Relationships between Height, Mass, Body Mass Index, and Trunk Muscle Activation during Seated Whole-Body Vibration Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electromyography
2.2. Whole-Body Vibration Exposure
2.3. Fatigue Protocol
2.4. Data Processing
2.5. Statistical Analyses
3. Results
3.1. Spearman’s Rank Coefficient of Correlation
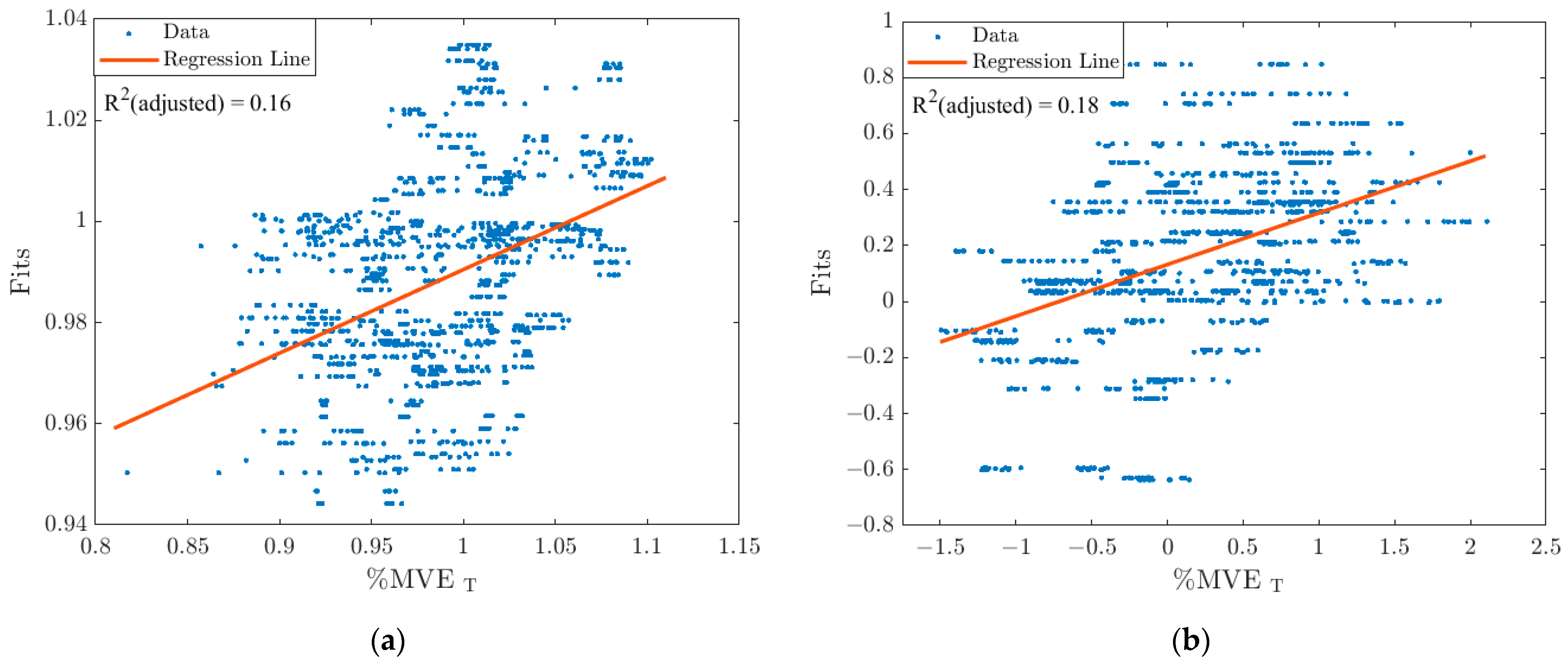
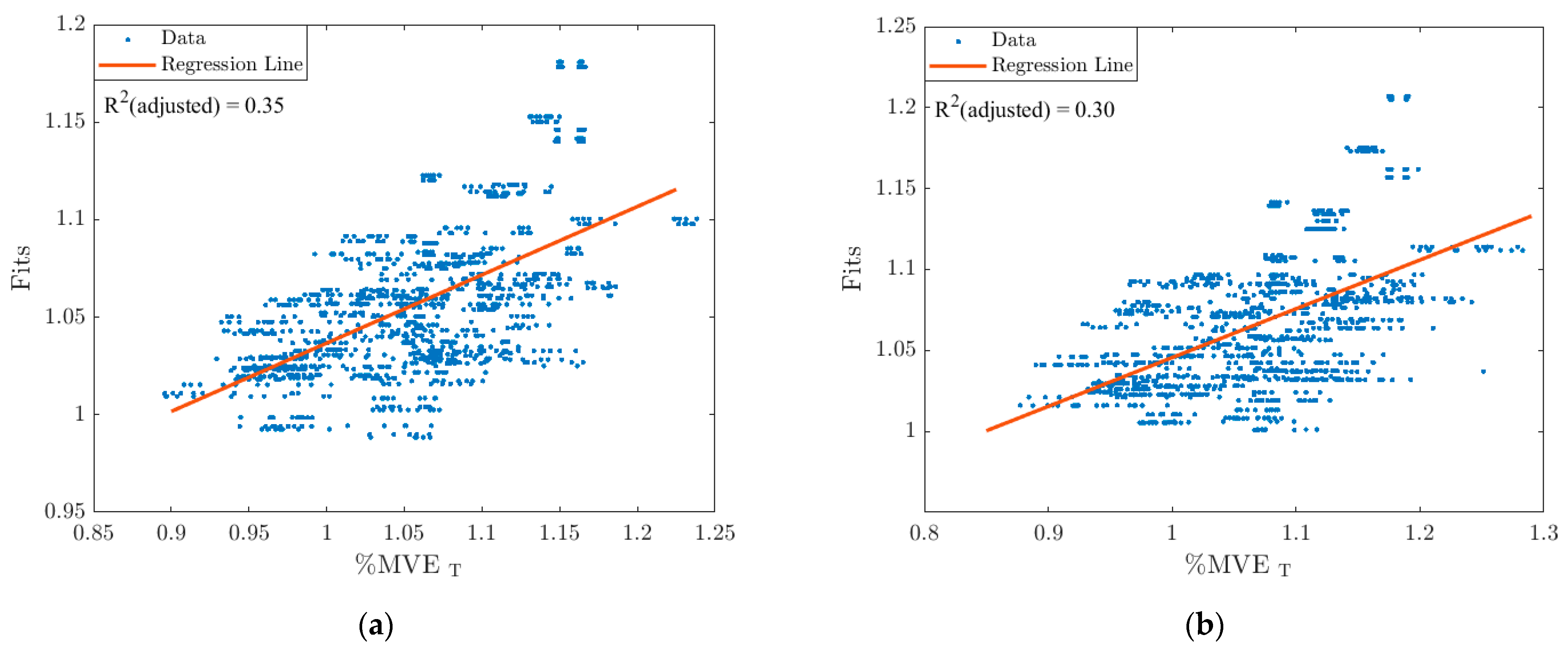
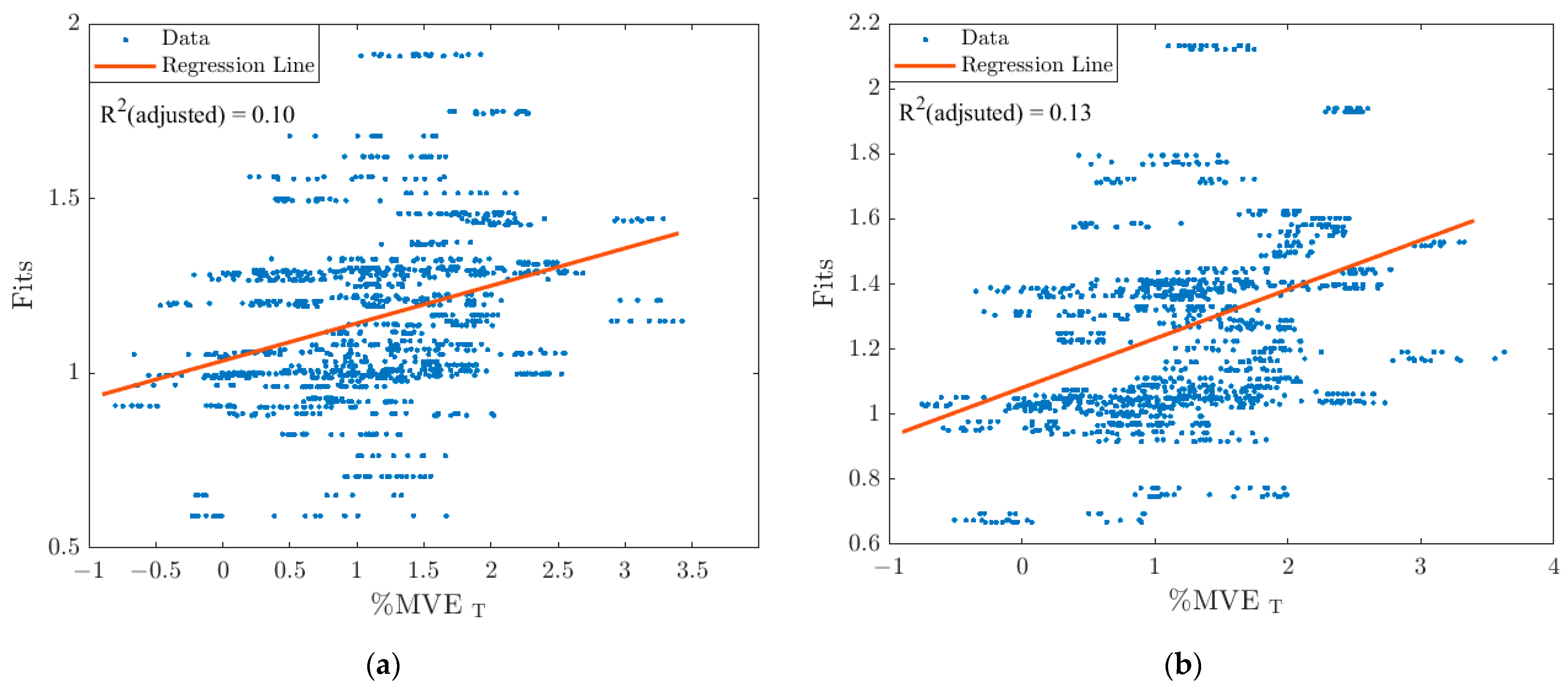
3.2. Stepwise Linear Regression Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, X.; Kociolek, A.M.; Khan, M.I.; Milosavljevic, S.; Bath, B.; Trask, C. Whole Body Vibration Exposure Patterns in Canadian Prairie Farmers. Ergonomics 2017, 60, 1064–1073. [Google Scholar] [CrossRef]
- Brammer, A.J.; Peterson, D.R. Vibration, mechanical shock, and impact. In Standard Handbook of Biomedical Engineering and Design; McGraw-Hill: New York, NY, USA, 2004; pp. 10.1–10.25. [Google Scholar]
- Chaudhary, D.K.; Bhattacherjee, A.; Patra, A.K.; Upadhyay, R.; Chau, N. Associations between Whole-Body Vibration Exposure and Occupational and Personal Factors in Drill Operators in Indian Iron Ore Mines. Min. Metall. Explor. 2019, 36, 495–511. [Google Scholar] [CrossRef]
- Gustafsson, B.; Pinzke, S.; Isberg, P. Musculoskeleteal Symptoms in Swedish Dairy Farmers. Swed. J. Agric. Res. 1994, 24, 177–188. [Google Scholar]
- Milosavljevic, S.; Mani, R.; Ribeiro, D.C.; Vasiljev, R.; Rehn, B. Exploring How Anthropometric, Vehicle and Workplace Factors Influence Whole-Body Vibration Exposures during on-Farm Use of a Quad Bike. Int. J. Ind. Ergon. 2012, 42, 392–396. [Google Scholar] [CrossRef]
- Schwarze, S.; Notbohm, G.; Dupuis, H.; Härtung, E. Dose-Response Relationships between Whole-Body Vibration and Lumbar Disk Disease-A Field Study on 388 Drivers of Different Vehicles. J. Sound Vib. 1998, 215, 613–628. [Google Scholar] [CrossRef]
- Bovenzi, M.; Hulshof, C.T.J. An Updated Review of Epidemiologic Studies on the Relationship between Exposure to Whole-Body Vibration and Low Back Pain. J. Sound Vib. 1998, 215, 595–611. [Google Scholar] [CrossRef]
- Wikström, B.O.; Kjellberg, A.; Landström, U. Health Effects of Long-Term Occupational Exposure to Whole-Body Vibration: A Review. Int. J. Ind. Ergon. 1994, 14, 273–292. [Google Scholar] [CrossRef]
- Burström, L.; Nilsson, T.; Wahlström, J. Whole-Body Vibration and the Risk of Low Back Pain and Sciatica: A Systematic Review and Meta-Analysis. Int. Arch. Occup. Environ. Health 2015, 88, 403–418. [Google Scholar] [CrossRef]
