Astrogliopathy in Tauopathies
Abstract
:1. Introduction
2. Human Tauopathies
3. Non-Human Primate Tauopathies in Old Age
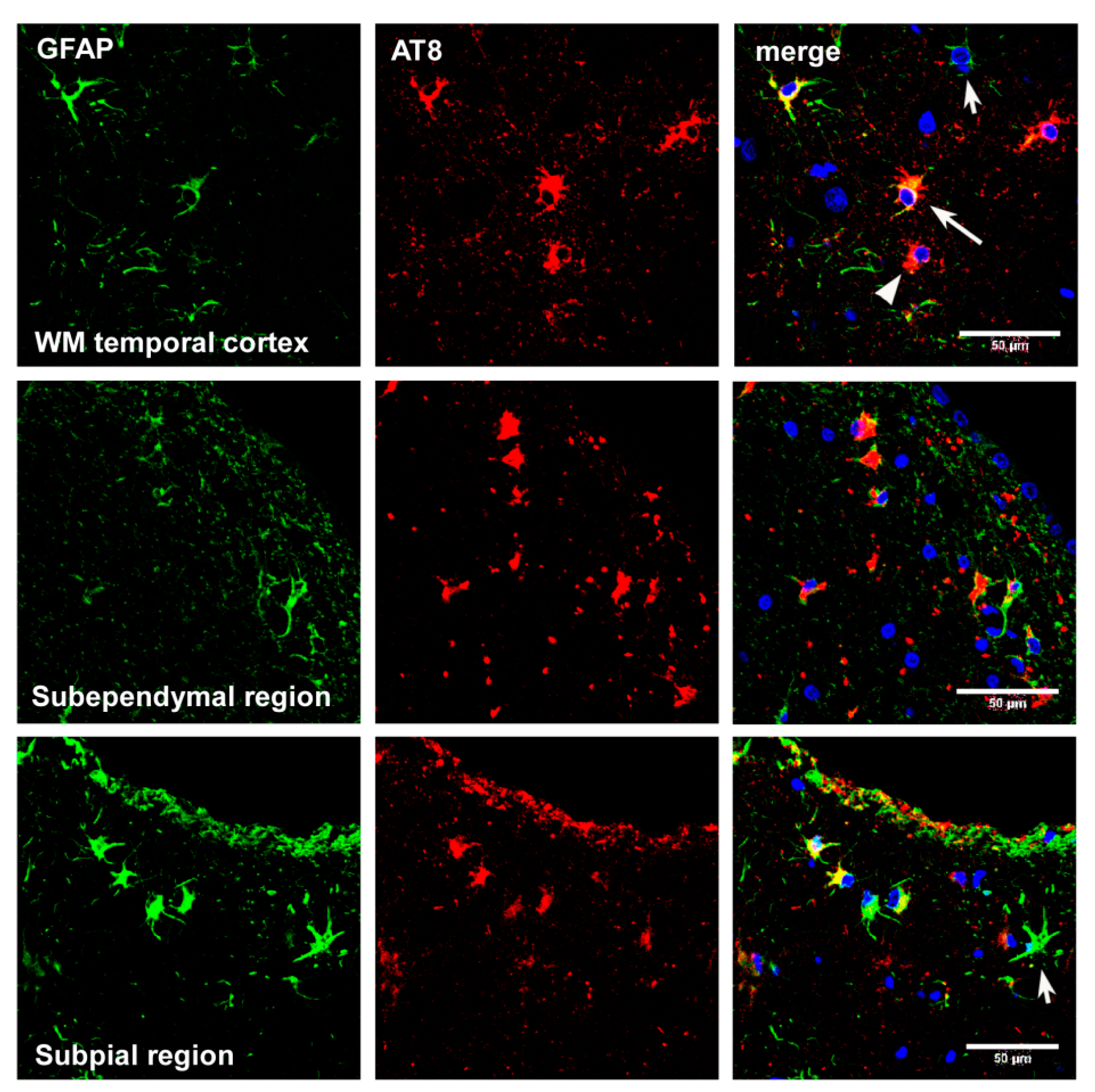
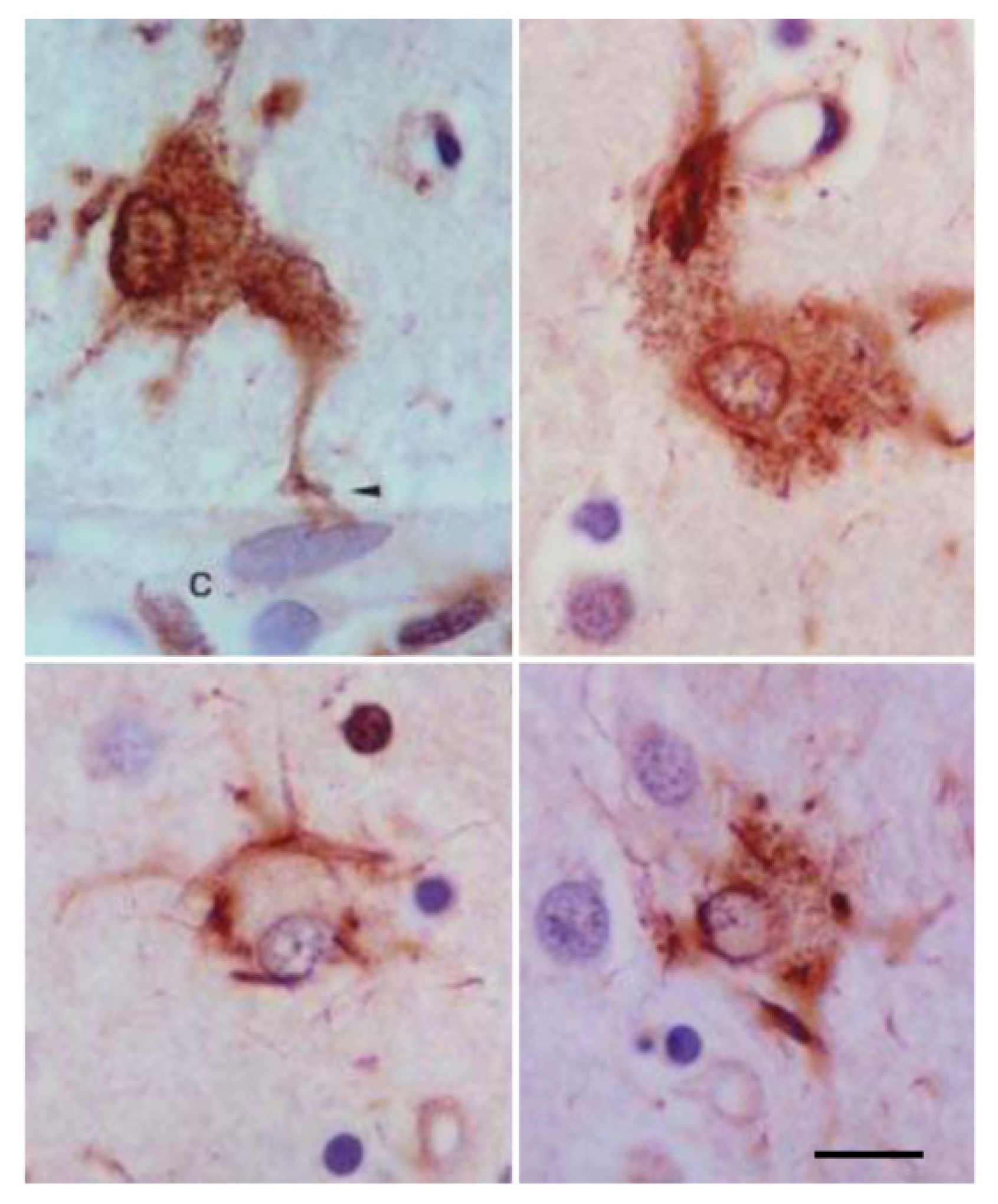
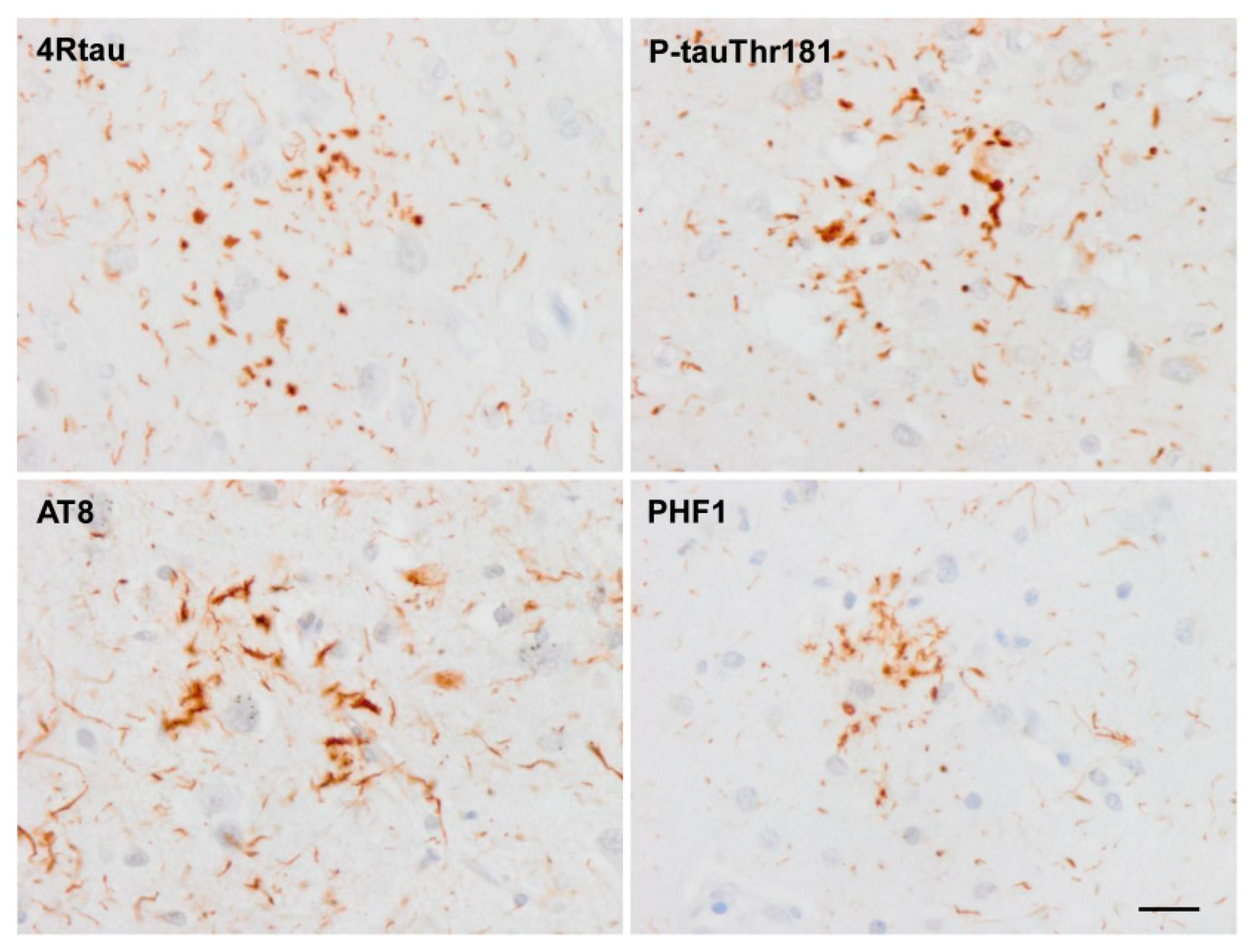
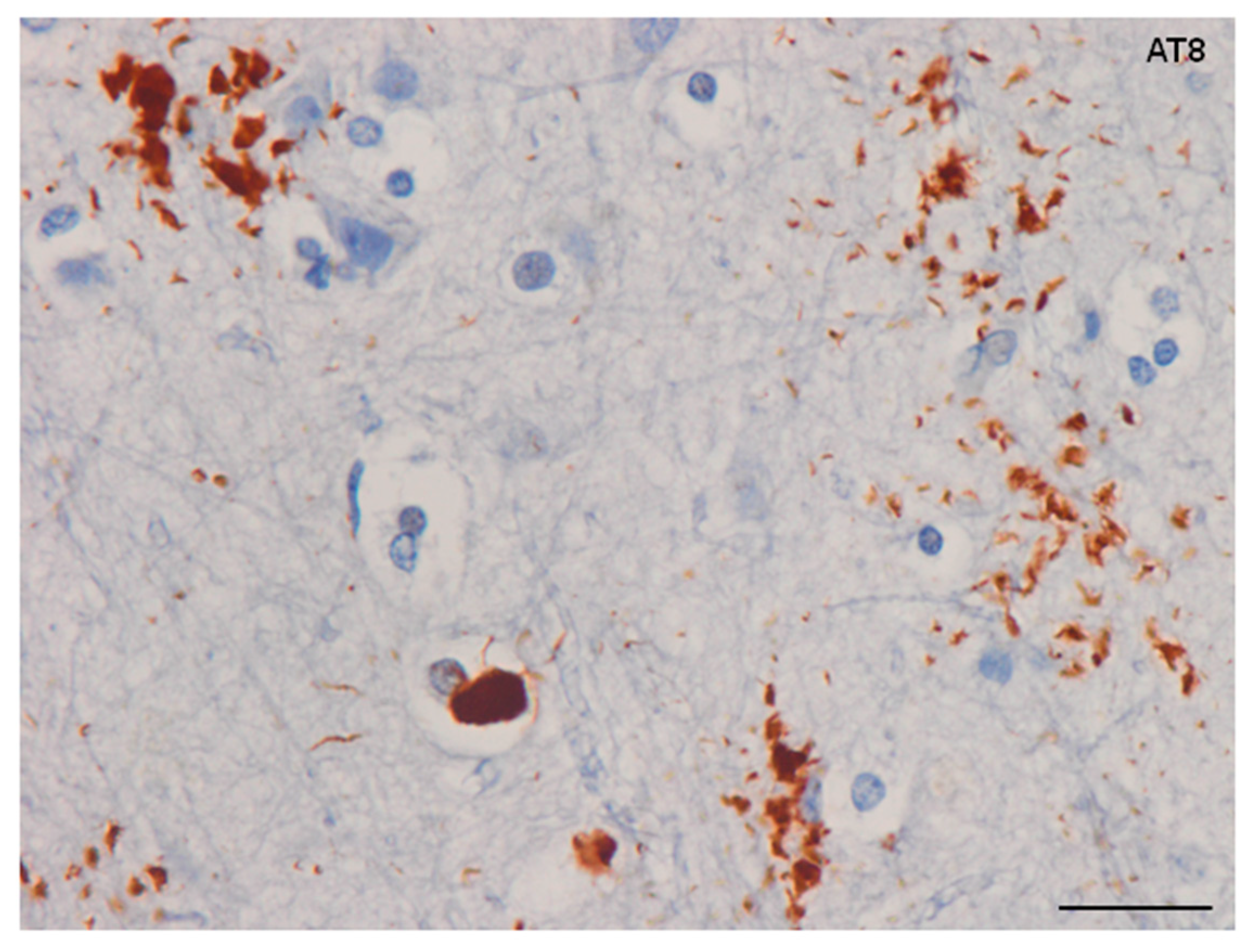
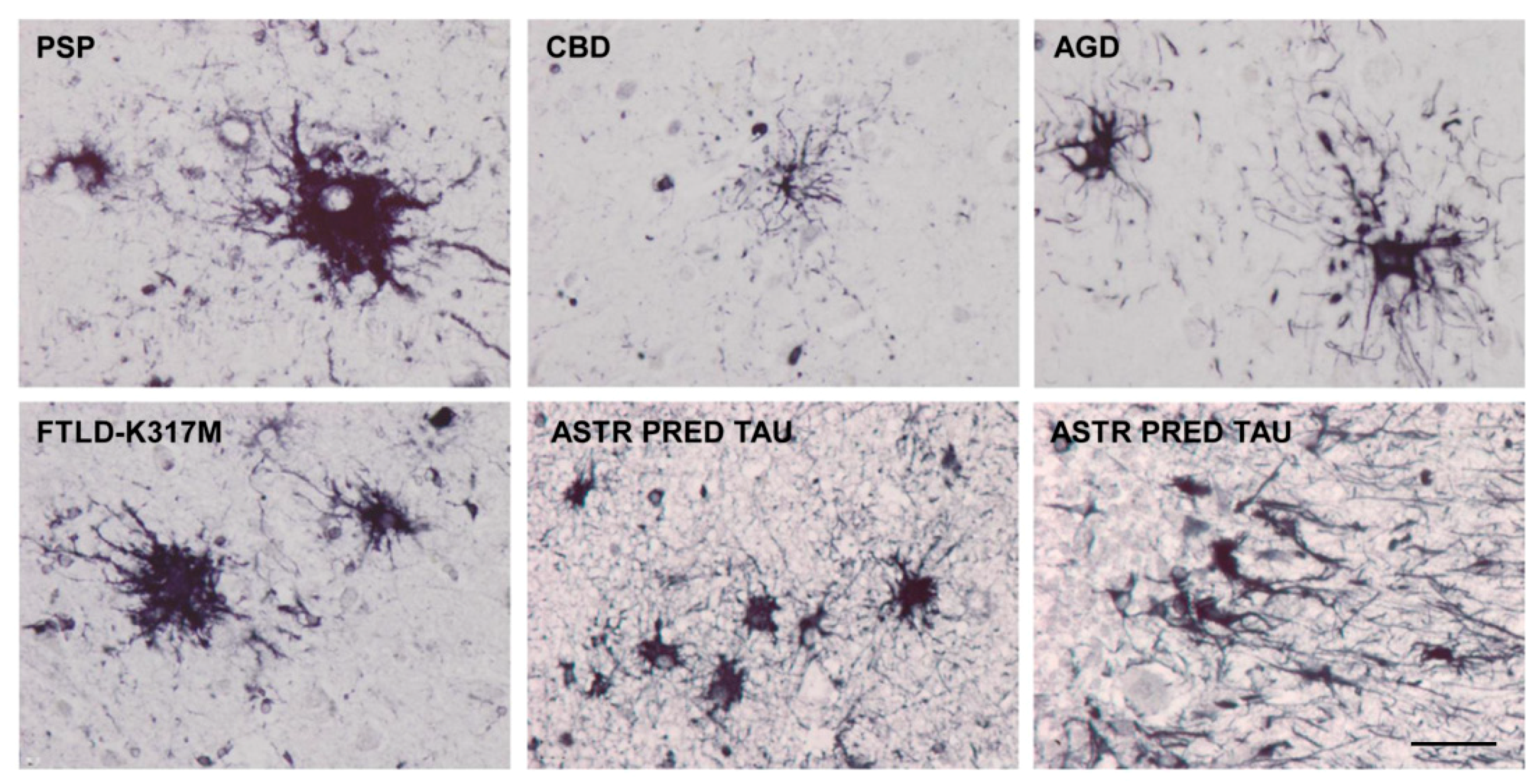
4. Main Types of Tau-Containing Astrocytes
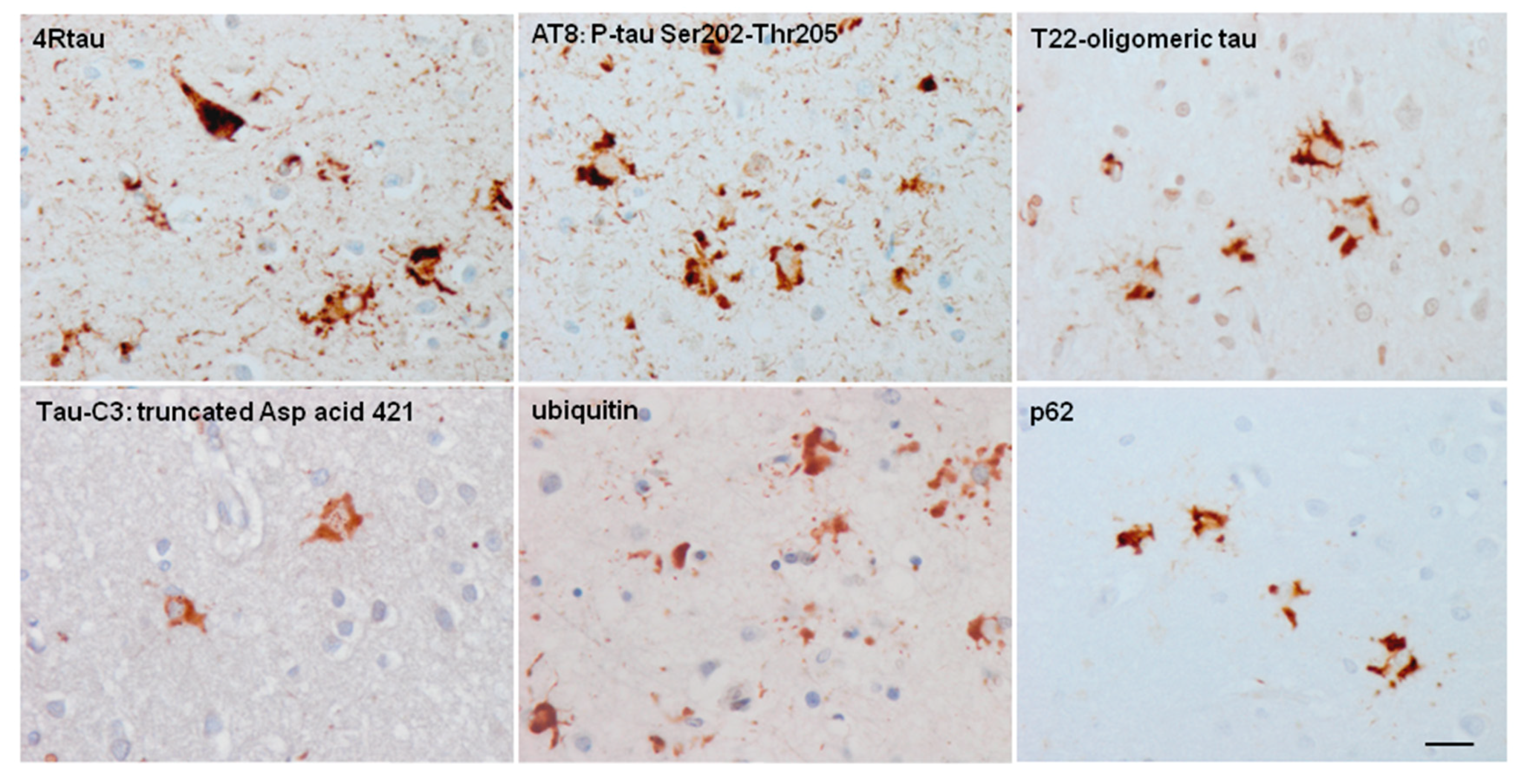
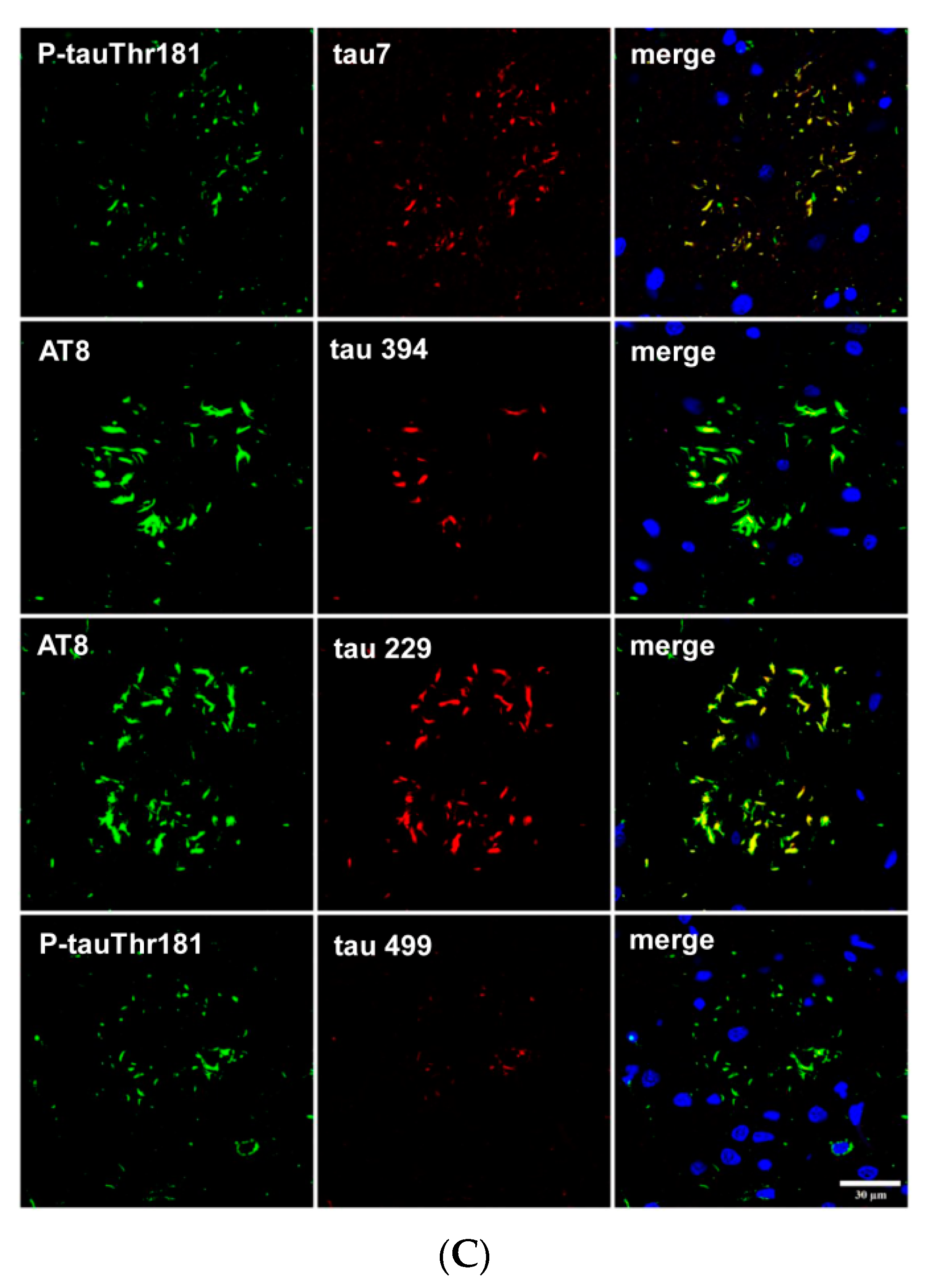
5. Post-Translational Tau Modifications and Tau Kinases in Tau-Containing Astrocytes in Tauopathies
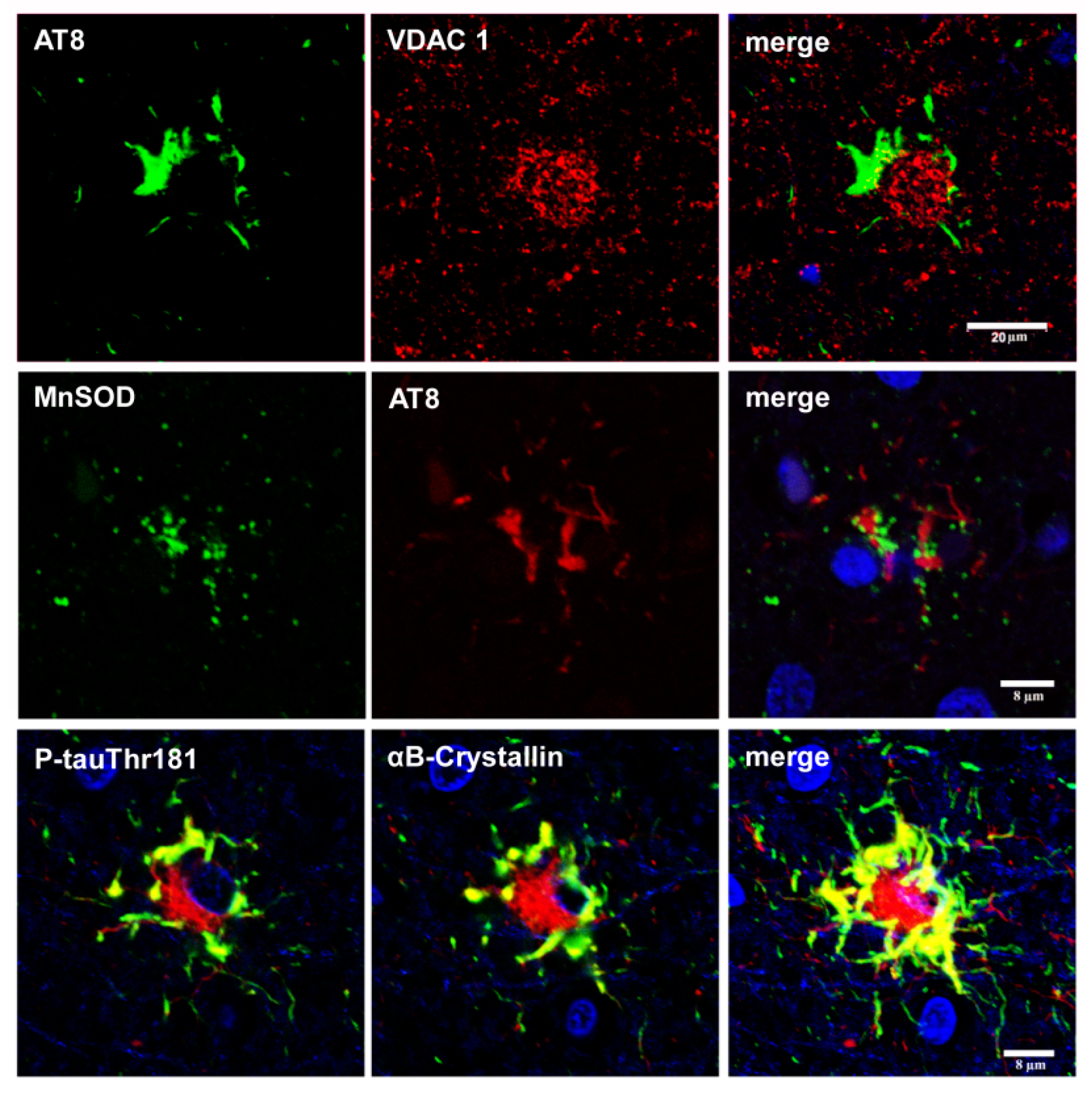
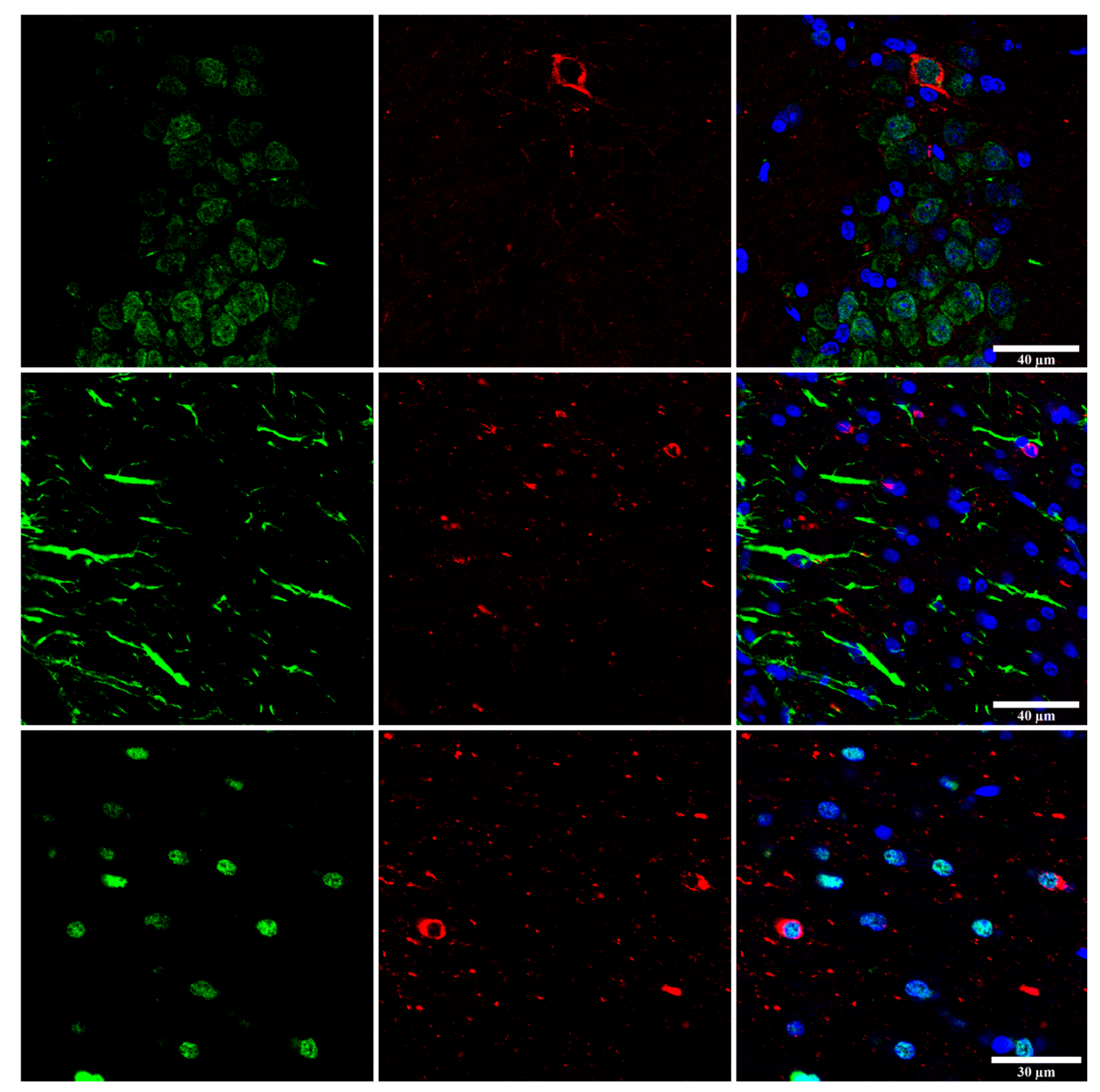
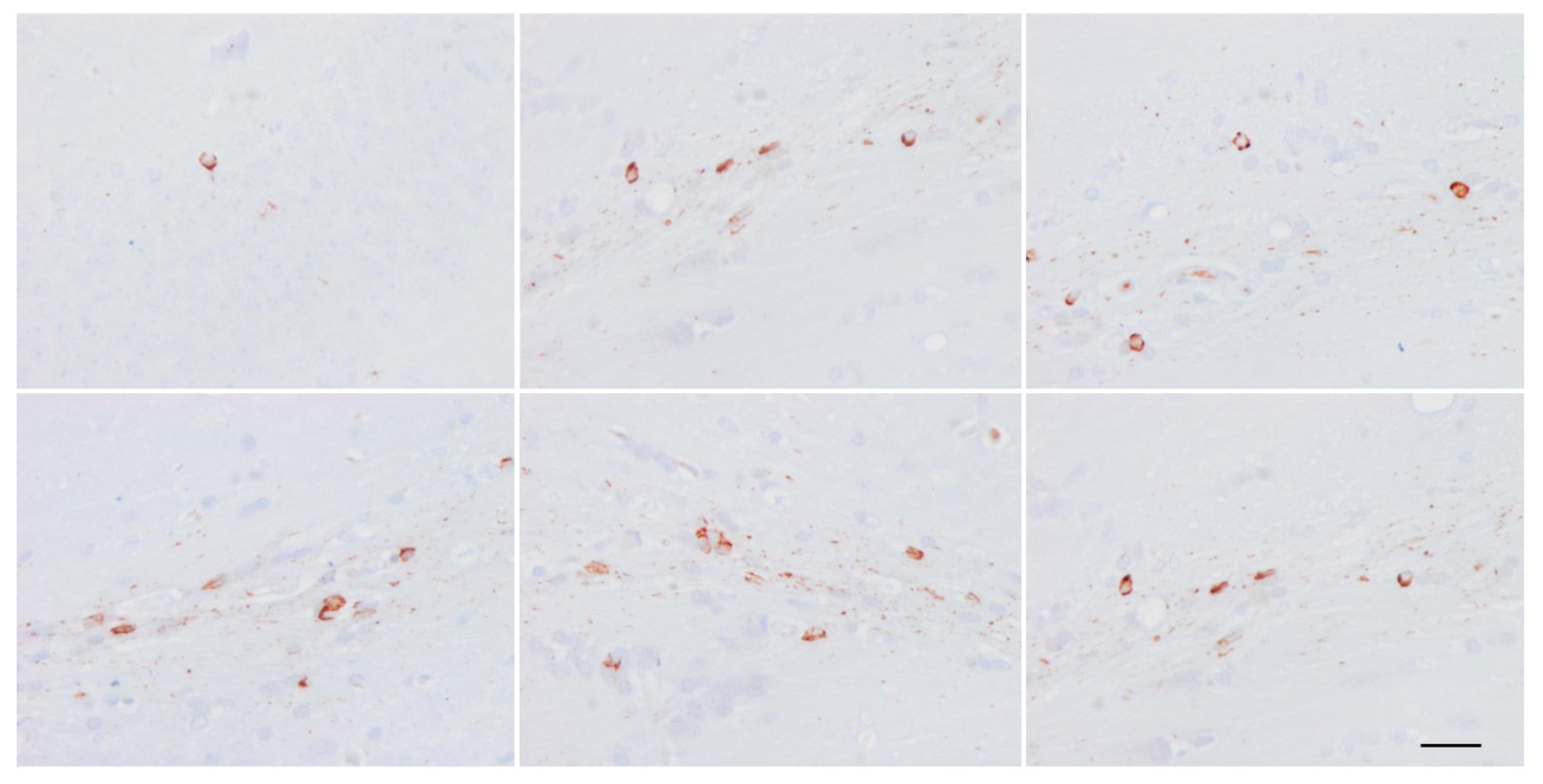
6. Cytoarchitectonic Changes Linked to Tau Deposits in Astrocytes
7. Astrogliopathy
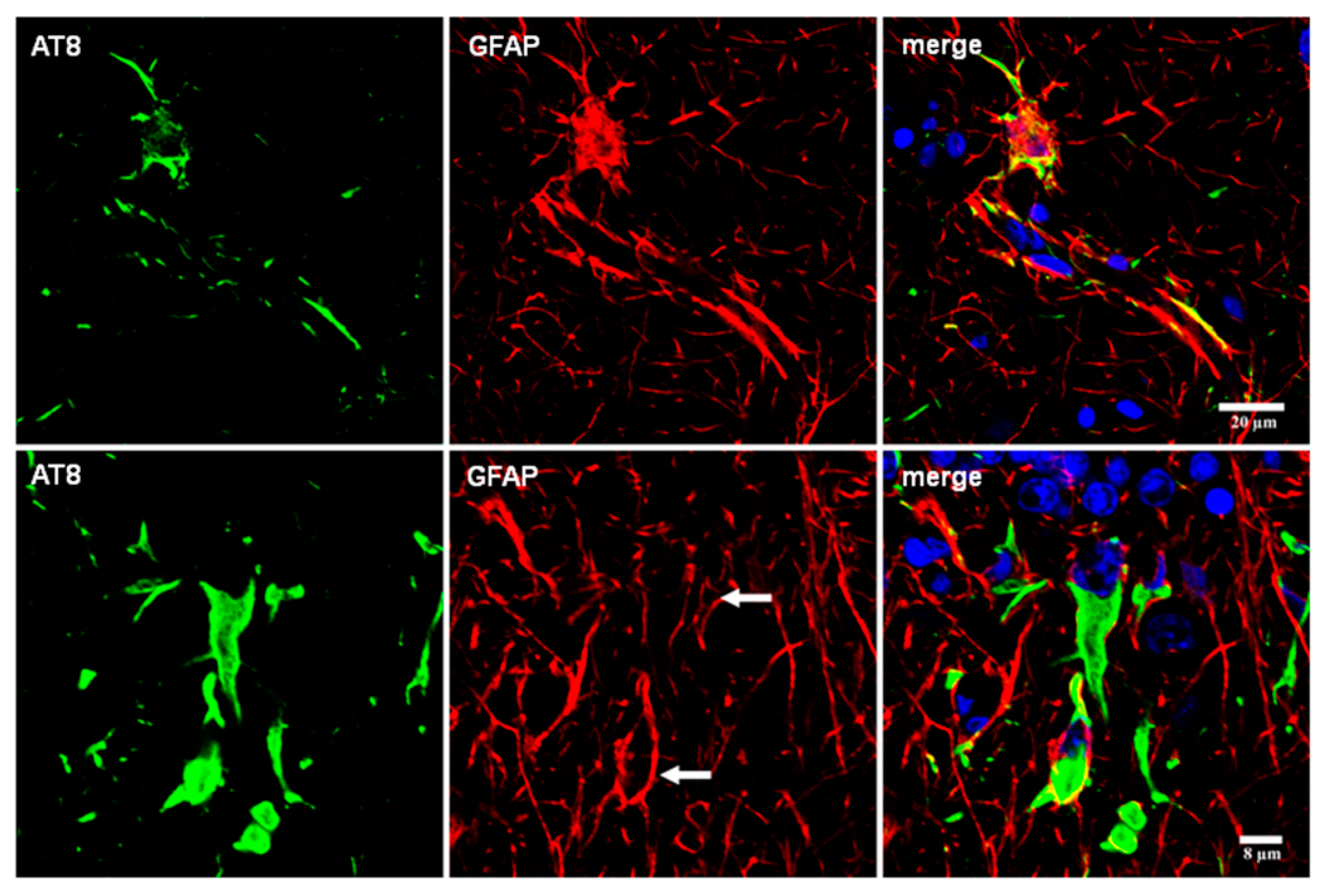
8. Reactive Astrogliosis
9. Astrocytopathy
10. Disease Progression: Seeding and Spreading; Role of Astrocytes
Funding
Acknowledgments
Conflicts of Interest
References
- Tolnay, M.; Spillantini, M.G.; Goedert, M.; Ulrich, J.; Langui, D.; Probst, A. Argyrophilic grain disease: Widespread hyperphosphorylation of tau protein in limbic neurons. Acta Neuropathol. 1997, 93, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.A.; Probst, A.; Villanueva, C.; Jimenez-Jimenez, F.J.; Madero, S.; Torres, N.; Bermejo, F. Primary progressive aphasia with glial cytoplasmic inclusions. Eur. Neurol. 1998, 40, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Dementia with grains (argyrophilic grain disease). Brain Pathol. 1998, 8, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Bigio, E.H.; Lipton, A.M.; Yen, S.H.; Hutton, M.L.; Baker, M.; Nacharaju, P.; White, C.L., 3rd; Davies, P.; Lin, W.; Dickson, D.W. Frontal lobe dementia with novel tauopathy: Sporadic multiple system tauopathy with dementia. J. Neuropathol. Exp. Neurol. 2001, 60, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.W.; Quinn, B.; Johnson, N.; Cochran, E.J.; Ghoshal, N.; Binder, L.I. Pathological glial tau accumulations in neurodegenerative disease: Review and case report. Neurochem. Int. 2001, 39, 469–479. [Google Scholar] [CrossRef]
- Ferrer, I.; Hernandez, I.; Boada, M.; Llorente, A.; Rey, M.J.; Cardozo, A.; Ezquerra, M.; Puig, B. Primary progressive aphasia as the initial manifestation of corticobasal degeneration and unusual tauopathies. Acta Neuropathol. 2003, 106, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.M.; Byrne, N.P.; Ito, M.; Takao, M.; Yankopoulou, D.; Spillantini, M.G.; Ghetti, B. A novel leukoencephalopathy associated with tau deposits primarily in white matter glia. Acta Neuropathol. 2003, 106, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.N.; Lashley, T.; Mahoney, C.J.; Warren, J.D.; Revesz, T.; Rohrer, J.D. Temporal Variant Frontotemporal dementia is associated with globular glial tauopathy. Cogn. Behav. Neurol. 2015, 28, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.S.; Tan, C.F.; Iwanaga, K.; Kakita, A.; Takano, H.; Nishizawa, M.; Lashley, T.; Revesz, T.; Lees, A.; de Silva, R.; et al. Sporadic four-repeat tauopathy with frontotemporal degeneration, parkinsonism and motor neuron disease. Acta Neuropathol. 2005, 110, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Josephs, K.A.; Katsuse, O.; Beccano-Kelly, D.A.; Lin, W.L.; Uitti, R.J.; Fujino, Y.; Boeve, B.F.; Hutton, M.L.; Baker, M.C.; Dickson, D.W. Atypical progressive supranuclear palsy with corticospinal tract degeneration. J. Neuropathol. Exp. Neurol. 2006, 65, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R. Tauopathies: Classification and clinical update on neurodegenerative diseases associated with microtubule-associated protein tau. Intern. Med. J. 2006, 36, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Majtenyi, K.; Spina, S.; Murrell, J.R.; Gelpi, E.; Hoftberger, R.; Fraser, G.; Crowther, R.A.; Goedert, M.; Budka, H.; et al. White matter tauopathy with globular glial inclusions: A distinct sporadic frontotemporal lobar degeneration. J. Neuropathol. Exp. Neurol. 2008, 67, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Marcon, G.; Mangieri, M.; Morbin, M.; Rossi, G.; Fetoni, V.; Patriarca, C.; Catania, M.; Di Fede, G.; Tagliavini, F.; et al. Atypical tauopathy with massive involvement of the white matter. Neuropathol. Appl. Neurobiol. 2008, 34, 468–472. [Google Scholar] [PubMed]
- Ferrer, I.; Santpere, G.; van Leeuwen, F.W. Argyrophilic grain disease. Brain 2008, 146, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.J.; Nishihira, Y.; Kuroda, S.; Toyoshima, Y.; Ishihara, T.; Shinozaki, M.; Miyashita, A.; Piao, Y.S.; Tan, C.F.; Tani, T.; et al. Sporadic four-repeat tauopathy with frontotemporal lobar degeneration, parkinsonism, and motor neuron disease: A distinct clinicopathological and biochemical disease entity. Acta Neuropathol. 2010, 120, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Doherty, K.M.; Silveira-Moriyama, L.; Bandopadhyay, R.; Lashley, T.; Mamais, A.; Hondhamuni, G.; Wray, S.; Newcombe, J.; O’Sullivan, S.S.; et al. Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: An emerging group of 4-repeat tauopathies. Acta Neuropathol. 2011, 122, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W.; Hauw, J.J.; Agid, Y.; Litvan, I. Progressive supranuclear palsy and corticobasal degeneration. In Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders, 2nd ed.; Dickson, D.W., Weller, R.O., Eds.; Willey-Blackwell: Chichester, UK, 2011; pp. 135–155. [Google Scholar]
- Muñoz, D.G.; Morris, H.R.; Rossor, M. Pick’s disease. In Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders, 2nd ed.; Dickson, D.W., Weller, R.O., Eds.; Willey-Blackwell: Chichester, UK, 2011; pp. 156–164. [Google Scholar]
- Tolnay, M.; Braak, H. Argyrophilic grain disease. In Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders, 2nd ed.; Dickson, D.W., Weller, R.O., Eds.; Wiley-Blackwell: Chichester, UK, 2011; pp. 165–170. [Google Scholar]
