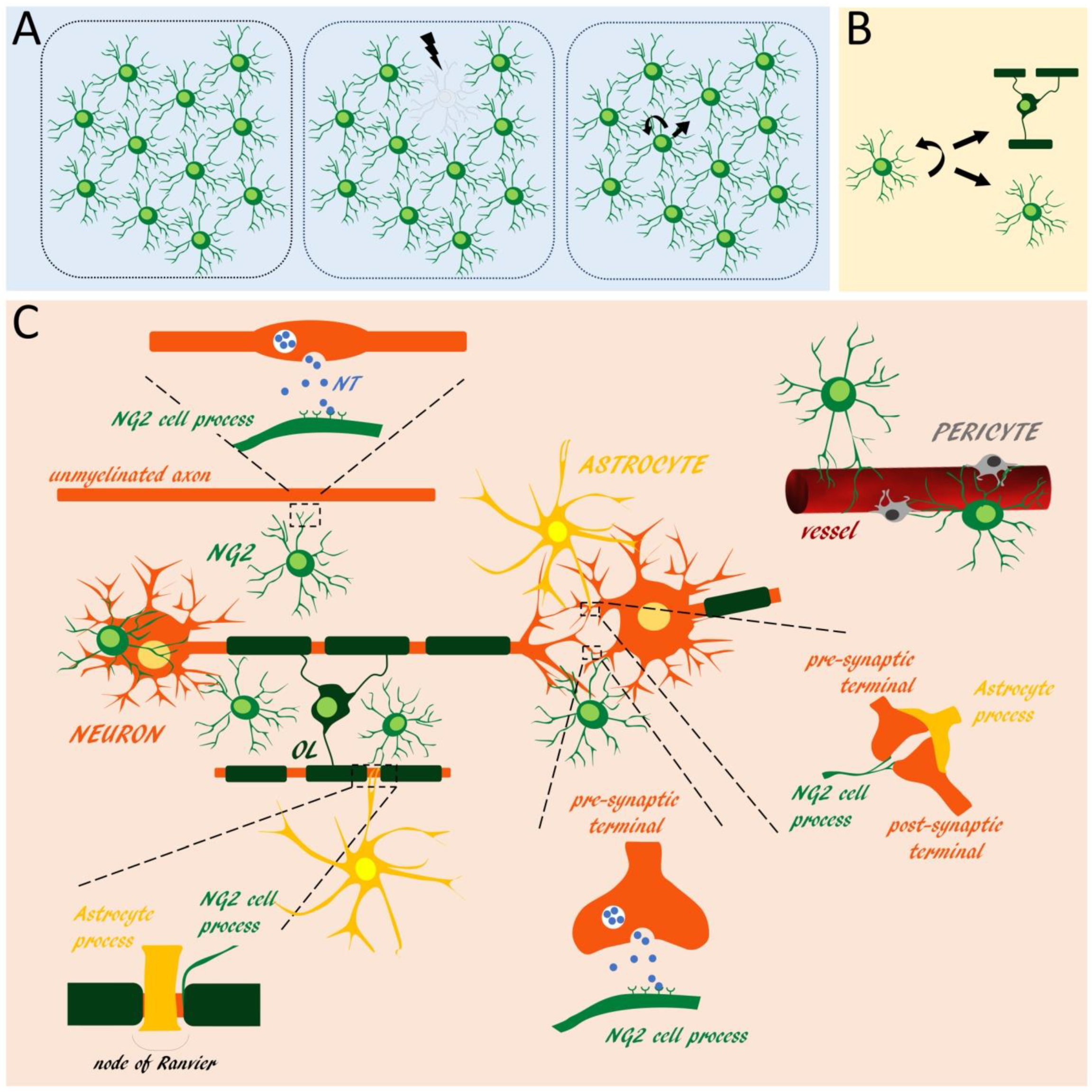
2. NG2 Glia Distribution, Self-Maintenance and Anatomical Relationships with Central Nervous System Cells
3. NG2 Glia as Sensors of Neuronal Activity
4. Maintenance of NG2 Glia Is Required for Central Nervous System Homeostasis and Development
5. NG2 Glia-Derived Signals Can Modulate Neuronal and Non-Neuronal Cell Functions in the Central Nervous System
6. NG2 Glia Upon Central Nervous System Injury and Stress
7. Concluding Remarks and Open Issues
Funding
Acknowledgments
Conflicts of Interest
References
- Boda, E.; Buffo, A. Glial cells in non-germinal territories: Insights into their stem/progenitor properties in the intact and injured nervous tissue. Arch. Ital. Biol. 2010, 148, 119–136. [Google Scholar] [PubMed]
- Dawson, M.R.L.; Polito, A.; Levine, J.M.; Reynolds, R. NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 2003, 24, 476–488. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Plemel, J.R.; Lee, M.J.; Stratton, J.A.; Manesh, S.B.; Liu, J.; Ramer, L.M.; Kang, S.H.; Bergles, D.E.; et al. Myelinogenic plasticity of oligodendrocyte precursor cells following spinal cord contusion injury. J. Neurosci. 2017, 37, 8635–8654. [Google Scholar] [CrossRef] [PubMed]
- Baxi, E.G.; DeBruin, J.; Jin, J.; Strasburger, H.J.; Smith, M.D.; Orthmann-Murphy, J.L.; Schott, J.T.; Fairchild, A.N.; Bergles, D.E.; Calabresi, P.A. Lineage tracing reveals dynamic changes in oligodendrocyte precursor cells following cuprizone-induced demyelination. Glia 2017, 65, 2087–2098. [Google Scholar] [CrossRef] [PubMed]
- Boda, E.; Di Maria, S.; Rosa, P.; Taylor, V.; Abbracchio, M.P.; Buffo, A. Early phenotypic asymmetry of sister oligodendrocyte progenitor cells after mitosis and its modulation by aging and extrinsic factors. Glia 2015, 63, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Patel, K.D.; Goncalves, C.M.; Grutzendler, J.; Nishiyama, A. Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat. Neurosci. 2014, 17, 1518–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, I.A.; Ohayon, D.; Li, H.; Paes de Faria, J.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor skill learning requires active central myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ohayon, D.; McKenzie, I.A.; Sinclair-Wilson, A.; Wright, J.L.; Fudge, A.D.; Emery, B.; Li, H.; Richardson, W.D. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci. 2016, 19, 1210–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, E.G.; Orthmann-Murphy, J.L.; Langseth, A.J.; Bergles, D.E. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 2018, 21, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Li, A.M.; Grutzendler, J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci. 2018, 21, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Young, K.M. White matter plasticity in adulthood. Neuroscience 2014, 276, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Gruart, A.; Grade, S.; Zhang, Y.; Kröger, S.; Kirchhoff, F.; Eichele, G.; Delgado García, J.M.; Dimou, L. Decrease in newly generated oligodendrocytes leads to motor dysfunctions and changed myelin structures that can be rescued by transplanted cells. Glia 2016, 64, 2201–2218. [Google Scholar] [CrossRef] [PubMed]
- Psachoulia, K.; Jamen, F.; Young, K.M.; Richardson, W.D. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009, 5, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boda, E.; Buffo, A. Beyond cell replacement: Unresolved roles of NG2-expressing progenitors. Front. Neurosci. 2014, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Birey, F.; Kokkosis, A.; Aguirre, A. Oligodendroglia-lineage cells in brain plasticity, homeostasis and psychiatric disorders. Curr. Opin. Neurobiol. 2017, 47, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Boda, E.; Viganò, F.; Rosam, P.; Fumagalli, M.; Labat-Gest, V.; Tempia, F.; Abbracchio, M.P.; Dimou, L.; Buffo, A. The GPR17 receptor in NG2 expressing cells: Focus on in vivocell maturation and participation in acute trauma and chronic damage. Glia 2011, 59, 1958–1973. [Google Scholar] [CrossRef] [PubMed]
- Levine, J. The reactions and role of NG2 glia in spinal cord injury. Brain Res. 2016, 1638, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Riew, T.R.; Kim, H.L.; Choi, J.H.; Lee, M.Y. Morphological characterization of NG2 glia and their association with neuroglial cells in the 3-nitropropionic acid–lesioned striatum of rat. Sci. Rep. 2018, 8, 5942. [Google Scholar] [CrossRef] [PubMed]
- Valny, M.; Honsa, P.; Waloschkova, E.; Matuskova, H.; Kriska, J.; Kirdajova, D.; Androvic, P.; Valihrach, L.; Kubista, M.; Anderova, M. A single-cell analysis reveals multiple roles of oligodendroglial lineage cells during post-ischemic regeneration. Glia 2018, 66, 1068–1081. [Google Scholar] [CrossRef] [PubMed]
- Kazanis, I.; Evans, K.A.; Andreopoulou, E.; Dimitriou, C.; Koutsakis, C.; Karadottir, R.T.; Franklin, R.J.M. Subependymal zone-derived oligodendroblasts respond to focal demyelination but fail to generate myelin in young and aged mice. Stem Cell Rep. 2017, 8, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, C.; Praet, J.; Rangarajan, J.R.; Vreys, R.; De Vocht, N.; Maes, F.; Verhoye, M.; Ponsaerts, P.; Van der Linden, A. Multimodal imaging of subventricular zone neural stem/progenitor cells in the cuprizone mouse model reveals increased neurogenic potential for the olfactory bulb pathway, but no contribution to remyelination of the corpus callosum. Neuroimage 2014, 86, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.L.; Roth, P.T.; Stratton, J.A.S.; Chuang, B.H.A.; Danne, J.; Ellis, S.L.; Ng, S.W.; Kilpatrick, T.J.; Merson, T.D. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J. Neurosci. 2014, 34, 14128–14146. [Google Scholar] [CrossRef] [PubMed]
- Van Tilborg, E.; de Theije, C.G.M.; van Hal, M.; Wagenaar, N.; de Vries, L.S.; Benders, M.J.; Rowitch, D.H.; Nijboer, C.H. Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia 2018, 66, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hill, R.A.; Nishiyama, A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol. 2009, 4, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Bergles, D.E.; Nishiyama, A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 2007, 135, 145–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Zhao, N.; Bai, X.; Karram, K.; Trotter, J.; Goebbels, S.; Scheller, A.; Kirchhoff, F. Novel NG2-CreERT2 knock-in mice demonstrate heterogeneous differentiation potential of NG2 glia during development. Glia 2014, 62, 896–913. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.B.; Rivers, L.E.; Young, K.M.; Jamen, F.; Richardson, W.D. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J. Neurosci. 2010, 30, 16383–16390. [Google Scholar] [CrossRef] [PubMed]
