Comparative Analysis of Chemical Reaction Mechanisms of Ammonia-n-Heptane Mixtures: From Ignition, Oxidation, and Laminar Flame Propagation to Engine Applications
Abstract
1. Introduction
2. Research Methodology
2.1. Simulation Methods for Ignition, Oxidation and Flame Processes
2.2. Methods for Reaction Pathway and Sensitivity Analyses
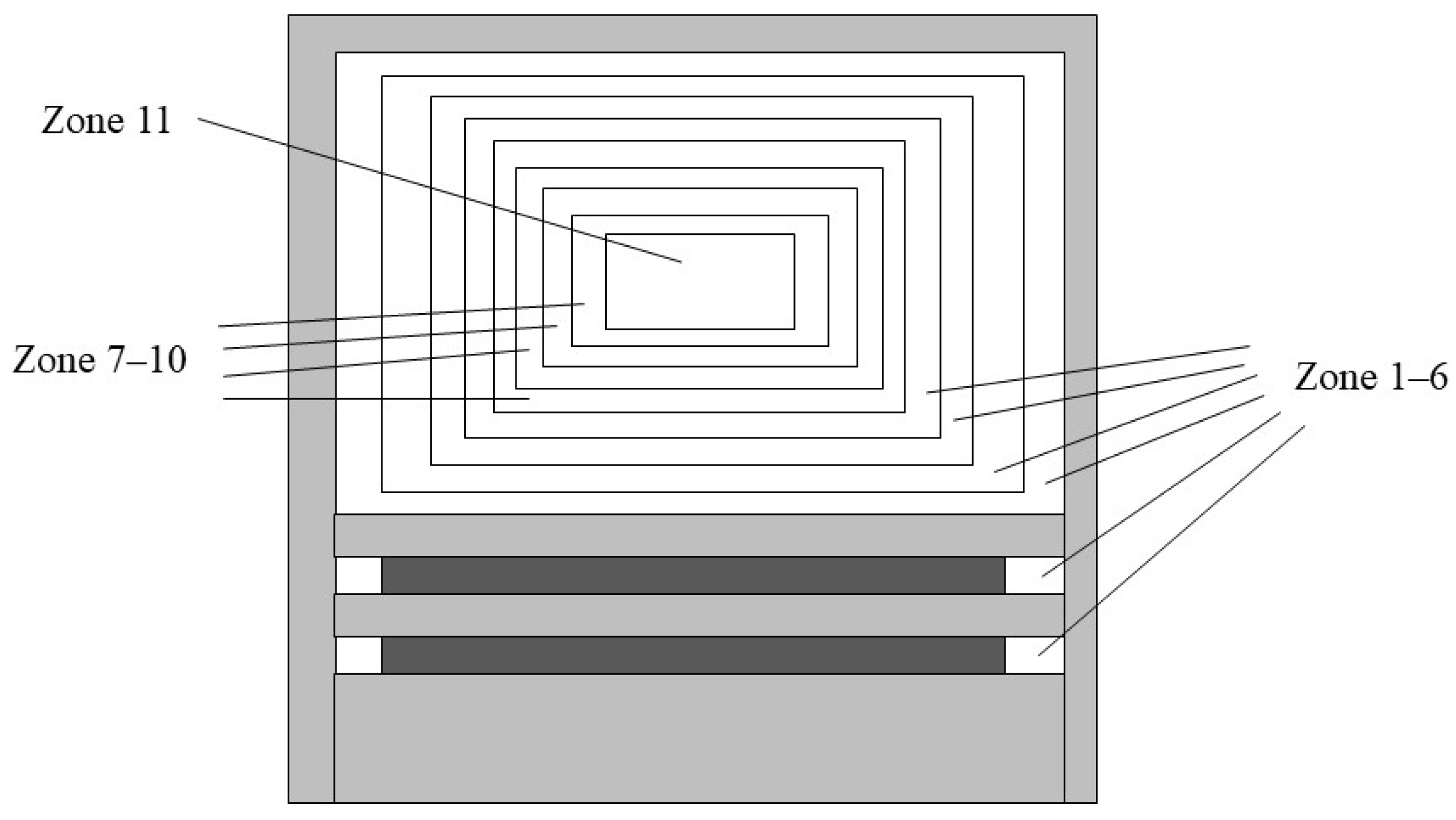
2.3. Multi-Zone Engine Simulation Method
3. Results and Discussion
3.1. Comparative Analysis of Ammonia-n-Heptane Reaction Mechanisms from the Points of Ignition, Oxidation and Flame Propagation
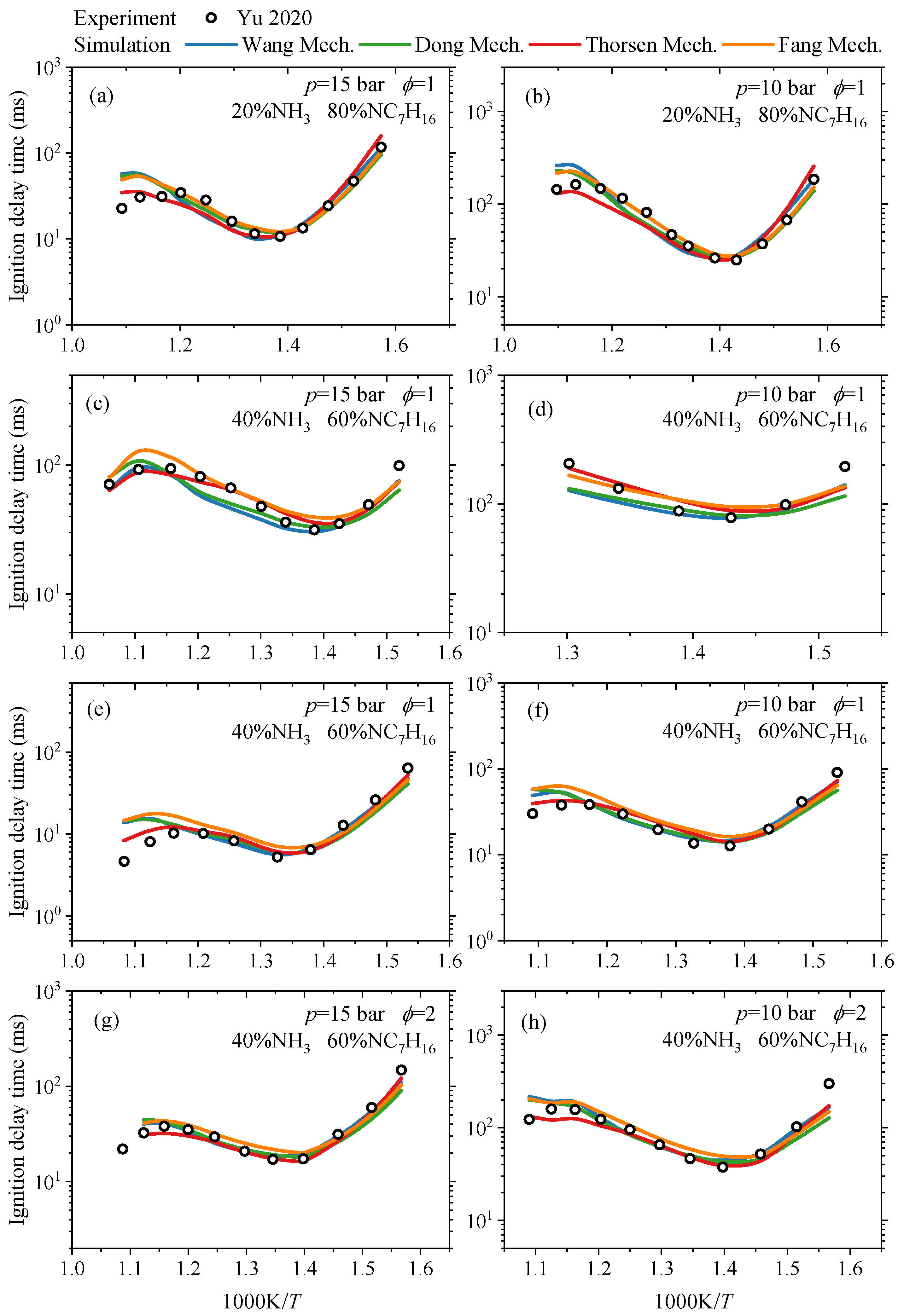
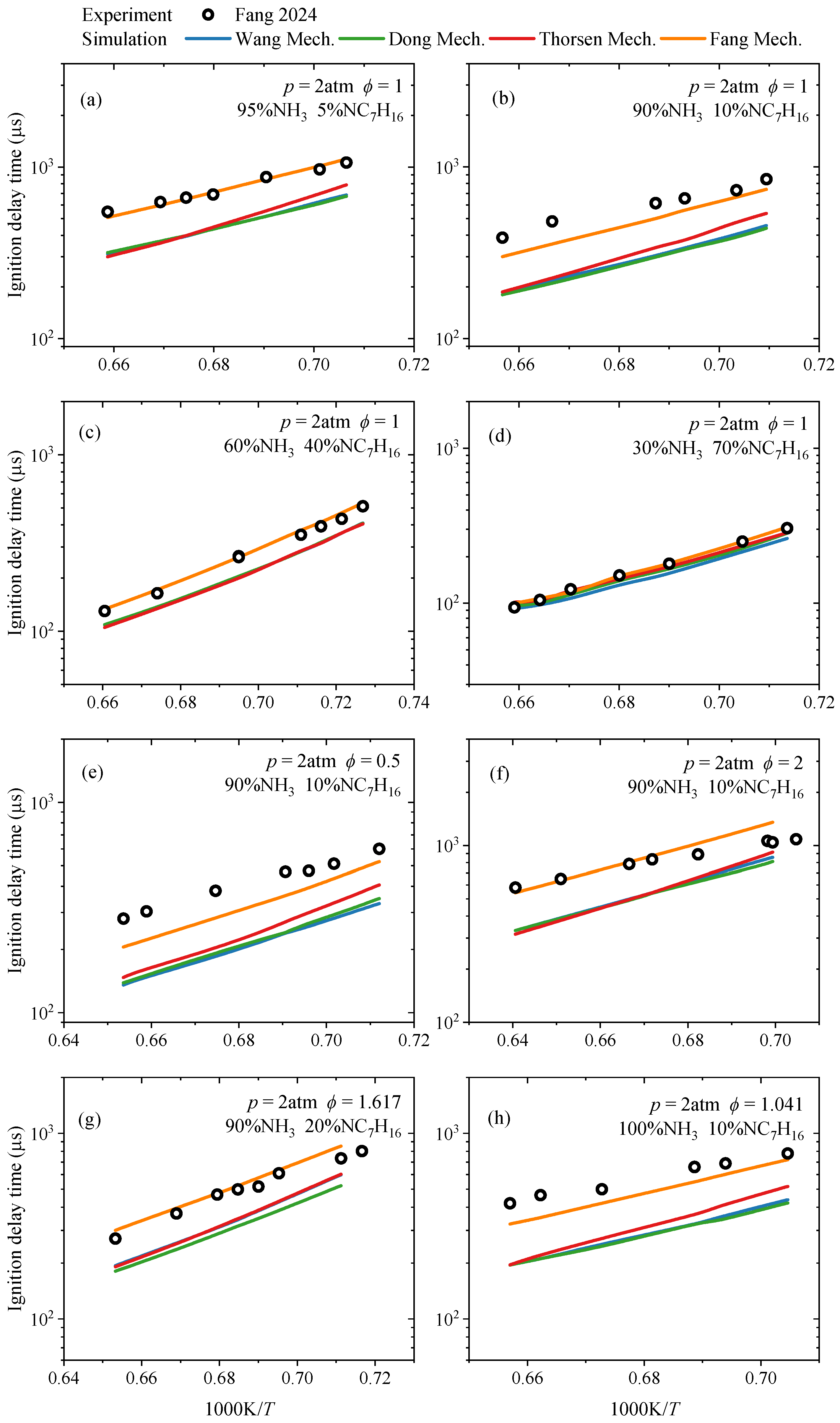
3.1.1. Ignition Delay Time
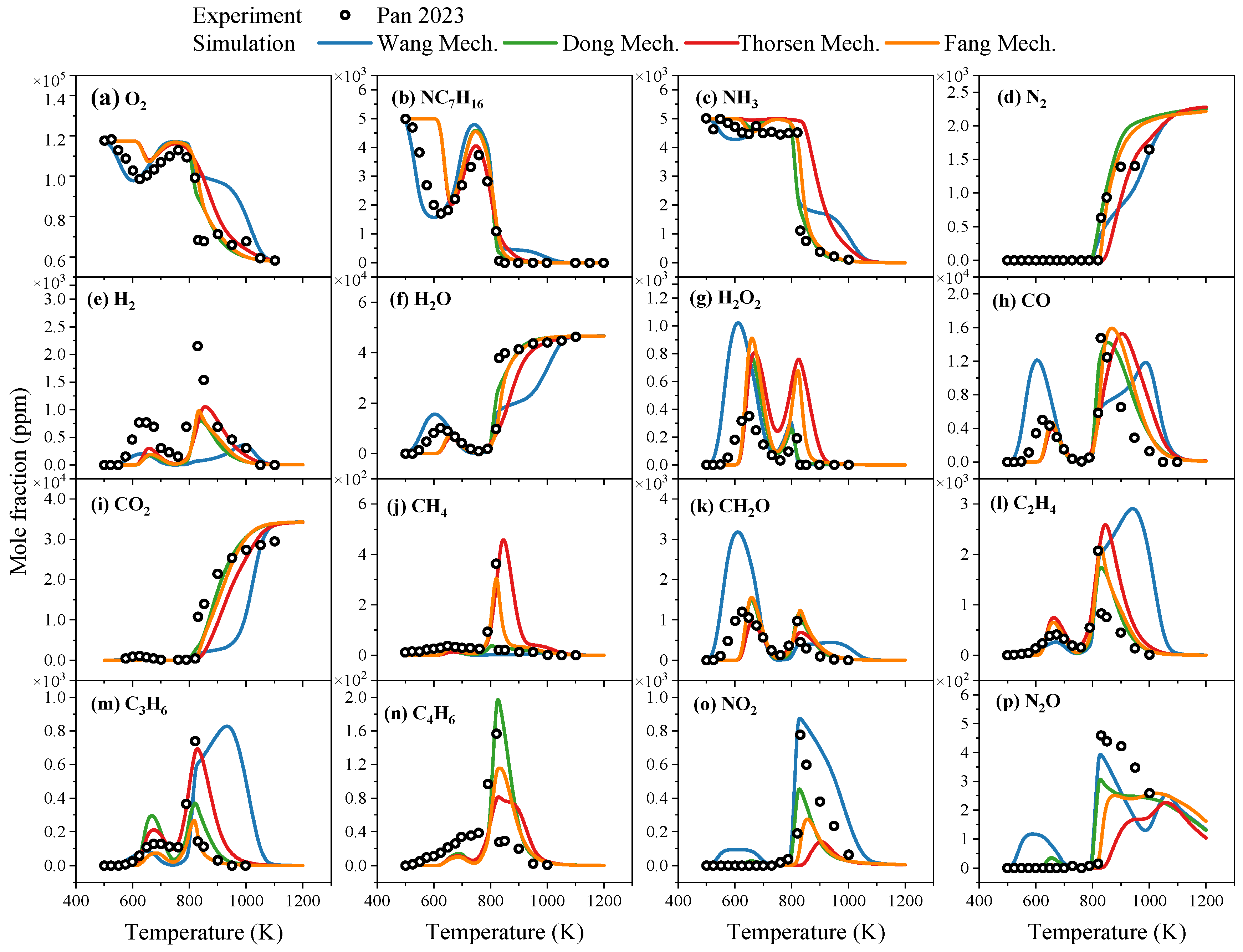
3.1.2. Oxidation
3.1.3. Laminar Flame Propagation
3.2. Analyses of Reaction Pathways and Consumption Processes for Ammonia-n-Heptane Mixtures
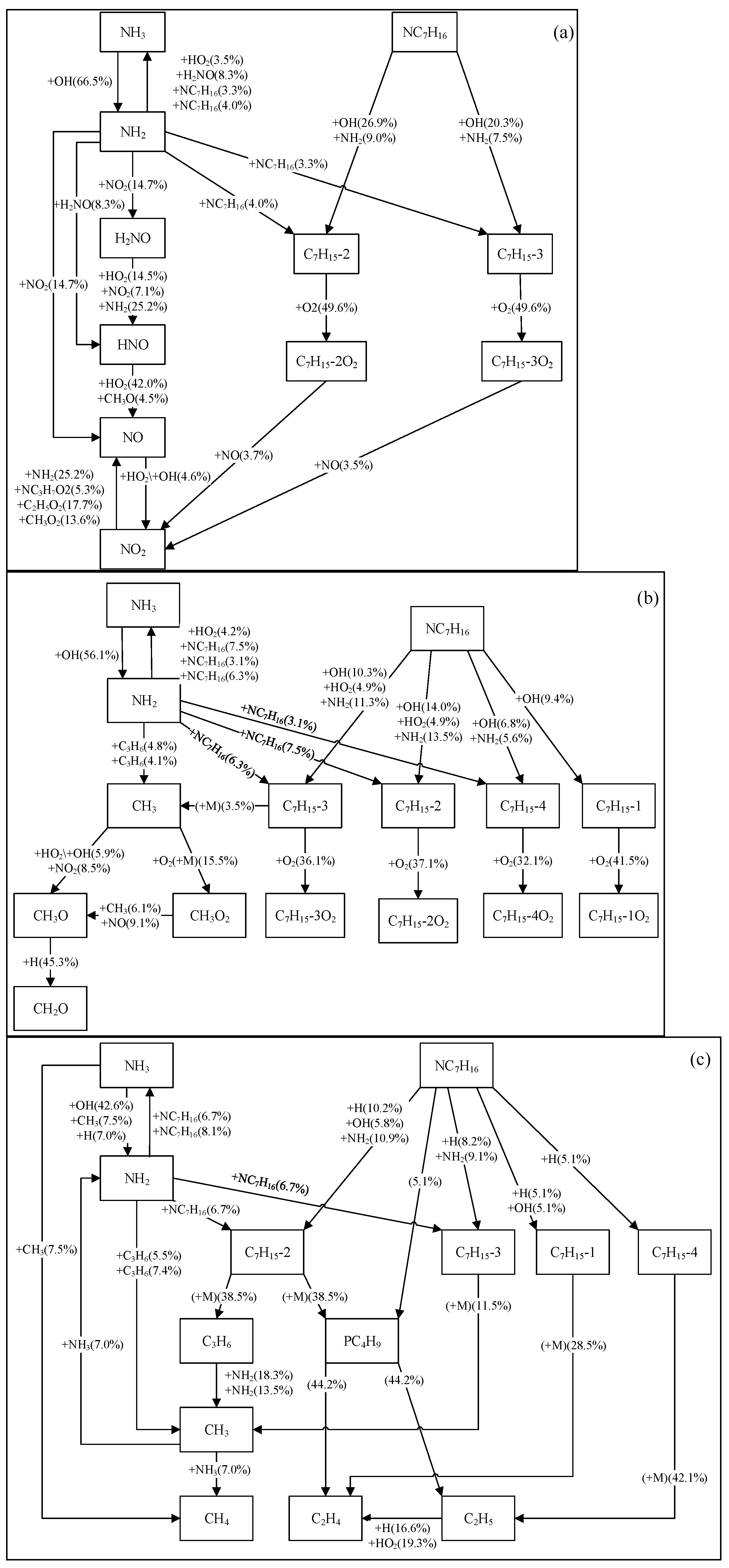
3.2.1. Reaction Pathway Analysis
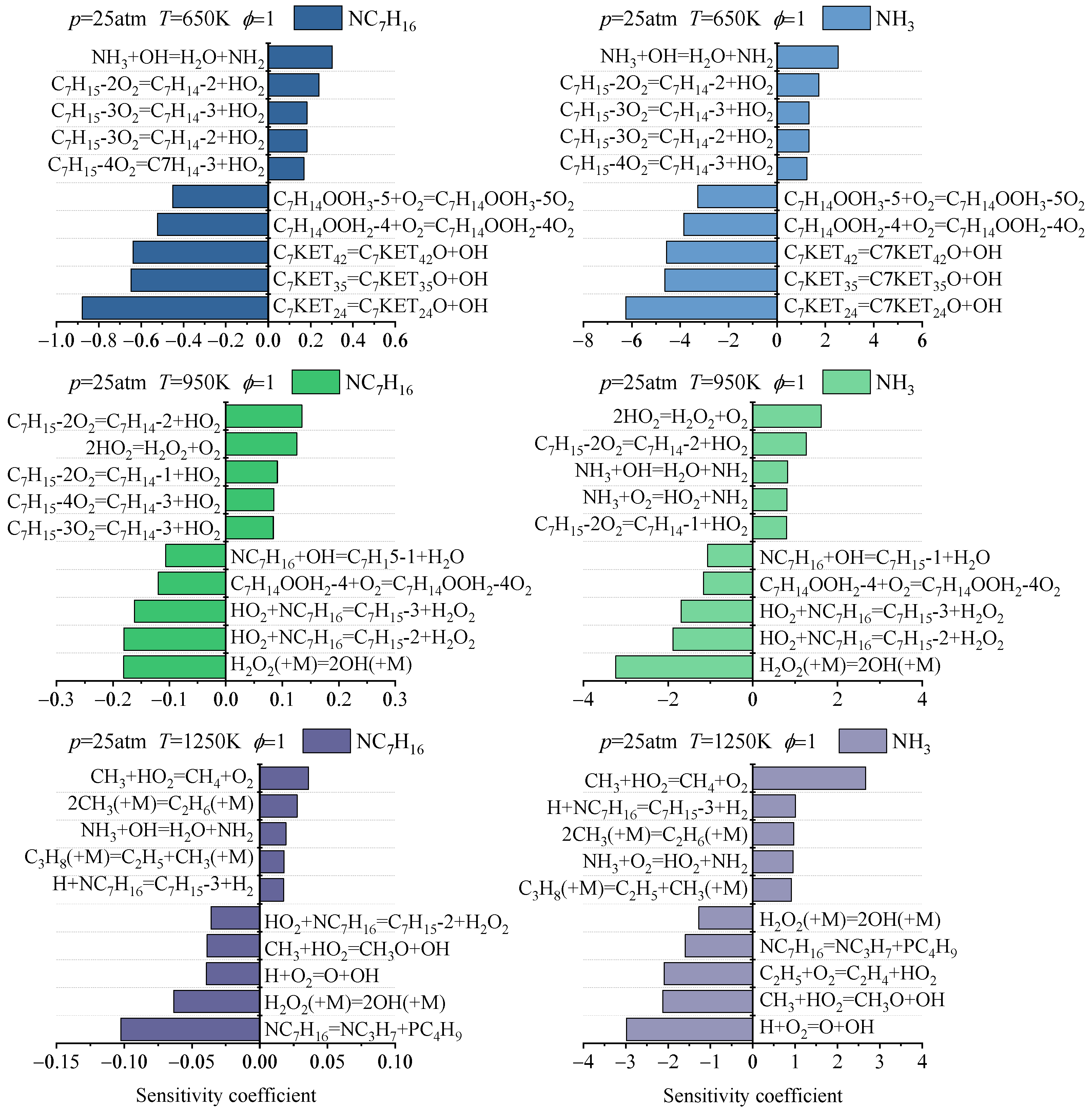
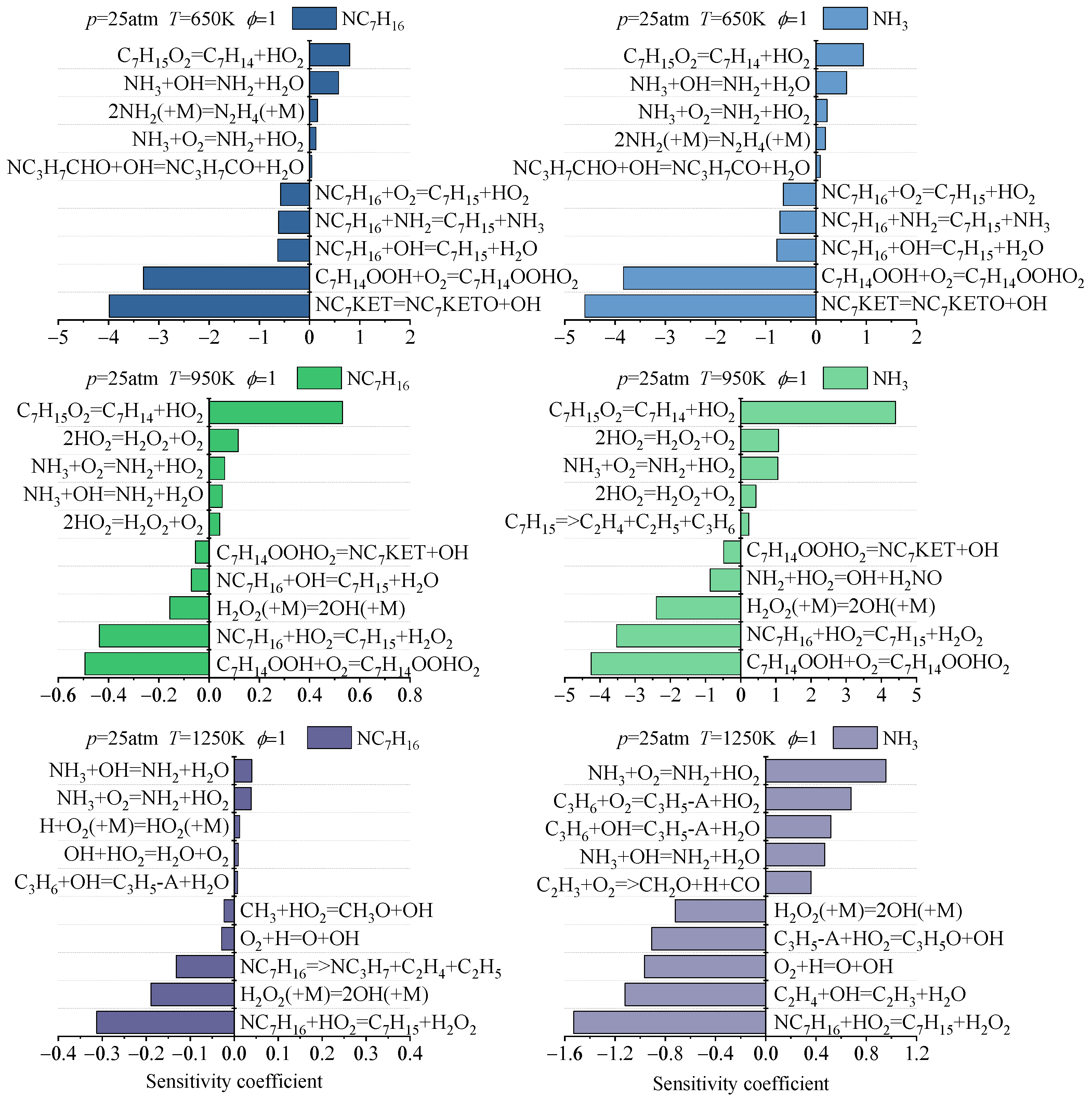
3.2.2. Consumption Process Analysis
3.3. Comparisons of Ammonia-n-Heptane Reaction Mechanisms Based on Engine Simulation
4. Conclusions
- (1)
- In terms of ignition delay time, under conditions of low ammonia blending ratios (≤40%), the simulated values of each mechanism show good agreement with experimental data. Under high ammonia blending ratios and high-temperature conditions, the Fang mechanism exhibits relatively higher prediction accuracy, while the predictions of other mechanisms are lower than experimental values. In the oxidation process, each reaction mechanism can generally predict the concentrations of reactants and