- Ahmed, Z.; Bigio, E.H.; Budka, H.; Dickson, D.W.; Ferrer, I.; Ghetti, B.; Giaccone, G.; Hatanpaa, K.J.; Holton, J.L.; Josephs, K.A.; et al. Globular glial tauopathies (GGT): Consensus recommendations. Acta Neuropathol. 2013, 126, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.; Kalaria, R. Dementia. In Greenfield’s Neuropathology, 9th ed.; Love, S., Budka, H., Ironside, J., Perry, A., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 858–973. [Google Scholar]
- Crary, J.F.; Trojanowski, J.Q.; Schneider, J.A.; Abisambra, J.F.; Abner, E.L.; Alafuzoff, I.; Arnold, S.E.; Attems, J.; Beach, T.G.; Bigio, E.H.; et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol. 2014, 128, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A.; Alafuzoff, I.; Attems, J.; Beach, T.G.; Cairns, N.J.; Crary, J.F.; Dickson, D.W.; Hof, P.R.; Hyman, B.T.; Jack, C.R., Jr.; et al. PART, a distinct tauopathy, different from classical sporadic Alzheimer disease. Acta Neuropathol. 2015, 129, 757–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, G.G. Invited review: Neuropathology of tauopathies: Principles and practice. Neuropathol. Appl. Neurobiol. 2015, 41, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G. Tauopathies. In Neuropathology of Neurodegenerative Diseases: A Practical Guide; Kovacs, G.G., Ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 109–148. [Google Scholar]
- Kovacs, G.G.; Ferrer, I.; Grinberg, L.T.; Alafuzoff, I.; Attems, J.; Budka, H.; Cairns, N.J.; Crary, J.F.; Duyckaerts, C.; Ghetti, B.; et al. Aging-related tau astrogliopathy (ARTAG): Harmonized evaluation strategy. Acta Neuropathol. 2016, 131, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Graff-Radford, J.; Josephs, K.A.; Parisi, J.E.; Dickson, D.W.; Giannini, C.; Boeve, B.F. Globular glial tauopathy presenting as semantic variant PPA. JAMA Neurol. 2016, 73, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Lee, V.M.; Trojanowski, J.Q. Protein astrogliopathies in human neurodegenerative diseases and aging. Brain Pathol. 2017, 27, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Robinson, J.L.; Xie, S.X.; Lee, E.B.; Grossman, M.; Wolk, D.A.; Irwin, D.J.; Weintraub, D.; Kim, C.F.; Schuck, T.; et al. Evaluating the patterns of aging-related tau astrogliopathy unravels novel insights into brain aging and neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2017, 76, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Goedert, M.; Crowther, R.A.; Murrell, J.R.; Farlow, M.R.; Ghetti, B. Familial multiple system tauopathy with presenile dementia: A disease with abundant neuronal and glial tau filaments. Proc. Natl. Acad. Sci. USA 1997, 94, 4113–4118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iseki, E.; Matsumura, T.; Marui, W.; Hino, H.; Odawara, T.; Sugiyama, N.; Suzuki, K.; Sawada, H.; Arai, T.; Kosaka, K. Familial frontotemporal dementia and parkinsonism with a novel N296H mutation in exon 10 of the tau gene and a widespread tau accumulation in the glial cells. Acta Neuropathol. 2001, 102, 285–292. [Google Scholar] [PubMed]
- Spina, S.; Farlow, M.R.; Unverzagt, F.W.; Kareken, D.A.; Murrell, J.R.; Fraser, G.; Epperson, F.; Crowther, R.A.; Spillantini, M.G.; Goedert, M.; et al. The tauopathy associated with mutation +3 in intron 10 of tau: Characterization of the MSTD family. Brain 2008, 131, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Ghetti, B.; Wszolek, Z.K.; Boeve, B.F.; Spina, S.; Goedert, M. Frontotemporal dementia and parkinsonism linked to chromosome 17. In Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders, 2nd ed.; Dickson, D.W., Weller, R.O., Eds.; Willey-Blackwell: Chichester, UK, 2011; pp. 110–134. [Google Scholar]
- Tacik, P.; Sanchez-Contreras, M.; Rademakers, R.; Dickson, D.W.; Wszolek, Z.K. Genetic disorders with tau pathology: A review of the literature and report of two patients with tauopathy and positive family histories. Neurodegener. Dis. 2016, 16, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Tacik, P.; DeTure, M.; Lin, W.L.; Sanchez-Contreras, M.; Wojtas, A.; Hinkle, K.M.; Fujioka, S.; Baker, M.C.; Walton, R.L.; Carlomagno, Y.; et al. A novel tau mutation, p.K317N, causes globular glial tauopathy. Acta Neuropathol. 2015, 130, 199–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarranz, J.J.; Ferrer, I.; Lezcano, E.; Forcadas, M.I.; Eizaguirre, B.; Atares, B.; Puig, B.; Gomez-Esteban, J.C.; Fernandez-Maiztegui, C.; Rouco, I.; et al. A novel mutation (K317M) in the MAPT gene causes FTDP and motor neuron disease. Neurology 2005, 64, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; López-González, I.; Carmona, M.; Arregui, L.; Dalfó, E.; Torrejón-Escribano, B.; Diehl, R.; Kovacs, G.G. Glial and neuronal tau pathology in tauopathies: Characterization of disease-specific phenotypes and tau pathology progression. J. Neuropathol. Exp. Neurol. 2014, 73, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Écija, S.; Morgado, J.; Palencia-Madrid, L.; Grau-Rivera, O.; Reñé, R.; Hernández, I.; Almenar, C.; Balasa, M.; Antonell, A.; Molinuevo, J.L.; et al. Frontotemporal dementia caused by the P301L mutation in the MAPT gene: Clinicopathological features of 13 cases from the same geographical origin in Barcelona, Spain. Dement. Geriatr. Cogn. Disord. 2017, 44, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Duyckaerts, C.; Dickson, D. Neuropathology of Alzheimer’s disease. In Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders, 2nd ed.; Dickson, D.W., Weller, R.O., Eds.; Willey-Blackwell: Chichester, UK, 2011; pp. 62–91. [Google Scholar]
- Revesz, T.; Rostagno, A.; Plant, G.; Lashley, T.; Frangione, B.; Ghiso, J.; Holton, J.L. Inherited amyloidoses and neurodegeneration: Familial British dementia and Familial Danish dementia. In Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders, 2nd ed.; Dickson, D.W., Weller, R.O., Eds.; Willey-Blackwell: Chichester, UK, 2011; pp. 439–445. [Google Scholar]