- Reeves, N.P.; Cholewicki, J.; Milner, T.; Lee, A.S. Trunk Antagonist Co-Activation Is Associated with Impaired Neuromuscular Performance. Exp. Brain Res. 2008, 188, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, N.; Grenier, S.G. Acute Effects of Whole Body Vibration on Directionality and Reaction Time Latency of Trunk Muscles: The Importance of Rest and Implications for Spine Stability. J. Electromyogr. Kinesiol. 2013, 23, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Graham, R.B.; Grenier, S.G. Effect of Whole Body Vibration on the Postural Control of the Spine in Sitting. Hum. Mov. Sci. 2015, 40, 77–88. [Google Scholar] [CrossRef]
- ISO-ISO 2631-1:1997-Mechanical Vibration and Shock—Evaluation of Human Exposure to Whole-Body Vibration—Part 1: General Requirements; International Organization for Standardization: Geneva, Switzerland, 1997.
- Milosavljevic, S.; Mcbride, D.I.; Bagheri, N.; Vasiljev, R.M.; Mani, R.; Carman, A.B.; Rehn, B. Exposure to Whole-Body Vibration and Mechanical Shock: A Field Study of Quad Bike Use in Agriculture. Ann. Occup. Hyg. 2011, 55, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caffaro, F.; Cremasco, M.M.; Preti, C.; Cavallo, E. Ergonomic Analysis of the Effects of a Telehandler’s Active Suspended Cab on Whole Body Vibration Level and Operator Comfort. Int. J. Ind. Ergon. 2016, 53, 19–26. [Google Scholar] [CrossRef]
- Rakheja, S.; Mandapuram, S.; Dong, R.G. Energy Absorption of Seated Occupants Exposed to Horizontal Vibration and Role of Back Support Condition. Ind. Health 2008, 46, 550–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Rakheja, S.; Boileau, P.É. The Role of Seat Geometry and Posture on the Mechanical Energy Absorption Characteristics of Seated Occupants under Vertical Vibration. Int. J. Ind. Ergon. 2006, 36, 171–184. [Google Scholar] [CrossRef]
- Holmlund, P.; Lundström, R.; Lindberg, L. Mechanical Impedance of the Human Body in Vertical Direction. Appl. Ergon. 2000, 31, 415–422. [Google Scholar] [CrossRef]
- Huston, D.R.; Johnson, C.C.; Wood, M.A.; Zhao, X. Vibration Attenuating Characteristics of Air Filled Seat Cushions. J. Sound Vib. 1999, 222, 333–340. [Google Scholar] [CrossRef]
- Dewangan, K.N.; Rakheja, S.; Marcotte, P. Gender and Anthropometric Effects on Whole-Body Vibration Power Absorption of the Seated Body. J. Low Freq. Noise Vib. Act. Control 2018, 37, 167–190. [Google Scholar] [CrossRef] [Green Version]
- Toward, M.G.R.; Griffin, M.J. Apparent Mass of the Human Body in the Vertical Direction: Inter-Subject Variability. J. Sound Vib. 2011, 330, 827–841. [Google Scholar] [CrossRef] [Green Version]