- Boda, E.; Nato, G.; Buffo, A. Emerging pharmacological approaches to promote neurogenesis from endogenous glial cells. Biochem. Pharmacol. 2017, 141, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Wood, W.M.; Sherafat, A.; Hill, R.A.; Lu, Q.R.; Nishiyama, A. Age-dependent decline in fate switch from NG2 cells to astrocytes after Olig2 deletion. J. Neurosci. 2018, 38, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Valny, M.; Honsa, P.; Kriska, J.; Anderova, M. Multipotency and therapeutic potential of NG2 cells. Biochem. Pharmacol. 2017, 141, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.G.; Kang, S.H.; Fukaya, M.; Bergles, D.E. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 2013, 16, 668–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirby, B.B.; Takada, N.; Latimer, A.J.; Shin, J.; Carney, T.J.; Kelsh, R.N.; Appel, B. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat. Neurosci. 2006, 9, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Birey, F.; Aguirre, A. Age-dependent netrin-1 signaling regulates NG2+ glial cell spatial homeostasis in normal adult gray matter. J. Neurosci. 2015, 35, 6946–6951. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.; Gontran, E.; Deroulers, C.; Varlet, P.; Pallud, J.; Grammaticos, B.; Badoual, M. Modeling the dynamics of oligodendrocyte precursor cells and the genesis of gliomas. PLoS Comput. Biol. 2018, 14, e1005977. [Google Scholar] [CrossRef] [PubMed]
- Sugiarto, S.; Persson, A.I.; Munoz, E.G.; Waldhuber, M.; Lamagna, C.; Andor, N.; Hanecker, P.; Ayers-Ringler, J.; Phillips, J.; Siu, J.; et al. Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer Cell 2011, 20, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Balia, M.; Benamer, N.; Angulo, M.C. A specific GABAergic synapse onto oligodendrocyte precursors does not regulate cortical oligodendrogenesis. Glia 2017, 65, 1821–1832. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkhratsky, A.; Bush, N.; Nedergaard, M.; Butt, A. The special case of human astrocytes. Neuroglia 2018, 1, 4. [Google Scholar] [CrossRef]
- Casano, A.M.; Peri, F. Microglia: Multitasking specialists of the brain. Dev. Cell 2015, 32, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Réu, P.; Khosravi, A.; Bernard, S.; Mold, J.E.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; et al. The lifespan and turnover of microglia in the human brain. Cell Rep. 2017, 20, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Wigley, R.; Butt, A.M. Integration of NG2-glia (synantocytes) into the neuroglial network. Neuron Glia Biol. 2009, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Djogo, T.; Robins, S.C.; Schneider, S.; Kryzskaya, D.; Liu, X.; Mingay, A.; Gillon, C.J.; Kim, J.H.; Storch, K.F.; Boehm, U.; et al. Adult NG2-glia are required for median eminence-mediated leptin sensing and body weight control. Cell Metab. 2016, 23, 797–810. [Google Scholar] [CrossRef] [PubMed]
- März, M.; Schmidt, R.; Rastegar, S.; Strähle, U. Expression of the transcription factor Olig2 in proliferating cells in the adult zebrafish telencephalon. Dev. Dyn. 2010, 239, 3336–3349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulanger, J.J.; Messier, C. Oligodendrocyte progenitor cells are paired with GABA neurons in the mouse dorsal cortex: Unbiased stereological analysis. Neuroscience 2017, 362, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Duncan, A.; Hornby, M.F.; Kirvell, S.L.; Hunter, A.; Levine, J.M.; Berry, M. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia 1999, 26, 84–91. [Google Scholar] [CrossRef]
- Serwanski, D.R.; Jukkola, P.; Nishiyama, A. Heterogeneity of astrocyte and NG2 cell insertion at the node of Ranvier. J. Comp. Neurol. 2017, 525, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.Y.; Levine, J.M. A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan-positive oligodendrocyte precursor cells in the normal and kainate-lesioned rat hippocampus. Neuroscience 1999, 92, 83–95. [Google Scholar] [CrossRef]
- Maldonado, P.P.; Angulo, M.C. Multiple modes of communication between neurons and oligodendrocyte precursor cells. Neuroscientist 2015, 21, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Orduz, D.; Maldonado, P.P.; Balia, M.; Vélez-Fort, M.; de Sars, V.; Yanagawa, Y.; Emiliani, V.; Angulo, M.C. Interneurons and oligodendrocyte progenitors form a structured synaptic network in the developing neocortex. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Huck, J.H.J.; Roberts, J.D.B.; Macklin, W.B.; Somogyi, P.; Bergles, D.E. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron 2005, 46, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Reyes-Haro, D.; Pivneva, T.; Nolte, C.; Schaette, R.; Lübke, J.; Kettenmann, H. The principal neurons of the medial nucleus of the trapezoid body and NG2+ glial cells receive coordinated excitatory synaptic input. J. Gen. Physiol. 2009, 134, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Mangin, J.M.; Li, P.; Scafidi, J.; Gallo, V. Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat. Neurosci. 2012, 15, 1192–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Bergles, D.E. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci. 2004, 7, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Mangin, J.M.; Kunze, A.; Chittajallu, R.; Gallo, V. Satellite NG2 progenitor cells share common glutamatergic inputs with associated interneurons in the mouse Dentate gyrus. J. Neurosci. 2008, 28, 7610–7623. [Google Scholar] [CrossRef] [PubMed]
- Balia, M.; Vélez-Fort, M.; Passlick, S.; Schäfer, C.; Audinat, E.; Steinhäuser, C.; Seifert, G.; Angulo, M.C. Postnatal down-regulation of the GABAA receptor γ2 subunit in neocortical NG2 cells accompanies synaptic-to-extrasynaptic switch in the GABAergic transmission mode. Cereb. Cortex 2015, 25, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, N.; Vayro, S.; Wigley, R.; Butt, A.M. Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia 2010, 58, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wang, W.; Zhou, M. Spatial organization of NG2 glial cells and astrocytes in rat hippocampal CA1 region. Hippocampus 2014, 24, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Yu, M.; Drazba, J.A.; Tuohy, V.K. Normal and reactive NG2+ glial cells are distinct from resting and activated microglia. J. Neurosci. Res. 1997, 48, 299–312. [Google Scholar] [CrossRef]
- Wallraff, A.; Odermatt, B.; Willecke, K.; Steinhäuser, C. Distinct types of astroglial cells in the hippocampus differ in gap junction coupling. Glia 2004, 48, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Hamilton, N.; Hubbard, P.; Pugh, M.; Ibrahim, M. Synantocytes: The fifth element. J. Anat. 2005, 207, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Melanson-Drapeau, L.; Beyko, S.; Davé, S.; Hebb, A.L.O.; Franks, D.J.; Sellitto, C.; Paul, D.L.; Bennett, S.A. Oligodendrocyte progenitor enrichment in the connexin32 null-mutant mouse. J. Neurosci. 2003, 23, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.; Tress, O.; Haas, B.; Karram, K.; Trotter, J.; Willecke, K.; Kettenmann, H. Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia 2010, 58, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Minocha, S.; Valloton, D.; Brunet, I.; Eichmann, A.; Hornung, J.P.; Lebrand, C. NG2 glia are required for vessel network formation during embryonic development. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, H.H.; Niu, J.; Munji, R.; Davalos, D.; Chang, J.; Zhang, H.; Tien, A.C.; Kuo, C.J.; Chan, J.R.; Daneman, R.; et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 2016, 351, 379–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.H.; Maki, T.; Maeda, M.; Miyamoto, N.; Liang, A.C.; Hayakawa, K.; Pham, L.D.; Suwa, F.; Taguchi, A.; Matsuyama, T.; et al. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-β signaling. PLoS ONE 2014, 9, e103174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maki, T.; Maeda, M.; Uemura, M.; Lo, E.K.; Terasaki, Y.; Liang, A.C.; Shindo, A.; Choi, Y.K.; Taguchi, A.; Matsuyama, T.; et al. Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci. Lett. 2015, 597, 164–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.H.; Miyamoto, N.; Hayakawa, K.; Pham, L.D.D.; Maki, T.; Ayata, C.; Kim, K.W.; Lo, E.H.; Arai, K. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J. Clin. Investig. 2013, 123, 782–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, N.; Pham, L.D.D.; Seo, J.H.; Kim, K.W.; Lo, E.H.; Arai, K. Crosstalk between cerebral endothelium and oligodendrocyte. Cell. Mol. Life Sci. 2014, 71, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Nishiyama, A. NG2 cells (polydendrocytes): Listeners to the neural network with diverse properties. Glia 2014, 62, 1195–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, D.; Eto, K.; Nabekura, J.; Wake, H. Activity-dependent functions of non-electrical glial cells. J. Biochem. 2018, 163, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Barres, B.A.; Raff, M.C. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 1993, 361, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Brus-Ramer, M.; Martin, J.H.; McDonald, J.W. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci. Lett. 2010, 479, 128–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, E.M.; Purger, D.; Mount, C.W.; Goldstein, A.K.; Lin, G.L.; Wood, L.S.; Inema, I.; Miller, S.E.; Bieri, G.; Zuchero, J.B.; et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 2014, 344, 1252304. [Google Scholar] [CrossRef] [PubMed]
- Ehninger, D.; Wang, L.P.; Klempin, F.; Römer, B.; Kettenmann, H.; Kempermann, G. Enriched environment and physical activity reduce microglia and influence the fate of NG2 cells in the amygdala of adult mice. Cell Tissue Res. 2011, 345, 69–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, C.; Götz, M.; Dimou, L. Progenitors in the adult cerebral cortex: Cell cycle properties and regulation by physiological stimuli and injury. Glia 2011, 59, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, L.; Huang, P.H.; Colognato, H. Prefrontal cortex NG2 glia undergo a developmental switch in their responsiveness to exercise. Dev. Neurobiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Hovhannisyan, A.; Barzan, R.; Chen, T.J.; Kukley, M. Different patterns of neuronal activity trigger distinct responses of oligodendrocyte precursor cells in the corpus callosum. PLoS Biol. 2017, 15, e2001993. [Google Scholar] [CrossRef] [PubMed]
- Larson, V.A.; Zhang, Y.; Bergles, D.E. Electrophysiological properties of NG2+ cells: Matching physiological studies with gene expression profiles. Brain Res. 2016, 1638, 138–160. [Google Scholar] [CrossRef] [PubMed]
- Ziskin, J.L.; Nishiyama, A.; Rubio, M.; Fukaya, M.; Bergles, D.E. Vesicular release of glutamate from unmyelinated axons in white matter. Nat. Neurosci. 2007, 10, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado, P.P.; Velez-Fort, M.; Levavasseur, F.; Angulo, M.C. Oligodendrocyte precursor cells are accurate sensors of local K+ in mature gray matter. J. Neurosci. 2013, 33, 2432–2442. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Matthews, E.A.; Nicolas, V.; Schoch, S.; Dietrich, D. NG2 glial cells integrate synaptic input in global and dendritic calcium signals. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Hines, J.H.; Ravanelli, A.M.; Schwindt, R.; Scott, E.K.; Appel, B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 2015, 18, 683–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensch, S.; Baraban, M.; Almeida, R.; Czopka, T.; Ausborn, J.; El Manira, A.; Lyons, D.A. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 2015, 18, 628–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wake, H.; Lee, P.R.; Fields, R.D. Control of local protein synthesis and initial events in myelination by action potentials. Science 2011, 333, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Haberlandt, C.; Derouiche, A.; Wyczynski, A.; Haseleu, J.; Pohle, J.; Karram, K.; Trotter, J.; Seifert, G.; Frotscher, M.; Steinhäuser, C.; et al. Gray matter NG2 cells display multiple Ca2+-signaling pathways and highly motile processes. PLoS ONE 2011, 6, e17575. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, S.; Dimou, L. Glial cells and their function in the adult brain: A journey through the history of their ablation. Front. Cell. Neurosci. 2017, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Birey, F.; Kloc, M.; Chavali, M.; Hussein, I.; Wilson, M.; Christoffel, D.J.; Chen, T.; Frohman, M.A.; Robinson, J.K.; Russo, S.J.; et al. Genetic and stress-induced loss of NG2 glia triggers emergence of depressive-like behaviors through reduced secretion of FGF2. Neuron 2015, 88, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Tamura, Y.; Yamato, M.; Kume, S.; Eguchi, A.; Takata, K.; Watanabe, Y.; Kataoka, Y. NG2 glial cells regulate neuroimmunological responses to maintain neuronal function and survival. Sci. Rep. 2017, 7, 42041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessaris, N.; Fogarty, M.; Iannarelli, P.; Grist, M.; Wegner, M.; Richardson, W.D. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 2006, 9, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Van Tilborg, E.; Achterberg, E.J.M.; van Kammen, C.M.; van der Toorn, A.; Groenendaal, F.; Dijkhuizen, R.M.; Heijnen, C.J.; Vanderschuren, L.J.M.J.; Benders, M.N.J.L.; Nijboer, C.H.A. Combined fetal inflammation and postnatal hypoxia causes myelin deficits and autism-like behavior in a rat model of diffuse white matter injury. Glia 2018, 66, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Doretto, S.; Malerba, M.; Ramos, M.; Ikrar, T.; Kinoshita, C.; De Mei, C.; Tirotta, E.; Xu, X.; Borrelli, E. Oligodendrocytes as regulators of neuronal networks during early postnatal development. PLoS ONE 2011, 6, e19849. [Google Scholar] [CrossRef] [PubMed]
- Clemente, D.; Ortega, M.C.; Melero-Jerez, C.; de Castro, F. The effect of glia-glia interactions on oligodendrocyte precursor cell biology during development and in demyelinating diseases. Front. Cell. Neurosci. 2013, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Sypecka, J.; Sarnowska, A. The neuroprotective effect exerted by oligodendroglial progenitors on ischemically impaired hippocampal cells. Mol. Neurobiol. 2014, 49, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, A.; Chandran, S.; Compston, A. A role for oligodendrocyte-derived IGF-1 in trophic support of cortical neurons. Glia 2001, 36, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, A.; Majed, H.; Layfield, R.; Compston, A.; Chandran, S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: A novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J. Neurosci. 2003, 23, 4967–4974. [Google Scholar] [CrossRef] [PubMed]
- Yuen, T.J.; Silbereis, J.C.; Griveau, A.; Chang, S.M.; Daneman, R.; Fancy, S.P.J.; Zahed, H.; Maltepe, E.; Rowitch, D.H. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 2014, 158, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiao, L.; Gong, X.; Shao, W.; Yin, Y.; Liao, Q.; Meng, Y.; Zhang, Y.; Ma, D.; Qiu, X. Cytokine-like molecule CCDC134 Contributes to CD8+ T-cell effector functions in cancer immunotherapy. Cancer Res. 2014, 74, 5734–5745. [Google Scholar] [CrossRef] [PubMed]