- Ghetti, B.; Tagliavini, F.; Kovacs, G.G.; Piccardo, P. Gerstmann-Straüssler-Scheinker. In Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders, 2nd ed.; Dickson, D.W., Weller, R.O., Eds.; Willey-Blackwell: Chichester, UK, 2011; pp. 364–377. [Google Scholar]
- Rahimi, J.; Kovacs, G.G. Prevalence of mixed pathologies in the aging brain. Alzheimer’s Res. Ther. 2014, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; von Arnim, C.A.; Griffin, W.S.; Mrak, R.E.; Walker, L.; Attems, J.; Arzberger, T. Frontotemporal lobar degeneration FTLD-tau: Preclinical lesions, vascular, and Alzheimer-related co-pathologies. J. Neural Transm. 2015, 122, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, N.; Kimura, N.; Yanagisawa, K. Alzheimer-type tau pathology in advanced aged nonhuman primate brains harboring substantial amyloid deposition. Brain Res. 2010, 1315, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.E.; Raghanti, M.A.; Hof, P.R.; Kramer, L.; Ikonomovic, M.D.; Lacor, P.N.; Erwin, J.M.; Sherwood, C.C.; Mufson, E.J. Alzheimer’s disease pathology in the neocortex and hippocampus of the western lowland gorilla (Gorilla gorilla gorilla). J. Comp. Neurol. 2013, 521, 4318–4338. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.E.; Sherwood, C.C.; Cranfield, M.R.; Erwin, J.M.; Mudakikwa, A.; Hof, P.R.; Mufson, E.J. Early Alzheimer’s disease-type pathology in the frontal cortex of wild mountain gorillas (Gorilla beringei beringei). Neurobiol. Aging 2016, 39, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.; Dehghani, F.; Hubbard, G.B.; Thal, D.R.; Struckhoff, G.; Braak, E.; Braak, H. Filamentous tau pathology in nerve cells, astrocytes, and oligodendrocytes of aged baboons. J. Neuropathol. Exp. Neurol. 2000, 59, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.F.; Farberg, A.S.; Gearing, M.; Dooyema, J.; Long, P.M.; Anderson, D.C.; Davis-Turak, J.; Coppola, G.; Geschwind, D.H.; Paré, J.F.; et al. Tauopathy with paired helical filaments in an aged chimpanzee. J. Comp. Neurol. 2008, 509, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edler, M.K.; Sherwood, C.C.; Meindl, R.S.; Hopkins, W.D.; Ely, J.J.; Erwin, J.M.; Mufson, E.J.; Hof, P.R.; Raghanti, M.A. Aged chimpanzees exhibit pathologic hallmarks of Alzheimer’s disease. Neurobiol. Aging 2017, 59, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Lemere, C.A.; Oh, J.; Stanish, H.A.; Peng, Y.; Pepivani, I.; Fagan, A.M.; Yamaguchi, H.; Westmoreland, S.V.; Mansfield, K.G. Cerebral amyloid-β protein accumulation with aging in cotton-top tamarins: A model of early Alzheimer’s disease? Rejuvenation Res. 2008, 11, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Craxton, M.; Jakes, R.; Arendt, T.; Goedert, M. Tau gene (MAPT) sequence variation among primates. Gene 2004, 341, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Namba, Y.; Ikeda, K.; Oda, M. Glial fibrillary tangles with straight tubules in the brains of patients with progressive supranuclear palsy. Neurosci. Lett. 1992, 143, 35–38. [Google Scholar] [CrossRef]
- Yamada, T.; McGeer, P.L.; McGeer, E.G. Appearance of paired nucleated, tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci. Lett. 1992, 135, 99–102. [Google Scholar] [CrossRef]
- Nishimura, T.; Ikeda, K.; Akiyama, H.; Kondo, H.; Kato, M.; Li, F.; Iseki, E.; Kosaka, K. Immunohistochemical investigation of tau-positive structures in the cerebral cortex of patients with progressive supranuclear palsy. Neurosci. Lett. 1995, 201, 123–126. [Google Scholar] [CrossRef]
- Feany, M.B.; Dickson, D.W. Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am. J. Pathol. 1995, 146, 1388–1396. [Google Scholar] [PubMed]
- Ikeda, K.; Akiyama, H.; Arai, T.; Nishimura, T. Glial tau pathology in neurodegenerative diseases: Their nature and comparison with neuronal tangles. Neurobiol. Aging 1998, 19 (Suppl. 1), S85–S91. [Google Scholar] [CrossRef]
- Komori, T.; Arai, N.; Oda, M.; Nakayama, H.; Mori, H.; Yagishita, S.; Takahashi, T.; Amano, N.; Murayama, S.; Murakami, S.; et al. Astrocytic plaques and tufts of abnormal fibers do not coexist in corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. 1998, 96, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol. 1999, 9, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Ikeda, K.; Akiyama, H.; Shikamoto, Y.; Tsuchiya, K.; Yagishita, S.; Beach, T.; Rogers, J.; Schwab, C.; McGeer, P.L. Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick’s disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. 2001, 101, 167–173. [Google Scholar] [PubMed]
- Arai, T.; Ikeda, K.; Akiyama, H.; Tsuchiya, K.; Yagishita, S.; Takamatsu, J. Intracellular processing of aggregated tau differs between corticobasal degeneration and progressive supranuclear palsy. Neuroreport 2001, 12, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Hashizume, Y.; Yoshida, M.; Iwasaki, Y.; Hishikawa, N.; Ueda, R.; Ojika, K. Distribution of astrocytic plaques in the corticobasal degeneration brain and comparison with tuft-shaped astrocytes in the progressive supranuclear palsy brain. Acta Neuropathol. 2003, 106, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Yoshida, M.; Hattori, M.; Goto, A.; Aiba, I.; Hashizume, Y.; Sobue, G. Distribution of tuft-shaped astrocytes in the cerebral cortex in progressive supranuclear palsy. Acta Neuropathol. 2004, 108, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Arima, K. Ultrastructural characteristics of tau filaments in tauopathies: Immuno-electron microscopic demonstration of tau filaments in tauopathies. Neuropathology 2006, 26, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Mimuro, M.; Yoshida, M.; Miyao, S.; Harada, T.; Ishiguro, K.; Hashizume, Y. Neuronal and glial tau pathology in early frontotemporal lobar degeneration-tau, Pick’s disease subtype. J. Neurol. Sci. 2010, 290, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M. Astrocytic inclusions in progressive supranuclear palsy and corticobasal degeneration. Neuropathology 2014, 34, 555–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghetti, B.; Oblak, A.L.; Boeve, B.F.; Johnson, K.A.; Dickerson, B.C.; Goedert, M. Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: A chameleon for neuropathology and neuroimaging. Neuropathol. Appl. Neurobiol. 2015, 41, 24–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, K.; Akiyama, H.; Kondo, H.; Haga, C.; Tanno, E.; Tokuda, T.; Ikeda, S. Thorn-shaped astrocytes: Possibly secondarily induced tau-positive glial fibrillary tangles. Acta Neuropathol. 1995, 90, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.; Ghebremedhin, E.; Del Tredici, K.; Rüb, U.; Braak, H. High prevalence of thorn-shaped astrocytes in the aged human medial temporal lobe. Neurobiol. Aging 2004, 25, 397–405. [Google Scholar] [CrossRef]