- Blood, R.P.; Ploger, J.D.; Johnson, P.W. Whole Body Vibration Exposures in Forklift Operators: Comparison of a Mechanical and Air Suspension Seat. Ergonomics 2010, 53, 1385–1394. [Google Scholar] [CrossRef]
- Holmberg, S.; Thelin, A.; Stiernstrom, E.-L.; Svardsudd, K. Psychosocial Factors and Low Back Pain, Consultations, and Sick Leave Among Farmers and Rural Referents: A Population-Based Study. J. Occup. Environ. Med. 2004, 46, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Seidel, H.; Blüthner, R.; Hinz, B. Application of Finite-Element Models to Predict Forces Acting on the Lumbar Spine during Whole-Body Vibration. Clin. Biomech. 2001, 16, 57–63. [Google Scholar] [CrossRef]
- Fritz, M. Description of the Relation between the Forces Acting in the Lumbar Spine and Whole-Body Vibrations by Means of Transfer Functions. Clin. Biomech. 2000, 15, 234–240. [Google Scholar] [CrossRef]
- van der Windt, D.A.W.; Thomas, E.; Pope, D.P.; de Winter, A.F.; Macfarlance, G.J.; Bouter, L.M.; Silman, A.J. Occupational Risk Factors for Shoulder Pain: A Systemic Review. Occup. Environ. Med. 2000, 57, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baig, H.A.; Dorman, D.B.; Bulka, B.A.; Shivers, B.L.; Chancey, V.C.; Winkelstein, B.A. Characterization of the Frequency and Muscle Responses of the Lumbar and Thoracic Spines of Seated Volunteers during Sinusoidal Whole Body Vibration. J. Biomech. Eng. 2014, 136, 101002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazrgari, B.; Shirazi-Adl, A.; Kasra, M. Seated Whole Body Vibrations with High-Magnitude Accelerations-Relative Roles of Inertia and Muscle Forces. J. Biomech. 2008, 41, 2639–2646. [Google Scholar] [CrossRef] [PubMed]
- Grondin, D.E.; Potvin, J.R. Effects of Trunk Muscle Fatigue and Load Timing on Spinal Responses during Sudden Hand Loading. J. Electromyogr. Kinesiol. 2009, 19, e237–e245. [Google Scholar] [CrossRef] [PubMed]
- Granata, K.P.; Gottipati, P. Fatigue Influences the Dynamic Stability of the Torso. Ergonomics 2008, 51, 1258–1271. [Google Scholar] [CrossRef]
- Nolan, A.J.; Govers, M.E.; Oliver, M.L. Effect of Fatigue on Muscle Latency, Muscle Activation and Perceived Discomfort When Exposed to Whole-Body Vibration. Ergonomics 2021, 64, 1281–1296. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M. The Stabilizing System of the Spine. Part II. Neutral Zone and Instability Hypothesis. J. Spinal Disord. 1992, 5, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M. The Stabilizing System of the Spine. Part I. Function, Dysfunction, Adaptation and Enhancement. J. Spinal Disord. 