Acute Whole-Body Vibration Does Not Alter Passive Muscle Stiffness in Physically Active Males
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Experimental Protocol
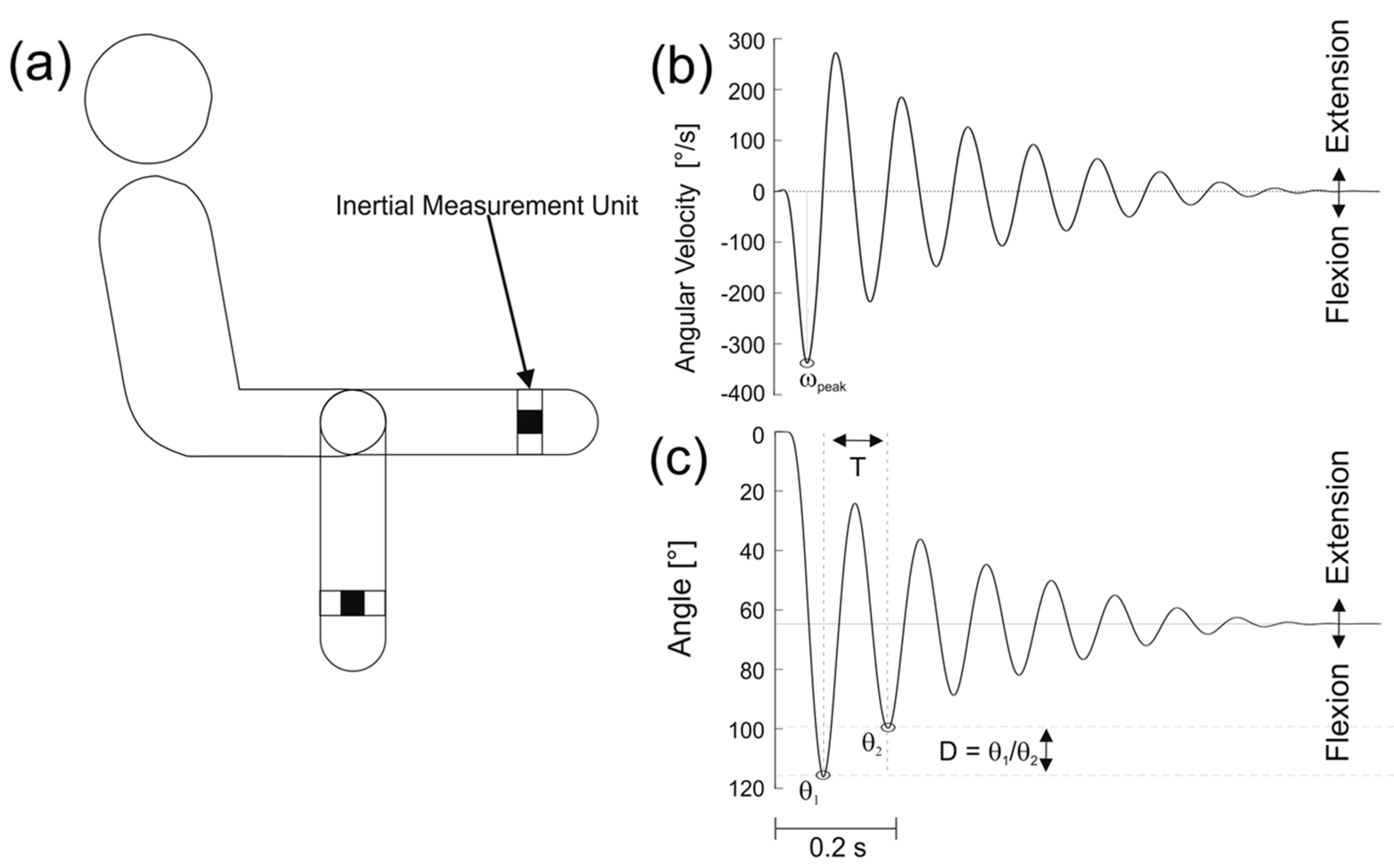
2.4. Data Analysis
2.4.1. Wartenberg Pendulum Test
2.4.2. Countermovement Jump
2.5. Statistical Analysis
3. Results
3.1. Wartenberg Pendulum Test
3.2. Countermovement Jump
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cardinale, M.; Wakeling, J. Whole Body Vibration Exercise: Are Vibrations Good for You? Br. J. Sport. Med. 2005, 39, 585–589; discussion 589. [Google Scholar] [CrossRef] [PubMed]
- Rittweger, J. Vibration as an Exercise Modality: How It May Work, and What Its Potential Might Be. Eur. J. Appl. Physiol. 2010, 108, 877–904. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.J. The Potential Neural Mechanisms of Acute Indirect Vibration. J. Sport. Sci. Med. 2011, 10, 19–30. [Google Scholar]
- Manimmanakorn, N.; Hamlin, M.J.; Ross, J.J.; Manimmanakorn, A. Long-Term Effect of Whole Body Vibration Training on Jump Height: Meta-Analysis. J. Strength Cond. Res. 2014, 28, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Edwards, D.; Serravite, D.H.; Bedient, A.M.; Huntsman, E.; Jacobs, K.A.; Del Rossi, G.; Roos, B.A.; Signorile, J.F. Optimal Frequency, Displacement, Duration, and Recovery Patterns to Maximize Power Output Following Acute Whole-Body Vibration. J. Strength Cond. Res. 2009, 23, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Bosco, C. The Use of Vibration as an Exercise Intervention. Exerc. Sport Sci. Rev. 2003, 31, 3–7. [Google Scholar] [CrossRef]
- Wu, C.-C.; Wang, M.-H.; Chang, C.-Y.; Hung, M.-H.; Wang, H.-H.; Chen, K.-C.; Ger, T.-R.; Lin, K.-C. The Acute Effects of Whole Body Vibration Stimulus Warm-up on Skill-Related Physical Capabilities in Volleyball Players. Sci. Rep. 2021, 11, 5606. [Google Scholar] [CrossRef] [PubMed]
- Cormie, P.; Deane, R.S.; Triplett, N.T.; McBride, J.M. Acute Effects of Whole-Body Vibration on Muscle Activity, Strength, and Power. J. Strength Cond. Res. 2006, 20, 257–261. [Google Scholar] [CrossRef]
- Greco, F.; Quinzi, F.; Folino, K.; Spadafora, M.; Cosco, L.F.; Tarsitano, M.G.; Emerenziani, G.P. Acute Effects of Whole-Body Vibration on Quadriceps Isometric Muscular Endurance in Middle-Aged Adults: A Pilot Study. Vibration 2023, 6, 399–406. [Google Scholar] [CrossRef]
- Fowler, B.D.; Palombo, K.T.M.; Feland, J.B.; Blotter, J.D. Effects of Whole-Body Vibration on Flexibility and Stiffness: A Literature Review. Int. J. Exerc. Sci. 2019, 12, 735–747. [Google Scholar]