- Sakry, D.; Neitz, A.; Singh, J.; Frischknecht, R.; Marongiu, D.; Binamé, F.; Perera, S.S.; Endres, K.; Lutz, B.; Radyushkin, K.; et al. Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLoS Biol. 2014, 12, e1001993. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.B.; Wu, Y.; Trigo, D.; Clarke, E.; Malmqvist, T.; Grist, J.; Hobbs, C.; Carlstedt, T.P.; Corcoran, J.P.T. Retinoic acid synthesis by NG2 expressing cells promotes a permissive environment for axonal outgrowth. Neurobiol. Dis. 2018, 111, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef] [PubMed]
- Zechel, S.; Unsicker, K.; von Bohlen und Halbach, O. Fibroblast growth factor-2 deficiency affects hippocampal spine morphology, but not hippocampal catecholaminergic or cholinergic innervation. Dev. Dyn. 2009, 238, 343–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goddard, D.R.; Berry, M.; Kirvell, S.L.; Butt, A.M. Fibroblast growth factor-2 induces astroglial and microglial reactivity in vivo. J. Anat. 2002, 200, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, B.; Mattson, M.P. PDGFs protect hippocampal neurons against energy deprivation and oxidative injury: Evidence for induction of antioxidant pathways. J. Neurosci. 1995, 15, 7095–7104. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Yao, H.; Bai, X.; Zhu, X.; Reiner, B.C.; Beazely, M.; Funa, K.; Xiong, H.; Buch, S. Platelet-derived growth factor-mediated induction of the synaptic plasticity gene Arc/Arg3.1. J. Biol. Chem. 2010, 285, 21615–21624. [Google Scholar] [CrossRef] [PubMed]
- Risau, W.; Drexler, H.; Mironov, V.; Smits, A.; Siegbahn, A.; Funa, K.; Heldin, C.H. Platelet-derived growth factor is angiogenic in vivo. Grow. Factors 1992, 7, 261–266. [Google Scholar] [CrossRef]
- Sasahara, A.; Kott, J.N.; Sasahara, M.; Raines, E.W.; Ross, R.; Westrum, L.E. Platelet-derived growth factor B-chain-like immunoreactivity in the developing and adult rat brain. Dev. Brain Res. 1992, 68, 41–53. [Google Scholar] [CrossRef]
- Rosenstein, J.M.; Krum, J.M.; Ruhrberg, C. VEGF in the nervous system. Organogenesis 2010, 6, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tovar-y-Romo, L.B.; Tapia, R. Delayed administration of VEGF rescues spinal motor neurons from death with a short effective time frame in excitotoxic experimental models in vivo. ASN Neuro 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Estévez, V.; Defterali, Ç.; Vicario-Abejón, C. IGF-I: A key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front. Neurosci. 2016, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Rivkees, S.A. Hepatocyte growth factor stimulates the proliferation and migration of oligodendrocyte precursor cells. J. Neurosci. Res. 2002, 69, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Funakoshi, H.; Kadoyama, K.; Noma, S.; Kanai, M.; Ohya-Shimada, W.; Mizuno, S.; Doe, N.; Taniguchi, T.; Nakamura, T. Hepatocyte growth factor overexpression in the nervous system enhances learning and memory performance in mice. J. Neurosci. Res. 2012, 90, 1743–1755. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Tozuka, Y.; Takata, T.; Shimazu, N.; Matsumura, N.; Ohta, A.; Hisatsune, T. Excitatory GABAergic activation of cortical dividing glial cells. Cereb. Cortex 2009, 19, 2181–2195. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; The, D.B.L.; Blasiak, A.; Chai, C.; Wu, Y.; Gharibani, P.M.; Yang, I.H.; Phan, T.T.; Lim, K.L.; Yang, H.; et al. Static magnetic field stimulation enhances oligodendrocyte differentiation and secretion of neurotrophic factors. Sci. Rep. 2017, 7, 6743. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Byravan, S.; Foster, L.M.; Phan, T.; Verity, A.N.; Campagnoni, A.T. Murine oligodendroglial cells express nerve growth factor. Proc. Natl. Acad. Sci. USA 1994, 91, 8812–8816. [Google Scholar] [CrossRef] [PubMed]
- Varon, S.; Conner, J.M. Nerve growth factor in CNS repair. J. Neurotrauma 1994, 11, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Hodgetts, S.I.; Harvey, A.R. Neurotrophic factors used to treat spinal cord injury. Vitam. Horm. 2017, 104, 405–457. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.Q.; Dybdal, N.; Shinsky, N.; Murnane, A.; Schmelzer, C.; Siegel, M.; Keller, G.; Hefti, F.; Phillips, H.S.; Winslow, J.W. Neurotrophin-3 reverses experimental cisplatin-induced peripheral sensory neuropathy. Ann. Neurol. 1995, 38, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Rabacchi, S.A.; Kruk, B.; Hamilton, J.; Carney, C.; Hoffman, J.R.; Meyer, S.L.; Springer, J.E.; Baird, D.H. BDNF and NT4/5 promote survival and neurite outgrowth of pontocerebellar mossy fiber neurons. J. Neurobiol. 1999, 40, 254–269. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Barres, B.A.; Burne, J.F.; Holtmann, B.; Thoenen, H.; Sendtner, M.; Raff, M.C. Ciliary neurotrophic factor enhances the rate of oligodendrocyte generation. Mol. Cell. Neurosci. 1996, 8, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Giess, R.; Holtmann, B.; Braga, M.; Grimm, T.; Müller-Myhsok, B.; Toyka, K.V.; Sendtner, M. Early onset of Severe Familial Amyotrophic Lateral Sclerosis with a SOD-1 mutation: Potential impact of CNTF as a candidate modifier gene. Am. J. Hum. Genet. 2002, 70, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Talbott, J.F.; Cao, Q.; Bertram, J.; Nkansah, M.; Benton, R.L.; Lavik, E.; Whittemore, S.R. CNTF promotes the survival and differentiation of adult spinal cord-derived oligodendrocyte precursor cells in vitro but fails to promote remyelination in vivo. Exp. Neurol. 2007, 204, 485–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, F.J.; Lang, J.K.; Waldau, B.; Roy, N.S.; Schwartz, T.E.; Pilcher, W.H.; Chandross, K.J.; Natesan, S.; Merrill, J.E.; Goldman, S.A. Complementary patterns of gene expression by human oligodendrocyte progenitors and their environment predict determinants of progenitor maintenance and differentiation. Ann. Neurol. 2006, 59, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Amor, V.; Feinberg, K.; Eshed-Eisenbach, Y.; Vainshtein, A.; Frechter, S.; Grumet, M.; Rosenbluth, J.; Peles, E. Long-term maintenance of Na+ channels at nodes of ranvier depends on glial contact mediated by gliomedin and NrCAM. J. Neurosci. 2014, 34, 5089–5098. [Google Scholar] [CrossRef] [PubMed]
- Demyanenko, G.P.; Mohan, V.; Zhang, X.; Brennaman, L.H.; Dharbal, K.E.S.; Tran, T.S.; Manis, P.B.; Maness, P.F. Neural cell adhesion molecule NrCAM regulates Semaphorin 3F-induced dendritic spine remodeling. J. Neurosci. 2014, 34, 11274–11287. [Google Scholar] [CrossRef] [PubMed]
- Rønn, L.C.; Bock, E.; Linnemann, D.; Jahnsen, H. NCAM-antibodies modulate induction of long-term potentiation in rat hippocampal CA1. Brain Res. 1995, 677, 145–151. [Google Scholar] [CrossRef]
