Effects of a Single Session of Whole Body Vibration Compared to Multiple Sessions—An Updated Review and Meta-Analysis
Abstract
:1. Background
2. Methods
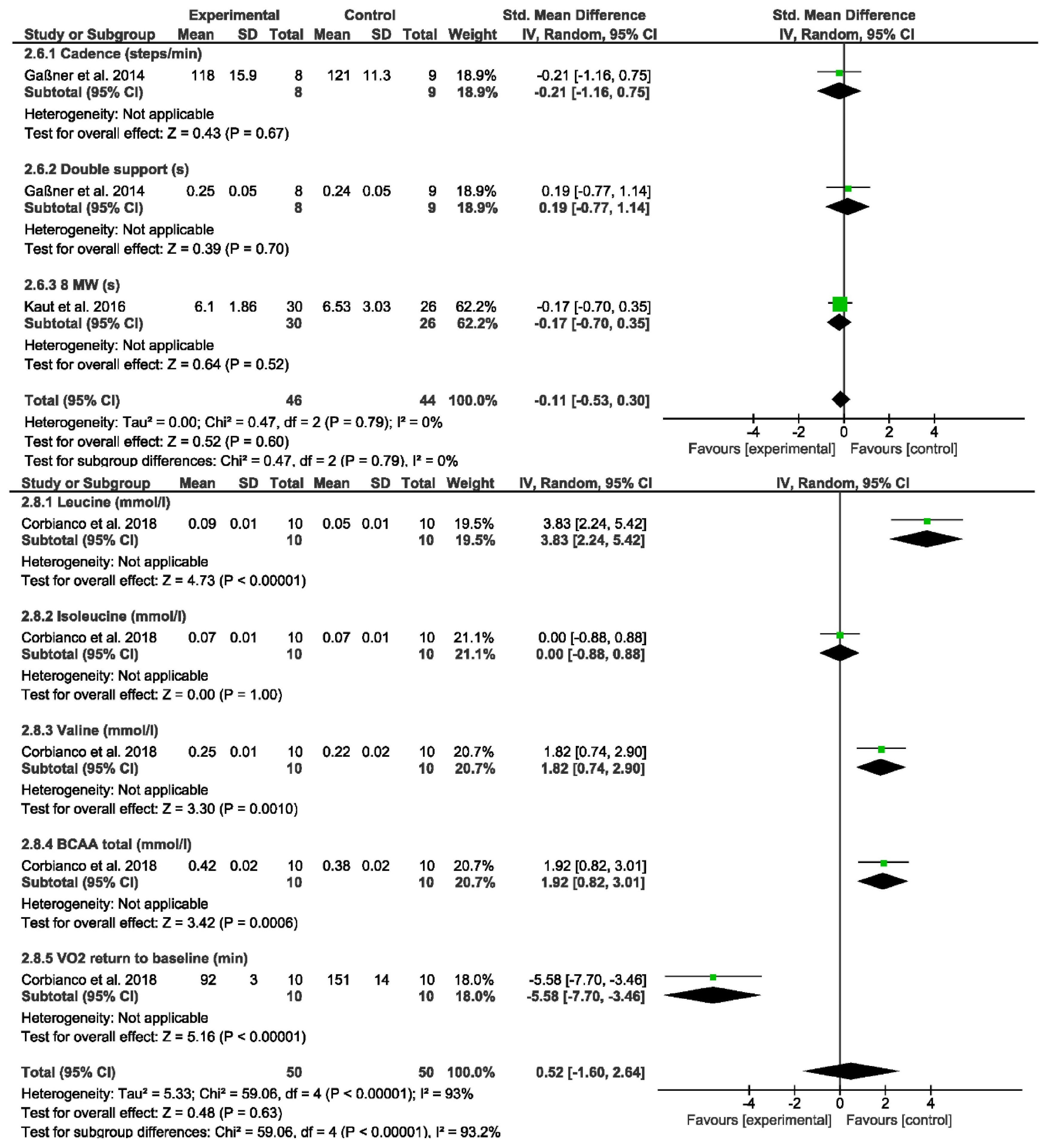
3. Results
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maserejian, N.; Vinikoor-Imler, L.; Dilley, A. Schätzung der Globalen Population der Parkinson-Krankheit (PD) für 2020 [Abstract]. Mov. Disord. 2020, 35 (Suppl. S1). Available online: https://www.mdsabstracts.org/abstract/estimation-of-the-2020-global-population-of-parkinsons-disease-pd/ (accessed on 26 July 2023).