complete combustion end products. However, for other end products and intermediate species, the discrepancies in the prediction results of each mechanism vary, depending on the type of substance and reaction conditions. Regarding laminar burning velocities, existing reaction mechanisms can predict the variation trend of laminar burning velocities with the equivalence ratio, and the predictions at low and high equivalence ratios show small differences from the experimental values.
- (2)
- The main reaction pathways of ammonia and n-heptane predicted by the Dong, Thorsen, and Fang mechanisms are basically consistent, but there are certain differences in the reaction fluxes of each pathway. The Wang mechanism can predict the main pathways of ammonia and n-heptane reactions, but there are significant differences in specific pathways compared with the prediction results of the other three mechanisms. Under different temperatures and pressures, the fuel consumption rates corresponding to each ammonia-n-heptane reaction mechanism are somewhat different. From the perspective of reaction classes, the reactions significantly affecting the consumption of ammonia and n-heptane in each mechanism have certain similarities, but in terms of specific reactions and the sensitivity coefficients, the sensitivity analysis results of each mechanism present certain differences.
- (3)
- As a consequence, the Fang mechanism can be selected to understand more accurately ignition, oxidation and flame processes of ammonia-n-heptane mixtures, while to reduce the engineering computational cost of the engine simulation, the Wang mechanism tends to be a good choice.