- Tessier-Lavigne, M.; Goodman, C.S. The molecular biology of axon guidance. Science 1996, 274, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Gascon, E.; Vutskits, L.; Kiss, J.Z. The role of PSA-NCAM in adult neurogenesis. Adv. Exp. Med. Biol. 2010, 663, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Hattori, D.; Demir, E.; Kim, H.W.; Viragh, E.; Zipursky, S.L.; Dickson, B.J. Dscam diversity is essential for neuronal wiring and self-recognition. Nature 2007, 449, 223–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, D.; Chen, Y.; Matthews, B.J.; Salwinski, L.; Sabatti, C.; Grueber, W.B.; Zipursky, S.L. Robust discrimination between self and non-self neurites requires thousands of Dscam1 isoforms. Nature 2009, 461, 644–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soba, P.; Zhu, S.; Emoto, K.; Younger, S.; Yang, S.J.; Yu, H.H.; Lee, T.; Jan, L.Y.; Jan, Y.N. Drosophila sensory neurons require dscam for dendritic self-avoidance and proper dendritic field organization. Neuron 2007, 54, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.E.; Bortnick, R.; Tsubouchi, A.; Bäumer, P.; Kondo, M.; Uemura, T.; Schmucker, D. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron 2007, 54, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Bosse, F.; D’Urso, D.; Muller, H.; Sereda, M.W.; Nave, K.; Niehaus, A.; Kempf, T.; Schnolzer, M.; Trotter, J. The AN2 protein is a novel marker for the Schwann cell lineage expressed by immature and nonmyelinating Schwann cells. J. Neurosci. 2001, 21, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Hunanyan, A.S.; Garcia-Alias, G.; Alessi, V.; Levine, J.M.; Fawcett, J.W.; Mendell, L.M.; Arvanian, V.L. Role of chondroitin sulfate proteoglycans in axonal conduction in mammalian spinal cord. J. Neurosci. 2010, 30, 7761–7769. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.H.; Katagiri, Y.; Susarla, B.; Figge, D.; Symes, A.J.; Geller, H.M. Alterations in sulfated chondroitin glycosaminoglycans following controlled cortical impact injury in mice. J. Comp. Neurol. 2012, 520, 3295–3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dours-Zimmermann, M.T.; Maurer, K.; Rauch, U.; Stoffel, W.; Fassler, R.; Zimmermann, D.R. Versican V2 assembles the extracellular matrix surrounding the nodes of Ranvier in the CNS. J. Neurosci. 2009, 29, 7731–7742. [Google Scholar] [CrossRef] [PubMed]
- Bekku, Y.; Rauch, U.; Ninomiya, Y.; Oohashi, T. Brevican distinctively assembles extracellular components at the large diameter nodes of Ranvier in the CNS. J. Neurochem. 2009, 108, 1266–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dityatev, A.; Schachner, M. Extracellular matrix molecules and synaptic plasticity. Nat. Rev. Neurosci. 2003, 4, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Hagino, S.; Iseki, K.; Mori, T.; Zhang, Y.; Sakai, N.; Yokoya, S.; Hikake, T.; Kikuchi, S.; Wanaka, A. Expression pattern of glypican-1 mRNA after brain injury in mice. Neurosci. Lett. 2003, 349, 29–32. [Google Scholar] [CrossRef]
- Hagino, S.; Iseki, K.; Mori, T.; Zhang, Y.; Hikake, T.; Yokoya, S.; Takeuchi, M.; Hasimoto, H.; Kikuchi, S.; Wanaka, A. Slit and glypican-1 mRNAs are coexpressed in the reactive astrocytes of the injured adult brain. Glia 2003, 42, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, M.; Nakayama, T.; Sato, N.; Naganuma, T.; Yamaguchi, M.; Aoi, N.; Sato, M.; Izumi, Y.; Soma, M.; Matsumoto, K. Association Study of the elastin microfibril interfacer 1 (EMILIN1) gene in essential hypertension. Am. J. Hypertens. 2010, 23, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Pavlov, I.; Võikar, V.; Lauri, S.E.; Hienola, A.; Riekki, R.; Lakso, M.; Taira, T.; Rauvala, H. Syndecan-3-deficient mice exhibit enhanced LTP and impaired hippocampus-dependent memory. Mol. Cell. Neurosci. 2002, 21, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, Y.; Borrell, V.; Garcia, C.; Burstyn-Cohen, T.; Tzarfaty, V.; Frumkin, A.; Nose, A.; Okamoto, H.; Higashijima, S.; Soriano, E.; et al. F-spondin and mindin: Two structurally and functionally related genes expressed in the hippocampus that promote outgrowth of embryonic hippocampal neurons. Development 1999, 126, 3637–3648. [Google Scholar] [PubMed]
- Wang, B.; Guo, W.; Huang, Y. Thrombospondins and synaptogenesis. Neural Regen. Res. 2012, 7, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Sawyer, A.; Kocaoglu, F.B.; Kyriakides, T.R. Astrocyte-derived thrombospondin-2 is critical for the repair of the blood-brain barrier. Am. J. Pathol. 2011, 179, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Yuzaki, M. Cbln family proteins promote synapse formation by regulating distinct neurexin signaling pathways in various brain regions. Eur. J. Neurosci. 2011, 33, 1447–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuzaki, M. Two classes of secreted synaptic organizers in the central nervous system. Annu. Rev. Physiol. 2018, 80, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Awwad, K.; Hu, J.; Shi, L.; Mangels, N.; Abdel Malik, R.; Zippel, N.; Fisslthaler, B.; Eble, J.A.; Pfeilschifter, J.; Popp, R.; et al. Role of secreted modular calcium-binding protein 1 (SMOC1) in transforming growth factor β signalling and angiogenesis. Cardiovasc. Res. 2015, 106, 284–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettem, K.L. New Synaptic Organizing Proteins and Their Roles in Excitatory and Inhibitory Synapse Development. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2012. [Google Scholar]
- Dean, C.; Dresbach, T. Neuroligins and neurexins: Linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006, 29, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Sakry, D.; Yigit, H.; Dimou, L.; Trotter, J. Oligodendrocyte precursor cells synthesize neuromodulatory factors. PLoS ONE 2015, 10, e0127222. [Google Scholar] [CrossRef] [PubMed]
- Tucsek, Z.; Noa Valcarcel-Ares, M.; Tarantini, S.; Yabluchanskiy, A.; Fülöp, G.; Gautam, T.; Orock, A.; Csiszar, A.; Deak, F.; Ungvari, Z. Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: Implications for the pathogenesis of vascular cognitive impairment. GeroScience 2017, 39, 385. [Google Scholar] [CrossRef] [PubMed]
- Pelkey, K.A.; Barksdale, E.; Craig, M.T.; Yuan, X.; Sukumaran, M.; Vargish, G.A.; Mitchell, R.M.; Wyeth, M.S.; Petralia, R.S.; Chittajallu, R.; et al. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron 2016, 90, 661. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, I.; Lerer-Goldshtein, T.; Okamoto, H.; Appelbaum, L. Reduced synaptic density and deficient locomotor response in neuronal activity-regulated pentraxin 2a mutant zebrafish. FASEB J. 2015, 29, 1220–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Z.; Wang, C.; Zepp, J.; Wu, L.; Sun, K.; Zhao, J.; Chandrasekharan, U.; DiCorleto, P.E.; Trapp, B.D.; Ransohoff, R.M.; et al. Act1 mediates IL-17–induced EAE pathogenesis selectively in NG2+ glial cells. Nat. Neurosci. 2013, 16, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Van Hove, I.; Lemmens, K.; Van de Velde, S.; Verslegers, M.; Moons, L. Matrix metalloproteinase-3 in the central nervous system: A look on the bright side. J. Neurochem. 2012, 123, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.D.D.; Hayakawa, K.; Seo, J.H.; Nguyen, M.N.; Som, A.T.; Lee, B.J.; Guo, S.; Kim, K.W.; Lo, E.H.; Arai, K. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia 2012, 60, 875–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhard, S.M.; Razak, K.; Ethell, I.M. A delicate balance: Role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front. Cell. Neurosci. 2015, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- Jourquin, J.; Tremblay, E.; Bernard, A.; Charton, G.; Chaillan, F.A.; Marchetti, E.; Roman, F.S.; Soloway, P.D.; Dive, V.; Yiotakis, A.; et al. Tissue inhibitor of metalloproteinases-1 (TIMP-1) modulates neuronal death, axonal plasticity, and learning and memory. Eur. J. Neurosci. 2005, 22, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Leco, K.J.; Apte, S.S.; Taniguchi, G.T.; Hawkes, S.P.; Khokha, R.; Schultz, G.A.; Edwards, D.R. Murine tissue inhibitor of metalloproteinases-4 (Timp-4): CDNA isolation and expression in adult mouse tissues. FEBS Lett. 1997, 401, 213–217. [Google Scholar] [CrossRef]