- Trepel, M. (Ed.) Neuroanatomie; Urban & Fischer: München, Germany, 2008. [Google Scholar]
- Kaut, O.; Brenig, D.; Marek, M.; Allert, N.; Wüllner, U. Postural Stability in Parkinson’s Disease Patients Is Improved after Stochastic Resonance Therapy. Park. Dis. 2016, 2016, 7948721. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; He, L.X.; Huang, S.N.; Gong, L.J.; Li, L.; Lv, Y.Y.; Qian, Z.M. Protection of Dopamine Neurons by Vibration Training and Up-Regulation of Brain-Derived Neurotrophic Factor in a MPTP Mouse Model of Parkinson’s Disease. Physiol. Res. 2014, 63, 649–657. [Google Scholar] [CrossRef] [PubMed]
- del Pozo-Cruz, B.; Adsuar, J.C.; Parraca, J.A.; Olivares, P.R.; Herrera, E.; Gusi, N. Whole-body vibration effects in patients affected with Parkinson’s disease: A systematic literature review. Rev. Andal. Med. Deport. 2011, 4, 63–70. [Google Scholar]
- Tomlinson, C.L.; Patel, S.; Meek, C.; Clarke, C.E.; Stowe, R.; Shah, L.; Sackley, C.M.; Deane, K.H.; Herd, C.P.; Wheatley, K.; et al. Physiotherapy versus placebo or no intervention in Parkinson’s disease (Review). Cochrane Database Syst. Rev. 2013, 9, 63. [Google Scholar]
- Swe, M.; Benjamin, B.; Tun, A.A.; Sugathan, S. Role of the whole body vibration machine in the prevention and management of osteoporosis in old age: A systematic review. Malays. J. Med. Sci. 2016, 23, 8–16. [Google Scholar] [CrossRef]
- Cochrane, D.J. Vibration exercise, the potential benefits. Int. J. Sports Med. 2011, 32, 75–99. [Google Scholar] [CrossRef]
- Haas, C.T. Vibrationstraining, biomechanische Stimulation und stochastische Resonanztherapie. Zeitsch Physiother. 2008, 60, 728–740. [Google Scholar]
- Madou, K.H.; Cronin, J.B. The effects of whole body vibration on physical and physiological capability in special populations. Hong Kong Physiother. J. 2008, 26, 24–38. [Google Scholar] [CrossRef]
- De Domenico, G. Tonic vibratory reflex. What is it? Can we use it? Physiotherapy 1979, 65, 44–48. [Google Scholar]
- Arenales Arauz, Y.L.; Ahuja, G.; Kamsma, Y.P.T.; Kortholt, A.; van der Zee, E.A.; van Heuvelen, M.J.G. Potential of Whole-Body Vibration in Parkinson’s Disease: A Systematic Review and Meta-Analysis of Human and Animal Studies. Biology 2022, 11, 1238. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.T.; Turbanski, S.; Kessler, K.; Schmidtbleicher, D. The Effects of Random Whole-Body-Vibration on Motor Symptoms in Parkinson’s Disease. NeuroRehabilitation 2006, 21, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Boecker, H.; Ceballos-Baumann, A.; Bartenstein, P.; Weindl, A.; Siebner, H.R.; Fassbender, T.; Munz, F.; Schwaiger, M.; Conrad, B. Sensory processing in Parkinson’s and Huntington’s disease: Investigations with 3D H215O-PET. Brain 1999, 122, 1651–1665. [Google Scholar] [CrossRef]
- Haas, C.T.; Turbanski, S.; Kaiser, I.; Schmidtbleicher, D. Influences of whole-body vibration on symptom structure in Parkinson’s disease. J. Neurol. 2004, 3 (Suppl. III/18), 56. [Google Scholar]
- King, L.K.; Almeida, Q.J.; Ahonen, H. Short-term effects of vibration therapy on motor impairments in Parkinson’s disease. NeuroRehabilitation 2009, 25, 297–306. [Google Scholar] [CrossRef]
- Schmidtbleicher, D.; Turbanski, S.; Haas, C.T. Effects of whole-body vibration on postural control in Parkinson’s disease. Mov. Disord. 2004, 19 (Suppl. S9), s185. [Google Scholar]
- Turbanski, S.; Haas, C.T.; Schmidtbleicher, D.; Friedrich, A.; Duisberg, P. Effects of random whole-body vibration on postural control in Parkinson’s disease. Res. Sports Med. 2005, 13, 243–256. [Google Scholar] [CrossRef]
- Gaßner, H.; Janzen, A.; Schwirtz, A.; Jansen, P. Random Whole Body Vibration over 5 Weeks Leads to Effects Similar to Placebo: A Controlled Study in Parkinson’s Disease. Park. Dis. 2014, 2014, 386495. [Google Scholar] [CrossRef]
- Sharififar, S.; Coronado, R.A.; Romero, S.; Azari, H.; Thigpen, M. The effects of whole body vibration on mobility and balance in Parkinson disease: A systematic review. Iran. J. Med. Sci. 2014, 4, 318–326. [Google Scholar]
- Verhagen, A.P.; De Vet, H.C.; De Bie, R.A.; Kessels, A.G.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi List: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J. Clin. Epidemiol. 1998, 12, 1235–1241. [Google Scholar] [CrossRef]
- Verhagen, A.P.; Ferreira, M.L. Forest plots. J. Physiother. 2014, 60, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0 [Updated March 2011]; The Cochrane Collaboration: London, UK, 2011; Available online: www.handbook-5-1.cochrane.org (accessed on 17 July 2023).
- Dincher, A.; Schwarz, M.; Wydra, G. Analysis of the Effects of Whole-Body Vibration in Parkinson Disease—Systematic Review and Meta-Analysis. PM&R 2019, 11, 640–653. [Google Scholar] [CrossRef]