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for Power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A Review of Ammonia as a Compression Ignition Engine Fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Niki, Y. Reductions in Unburned Ammonia and Nitrous Oxide Emissions From an Ammonia-Assisted Diesel Engine With Early Timing Diesel Pilot Injection. J. Eng. Gas Turbines Power 2021, 143, 91014. [Google Scholar] [CrossRef]
- Yousefi, A.; Guo, H.; Dev, S.; Lafrance, S.; Liko, B. A Study on Split Diesel Injection on Thermal Efficiency and Emissions of an Ammonia/Diesel Dual-Fuel Engine. Fuel 2022, 316, 123412. [Google Scholar] [CrossRef]
- Jin, S.; Wu, B.; Zi, Z.; Yang, P.; Shi, T.; Zhang, J. Effects of Fuel Injection Strategy and Ammonia Energy Ratio on Combustion and Emissions of Ammonia-Diesel Dual-Fuel Engine. Fuel 2023, 341, 127668. [Google Scholar] [CrossRef]
- Zhang, K.; Banyon, C.; Bugler, J.; Curran, H.J.; Rodriguez, A.; Herbinet, O.; Battin-Leclerc, F.; B’Chir, C.; Heufer, K.A. An Updated Experimental and Kinetic Modeling Study of N-Heptane Oxidation. Combust. Flame 2016, 172, 116–135. [Google Scholar] [CrossRef]
- Chang, Y.; Jia, M.; Wang, P.; Niu, B.; Liu, J. Construction and Derivation of a Series of Skeletal Chemical Mechanisms for N-Alkanes with Uniform and Decoupling Structure Based on Reaction Rate Rules. Combust. Flame 2022, 236, 111785. [Google Scholar] [CrossRef]