- Melendez-Zajgla, J.; Del Pozo, L.; Ceballos, G.; Maldonado, V. Tissue inhibitor of metalloproteinases-4. The road less traveled. Mol. Cancer 2008, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Raabe, T.D.; Clive, D.R.; Wen, D.; DeVries, G.H. Neonatal oligodendrocytes contain and secrete neuregulins in vitro. J. Neurochem. 2002, 69, 1859–1863. [Google Scholar] [CrossRef]
- Buonanno, A.; Kwon, O.B.; Yan, L.; Gonzalez, C.; Longart, M.; Hoffman, D.; Vullhorst, D. Neuregulins and neuronal plasticity: Possible relevance in Schizophrenia. Novartis Found. Symp. 2008, 289, 165–177, discussion 177–179, 193–195. [Google Scholar] [PubMed]
- Canoll, P.D.; Musacchio, J.M.; Hardy, R.; Reynolds, R.; Marchionni, M.A.; Salzer, J.L. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron 1996, 17, 229–243. [Google Scholar] [CrossRef]
- Bermingham-McDonogh, O.; McCabe, K.L.; Reh, T.A. Effects of GGF/neuregulins on neuronal survival and neurite outgrowth correlate with erbB2/neu expression in developing rat retina. Development 1996, 122, 1427–1438. [Google Scholar] [PubMed]
- Shen, K.Z.; Zhu, Z.T.; Munhall, A.; Johnson, S.W. Dopamine receptor supersensitivity in rat subthalamus after 6-hydroxydopamine lesions. Eur. J. Neurosci. 2003, 18, 2967–2974. [Google Scholar] [CrossRef] [PubMed]
- Hökfelt, T.; Broberger, C.; Diez, M.; Xu, Z.Q.; Shi, T.; Kopp, J.; Zhang, X.; Holmberg, K.; Landry, M.; Koistinaho, J. Galanin and NPY, two peptides with multiple putative roles in the nervous system. Horm. Metab. Res. 1999, 31, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Biswas, N.; Gayen, J.R.; Miramontes-Gonzalez, J.P.; Hightower, C.M.; Mustapic, M.; Mahata, M.; Huang, C.T.; Hook, V.Y.; Mahata, S.K.; et al. Chromogranin B: Intra- and extra-cellular mechanisms to regulate catecholamine storage and release, in catecholaminergic cells and organisms. J. Neurochem. 2014, 129, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.G.; Kim, J.H.; Park, C.S.; Kim, B.J.; Kim, J.W.; Choi, I.G.; Hwang, J.; Shin, H.D.; Woo, S.I. Gender-specific associations between CHGB genetic variants and Schizophrenia in a Korean population. Yonsei Med. J. 2017, 58, 619. [Google Scholar] [CrossRef] [PubMed]
- Gasser, M.C.; Berti, I.; Hauser, K.F.; Fischer-Colbrie, R.; Saria, A. Secretoneurin promotes pertussis toxin-sensitive neurite outgrowth in cerebellar granule cells. J. Neurochem. 2003, 85, 662–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchmair, R.; Gander, R.; Egger, M.; Hanley, A.; Silver, M.; Ritsch, A.; Murayama, T.; Kaneider, N.; Sturm, W.; Kearny, M.; et al. The neuropeptide secretoneurin acts as a direct angiogenic cytokine in vitro and in vivo. Circulation 2004, 109, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Milbrandt, J.; de Sauvage, F.J.; Fahrner, T.J.; Baloh, R.H.; Leitner, M.L.; Tansey, M.G.; Lampe, P.A.; Heuckeroth, R.O.; Kotzbauer, P.T.; Simburger, K.S.; et al. Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron 1998, 20, 245–253. [Google Scholar] [CrossRef]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Farías, G.G.; Godoy, J.A.; Cerpa, W.; Varela-Nallar, L.; Inestrosa, N.C. Wnt signaling modulates pre- and postsynaptic maturation: Therapeutic considerations. Dev. Dyn. 2009, 239, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.C.; Lucas, F.R.; Salinas, P.C. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 2000, 100, 525–535. [Google Scholar] [CrossRef]
- Oliva, C.A.; Vargas, J.Y.; Inestrosa, N.C. Wnts in adult brain: From synaptic plasticity to cognitive deficiencies. Front. Cell. Neurosci. 2013, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Annuar, A.; Ciani, L.; Simeonidis, I.; Herreros, J.; Fredj, N.B.; Rosso, S.B.; Hall, A.; Brickley, S.; Salinas, P.C. Signaling across the synapse: A role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J. Cell Biol. 2006, 174, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Katayama, M.; Iwasaki, S.; Ishii, K.; Tsujimoto, M.; Kohno, M. Bone morphogenetic protein-2 promotes survival and differentiation of striatal GABAergic neurons in the absence of glial cell proliferation. J. Neurochem. 2002, 72, 2264–2271. [Google Scholar] [CrossRef]
- Kusakawa, Y.; Mikawa, S.; Sato, K. BMP7 expression in the adult rat brain. IBRO Rep. 2017, 3, 72–86. [Google Scholar] [CrossRef]
- Nguyen-Ba-Charvet, K.T.; Picard-Riera, N.; Tessier-Lavigne, M.; Baron-Van Evercooren, A.; Sotelo, C.; Chédotal, A. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J. Neurosci. 2004, 24, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, R.; Camand, E.; Chedotal, A.; Sotelo, C.; Dusart, I. Expression of netrin-1, slit-1 and slit-3 but not of slit-2 after cerebellar and spinal cord lesions. Eur. J. Neurosci. 2005, 22, 2134–2144. [Google Scholar] [CrossRef] [PubMed]
- Hewett, S.J.; Jackman, N.A.; Claycomb, R.J. Interleukin-1β in central nervous system injury and repair. Eur. J. Neurodegener. Dis. 2012, 1, 195–211. [Google Scholar] [PubMed]
- Bauer, S.; Kerr, B.J.; Patterson, P.H. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat. Rev. Neurosci. 2007, 8, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Deverman, B.E.; Patterson, P.H. Cytokines and CNS development. Neuron 2009, 64, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Strle, K.; Zhou, J.H.; Shen, W.H.; Broussard, S.R.; Johnson, R.W.; Freund, G.G.; Dantzer, R.; Kelley, K.W. Interleukin-10 in the brain. Crit. Rev. Immunol. 2001, 21, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Stillings, S.A.; Leclerc, J.L.; Phillips, H.; Edwards, N.J.; Robicsek, S.A.; Hoh, B.L.; Blackburn, S.; Doré, S.L. Role of interleukin-10 in acute brain injuries. Front. Neurol. 2017, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Dobolyi, A.; Vincze, C.; Pál, G.; Lovas, G. The neuroprotective functions of transforming growth factor βproteins. Int. J. Mol. Sci. 2012, 13, 8219–8258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.E.; Lipsky, B.P.; Russell, C.; Ketchem, R.R.; Kirchner, J.; Hensley, K.; Huang, Y.; Friedman, W.J.; Boissonneault, V.; Plante, M.M.; et al. A central nervous system-restricted isoform of the interleukin-1 receptor accessory protein modulates neuronal responses to interleukin-1. Immunity 2009, 30, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, C.J.; Martin, B.N.; Bulek, K.; Kang, Z.; Zhao, J.; Bian, G.; Carman, J.A.; Gao, J.; Dongre, A.; et al. IL-17 induced NOTCH1 activation in oligodendrocyte progenitor cells enhances proliferation and inflammatory gene expression. Nat. Commun. 2017, 8, 15508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marro, B.S.; Grist, J.J.; Lane, T.E. Inducible expression of CXCL1 within the central nervous system amplifies viral-induced demyelination. J. Immunol. 2016, 196, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.S. Regulation of neuroinflammation: The role of CXCL10 in lymphocyte infiltration during autoimmune encephalomyelitis. J. Cell. Biochem. 2004, 92, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bisht, K.; Tremblay, M.È. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front. Cell. Neurosci. 2014, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Hesselgesser, J.; Horuk, R. Chemokine and chemokine receptor expression in the central nervous system. J. Neurovirol. 1999, 5, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Tani, M. Do chemokines mediate leukocyte recruitment in post-traumatic CNS inflammation? Trends Neurosci. 1998, 21, 154–159. [Google Scholar] [CrossRef]