- Valle, M.S.; Casabona, A.; Sgarlata, R.; Garozzo, R.; Vinci, M.; Cioni, M. The Pendulum Test as a Tool to Evaluate Passive Knee Stiffness and Viscosity of Patients with Rheumatoid Arthritis. BMC Musculoskelet. Disord. 2006, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Başol, F.; Kara, İ.; Saldıran, T.Ç. The Effects of Vibration Exposure on Lower-Limb Extensor Muscles’ Stiffness, Elasticity, and Strength Responses in Untrained Young Individuals: A Randomized Controlled Trial. J. Sport Rehabil. 2023, 32, 415–423. [Google Scholar] [CrossRef]
- Kipp, K.; Kim, H.; Wolf, W.I. Muscle Forces During the Squat, Split Squat, and Step-Up Across a Range of External Loads in College-Aged Men. J. Strength Cond. Res. 2022, 36, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.; Bull, F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J. Public Health 2006, 14, 66–70. [Google Scholar] [CrossRef]
- Fox, S.; Naughton, J.; Haskell, W. Physical Activity and the Prevention of Coronary Heart Disease. Ann. Clin. Res. 1971, 3, 404–432. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.J.; Goss, F.L.; Dube, J.; Rutkowski, J.; Dupain, M.; Brennan, C.; Andreacci, J. Validation of the Adult OMNI Scale of Perceived Exertion for Cycle Ergometer Exercise. Med. Sci. Sport. Exerc. 2004, 36, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Wartenberg, R. Pendulousness of the Legs as a Diagnostic Test. Neurology 1951, 1, 18–24. [Google Scholar] [CrossRef] [PubMed]
- González-Badillo, J.J.; Marques, M.C. Relationship between Kinematic Factors and Countermovement Jump Height in Trained Track and Field Athletes. J. Strength Cond. Res. 2010, 24, 3443–3447. [Google Scholar] [CrossRef]
- Casabona, A.; Valle, M.S.; Pisasale, M.; Pantò, M.R.; Cioni, M. Functional Assessments of the Knee Joint Biomechanics by Using Pendulum Test in Adults with Down Syndrome. J. Appl. Physiol. 2012, 113, 1747–1755. [Google Scholar] [CrossRef]
- De Leva, P. Adjustments to Zatsiorsky-Seluyanov’s Segment Inertia Parameters. J. Biomech. 1996, 29, 1223–1230. [Google Scholar] [CrossRef]
- Claudino, J.G.; Cronin, J.; Mezêncio, B.; McMaster, D.T.; McGuigan, M.; Tricoli, V.; Amadio, A.C.; Serrão, J.C. The Countermovement Jump to Monitor Neuromuscular Status: A Meta-Analysis. J. Sci. Med. Sport 2017, 20, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.J.; Stannard, S.R. Acute Whole Body Vibration Training Increases Vertical Jump and Flexibility Performance in Elite Female Field Hockey Players. Br. J. Sport. Med. 2005, 39, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.; Sanderson, M.F.; Attwood, L.A. The Acute Effect of Different Frequencies of Whole-Body Vibration on Countermovement Jump Performance. J. Strength Cond. Res. 2011, 25, 1592–1597. [Google Scholar] [CrossRef]
- Cronin, J.B.; Oliver, M.; McNair, P.J. Muscle Stiffness and Injury Effects of Whole Body Vibration. Phys. Ther. Sport 2004, 5, 68–74. [Google Scholar] [CrossRef]