- Frassoldati, A.; Cuoci, A.; Stagni, A.; Faravelli, T.; Ranzi, E. Skeletal Kinetic Mechanism for Diesel Combustion. Combust. Theory Model. 2017, 21, 79–92. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y.; Wang, X.; Wang, P. Development of a Skeletal Mechanism for Tri-Component Diesel Surrogate Fuel: N-Hexadecane/Iso-Cetane/1-Methylnaphthalene. Fuel 2020, 259, 116217. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, D.; Yu, L.; Qian, Y.; Lu, X. Construction of a Skeletal Multi-Component Diesel Surrogate Model by Integrating Chemical Lumping and Genetic Algorithm. Fuel 2022, 313, 122711. [Google Scholar] [CrossRef]
- Otomo, J.; Koshi, M.; Mitsumori, T.; Iwasaki, H.; Yamada, K. Chemical Kinetic Modeling of Ammonia Oxidation with Improved Reaction Mechanism for Ammonia/Air and Ammonia/Hydrogen/Air Combustion. Int. J. Hydrogen Energy 2018, 43, 3004–3014. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Lhuillier, C.; Barbosa, A.A.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C.; Seidel, L.; Mauss, F. An Experimental and Modeling Study of Ammonia with Enriched Oxygen Content and Ammonia/Hydrogen Laminar Flame Speed at Elevated Pressure and Temperature. Proc. Combust. Inst. 2021, 38, 2163–2174. [Google Scholar] [CrossRef]
- Zhou, S.; Cui, B.; Yang, W.; Tan, H.; Wang, J.; Dai, H.; Li, L.; Rahman, Z.U.; Wang, X.; Deng, S.; et al. An Experimental and Kinetic Modeling Study on NH3/Air, NH3/H2/Air, NH3/CO/Air, and NH3/CH4/Air Premixed Laminar Flames at Elevated Temperature. Combust. Flame 2023, 248, 112536. [Google Scholar] [CrossRef]
- Dong, S.; Wang, B.; Jiang, Z.; Li, Y.; Gao, W.; Wang, Z.; Cheng, X.; Curran, H.J. An Experimental and Kinetic Modeling Study of Ammonia/n-Heptane Blends. Combust. Flame 2022, 246, 112428. [Google Scholar] [CrossRef]