- Muñoz, D.G.; Woulfe, J.; Kertesz, A. Argyrophilic thorny astrocyte clusters in association with Alzheimer’s disease pathology in possible primary progressive aphasia. Acta Neuropathol. 2007, 114, 347–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, G.G.; Molnár, K.; László, L.; Ströbel, T.; Botond, G.; Hönigschnabl, S.; Reiner-Concin, A.; Palkovits, M.; Fischer, P.; Budka, H. A peculiar constellation of tau pathology defines a subset of dementia in the elderly. Acta Neuropathol. 2011, 122, 205–222. [Google Scholar] [CrossRef] [PubMed]
- López-González, I.; Carmona, M.; Blanco, R.; Luna-Muñoz, J.; Martínez-Mandonado, A.; Mena, R.; Ferrer, I. Characterization of thorn-shaped astrocytes in white matter of temporal lobe in Alzheimer’s disease brains. Brain Pathol. 2013, 23, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.K.; Goldfinger, M.H.; Questari, H.E.; Pearce, R.K.; Gentleman, S.M. ARTAG in the basal forebrain: Widening the constellation of astrocytic tau pathology. Acta Neuropathol. Commun. 2016, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Lingh, H.; Neal, J.W.; Revesz, T. Evolving concepts of chronic traumatic encephalopathy as a neuropathklogical entity. Neuropathol. Appl. Neurobiol. 2017, 43, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Santpere, G.; Ferrer, I. Delineation of early changes in cases with progressive supranuclear palsy-like pathology. Astrocytes in striatum are primary targets of tau phosphorylation and GFAP oxidation. Brain Pathol. 2009, 19, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Kovacs, G.G.; Vonsattel, J.P.; Davey, K.; Mok, K.Y.; Hardy, J.; Morris, H.R.; Warner, T.T.; Holton, J.L.; Revesz, T. Astrogliopathy predominates the earliest stage of corticobasal degeneration pathology. Brain 2016, 139, 3237–3252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Maldonado, A.; Luna-Munoz, J.; Ferrer, I. Incidental corticobasal degeneration. Neuropathol. Appl. Neurobiol. 2016, 42, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Tabira, T.; Poorkaj, P.; Schellenberg, G.D.; Trojanowski, J.Q.; Lee, V.M.; Schmidt, M.L.; Takahashi, K.; Nabika, T.; Matsumoto, T.; et al. A distinct familial presenile dementia with a novel missense mutation in the tau gene. Neuroreport 1999, 10, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Stanford, P.M.; Halliday, G.M.; Brooks, W.S.; Kwok, J.B.; Storey, C.E.; Creasey, H.; Morris, J.G.; Fulham, M.J.; Schofield, P.R. Progressive supranuclear palsy pathology caused by a novel silent mutation in exon 10 of the tau gene: Expansion of the disease phenotype caused by tau gene mutations. Brain 2000, 123, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Toyoshima, Y.; Hasegawa, M.; Umeda, Y.; Wakabayashi, K.; Tokiguchi, S.; Iwatsubo, T.; Takahashi, H. Late-onset frontotemporal dementia with a novel exon 1 (Arg5His) tau gene mutation. Ann. Neurol. 2002, 51, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Poorkaj, P.; Muma, N.A.; Zhukareva, V.; Cochran, E.J.; Shannon, K.M.; Hurtig, H.; Koller, W.C.; Bird, T.D.; Trojanowski, J.Q.; Lee, V.M.; et al. An R5L tau mutation in a subject with a progressive supranuclear palsy phenotype. Ann. Neurol. 2002, 52, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Ota, S.; Tanaka, K.; Ito, Y.; Hasegawa, M.; Umeda, Y.; Motoi, Y.; Takanashi, M.; Yasuhara, M.; Anno, M.; et al. A novel L266V mutation of the tau gene causes frontotemporal dementia with a unique tau pathology. Ann. Neurol. 2003, 53, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Pastor, P.; Rey, M.J.; Muñoz, E.; Puig, B.; Pastor, E.; Oliva, R.; Tolosa, E. Tau phosphorylation and kinase activation in familial tauopathy linked to deln296 mutation. Neuropathol. Appl. Neurobiol. 2003, 29, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Van Herpen, E.; Rosso, S.M.; Serverijnen, L.A.; Yoshida, H.; Breedveld, G.; van de Graaf, R.; Kamphorst, W.; Ravid, R.; Willemsen, R.; Dooijes, D.; et al. Variable phenotypic expression and extensive tau pathology in two families with the novel tau mutation L315R. Ann. Neurol. 2003, 54, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M.; Song, Y.J.; Creasey, H.; Morris, J.G.; Brooks, W.S.; Kril, J.J. Neuropathology in the S305S tau gene mutation. Brain 2006, 129, E40. [Google Scholar] [CrossRef] [PubMed]
- Ros, R.; Thobois, S.; Streichenberger, N.; Kopp, N.; Sanchez, M.P.; Perez, M.; Hoenicka, J.; Avila, J.; Honnorat, J.; de Yébenes, J.G. A new mutation of the tau gene, G303V, in early-onset familial progressive supranuclear palsy. Arch. Neurol. 2005, 62, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Malkani, R.; D’Souza, I.; Gwinn-Hardy, K.; Schellenberg, G.D.; Hardy, J.; Momeni, P. A MAPT mutation in a regulatory element upstream of exon 10 causes frontotemporal dementia. Neurobiol. Dis. 2006, 22, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Legati, A.; García-Monco, J.C.; Gomez-Beldarrain, M.; Carmona, M.; Blanco, R.; Seeley, W.W.; Coppola, G. Familial behavioral variant frontotemporal dementia associated with astrocyte-predominant tauopathy. J. Neuropathol. Exp. Neurol. 2015, 74, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Botez, G.; Probst, A.; Ipsen, S.; Tolnay, M. Astrocytes expressing hyperphosphorylated tau protein without glial fibrillary tangles in argyrophilicgrain disease. Acta Neuropathol. 