1992, 5, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Hamberg-van Reenen, H.H.; van der Beek, A.J.; Blatter, B.; van der Grinten, M.P.; van Mechelen, W.; Bongers, P.M. Does Musculoskeletal Discomfort at Work Predict Future Musculoskeletal Pain? Ergonomics 2008, 51, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Wilder, D.G.; Aleksiev, A.R.; Magnusson, M.L.; Pope, M.H.; Spratt, K.F.; Goel, V.K. Muscular Response to Sudden Load: A Tool to Evaluate Fatigue and Rehabilitation. Spine 1996, 21, 2628–2639. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bazrgari, B.; Shirazi-Adl, A.; Rakheja, S.; Boileau, P.-E. Biodynamic Response and Spinal Load Estimation of Seated Body in Vibration Using Finite Element Modeling. Ind. Health 2010, 48, 557–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milosavljevic, S.; Bagheri, N.; Vasiljev, R.M.; McBride, D.I.; Rehn, B. Does Daily Exposure to Whole-Body Vibration and Mechanical Shock Relate to the Prevalence of Low Back and Neck Pain in a Rural Workforce? Ann. Occup. Hyg. 2012, 56, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Kingma, I.; Staudenmann, D.; van Dieën, J.H. Trunk Muscle Activation and Associated Lumbar Spine Joint Shear Forces under Different Levels of External Forward Force Applied to the Trunk. J. Electromyogr. Kinesiol. 2007, 17, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Cholewicki, J.; Juluru, K.; Mcgill, S.M. Intra-Abdominal Pressure Mechanism for Stabilizing the Lumbar Spine. J. Biomech. 1999, 32, 13–17. [Google Scholar] [CrossRef]
- Cram, J.R.; Kasman, G.S.; Holtz, J. Introduction to Surface Electromyography; Aspen Publishers Inc.: Chicago, IL, USA, 1998. [Google Scholar]
- O’Sullivan, K.; McCarthy, R.; White, A.; O’Sullivan, L.; Dankaerts, W. Lumbar Posture and Trunk Muscle Activation during a Typing Task When Sitting on a Novel Dynamic Ergonomic Chair. Ergonomics 2012, 55, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Moreside, J.M.; Vera-garcia, F.J.; Mcgill, S.M. Trunk Muscle Activation Patterns, Lumbar Compressive Forces, and Spine Stability When Using the Bodyblade. Phys. Ther. 2007, 87, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Mathiassen, S.E.; Winkel, J.; Hagg, G.M. Normalization of Surface EMG Amplitude from the Upper Trapezius Muscle in Ergonomic Studies-A Review. J. Electromyogr. Kinesiol 1995, 5, 197–226. [Google Scholar] [CrossRef]
- Dickey, J.P.; Oliver, M.L.; Boileau, P.-E.; Eger, T.R.; Trick, L.M.; Edwards, A.M. Multi-Axis Sinusoidal Whole-Body Vibrations: Part I-How Long Should the Vibration and Rest Exposures Be for Reliable Discomfort Measures? J. Low Freq. Noise Vib. Act. Control 2006, 25, 175–184. [Google Scholar] [CrossRef]