- Wang, B.; Dong, S.; Jiang, Z.; Gao, W.; Wang, Z.; Li, J.; Yang, C.; Wang, Z.; Cheng, X. Development of a Reduced Chemical Mechanism for Ammonia/n-Heptane Blends. Fuel 2023, 338, 127358. [Google Scholar] [CrossRef]
- Thorsen, L.S.; Jensen, M.S.T.; Pullich, M.S.; Christensen, J.M.; Hashemi, H.; Glarborg, P.; Alekseev, V.A.; Nilsson, E.J.K.; Wang, Z.; Mei, B.; et al. High Pressure Oxidation of NH3/n-Heptane Mixtures. Combust. Flame 2023, 254, 112785. [Google Scholar] [CrossRef]
- Fang, Y.; Qu, W.; Feng, L. Experimental and Kinetic Modeling Study on Auto-Ignition of Ammonia/n-Heptane Mixtures at Intermediate Temperatures. Combust. Flame 2024, 265, 113488. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, W.; Feng, Y.; Wang, W.; Zhu, J.; Qian, Y.; Lu, X. The Effect of Ammonia Addition on the Low-Temperature Autoignition of n-Heptane: An Experimental and Modeling Study. Combust. Flame 2020, 217, 4–11. [Google Scholar] [CrossRef]
- Pan, J.; Tang, R.; Wang, Z.; Gao, J.; Xu, Q.; Shu, G.; Wei, H. An Experimental and Modeling Study on the Oxidation of Ammonia and N-Heptane with JSR. Proc. Combust. Inst. 2023, 39, 477–485. [Google Scholar] [CrossRef]
- Lubrano Lavadera, M.; Han, X.; Konnov, A.A. Comparative Effect of Ammonia Addition on the Laminar Burning Velocities of Methane, n -Heptane, and Iso-Octane. Energy Fuels 2021, 35, 7156–7168. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling Nitrogen Chemistry in Combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Song, S.; Golden, D.; Hanson, R.; Bowman, C.; Senosiain, J.; Musgrave, C.; Friedrichs, G. A Shock Tube Study of the Reaction NH2 + CH4 → NH3 + CH3 and Comparison with Transition State Theory. Int. J. Chem. Kinet. 2003, 35, 304–309. [Google Scholar] [CrossRef]