1999, 98, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A.; Cairns, N.J. Spatial patterns of the tau pathology in progressive supranuclear palsy. Neurol. Sci. 2013, 34, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, I.; Kovacs, G.G. Incidental corticobasal degeneration in a 76-year-old woman. Clin. Neuropathol. 2013, 32, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J.; Brettschneider, J.; McMillan, C.T.; Cooper, F.; Olm, C.; Arnold, S.E.; van Deerlin, V.M.; Seeley, W.W.; Miller, B.L.; Lee, E.B.; et al. Deep clinical and neuropathological phenotyping of Pick disease. Ann. Neurol. 2016, 79, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I. Diversity of astroglial responses across human neurodegenerative disorders and brain aging. Brain Pathol. 2017, 27, 645–674. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Yagishita, S.; Nakamura, A.; Uchihara, T. Perivascular orientation of astrocytic plaques and tuft-shaped astrocytes. Brain Res. 2011, 1404, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Ishihara, T.; Zhang, B.; Hong, M.; Andreadis, A.; Trojanowski, J.; Lee, V.M. Transgenic mouse model of tauopathies with glial pathology and nervous system degeneration. Neuron 2002, 35, 433–446. [Google Scholar] [CrossRef]
- Lin, W.L.; Lewis, J.; Yen, S.H.; Hutton, M.; Dickson, D.W. Filamentous tau oligodendrocytes and astrocytes of transgenic mice expressing the human tau isoform with the P301L mutation. Am. J. Pathol. 2003, 162, 213–218. [Google Scholar] [CrossRef]
- Atzori, C.; Ghetti, B.; Piva, R.; Srinivasan, A.N.; Zolo, P.; Delisle, M.B.; Mirra, S.S.; Migheli, A. Activation of the JNK/p38 pathway occurs in diseases characterized by tau protein pathology and is related to tau phosphorylation but not to apoptosis. J. Neuropathol. Exp. Neurol. 2001, 60, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Blanco, R.; Carmona, M.; Puig, B. Phosphorylated mitogen-activated protein kinase (MAPK/ERK-P), protein kinase of 38 kDa (p38-P), stress-activated protein kinase (SAPK/JNK-P), and calcium/calmodulin-dependent kinase II (CaM kinase II) are differentially expressed in tau deposits in neurons and glial cells in tauopathies. J. Neural Transm. 2001, 108, 1397–1415. [Google Scholar] [PubMed]
- Ferrer, I.; Blanco, R.; Carmona, M.; Ribera, R.; Goutan, E.; Puig, B.; Rey, M.J.; Cardozo, A.; Viñals, F.; Ribalta, T. Phosphorylated map kinase (ERK1, ERK2) expression is associated with early tau deposition in neurones and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer disease, Pick’s disease, progressive supranuclear palsy and corticobasal degeneration. Brain Pathol. 2001, 11, 144–158. [Google Scholar] [PubMed]
- Ferrer, I.; Barrachina, M.; Puig, B. Glycogen synthase kinase-3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer’s disease, Pick’s disease, progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol. 2002, 104, 583–591. [Google Scholar] [PubMed]
- Ferrer, I.; Barrachina, M.; Tolnay, M.; Rey, M.J.; Vidal, N.; Carmona, M.; Blanco, R.; Puig, B. Phosphorylated protein kinases associated with neuronal and glial tau deposits in argyrophilic grain disease. Brain Pathol. 2003, 13, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Ikeda, K.; Akiyama, H.; Arai, T.; Kondo, H.; Okochi, M.; Furiya, Y.; Mori, H.; Oda, T.; Kato, M.; et al. Glial tau-positive structures lack the sequence encoded by exon 3 of the tau protein gene. Neurosci. Lett. 1997, 224, 169–172. [Google Scholar] [CrossRef]
- Irwin, D.J.; Cohen, T.J.; Grossman, M.; Arnold, S.E.; McCarty-Wood, E.; Van Deerlin, V.M.; Lee, V.M.; Trojanowski, J.Q. Acetylated tau neuropathology in sporadic and hereditary tauopathies. Am. J. Pathol. 2013, 183, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, L.T.; Wang, X.; Wang, C.; Sohn, P.D.; Theofilas, P.; Sidhu, M.; Arevalo, J.B.; Heinsen, H.; Huang, E.J.; Rosen, H.; et al. Argyrophilic grain disease differs from other tauopathies by lacking tau acetylation. Acta Neuropathol. 2013, 125, 581–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, S.W.; Cho, S.H.; Zhou, Y.; Schroeder, S.; Haroutunian, V.; Seeley, W.W.; Huang, E.J.; Shen, Y.; Masliah, E.; Mukherjee, C.; et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 2010, 67, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.J.; Guo, J.L.; Hurtado, D.E.; Kwong, L.K.; Mills, I.P.; Trojanowski, J.Q.; Lee, V.M. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2011, 2, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer, I.; Aguiló García, M.; López González, I.; Diaz Lucena, D.; Roig Villalonga, A.; Carmona, M.; Llorens, F.; Garcia-Esparcia, P.; Martinez-Maldonado, A.; Frau Mendez, M.; et al. Aging-related tau astrogliopathy (ARTAG): Not only tau phosphorylation in astrocytes. Brain Pathol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kimmelberg, H.K. The problem of astrocyte identity. Neurochem. Int. 2004, 45, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Avci, H.X.; Leist, M.; Kobolák, J.; Dinnyés, A. Astrocyte differentiation of human pluripotent stem cells: New tolos for neurological disorder research. Front. Cell. Neurosci. 2016, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Goldman, S.A.; Nedergaard, M. Heterogeneity of astrocytic form and function. Methods Mol. Biol. 2012, 814, 23–45. [Google Scholar] [PubMed]