- Mennicken, F.; Maki, R.; de Souza, E.B.; Quirion, R. Chemokines and chemokine receptors in the CNS: A possible role in neuroinflammation and patterning. Trends Pharmacol. Sci. 1999, 20, 73–78. [Google Scholar] [CrossRef]
- Schäbitz, W.R.; Krüger, C.; Pitzer, C.; Weber, D.; Laage, R.; Gassler, N.; Aronowski, J.; Mier, W.; Kirsch, F.; Dittgen, T.; et al. A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF). J. Cereb. Blood Flow Metab. 2008, 28, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Frischknecht, R.; Heine, M.; Perrais, D.; Seidenbecher, C.I.; Choquet, D.; Gundelfinger, E.D. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat. Neurosci. 2009, 12, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Michaluk, P.; Mikasova, L.; Groc, L.; Frischknecht, R.; Choquet, D.; Kaczmarek, L. Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin β1 signaling. J. Neurosci. 2009, 29, 6007–6012. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, H.A.; Hunanyan, A.S.; Alessi, V.; Schnell, L.; Levine, J.; Arvanian, V.L. Neutralization of inhibitory molecule NG2 improves synaptic transmission, retrograde transport, and locomotor function after spinal cord injury in adult rats. J. Neurosci. 2013, 33, 4032–4043. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dietz, K.; Hodes, G.E.; Russo, S.J.; Casaccia, P. Widespread transcriptional alternations in oligodendrocytes in the adult mouse brain following chronic stress. Dev. Neurobiol. 2018, 78, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.P.; Coulter, M.; Miotke, J.; Meyer, R.L.; Takemaru, K.I.; Levine, J.M. Abrogation of catenin signaling in oligodendrocyte precursor cells reduces glial scarring and promotes axon regeneration after CNS Injury. J. Neurosci. 2014, 34, 10285–10297. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.L.; Gallo, V. The diversity and disparity of the glial scar. Nat. Neurosci. 2018, 21, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Li, Y.; Fukaya, M.; Lorenzini, I.; Cleveland, D.W.; Ostrow, L.W.; Rothstein, J.D.; Bergles, D.E. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat. Neurosci. 2013, 16, 571–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampton, D.; Rhodes, K.; Zhao, C.; Franklin, R.J.; Fawcett, J. The responses of oligodendrocyte precursor cells, astrocytes and microglia to a cortical stab injury, in the brain. Neuroscience 2004, 127, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Lytle, J.M.; Chittajallu, R.; Wrathall, J.R.; Gallo, V. NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury. Glia 2009, 57, 270–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, S.; Zeisel, A.; Codeluppi, S.; van Bruggen, D.; Mendanha Falcão, A.; Xiao, L.; Häring, M.; Hochgerner, H.; Romanov, R.A.; Gyllborg, D.; et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 2016, 352, 1326–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakers, K.; Lake, A.M.; Khazanchi, R.; Ouwenga, R.; Vasek, M.J.; Dani, A.; Dougherty, J.D. Astrocytes locally translate transcripts in their peripheral processes. Proc. Natl. Acad. Sci. USA 2017, 114, E3830–E3838. [Google Scholar] [CrossRef] [PubMed]
- Crosetto, N.; Bienko, M.; van Oudenaarden, A. Spatially resolved transcriptomics and beyond. Nat. Rev. Genet. 2015, 16, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Dimou, L.; Simons, M. Diversity of oligodendrocytes and their progenitors. Curr. Opin. Neurobiol. 2017, 47, 73–79. [Google Scholar] [CrossRef] [PubMed]
| Type of Signal | Name and Abbreviation | Exp. Approach (Source) | Function |
|---|---|---|---|
| Growth factors | Fibroblast growth factor 2, FGF2 | Gene expr. study of cultured mouse NG2 glia and expr. in adult mouse NG2 glia in vivo [87] | NG2 glia modulation of glutamatergic neurotransmission and astrocytic extracellular glutamate uptake [87], neuronal protection and CNS repair [101], modulation of synaptic plasticity [102] and astroglial and microglial reactivity [103] |
| Platelet-derived growth factor AA, PDGF-AA | CM of cultured mouse NG2 glia [97] | Neuronal protection [104], synaptic plasticity [105] and angiogenesis [106] | |
| Platelet-derived growth factor BB, PDGF-BB | CM of cultured mouse NG2 glia [97] | Neurotrophic and neuroregulatory functions [107], synaptic plasticity [105] | |
| Vascular endothelial growth factor, VEGF | CM of cultured mouse NG2 glia [97] | Neurotrophic function [108,109], regulation of adult neurogenesis [108] | |
| Insulin-like growth factor 1, IGF-1 | CM of cultured rat NG2 glia [94]; Gene expr. study of cultured rat NG2 glia [93] | Synaptic maturation [110] | |
| Hepatocyte growth factor, HGF | In vivo expr. in rat NG2 glia [88]; Protein expr. in cultured rat NG2 glia [111] | Neuronal plasticity [112] | |
| Neurotrophins | Brain derived growth factor, BDNF | CM of cultured rat NG2 glia and protein expr. in adult mouse NG2 glia in vivo [113]; CM of cultured human NG2 glia [114]; Gene expr. study and CM of cultured rat NG2 glia [93] | Neurotrophic function, neurotransmitter modulation and in neuronal plasticity [115] |
| Nerve growth factor, NGF | Gene expr. study of cultured mouse NG2 glia [87,116] | Neurotrophic function, brain plasticity [117] | |
| Neurotrophin-3, NT-3 | CM of cultured human NG2 glia [114]; Gene expr. study of cultured rat NG2 glia [93] | Neurotrophic function, neuronal survival and differentiation [118,119] | |
| Neurotrophin 4/5, NT-4/5 | Gene expr. study of cultured mouse NG2 glia [87] | Neurotrophic function, neuronal survival and differentiation [120] | |
| Glial cell-derived neurotrophic factor, GDNF | Gene expr. study of cultured mouse NG2 glia [87]; Gene expr. study of cultured rat NG2 glia [93] | Neurotrophic function, survival and morphological differentiation of dopaminergic neurons [121] | |
| Ciliary neurotrophic factor, CNTF | Gene expr. study of cultured rat NG2 glia [93] | Neuronal and oligodendroglial survival [122,123,124] | |
| Cell adhesion and extracellular matrix molecules | Neuronal cell adhesion molecule, NrCAM | Gene expr. study of freshly sorted adult human NG2 glia [125] | Maintenance of Na+ channels at nodes of Ranvier [126], dendritic spine remodelling [127] |
| NCAM | Gene expr. study of freshly sorted adult human NG2 glia [125] | Neuronal plasticity [128], neuritogenesis and synaptogenesis [129], adult neurogenesis [130] | |
| Down Syndrome Cell Adhesion Molecule, Dscam | Gene expr. study of freshly sorted mouse NG2 glia [131] and freshly sorted adult human NG2 glia [125] | Neurite repulsion [132,133,134,135] | |
| Chondroitin sulphate proteoglycan4, Cspg4/NG2 | Gene expr. study of freshly sorted adult human NG2 glia [125,136] | Modulation of synaptic activity and action potential conduction [137], axonal guidance and regenerative processes [138] | |
| Versican, Vcan | Gene expr. study of freshly sorted adult human NG2 glia [125] and freshly sorted mouse NG2 glia [131] | Assembly, maintenance and function of the nodes of Ranvier [139] | |