- Mai, T.V.T.; Duong, M.v.; Huynh, L.K. Theoretical Kinetics of the C2H4 + NH2 Reaction. Combust. Flame 2020, 215, 193–202. [Google Scholar] [CrossRef]
- Siddique, K.; Altarawneh, M.; Gore, J.; Westmoreland, P.R.; Dlugogorski, B.Z. Hydrogen Abstraction from Hydrocarbons by NH2. J. Phys. Chem. A 2017, 121, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Siddique, K.; Altarawneh, M.; Saeed, A.; Zeng, Z.; Gore, J.; Dlugogorski, B.Z. Interaction of NH2 Radical with Alkylbenzenes. Combust. Flame 2019, 200, 85–96. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Yang, Y.; Brear, M.J.; He, X.; Wang, Z. Impact of Nitric Oxide (NO) on n-Heptane Autoignition in a Rapid Compression Machine. Combust. Flame 2017, 186, 94–104. [Google Scholar] [CrossRef]
- Almodovar, C.A.; Goldsmith, C.F. Laser Schlieren Study of the Thermal Decomposition of 2-Ethylhexyl-Nitrate. Proc. Combust. Inst. 2021, 38, 997–1005. [Google Scholar] [CrossRef]
- Fang, R.; Saggese, C.; Wagnon, S.W.; Sahu, A.B.; Curran, H.J.; Pitz, W.J.; Sung, C.-J. Effect of Nitric Oxide and Exhaust Gases on Gasoline Surrogate Autoignition: Iso-Octane Experiments and Modeling. Combust. Flame 2022, 236, 111807. [Google Scholar] [CrossRef]
- Meng, Q.; Lei, L.; Lee, J.; Burke, M.P. On the Role of HNNO in NOx Formation. Proc. Combust. Inst. 2023, 39, 551–560. [Google Scholar] [CrossRef]
- Easley, W.; Agarwal, A.; Lavoie, G. Modeling of HCCI Combustion and Emissions Using Detailed Chemistry. SAE Tech. Pap. 2001, 110, 1045–1061. [Google Scholar] [CrossRef]