- Song, Y.J.; Halliday, G.M.; Holton, J.L.; Lashley, T.; O’Sullivan, S.S.; McCann, H.; Lees, A.J.; Ozawa, T.; Williams, D.R.; Lockhart, P.J.; et al. Degeneration in different parkinsonian syndromes relates to astrocyte type and astrocyte protein expression. J. Neuropathol. Exp. Neurol. 2009, 68, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Nilsson, M. Astrocyte activation and reactive gliosis. Glia 2005, 50, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.; Schilling, K.; Steinhauser, C. Astrocyte dysfunction in neurological disorders: A molecular perspective. Nat. Rev. Neurosci. 2006, 7, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Rodríguez, J.J.; Parpura, V. Astroglia in neurological diseases. Future Neurol. 2013, 8, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkhratsky, A.; Rodríguez, J.J.; Parpura, V. Neuroglia in ageing and disease. Cell Tissue Res. 2014, 357, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Zorec, R.; Rodrıguez, J.J.; Parpura, V. Astroglia dynamics in ageing and Alzheimer’s disease. Curr. Opin. Pharmacol. 2016, 26, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M. Astrocyte reactivity and reactive astrogliosis: Costs and benefits. Physiol. Rev. 2014, 94, 1077–1098. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim. Biophys. Acta 2016, 1862, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Zorec, R.; Parpura, V. Stratification of astrocytes in healthy and diseased brain. Brain Pathol. 2017, 27, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Zorec, R.; Rodriguez, J.J.; Parpura, V. Neuroglia: Functional paralysis and reactivity in Alzheimer’s disease and other neurodegenerative pathologies. Adv. Neurobiol. 2017, 15, 427–449. [Google Scholar] [PubMed]
- Osborn, L.M.; Kamphuis, W.; Wadman, W.J.; Hol, E.M. Astrogliosis: An integral player in the pathogenesis of Alzheimer’s disease. Prog. Neurobiol. 2016, 144, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M.; Messing, A.; Steinhäuser, C.; Lee, J.M.; Parpura, V.; Hol, E.M.; Sofroniew, M.V.; Verkhratsky, A. Astrocytes: A central element in neurological diseases. Acta Neuropathol. 2016, 131, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Kersaitis, C.; Halliday, G.M.; Kril, J.J. Regional and cellular pathology in frontotemporal dementia: Relationship to stage of disease in cases with and without Pick bodies. Acta Neuropathol. 2004, 108, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Togo, T.; Dickson, D.W. Tau accumulation in astrocytes in progressive supranuclear palsy is a degenerative rather than a reactive process. Acta Neuropathol. 2002, 104, 398–402. [Google Scholar] [PubMed]
- López-González, I.; Aso, E.; Carmona, M.; Armand-Ugon, M.; Blanco, R.; Naudí, A.; Cabré, R.; Portero-Otin, M.; Pamplona, R.; Ferrer, I. Neuroinflammatory gene regulation, mitochondrial function, oxidative stress, and brain lipid modifications with disease progression in Tau P301S transgenic mice as a model of frontotemporal lobar degeneration-Tau. J. Neuropathol. Exp. Neurol. 2015, 74, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Renkawek, K.; Bosman, G.J.; de Jong, W.W. Expression of small heat-shock protein Hsp27 in reactive gliosis in Alzheimer disease and other types of dementia. Acta Neuropathol. 1994, 87, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Dabir, D.V.; Trojanowski, J.Q.; Richter-Landsberg, C.; Lee, V.M.; Forman, M.S. Expression of the small heat-shock protein αB-crystallin in tauopathies with glial pathology. Am. J. Pathol. 2004, 164, 155–166. [Google Scholar] [CrossRef]
- Schwarz, L.; Vollmer, G.; Richter-Landsberg, C. The small heat shock protein HSP25/27 (HspB1) is abundant in cultured astrocytes and associated with astrocytic pathology in progressive supranuclear palsy and corticobasal degeneration. Int. J. Cell Biol. 2010, 2010, 717520. [Google Scholar] [CrossRef] [PubMed]
- López-González, I.; Carmona, M.; Arregui, L.; Kovacs, G.G.; Ferrer, I. αB-crystallin and HSP27 in glial cells in tauopathies. Neuropathology 2014, 34, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Filipcik, P.; Cente, M.; Zilka, N.; Smolek, T.; Hanes, J.; Kucerak, J.; Opattova, A.; Kovacech, B.; Novak, M. Intraneuronal accumulation of misfolded tau protein induces overexpression of Hsp27 in activated astrocytes. Biochim. Biophys. Acta 2015, 1852, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Kahlson, M.A.; Colodner, K.J. Glial tau pathology in tauopathies: Functional consequences. J. Exp. Neurosci. 2016, 9, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Nedergaard, M. Physiology of astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Orre, M.; Kamphuis, W.; Osborn, L.M.; Melief, J.; Kooijman, L.; Huitinga, I.; Klooster, J.; Bossers, K.; Hol, E.M. Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol. Aging 2014, 35, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Darmanis, S.; Sloan, S.A.; Zhang, Y.; Enge, M.; Caneda, C.; Shuer, L.M.; Gephart, M.G.H.; Barres, B.A.; Quake, S.R. A survey of human brain transcriptome diversity at the single cell level. PNAS 2015, 112, 7285–7290. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, A.; Muñoz-Manchado, A.B.; Codeluppi, S.; Lönnerberg, P.; La Manno, G.; Juréus, A.; Marques, S.; Munguba, H.; He, L.; Betsholtz, C.; et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 2015, 347, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.; Li, G.; et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences in mouse. Neuron 2016, 89, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.J.; Yu, K.; Hatcher, A.; Huang, T.W.; Lee, H.K.; Carlson, J.; Weston, M.C.; Chen, F.; Zhang, Y.; Zhu, W.; et al. Identification of diverse astrocyte populations and their malignant analogs. Nat. Neurosci. 2017, 20, 396–405. [Google Scholar] [Green Version]
- Habib, N.; Avraham-Davidi, I.; Basu, A.; Burks, T.; Shekhar, K.; Hofree, M.; Choudhury, S.R.; Aguet, F.; Gelfand, E.; Ardlie, K.; et al. Massively parallel single-nucleus RNA-seq with Dronc-seq. Nat. Methods 2017, 14, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Spaethling, J.M.; Na, Y.-J.; Lee, J.; Ulyanova, A.V.; Baltuch, G.H.; Bell, T.J.; Brem, S.; Chen, H.I.; Dueck, H.; Fisher, S.A.; et al. Primary cell culture of live neurosurgically-resected aged adult human brain cells and single cell transcriptomics. Cell Rep. 2017, 18, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.E.; Ince, P.G.; Shaw, P.J.; Heath, P.R.; Raman, R.; Garwood, C.J.; Gelsthorpe, C.; Baxter, L.; Forster, G.; Matthews, F.E.; et al. Microarray analysis of the astrocyte transcriptome in the aging brain: Relationship to Alzheimer’s pathology and APOE genotype. Neurobiol. Aging 2011, 32, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Orre, M.; Kamphuis, W.; Osborn, L.M.; Jansen, A.H.; Kooijman, L.; Bossers, K.; Hol, E.M. Isolation of glia from Alzheimer’s mice reveals inflammation and dysfunction. Neurobiol. Aging 2014, 35, 2746–2760. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, Y.; Zhang, B.; Bruce, J.; Trojanowski, J.Q.; Lee, V.M. Reduction of detyrosinated microtubules and Golgi fragmentation are linked to tau-induced degeneration in astrocytes. J. Neurosci. 2003, 23, 10662–10671. [Google Scholar] [CrossRef] [PubMed]