| Brevican | Gene expr. study of freshly sorted mouse NG2 glia [131] | Assembly, maintenance and function of the nodes of Ranvier [140] | |
| Tenascins | Gene expr. study of freshly sorted adult human NG2 glia [125]; Gene expr. study of freshly sorted mouse NG2 glia [131] | Synaptic plasticity [141] | |
| Glypican 5, Gpc5 | Gene expr. study of freshly sorted mouse NG2 glia [131] | Neuronal plasticity [142,143] | |
| Emilin And Multimerin Domain-Containing Protein 1, Emid1 | Gene expr. study of freshly sorted mouse NG2 glia [131] | Blood vessel maintenance [144] | |
| Syndecan 3, sdc3 | Gene expr. study of freshly sorted adult human NG2 glia [125] | Synaptic plasticity [145] | |
| Spondin 1, Spon1 | Gene expr. study of freshly sorted mouse NG2 glia [131] | Neural cell adhesion and neurite outgrowth [146] | |
| Thrombospondin 2 and 4 | Gene expr. study of freshly sorted adult human NG2 glia [125] | Synaptogenesis and synaptic plasticity [147], inflammatory response and repair of the blood brain barrier [148] | |
| Cerebellin 1, cbln 1 | Gene expr. study of cultured mouse NG glia [87] | Synaptic organizer, synapse integrity and synaptic plasticity [149,150] | |
| SPARC-Related Modular Calcium-Binding Protein 1, SMOC1 | Gene expr. study of freshly sorted mouse NG2 glia [131] | Angiogenesis [151] | |
| Olfactomedin 2, Olfm2 | Gene expr. study of freshly sorted mouse NG2 glia [131] | Synaptogenesis and synaptic plasticity [152] | |
| Neurexophilin 1, Nxph1 | Gene expr. study of freshly sorted mouse NG2 glia [131] | Synaptogenesis and synaptic plasticity [150,153] | |
| Neuronal Pentraxin 2, Nptx2 | Gene expr. study of freshly sorted mouse NG2 glia [131] and of cultured and freshly sorted mouse NG2 glia [154] | Synaptogenesis and synaptic plasticity [150,153,155,156], trafficking of glutamate receptors [157] | |
| Matrix metalloproteases and metalloprotease inhibitors | Matrix Metalloprotease 3, MMP3 | CM of cultured mouse NG2 glia [26]; Gene expr. study of cultured mouse NG2 glia exposed to Il17 or Tnf [158] | Neuronal and synaptic plasticity [159] |
| Matrix Metalloprotease 9, MMP9 | CM of cultured mouse NG2 glia [26]; CM of cultured rat NG2 glia and protein expr. in mouse NG2 glia in vivo—adult white matter [67] Gene expr. study of cultured mouse NG2 glia exposed to Il17 or Tnf [158] | Blood vessel remodeling after white matter injury [160]. Remodeling of synaptic networks in adult brain [161] | |
| Tissue Inhibitor of Metalloprotease 1, TIMP1 | CM of cultured mouse NG2 glia [26] | Neuronal death and axonal plasticity [162] | |
| Tissue Inhibitor of Metalloprotease 4, TIMP4 | Gene expr. study of freshly sorted mouse NG2 glia [131]; Gene expr. study of freshly sorted adult human NG2 glia [125] | Tissue remodelling [163,164] | |
| Neuromodulatory/neurosupportive factors | Neuregulins | Gene expr. study of cultured mouse NG glia [87]; Gene expr. study and CM of cultured rat NG glia [165] | Synaptogenesis and synaptic plasticity [166], oligodendroglial and neuronal survival [167,168] |
| Galanin | mRNA and protein expr. in adult mouse NG2 glia [169] | Modulation of neuronal activity [170] | |
| Chromogranin B, Chgb | Gene expr. study of freshly sorted adult human NG2 glia [125] | Neurotransmission [171,172] | |
| Chromogranin C, Chgc | Gene expr. study of freshly sorted adult human NG2 glia [125] | Neurite outgrowth [173] and angiogenesis [174]. | |
| Persephin, PSPN | Gene expr. study of cultured mouse NG glia [87] | Neuronal survival [175] | |
| Morphogens | Wingless-type MMTV integration site family 4, Wnt4 | Gene expr. study of freshly sorted mouse NG2 glia [176] | Synaptic plasticity [177] |
| Wingless-type MMTV integration site family 7a, Wnt7a | Gene expr. study of freshly sorted mouse NG2 glia [176]; Gene expr. study and CM of cultured mouse NG2 glia [96]; In vivo expr. in embryonic NG2 glia [64] | Synaptic plasticity [178,179]; NG2 glia regulation of angiogenesis [96] | |
| Wingless-type MMTV integration site family 7b, Wnt7b | Gene expr. study of freshly sorted mouse NG2 glia [176]; Gene expr. study and CM of cultured mouse NG2 glia [96]; In vivo expr. in embryonic NG2 glia [64] | Synaptic plasticity [180] | |
| Bone morphogenic protein 2, BMP2 | Gene expr. study of freshly sorted adult human NG2 glia [125] | Neuronal survival [181] | |
| Bone morphogenic protein 7, BMP7 | Gene expr. study of freshly sorted adult human NG2 glia [125] | Maintainance of the identity of catecholaminergic neurons and differentiation of astrocytes [182] | |
| Retinoic acid, RA | Application of pharmacological inhibitors in cultured mouse NG2 glia and in vivo assays in a model of rat spinal cord injury [99] | NG2 glia regulation of axonal outgrowth [99] | |
| Slit1 | Gene expr. study of freshly sorted adult human NG2 glia [125] | Regulation of adult SVZ neurogenesis [183], axon outgrowth and glial scar formation [184] | |
| Inflammatory cytokines/immunomodulatory factors | Interleukin 1 beta, Il-1b | Gene expr. study of cultured mouse NG2 glia [87]; Gene expr. study of cultured rat NG2 glia [93] | Synaptic plasticity [185] |
| Interleukin 6, Il-6 | Gene expr. study of cultured mouse NG2 glia [87]; Gene expr. study of cultured rat NG2 glia [93] Gene expr. study of cultured mouse NG2 glia exposed to Il17 or Tnf [158] | Regulation of adult neurogenesis [186,187], neuronal protection [187] | |
| Interleukin 10, Il-10 | Gene expr. study of cultured mouse NG2 glia [87]; Gene expr. study and CM of cultured rat NG2 glia [93] | Neuronal and glial cell survival [88,188]. Anti-inflammatory functions [189] | |
| Transforming growth factor beta, Tgf-b | Gene expr. study of cultured mouse NG2 glia [87]; Gene expr. study of cultured rat NG2 glia and protein expr. in mouse neonatal NG2 glia in vivo [65] | Neuronal survival and modulation of synaptic transmission [190] | |
| Interleukin 1 Receptor Accessory Protein, Il1rap | Gene expr. study of freshly sorted mouse NG2 glia [131] | Neuronal survival [191] | |
| C-X-C Motif Chemokine Ligand 10, CxCl1 | Gene expr. study of cultured mouse NG2 glia exposed to Il17 or Tnf [158,192] | Neuroinflammation [193] | |
| C-X-C Motif Chemokine Ligand 10, CxCl10 | CM of cultured mouse NG2 glia [97] | Neuroinflammation [194] | |
| C-X3-C Motif Chemokine Ligand 1, Cx3Cl1 | CM of cultured mouse NG2 glia [97] | Regulation of synapses activity and plasticity, brain functional connectivity and adult hippocampal neurogenesis [195] | |
| C-C Motif Chemokine Ligand 7, Ccl7 | Gene expr. study of cultured mouse NG2 glia exposed to Il17 [192] | Neuroinflammation [196,197,198] | |
| Granulocyte-Macrophage Colony Stimulating Factor, GM-CSF or Csf2 | Gene expr. study of cultured mouse NG2 glia exposed to Il17 or Tnf [158] | Neurotrophic function [199] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parolisi, R.; Boda, E. NG2 Glia: Novel Roles beyond Re-/Myelination. Neuroglia 2018, 1, 151-175. https://doi.org/10.3390/neuroglia1010011
Parolisi R, Boda E. NG2 Glia: Novel Roles beyond Re-/Myelination. Neuroglia. 2018; 1(1):151-175. https://doi.org/10.3390/neuroglia1010011
Chicago/Turabian StyleParolisi, Roberta, and Enrica Boda. 2018. "NG2 Glia: Novel Roles beyond Re-/Myelination" Neuroglia 1, no. 1: 151-175. https://doi.org/10.3390/neuroglia1010011
APA StyleParolisi, R., & Boda, E. (2018). NG2 Glia: Novel Roles beyond Re-/Myelination. Neuroglia, 1(1), 151-175. https://doi.org/10.3390/neuroglia1010011