- Huang, Y.; Lyu, L.; Liang, J.; Yang, H.; Zhu, N.; Sang, H.; Zhang, X. Performance and Emission Characteristics of Marine Ammonia/Diesel Dual-Fuel Engines at Different Diesel Substitution Rates. Fuel 2025, 379, 132967. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Z.; Li, G.; Wan, Q.; Xu, L.; Fan, S. Experimental and Kinetic Studies of Ignition Processes of the Methane–n-heptane Mixtures. Fuel 2019, 235, 522–529. [Google Scholar] [CrossRef]





| Number | Chemical Kinetic Models | Number of Species | Number of Reactions | Reference |
|---|---|---|---|---|
| 1 | Dong Mechanism | 2854 | 11,790 | [14] |
| 2 | Wang Mechanism | 74 | 495 | [15] |
| 3 | Thorsen Mechanism | 1367 | 6314 | [16] |
| 4 | Fang Mechanism | 2860 | 11,892 | [17] |
| Number | Parameter | Experimental Conditions | Reference |
|---|---|---|---|
| 1 | Ignition delay time | NH3: 30–95%, ϕ = 1–2, p = 2 atm, T = 1350–1560 K | [17] |
| NH3: 0–40%, ϕ = 1–2, p = 10–15 bar, T = 635–945 K | [18] | ||
| 2 | Species concentration of oxidation | NH3: 50%, ϕ = 1, p = 1 atm, T = 500–1200 K, τ = 3 s | [19] |
| 3 | Laminar burning velocity | NH3: 25–50%, ϕ = 0.7–1.3, p = 1 atm, T = 338 K | [20] |
| Parameters | Values |
|---|---|
| Number of cylinders | 1 |
| Bore × Stroke (mm) | 129 × 155 |
| Connecting Rod (mm) | 256 |
| Compression Ratio | 17.5:1 |
| Rated speed (RPM) | 1500 |
| IVC (°CA ATDC) | −153 |
| EVO (°CA ATDC) | 117 |
| Zone | Temperature (K) | Zone Volume Fraction | NC7H16 Mass (mg) | NH3 Mass (mg) | O2 Mass (mg) | N2 Mass (mg) |
|---|---|---|---|---|---|---|
| 1 | 850 | 3 | 0 | 1.195845 | 3.93023 | 14.7849185 |
| 2 | 855 | 10 | 0 | 1.195845 | 3.93023 | 14.7849185 |
| 3 | 860 | 10 | 0 | 1.195845 | 3.93023 | 14.7849185 |
| 4 | 865 | 10 | 0 | 1.195845 | 3.93023 | 14.7849185 |
| 5 | 870 | 10 | 0 | 1.195845 | 3.93023 | 14.7849185 |