- Arias, P.; Chouza, M.; Vivas, J.; Cudeiro, J. Effect of whole body vibration in Parkinson’s disease: A controlled study. Mov. Disord. 2009, 24, 891–898. [Google Scholar] [CrossRef]
- Corbianco, S.; Cavallini, G.; Baldereschi, G.; Carboncini, M.C.; Fiamingo, F.L.; Bongioanni, P.; Dini, M. Whole body vibration and treadmill training in Parkinson’s disease rehabilitation: Effects on energy costs and recovery phases. Neurol. Sci. 2018, 39, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Dincher, A. Effects of Whole Body Vibration on reaction time in Parkinson’s Disease Patients—A pilot study. Neurodegener. Dis. Curr. Res. 2021, 1, 1–6. [Google Scholar]
- Dincher, A.; Becker, P.; Wydra, G. Effect of whole-body vibration on freezing and flexibility in Parkinson’s disease—A pilot study. Neurol. Sci. 2021, 42, 2795–2801. [Google Scholar] [CrossRef]
- Dincher, A.; Wydra, G. Chapter 3: Effect of Whole Body Vibration on Balance in Parkinson’s Disease—A Randomized Controlled Pilot Study. In Alzheimer’s Disease and Treatment; MedDocs Publishers, Ed.; MedDocs Publishers: Reno, NV, USA, 2021; Volume 3, pp. 21–25. [Google Scholar]
- Ebersbach, G.; Edler, D.; Kaufhold, O.; Wissel, J. Whole body vibration versus conventional physiotherapy to improve balance and gait in Parkinson’s disease. Arch. Phys. Med. Rehabil. 2008, 89, 399–403. [Google Scholar] [CrossRef]
- Guadarrama-Molina, E.; Barrón-Gámez, C.E.; Estrada-Bellmann, I.; Meléndez-Flores, J.D.; Ramírez-Castañeda, P.; Hernández-Suárez, R.M.G.; Menchaca-Pérez, M.; Salas-Fraire, O. Comparison of the effect of whole-body vibration therapy versus conventional therapy on functional balance of patients with Parkinson’s disease: Adding a mixed group. Acta Neurol. Belg. 2021, 121, 721–728. [Google Scholar] [CrossRef]
- Haas, C.T.; Buhlmann, A.; Turbanski, S.; Schmidtbleicher, D. Proprioceptive and Sensorimotor Performance in Parkinson’s Disease. Res. Sports Med. 2006, 14, 273–287. [Google Scholar] [CrossRef]
- Kapur, S.S.; Stebbins, G.T.; Goetz, C.G. Vibration therapy for Parkinson’s disease: Charcot’s studies revisited. J. Park. Dis. 2012, 1, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Kaut, O.; Allert, N.; Coch, C.; Paus, S.; Grzeska, A.; Minnerop, M.; Wüllner, U. Stochastic resonance therapy in Parkinson’s disease. NeuroRehabilitation 2011, 28, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Koebel, A.K.; Almeida, Q.J.; Ahonen, H. The Efficacy of Long-Term Whole-Body Vibration in the Treatment of Parkinson’s Disease; Poster, Wilfried Laurier University: Waterloo, ON, Canada, 2015. [Google Scholar]