- Colodner, K.J.; Feany, M.B. Glial fibrillary tangles and JAK/STAT-mediated glial and neuronal cell death in a Drosophila model of glial tauopathy. J. Neurosci. 2010, 30, 16102–16113. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.D.; Dramiga, J.; Schütz, U.; Kril, J.J.; Ittner, L.M.; Schröder, H.; Götz, J. Tau-mediated nuclear depletion and cytoplasmic accumulation of SFPQ in Alzheimer’s and Pick’s disease. PLoS ONE 2012, 7, E35678. [Google Scholar] [CrossRef]
- Frost, B.; Hemberg, M.; Lewis, J.; Feany, M.B. Tau promotes neurodegeneration through global chromatin relaxation. Nat. Neurosci. 2014, 17, 357–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Ortega, K.; Garcia-Esparcia, P.; Gil, L.; Lucas, J.J.; Ferrer, I. Altered machinery of protein synthesis in Alzheimer’s: From the nucleolus to the ribosome. Brain Pathol. 2016, 26, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Forman, M.S.; Lal, D.; Zhang, B.; Dabir, D.V.; Swanson, E.; Lee, V.M.; Trojanowski, J.Q. Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J. Neurosci. 2005, 25, 3539–3550. [Google Scholar] [CrossRef] [PubMed]
- Dabir, D.V.; Robinson, M.B.; Swanson, E.; Zhang, B.; Trojanowski, J.Q.; Lee, V.M.; Forman, M.S. Impaired glutamate transport in a mouse model of tau pathology in astrocytes. J. Neurosci. 2006, 26, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, R.; Li Puma, D.D.; Mainardi, M.; Lazzarino, G.; Tavazzi, B.; Arancio, O.; Grassi, C. Reduced gliotransmitter release from astrocytes mediates tau-induced synaptic dysfunction in cultured hippocampal neurons. Glia 2017, 65, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, A.L.; Araúzo-Bravo, M.J.; Mavrommatis, L.; Ehrlich, M.; Röpke, A.; Brockhaus, J.; Missler, M.; Sterneckert, J.; Schöler, H.R.; Kuhlmann, T.; et al. Astrocyte pathology in a human neural stem cell model of frontotemporal dementia caused by mutant tau protein. Sci. Rep. 2017, 7, 42991. [Google Scholar] [CrossRef] [PubMed]
- Sidoryk-Wegrzynowicz, M.; Gerber, Y.N.; Ries, M.; Sastre, M.; Tolkovsky, A.M.; Spillantini, M.G. Astrocytes in mouse models of tauopathies acquire early deficits and lose neurosupportive functions. Acta Neuropathol. Commun. 2017, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Temporal sequence of Alzheimer’s disease-related pathology. In Cerebral Cortex Vol. 14, Neurodegenerative and Age-Related Changes in Structure and Function of Cerebral Cortex; Peters, A., Morrison, J.H., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 1999; pp. 475–512. [Google Scholar]
- Duyckaerts, C.; Braak, H.; Brion, J.P.; Buée, L.; Del Tredici, K.; Goedert, M.; Halliday, G.; Neumann, M.; Spillantini, M.G.; Tolnay, M.; et al. PART is part of Alzheimer disease. Acta Neuropathol. 2015, 129, 749–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giaccone, G. The existence of primary age-related tauopathy suggests that not all the cases with early Braak stages of neurofibrillary pathology are Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 48, 919–921. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I. Defining Alzheimer as a common age-related neurodegenerative process not inevitably leading to dementia. Prog. Neurobiol. 2012, 97, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 2015, 138, 2814–2833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Ruberu, N.N.; Sawabe, M.; Arai, T.; Tanaka, N.; Kakuta, Y.; Yamanouchi, H.; Murayama, S. Staging of argyrophilic grains: An age-associated tauopathy. J. Neuropathol. Exp. Neurol. 2004, 63, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Nogami, A.; Yamazaki, M.; Saito, Y.; Hatsuta, H.; Sakiyama, Y.; Takao, M.; Kimura, K.; Murayama, S. Early stage of progressive supranuclear palsy: A neuropathological study of 324 consecutive autopsy cases. J. Nippon Med. Sch. 2015, 82, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Hua, A.Y.; Trujllo, A.; Attygalle, S.; Binney, R.J.; Spina, S.; Lee, S.E.; Kramer, J.H.; Miller, B.L.; Rosen, H.J.; et al. Advancing functional disconnectivity and atrophy in progressive supranuclear palsy. Neuroimage Clin. 2017, 16, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.; Dickson, D.W. Propagation of tau pathology: Hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 2016, 131, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, S.; Lecolle, K.; Caillierez, R.; Begard, S.; Zommer, N.; Lachaud, C.; Carrier, S.; Dufour, N.; Aurégan, G.; Winderickx, J.; et al. Neuron-to-neuron wild-type tau protein transfer through a trans-synaptic mechanism: Relevance to sporadic tauopathies. Acta Neuropathol. Commun. 2014, 2, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calafate, S.; Buist, A.; Miskiewicz, K.; Vijayan, V.; Daneels, G.; de Strooper, B.; de Wit, J.; Verstreken, P.; Moechars, D. Synaptic contacts enhance cell-to-cell tau pathology propagation. Cell Rep. 2015, 11, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Balaji, V.; Kaniyappan, S.; Krüger, L.; Irsen, S.; Tepper, K.; Chandupatla, R.; Maetzler, W.; Schneider, A.; Mandelkow, E.; et al. The release and trans-synaptic transmission of Tau via exosomes. Mol. Neurodegener. 2017, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Polanco, J.C.; Scicluna, B.J.; Hill, A.F.; Gotz, J. Extracellular vesicles isolated from the brains of rTg4510 mice seed tau protein aggregation in a threshold-dependent manner. J. Biol. Chem. 2016, 291, 12445–12466. [Google Scholar] [CrossRef] [PubMed]