- Ji, X.; Eger, T.R.; Dickey, J.P. Development of a Seat Selection Algorithm to Match Industrial Seats with Specific Forestry Vibration Exposures. Int. J. For. Eng. 2015, 26, 48–59. [Google Scholar] [CrossRef]
- Mannion, A.F.; Connolly, B.; Dolan, P. The Use of Surface EMG Power Spectral Analysis in the Evaluation of Back Muscle Function. J. Rehabil. Res. Dev. 1997, 34, 427–447. [Google Scholar]
- Mansfield, N.J.; Holmlund, P.; Lundström, R. Apparent Mass and Absorbed Power during Exposure to Whole-Body Vibration and Repeated Shocks. J. Sound Vib. 2001, 248, 427–440. [Google Scholar] [CrossRef]
- Wang, W.; Rakheja, S.; Boileau, P.É. Effects of Sitting Postures on Biodynamic Response of Seated Occupants under Vertical Vibration. Int. J. Ind. Ergon. 2004, 34, 289–306. [Google Scholar] [CrossRef]
- Hardyck, C.D.; Petrinovich, L.F. Introduction to Statistics for the Behavioral Sciences; W. B. Saunders Company: Philadelphia, PA, USA, 1976. [Google Scholar]
- WHO/Europe | Nutrition-Body Mass Index-BMI. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 15 August 2021).
- Son, S.M. Influence of Obesity on Postural Stability in Young Adults. Osong Public Health Res. Perspect. 2016, 7, 378–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teasdale, N.; Hue, O.; Marcotte, J.; Berrigan, F.; Simoneau, M.; Doré, J.; Marceau, P.; Marceau, S.; Tremblay, A. Reducing Weight Increases Postural Stability in Obese and Morbid Obese Men. Int. J. Obes. 2007, 31, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, P.X.; Abu Osman, N.A.; Yusof, A.; Wan Abas, W.A.B. Biomechanical Evaluation of the Relationship between Postural Control and Body Mass Index. J. Biomech. 2012, 45, 1638–1642. [Google Scholar] [CrossRef] [Green Version]
- Corbeil, P.; Simoneau, M.; Rancourt, D.; Tremblay, A.; Teasdale, N. Increased Risk for Falling Associated with Obesity: Mathematical Modeling of Postural Control. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 126–136. [Google Scholar] [CrossRef]
- Vedel, J.P.; Roll, J.P. Response to Pressure and Vibration of Slowly Adapting Cutaneous Mechanoreceptors in the Human Foot. Neurosci. Lett. 1982, 34, 289–294. [Google Scholar] [CrossRef]
- Hue, O.; Simoneau, M.; Marcotte, J.; Berrigan, F.; Doré, J.; Marceau, P.; Marceau, S.; Tremblay, A.; Teasdale, N. Body Weight Is a Strong Predictor of Postural Stability. Gait Posture 2007, 26, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Reeves, N.P.; Everding, V.Q.; Cholewicki, J.; Morrisette, D.C. The Effects of Trunk Stiffness on Postural Control during Unstable Seated Balance. Exp. Brain Res. 2006, 174, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. BMI-Related Errors in the Measurement of Obesity. Int. J. Obes. 2008, 32, S56–S59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewangan, K.N.; Rakheja, S.; Marcotte, P.; Shahmir, A.; Patra, S.K. Comparisons of Apparent Mass Responses of Human Subjects Seated on Rigid and Elastic Seats under Vertical Vibration. Ergonomics 2013, 56, 1806–1822. [Google Scholar] [CrossRef] [PubMed]
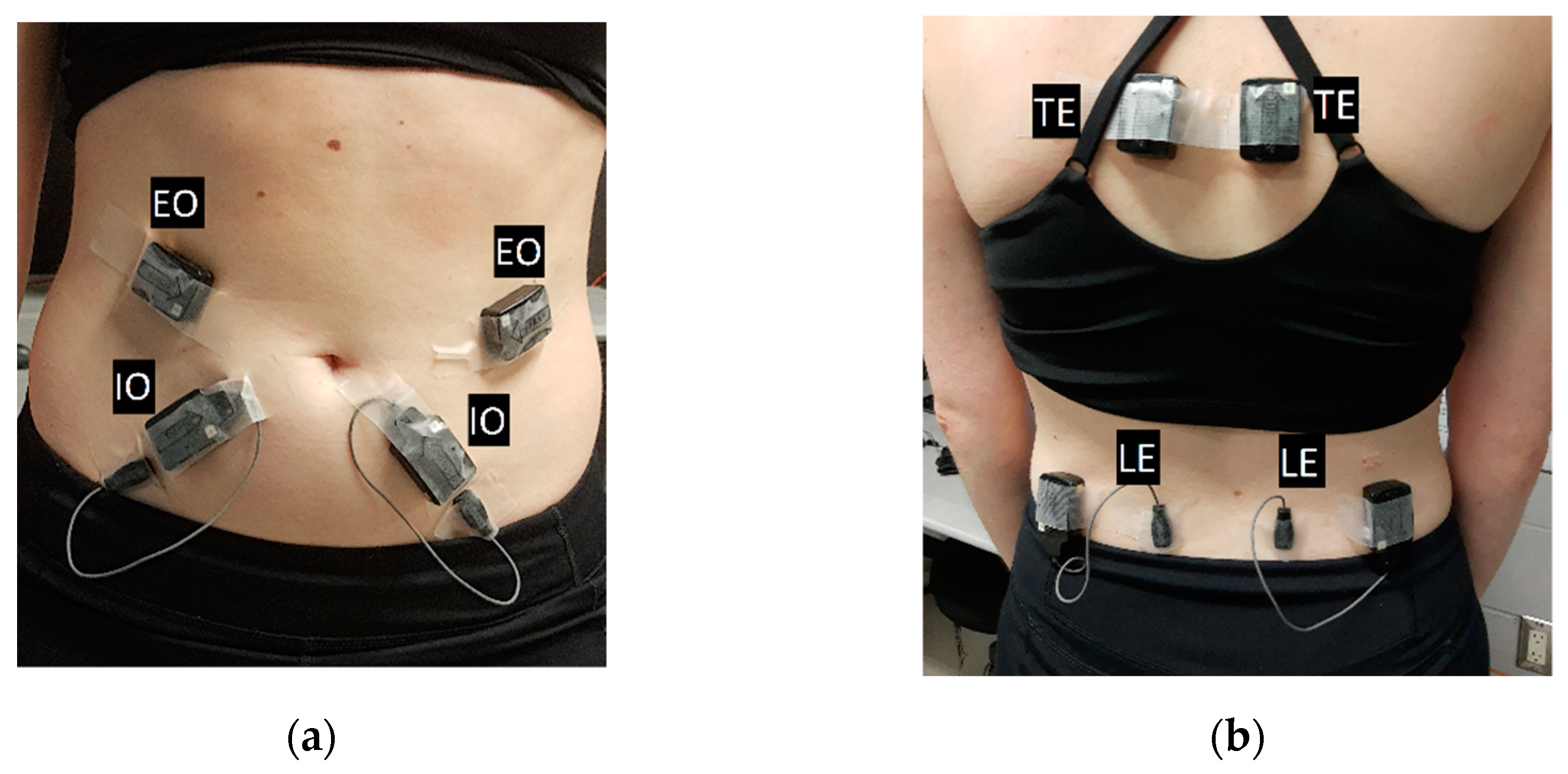


| Internal Oblique | |||||
| Pair | 1.25 Hz | 2.0 Hz | 2.5 Hz | 4.0 Hz | 4.5 Hz |
| Ρ | Ρ | ρ | ρ | ρ | |
| %MVET and mass | 0.26 (p < 0.0001) * | 0.24 (p < 0.0001) * | 0.21 (p = 0.001) * | 0.16 (p = 0.01) * | 0.14 (p = 0.02) * |
| %MVET and height | −0.34 (p < 0.0001) * | −0.35 (p < 0.0001) * | −0.36 (p < 0.0001) * | −0.37 (p < 0.0001) * | −0.35 (p < 0.0001) * |
| %MVET and BMI | 0.38 (p < 0.0001) * | 0.38 (p < 0.0001) * | 0.34 (p < 0.0001) * | 0.31 (p < 0.0001) * | 0.28 (p < 0.0001) * |
| External Oblique | |||||
| Pair | 1.25 Hz | 2.0 Hz | 2.5 Hz | 4.0 Hz | 4.5 Hz |
| Ρ | Ρ | ρ | ρ | ρ | |
| %MVET and mass | 0.11 (p = 0.08) | 0.14 (p = 0.02) * | 0.14 (p = 0.03) * | 0.14 (p = 0.03) * | 0.10 (p = 0.10) |
| %MVET and height | 0.37 (p < 0.0001) * | 0.38 (p < 0.0001) * | 0.38 (p < 0.0001) * | 0.39 (p < 0.0001) * | 0.38 (p < 0.0001) * |
| Lumbar Erector Spinae | |||||
| Pair | 1.25 Hz | 2.0 Hz | 2.5 Hz | 4.0 Hz | 4.5 Hz |
| Ρ | Ρ | ρ | ρ | ρ | |
| %MVET and mass | 0.36 (p < 0.0001) * | 0.43 (p < 0.0001) * | 0.39 (p < 0.0001) * | 0.44 (p < 0.0001) * | 0.42 (p < 0.0001) * |
| %MVET and height | 0.42 (p < 0.0001) * | 0.44 (p < 0.0001) * | 0.36 (p < 0.0001) * | 0.33 (p < 0.0001) * | 0.34 (p < 0.0001) * |
| %MVET and BMI | 0.08 (p = 0.23) | 0.15 (p = 0.01) * | 0.14 (p = 0.02) * | 0.21 (p = 0.001) * | 0.17 (p = 0.01) * |
| Thoracic Erector Spinae | |||||
| Pair | 1.25 Hz | 2.0 Hz | 2.5 Hz | 4.0 Hz | 4.5 Hz |
| Ρ | Ρ | ρ | ρ | ρ | |
| %MVET and mass | 0.08 (p = 0.21) | 0.08 (p = 0.19) | 0.09 (p = 0.15) | 0.07 (p = 0.26) | 0.09 (p = 0.13) |
| %MVET and height | −0.19 (p = 0.002) * | −0.18 (p = 0.003) * | −0.19 (p = 0.002) * | −0.21 (p = 0.001) * | −0.22 (p < 0.0001) * |
| %MVET and BMI | 0.10 (p = 0.10) | 0.10 (p = 0.12) | 0.12 (p = 0.06) | 0.13 (p = 0.04) * | 0.15 (p = 0.02) * |
| Internal Oblique | |||||
| Pair | 1.25 Hz | 2.0 Hz | 2.5 Hz | 4.0 Hz | 4.5 Hz |
| Ρ | ρ | ρ | Ρ | ρ | |
| %MVET and mass | 0.21 (p < 0.0001) * | 0.26 (p < 0.0001) * | 0.27 (p < 0.0001) * | 0.14 (p = 0.03) * | 0.13 (p = 0.03) * |
| %MVET and height | −0.29 (p < 0.0001) * | −0.28 (p < 0.0001) * | −0.29 (p < 0.0001) * | −0.27 (p < 0.0001) * | −0.29 (p < 0.0001) * |