- Spieß, A.E. Validierung der Therapieeffekte einer Repetitiven Stochastischen Resonanz-Therapie bei Symptomen des Idiopathischen Parkinson-Syndroms; Rheinische Friedrich-Wilhelms-Universität: Bonn, Germany, 2014; Available online: https://hdl.handle.net/20.500.11811/5928 (accessed on 26 July 2023).
- Karacan, I.; Cidem, M.; Yilmaz, G.; Sebik, O.; Cakar, H.I.; Türker, K.S. Renton reflex is suppressed during whole-body vibration. J. Electromyogr. Kinesiol. 2016, 30, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Corum, M.; Topkara, B.; Kokce, M.; Ozkan, M.; Bucak, O.F.; Ayture, L.; Karacan, I.; Türker, K.S. The reflex mechanism underlying the neuromuscular effects of whole-body vibration: Is it the tonic vibration reflex? J. Musculoskelet. Neuronal Interact. 2022, 22, 37–42. [Google Scholar]
- Shimomura, Y.; Murakami, T.; Nakai, N.; Nagasaki, M.; Harris, R.A. Exercise Promotes BCAA Catabolism: Effects of BCAA Supplementation on Skeletal Muscle during Exercise. J. Nutr. 2004, 134, 1583S–1587S. [Google Scholar] [CrossRef]
- Cardinale, M.; Pope, M.H. The effects of whole body vibration on humans: Dangerous or advantageous? Acta Physiol. Hung. 2003, 90, 195–206. [Google Scholar] [CrossRef]
- Dupuis, H.; Jansen, G. Immediate effects of vibration transmitted to the hand. In Man Under Vibration. Suffering and Protection; Bianchi, G., Frolov, K.V., Oledzki, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1981; pp. 76–86. [Google Scholar]
- Wakeling, J.M.; Nigg, B.M.; Rozitis, A.I. Muscle activity damps the sof tissue resonance that occurs in response to pulsed and continuous vibrations. J. Appl. Physiol. 2002, 93, 1093–1103. [Google Scholar] [CrossRef]
- Kreher, J.B.; Schwartz, J.B. Overtraining syndrome: A practical guide. Sports Health 2012, 4, 128–138. [Google Scholar] [CrossRef]
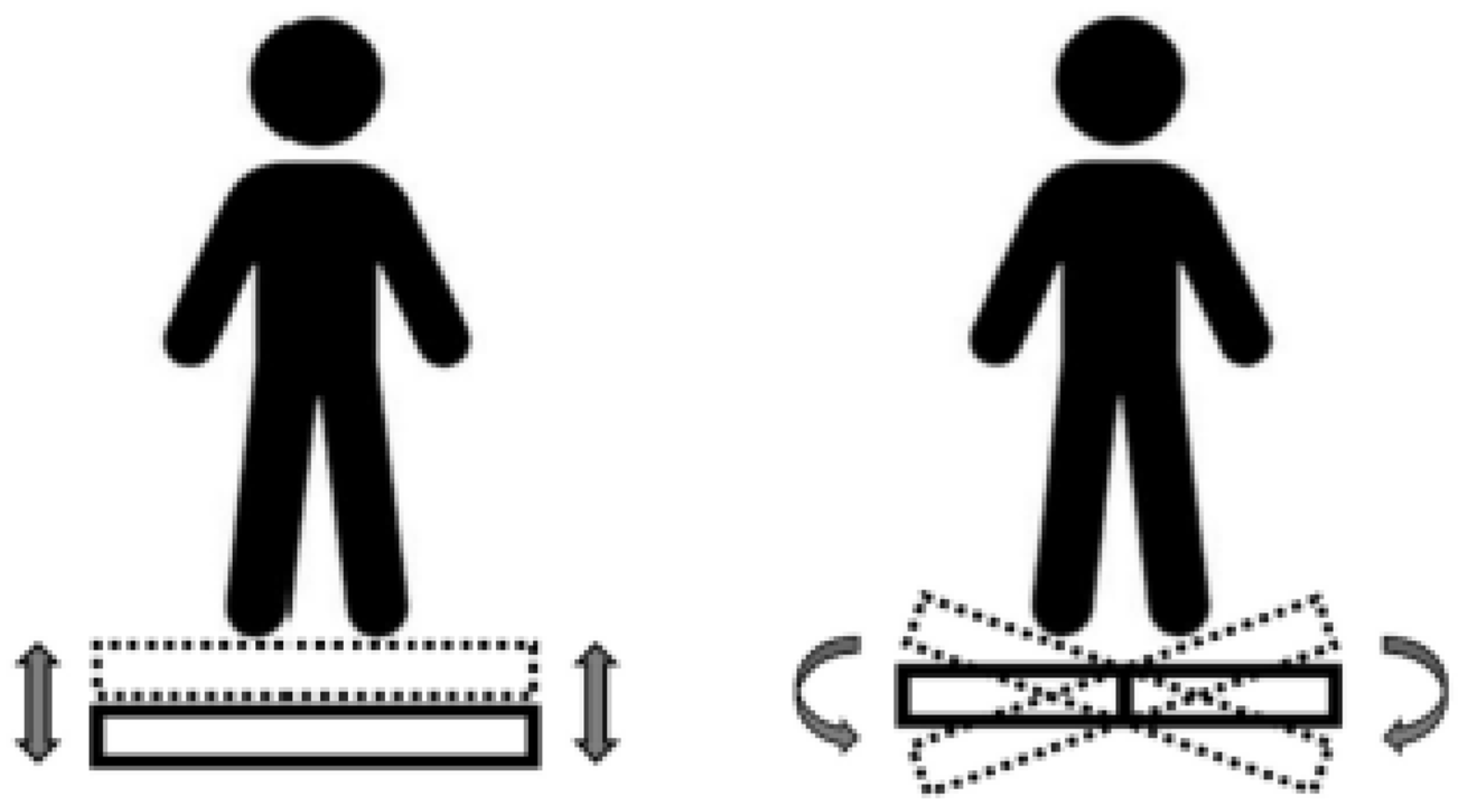






| Study | Sessions | Type | Frequency | Sets (Total Time) | Control Group | PEDro |
|---|---|---|---|---|---|---|
| Arias et al., 2009 [26] | multiple | WBV | 6 Hz | 5 × 60 s, 60 s rest × 12 sessions (3600 s) | Placebo | 5 |
| Corbianco et al., 2018 [27] | multiple | WBV | 26 Hz | 20 × 60 s, 60 s rest × 16 sessions (19,200 s) | Treadmill | 5 |