- Roschel, H.; Barroso, R.; Tricoli, V.; Batista, M.A.B.; Acquesta, F.M.; Serrão, J.C.; Ugrinowitsch, C. Effects of Strength Training Associated With Whole-Body Vibration Training on Running Economy and Vertical Stiffness. J. Strength Cond. Res. 2015, 29, 2215–2220. [Google Scholar] [CrossRef]
- Colson, S.S.; Petit, P.-D. Lower Limbs Power and Stiffness after Whole-Body Vibration. Int. J. Sport. Med. 2013, 34, 318–323. [Google Scholar] [CrossRef]
- Siu, P.M.; Tam, B.T.; Chow, D.H.; Guo, J.Y.; Huang, Y.P.; Zheng, Y.P.; Wong, S.H. Immediate Effects of 2 Different Whole-Body Vibration Frequencies on Muscle Peak Torque and Stiffness. Arch. Phys. Med. Rehabil. 2010, 91, 1608–1615. [Google Scholar] [CrossRef]

| Participants’ Characteristics | |
|---|---|
| N° | 24 |
| Age [years] | 25.1 ± 3.3 |
| Height [m] | 1.76 ± 0.10 |
| Body Mass [kg] | 74.7 ± 6.7 |
| BMI [kg/m2] | 24.6 ± 3.3 |
| % FM [%] | 17.4 ± 5.1 |
| SMM [kg] | 34.6 ± 2.9 |
| PAL [METs-min/week] | 1693.0 ± 1629.3 |
| Pre | Post | ||||
|---|---|---|---|---|---|
| CC | WBV | CC | WBV | ||
| CMJ | [m] | 0.42 ± 0.05 | 0.43 ± 0.05 | 0.43 ± 0.05 | 0.42 ± 0.05 |
| θPeak | [°] | 104.5 ± 10.1 | 103.5 ± 11.7 | 102.6 ± 12.1 | 103.8 ± 10.6 |
| ωPeak | [°/s] | 299.7 ± 39.5 | 297.7 ± 50.8 | 294.5 ± 44.5 | 296.2 ± 39.0 |
| Period | [s] | 1.022 ± 0.043 | 1.020 ± 0.045 | 1.013 ± 0.038 * | 1.019 ± 0.043 |
| Damping Coef # | [Nm/s/rad] | 0.056 ± 0.019 | 0.053 ± 0.019 | 0.061 ± 0.017 | 0.058 ± 0.019 |
| Stiffness Coef | [Nm/rad] | 7.22 ± 1.37 | 7.20 ± 1.37 | 7.09 ± 1.28 * | 7.18 ± 1.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spadafora, M.; Quinzi, F.; Lia, C.G.; Greco, F.; Folino, K.; Cosco, L.F.; Emerenziani, G.P. Acute Whole-Body Vibration Does Not Alter Passive Muscle Stiffness in Physically Active Males. Vibration 2024, 7, 595-604. https://doi.org/10.3390/vibration7020031
Spadafora M, Quinzi F, Lia CG, Greco F, Folino K, Cosco LF, Emerenziani GP. Acute Whole-Body Vibration Does Not Alter Passive Muscle Stiffness in Physically Active Males. Vibration. 2024; 7(2):595-604. https://doi.org/10.3390/vibration7020031
Chicago/Turabian StyleSpadafora, Marco, Federico Quinzi, Carmen Giulia Lia, Francesca Greco, Katia Folino, Loretta Francesca Cosco, and Gian Pietro Emerenziani. 2024. "Acute Whole-Body Vibration Does Not Alter Passive Muscle Stiffness in Physically Active Males" Vibration 7, no. 2: 595-604. https://doi.org/10.3390/vibration7020031
APA StyleSpadafora, M., Quinzi, F., Lia, C. G., Greco, F., Folino, K., Cosco, L. F., & Emerenziani, G. P. (2024). Acute Whole-Body Vibration Does Not Alter Passive Muscle Stiffness in Physically Active Males. Vibration, 7(2), 595-604. https://doi.org/10.3390/vibration7020031