| %MVET and BMI | 0.30 (p < 0.0001) * | 0.36 (p < 0.0001) * | 0.36 (p < 0.0001) * | 0.26 (p < 0.0001) * | 0.25 (p < 0.0001) * |
| External Oblique | |||||
| Pair | 1.25 Hz | 2.0 Hz | 2.5 Hz | 4.0 Hz | 4.5 Hz |
| Ρ | ρ | ρ | Ρ | ρ | |
| %MVET and mass | −0.20 (p = 0.001) * | −0.23 (p < 0.0001) * | −0.17 (p = 0.004) * | −0.17 (p = 0.01) * | −0.15 (p = 0.02) * |
| %MVET and height | −0.41 (p < 0.0001) * | −0.46 (p < 0.0001) * | −0.41 (p < 0.0001) * | −0.41 (p < 0.0001) * | −0.40 (p < 0.0001) * |
| Lumbar Erector Spinae | |||||
| Pair | 1.25 Hz | 2.0 Hz | 2.5 Hz | 4.0 Hz | 4.5 Hz |
| Ρ | ρ | ρ | Ρ | ρ | |
| %MVET and mass | 0.39 (p < 0.0001) * | 0.36 (p < 0.0001) * | 0.37 (p < 0.0001) * | 0.35 (p < 0.0001) * | 0.35 (p < 0.0001) * |
| %MVET and height | 0.41 (p < 0.0001) * | 0.39 (p < 0.0001) * | 0.39 (p < 0.0001) * | 0.31 (p < 0.0001) * | 0.29 (p < 0.0001) * |
| %MVET and BMI | 0.10 (p = 0.12) | 0.08 (p = 0.22) | 0.10 (p = 0.12) | 0.15 (p = 0.02) * | 0.12 (p = 0.05) * |
| Thoracic Erector Spinae | |||||
| Pair | 1.25 Hz | 2.0 Hz | 2.5 Hz | 4.0 Hz | 4.5 Hz |
| Ρ | ρ | ρ | Ρ | ρ | |
| %MVET and mass | 0.17 (p = 0.01) * | 0.12 (p = 0.05) * | 0.08 (p = 0.18) | 0.15 (p = 0.02) * | 0.08 (p = 0.19) |
| %MVET and height | −0.17 (p = 0.004) * | −0.16 (p = 0.01) * | −0.19 (p = 0.002) * | −0.16 (p = 0.01) * | −0.19 (p = 0.002) * |
| %MVET and BMI | 0.20 (p = 0.001) * | 0.15 (p = 0.02) * | 0.12 (p = 0.05) * | 0.19 (p = 0.002) * | 0.12 (p = 0.04) * |
| Frequency | Pre-Fatigue Regression Equations | Post-Fatigue Regression Equations |
|---|---|---|
| 1.25 Hz | %MVET = 1.35 − 0.01 mass + 0.20 height + 0.20 BMI | %MVET = 6.14 − 3.51 height |
| 2.00 Hz | %MVET = 1.35 − 0.01 mass + 0.20 height + 0.20 BMI | %MVET = 6.15 − 3.51 height |
| 2.50 Hz | %MVET = 1.35 − 0.01 mass + 0.20 height + 0.20 BMI | %MVET = 6.14 − 3.51 height |
| 4.00 Hz | %MVET = 1.34 − 0.01 mass + 0.20 height + 0.20 BMI | %MVET = 6.43 − 3.51 height |
| 4.50 Hz | %MVET = 1.33 − 0.01 mass + 0.20 height + 0.20 BMI | %MVET = 6.43 − 3.51 height |
| Frequency | Pre-Fatigue Regression Equations | Post-Fatigue Regression Equations |
|---|---|---|
| 1.25 Hz | %MVET = −0.46 − 0.01 mass + 0.80 height + 0.45 BMI | %MVET = −0.84 − 0.02 mass + 1. 01 height + 0.05 BMI |
| 2.00 Hz | %MVET = −0.46 − 0.01 mass + 0.80 height + 0.45 BMI | %MVET = −0.84 − 0.02 mass + 1. 01 height + 0.05 BMI |
| 2.50 Hz | %MVET = −0.46 − 0.01 mass + 0.80 height + 0.45 BMI | %MVET = −0.83 − 0.02 mass + 1. 01 height + 0.05 BMI |
| 4.00 Hz | %MVET = −0.42 − 0.01 mass + 0.80 height + 0.45 BMI | %MVET = −0.79 − 0.02 mass + 1. 01 height + 0.05 BMI |