| Dincher, 2021 [28] | single | WBV | 6, 12, 18 Hz | 5 × 60 s, 60 s rest (300 s) | Placebo | 9 |
| Dincher et al., 2021 [29] | single | WBV | 6, 12, 18 Hz | 5 × 60 s, 60 s rest (300 s) | Placebo | 10 |
| Dincher and Wydra, 2021 [30] | single | WBV | 6, 12, 18 Hz | 5 × 60 s, 60 s rest (300 s) | Placebo | 9 |
| Ebersbach et al., 2008 [31] | multiple | WBV | 25 Hz | 2 × 900 s × 30 sessions (27,000 s) | Physiotherapy | 5 |
| Gaßner et al., 2014 [19] | multiple | rWBV | 6 Hz | 5 × 60 s, 60 s rest × 12 sessions (3600 s) | Placebo | 8 |
| Guadarrama et al., 2021 [32] | multiple | WBV | 20 Hz | 8 × 20 s, 30–60 s rest × 20 sessions (3200 s) | Physiotherapy, Combi | 6 |
| Haas, Turbanski et al., 2006 [13] | single | rWBV | 6 Hz | 5 × 60 s, 60 s rest (300 s) | Control | 5 |
| Haas, Buhlmann et al., 2006 [33] | single | rWBV | 6 Hz | 5 × 60 s, 60 s rest (300 s) | Control | 6 |
| Kapur et al., 2012 [34] | multiple | WBV | 30–500 Hz | 1 × 1800 × 28 sessions (50,400 s) | Placebo | 8 |
| Kaut et al., 2011 [35] | multiple | rWBV | 6.5 Hz | 5 × 60 s, 60 s rest × 3 sessions (900 s) | Placebo | 7 |
| Kaut et al., 2016 [3] | multiple | rWBV | 7 Hz | 5 × 60 s, 60 s rest × 4 sessions (1200 s) | Placebo | 9 |
| Koebel et al., 2015 [36] | multiple | WBV | 40 Hz | 1 × 1500 × 36 sessions (54,000 s) | Placebo | 7 |
| Spieß, 2014 [37] | multiple | rWBV | 6–7 Hz | 5 × 60 s, 60 s rest × 3 sessions (900 s) | Placebo | 8 |
| Turbanski et al., 2005 [18] | Single | rWBV | 6 Hz | 5 × 60 s, 60 s rest (300 s) | Control | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dincher, A. Effects of a Single Session of Whole Body Vibration Compared to Multiple Sessions—An Updated Review and Meta-Analysis. Vibration 2023, 6, 932-944. https://doi.org/10.3390/vibration6040055
Dincher A. Effects of a Single Session of Whole Body Vibration Compared to Multiple Sessions—An Updated Review and Meta-Analysis. Vibration. 2023; 6(4):932-944. https://doi.org/10.3390/vibration6040055
Chicago/Turabian StyleDincher, Andrea. 2023. "Effects of a Single Session of Whole Body Vibration Compared to Multiple Sessions—An Updated Review and Meta-Analysis" Vibration 6, no. 4: 932-944. https://doi.org/10.3390/vibration6040055
APA StyleDincher, A. (2023). Effects of a Single Session of Whole Body Vibration Compared to Multiple Sessions—An Updated Review and Meta-Analysis. Vibration, 6(4), 932-944. https://doi.org/10.3390/vibration6040055