- Polanco, J.C.; Li, C.; Durisic, N.; Sullivan, R.; Götz, J. Exosomes taken up by neurons hijack the endosomal pathway to spread to interconnected neurons. Acta Neuropathol. Commun. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardivel, M.; Begard, S.; Bousset, L.; Dujardin, S.; Coens, A.; Melki, R.; Buée, L.; Colin, M. Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological tau protein assemblies. Acta Neuropathol. Commun. 2016, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.B.; Diamond, M.I. Prion-like properties of tau protein: The importance of extracellular tau as a therapeutic target. J. Biol. Chem. 2014, 289, 19855–19861. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Lee, V.M. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat. Med. 2014, 20, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Victoria, G.S.; Arkhipenko, A.; Zhu, S.; Syan, S.; Zurzolo, C. Astrocyte-to-neuron intercellular prion transfer is mediated by cell-cell contact. Sci. Rep. 2016, 6, 20762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clavaguera, F.; Bolmont, T.; Crowther, R.A.; Abramowski, D.; Frank, S.; Probst, A.; Fraser, G.; Stalder, A.K.; Beibel, M.; Staufenbiel, M.; et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009, 11, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Clavaguera, F.; Hench, J.; Goedert, M.; Tolnay, M. Invited review: Prion-like transmission and spreading of tau pathology. Neuropathol. Appl. Neurobiol. 2015, 41, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Boluda, S.; Iba, M.; Zhang, B.; Raible, K.M.; Lee, V.M.; Trojanowski, J.Q. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol. 2015, 129, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Clavaguera, F.; Akatsu, H.; Fraser, G.; Crowther, R.A.; Frank, S.; Hench, J.; Probst, A.; Winkler, D.T.; Reichwald, J.; Staufenbiel, M.; et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl. Acad. Sci. USA 2013, 110, 9535–9540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clavaguera, F.; Lavenir, I.; Falcon, B.; Frank, S.; Goedert, M.; Tolnay, M. “Prion-like” templated misfolding in tauopathies. Brain Pathol. 2013, 23, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, S.; Guo, J.L.; Changolkar, L.; Stieber, A.; McBride, J.D.; Silva, L.V.; He, Z.; Zhang, B.; Gathagan, R.J.; Trojanowski, J.Q.; et al. Pathological tau strains from human brains recapitulate the diversity of tauopathies in non-transgenic mouse brain. J. Neurosci. 2017, 37, 11406–11423. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Narasimhan, S.; Changolkar, L.; He, Z.; Stieber, A.; Zhang, B.; Gathagan, R.J.; Iba, M.; McBride, J.D.; Trojanowski, J.Q.; et al. Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J. Exp. Med. 2016, 213, 2635–2654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, S.K.; Sanders, D.W.; Thomas, T.L.; Ruchinskas, A.J.; Vaquer-Alicea, J.; Sharma, A.M.; Miller, T.M.; Diamond, M.I. Tau prion strains dictate patterns of cell pathology, progression rate, and regional vulnerability in vivo. Neuron 2016, 92, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Wharton, S.B.; Minett, T.; Drew, D.; Forster, G.; Matthews, F.; Brayne, C.; Ince, P.G.; MRC Cognitive Function and Ageing Neuropathology Study Group. Epidemiological pathology of tau in the ageing brain: Application of staging for neuropil threads (BrainNet Europe protocol) to the MRC cognitive function and ageing brain study. Acta Neuropathol. Commun. 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Cowan, N.; Kirschner, M. The primary structure and heterogeneity of tau protein from mouse brain. Science 1988, 239, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, F.; Sultana, R.; Barone, E.; Perluigi, M.; Cini, C.; Mancuso, C.; Cai, J.; Pierce, W.M.; Butterfield, D.A. Quantitative proteomics analysis of phosphorylated proteins in the hippocampus of Alzheimer’s disease subjects. J. Proteom. 2011, 74, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Zahid, S.; Oellerich, M.; Asif, A.R.; Ahmed, N. Phosphoproteome profiling of substantia nigra and cortex regions of Alzheimer’s disease patients. J. Neurochem. 2012, 121, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Triplett, J.C.; Swomley, A.M.; Cai, J.; Klein, J.B.; Butterfield, D.A. Quantitative phosphoproteomic analyses of the inferior parietal lobule from three different pathological stages of Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 49, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Muntané, G.; Dalfó, E.; Martínez, A.; Rey, M.J.; Avila, J.; Pérez, M.; Portero, M.; Pamplona, R.; Ayala, V.; Ferrer, I. Glial fibrillary acidic protein is a major target of glycoxidative and lipoxidative damage in Pick’s disease. J. Neurochem. 2006, 99, 177–185. [Google Scholar]
- Martínez, A.; Portero-Otin, M.; Pamplona, R.; Ferrer, I. Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol. 2010, 20, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, K.; Homma, H.; Saito, A.; Fujita, K.; Chen, X.; Imoto, S.; Oka, T.; Ito, H.; Motoki, K.; Yoshida, C.; et al. Comprehensive phosphoproteome analysis unravels the core signaling network that initiates the earliest synapse pathology in preclinical Alzheimer’s disease brain. Hum. Mol. Genet. 2015, 24, 540–558. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer, I. Astrogliopathy in Tauopathies. Neuroglia 2018, 1, 126-150. https://doi.org/10.3390/neuroglia1010010
Ferrer I. Astrogliopathy in Tauopathies. Neuroglia. 2018; 1(1):126-150. https://doi.org/10.3390/neuroglia1010010
Chicago/Turabian StyleFerrer, Isidro. 2018. "Astrogliopathy in Tauopathies" Neuroglia 1, no. 1: 126-150. https://doi.org/10.3390/neuroglia1010010
APA StyleFerrer, I. (2018). Astrogliopathy in Tauopathies. Neuroglia, 1(1), 126-150. https://doi.org/10.3390/neuroglia1010010