| 4.50 Hz | %MVET = −0.42 − 0.01 mass + 0.80 height + 0.45 BMI | %MVET = −0.79 − 0.02 mass + 1. 01 height + 0.05 BMI |
| Frequency | Pre-Fatigue Regression Equations | Post-Fatigue Regression Equations |
|---|---|---|
| 1.25 Hz | %MVET = −2.94 − 0.02 mass + 2.26 height + 0.08 BMI | %MVET = −1.08 − 0.01 mass + 1.20 height + 0.04 BMI |
| 2.00 Hz | %MVET = −2.94 − 0.02 mass + 2.26 height + 0.08 BMI | %MVET = −1.08 − 0.01 mass + 1.20 height + 0.04 BMI |
| 2.50 Hz | %MVET = −2.94 − 0.02 mass + 2.26 height + 0.08 BMI | %MVET = −1.07 − 0.01 mass + 1.20 height + 0.04 BMI |
| 4.00 Hz | %MVET = −2.91 − 0.02 mass + 2.26 height + 0.08 BMI | %MVET = −1.06 − 0.01 mass + 1.20 height + 0.04 BMI |
| 4.50 Hz | %MVET = −2.91 − 0.02 mass + 2.26 height + 0.08 BMI | %MVET = −1.06 − 0.01 mass + 1.20 height + 0.04 BMI |
| Frequency | Pre-Fatigue Regression Equations | Post-Fatigue Regression Equations |
|---|---|---|
| 1.25 Hz | %MVET = −21.65 − 0.18 mass + 12.97 height + 0.53 BMI | %MVET = −22.64 − 0.18 mass + 13.49 height + 0.56 BMI |
| 2.00 Hz | %MVET = −21.65 − 0.18 mass + 12.97 height + 0.53 BMI | %MVET = −22.65 − 0.18 mass + 13.49 height + 0.56 BMI |
| 2.50 Hz | %MVET = −21.59 − 0.18 mass + 12.97 height + 0.53 BMI | %MVET = −22.62 − 0.18 mass + 13.49 height + 0.56 BMI |
| 4.00 Hz | %MVET = −21.36 − 0.18 mass + 12.97 height + 0.53 BMI | %MVET = −22.29 − 0.18 mass + 13.49 height + 0.56 BMI |
| 4.50 Hz | %MVET = −21.36 − 0.18 mass + 12.97 height + 0.53 BMI | %MVET = −22.28 − 0.18 mass + 13.49 height + 0.56 BMI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govers, M.E.; Nolan, A.J.; Hassan, M.; Oliver, M.L. Relationships between Height, Mass, Body Mass Index, and Trunk Muscle Activation during Seated Whole-Body Vibration Exposure. Vibration 2021, 4, 822-835. https://doi.org/10.3390/vibration4040046
Govers ME, Nolan AJ, Hassan M, Oliver ML. Relationships between Height, Mass, Body Mass Index, and Trunk Muscle Activation during Seated Whole-Body Vibration Exposure. Vibration. 2021; 4(4):822-835. https://doi.org/10.3390/vibration4040046
Chicago/Turabian StyleGovers, Megan E., Alexander J. Nolan, Marwan Hassan, and Michele L. Oliver. 2021. "Relationships between Height, Mass, Body Mass Index, and Trunk Muscle Activation during Seated Whole-Body Vibration Exposure" Vibration 4, no. 4: 822-835. https://doi.org/10.3390/vibration4040046
APA StyleGovers, M. E., Nolan, A. J., Hassan, M., & Oliver, M. L. (2021). Relationships between Height, Mass, Body Mass Index, and Trunk Muscle Activation during Seated Whole-Body Vibration Exposure. Vibration, 4(4), 822-835. https://doi.org/10.3390/vibration4040046