| 6 | 875 | 10 | 0 | 1.195845 | 3.93023 | 14.7849185 |
| 7 | 880 | 10 | 0.5684056 | 1.1695 | 3.843639978 | 14.45917999 |
| 8 | 885 | 10 | 0.8526084 | 2.57938 | 8.477304905 | 31.89031182 |
| 9 | 900 | 10 | 1.1368112 | 2.78874 | 9.165380549 | 34.47874613 |
| 10 | 920 | 9 | 3.1262308 | 5.2514 | 17.25907737 | 64.92598357 |
| 11 | 925 | 8 | 0.715944 | 2.8179 | 9.26121684 | 34.83926745 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Lyu, L.; Chen, Q.; Chen, Y.; Liang, J.; Yang, H.; Zhu, N. Comparative Analysis of Chemical Reaction Mechanisms of Ammonia-n-Heptane Mixtures: From Ignition, Oxidation, and Laminar Flame Propagation to Engine Applications. Fire 2025, 8, 357. https://doi.org/10.3390/fire8090357
Huang Y, Lyu L, Chen Q, Chen Y, Liang J, Yang H, Zhu N. Comparative Analysis of Chemical Reaction Mechanisms of Ammonia-n-Heptane Mixtures: From Ignition, Oxidation, and Laminar Flame Propagation to Engine Applications. Fire. 2025; 8(9):357. https://doi.org/10.3390/fire8090357
Chicago/Turabian StyleHuang, Yongzhong, Lin Lyu, Qihang Chen, Yue Chen, Junjie Liang, He Yang, and Neng Zhu. 2025. "Comparative Analysis of Chemical Reaction Mechanisms of Ammonia-n-Heptane Mixtures: From Ignition, Oxidation, and Laminar Flame Propagation to Engine Applications" Fire 8, no. 9: 357. https://doi.org/10.3390/fire8090357
APA StyleHuang, Y., Lyu, L., Chen, Q., Chen, Y., Liang, J., Yang, H., & Zhu, N. (2025). Comparative Analysis of Chemical Reaction Mechanisms of Ammonia-n-Heptane Mixtures: From Ignition, Oxidation, and Laminar Flame Propagation to Engine Applications. Fire, 8(9), 357. https://doi.org/10.3390/fire8090357






