Abstract
The ammonia-n-heptane reaction mechanism is essential for simulation of the in-cylinder process for diesel-ignited ammonia engines. To gain insight into the differences in predictive performance among various ammonia-n-heptane reaction mechanisms, four mechanisms were comprehensively evaluated and analyzed based on the modeling of ignition, oxidation, laminar flame propagation and in-cylinder combustion processes. The result shows that only under high ammonia blending ratios and elevated temperatures are discrepancies in predicted ignition delay times observed among the studied reaction mechanisms. Regarding the oxidation process, on the whole, the concerned mechanisms can reasonably predict concentrations of reactants and complete combustion products. However, significant discrepancies exist among the mechanisms in predicting concentrations of intermediate species and other products. For laminar burning velocity, the modeled values from the studied mechanisms are consistent with experimental results under both fuel-lean and -rich conditions. The Wang mechanism exhibits significant deviations from the other three mechanisms in predicting reaction pathways of ammonia and n-heptane. From the perspective of reaction class, the studied mechanisms are similar to each other, to some extent, in the key reactions governing consumption of ammonia and n-heptane. For the engine simulation, the predicted in-cylinder pressure and temperature profiles show minimal variations across different reaction mechanisms. In conclusion, the Fang mechanism can be selected to understand more accurately ignition, oxidation and flame characteristics of ammonia-n-heptane mixtures, while to reduce the engineering computational cost of the engine simulation, the Wang mechanism tends to be a good choice.
1. Introduction
To effectively address greenhouse gas (GHG) emissions in the shipping industry, the 2023 Ship Greenhouse Gas Emission Strategy adopted by the International Maritime Organization (IMO) proposes that GHG emissions from international shipping should peak as soon as possible and achieve net-zero emissions around or by 2050. In the field of marine engines, one of the main measures for carbon reduction is the use of low-carbon/zero-carbon fuels. Ammonia (NH3) is a zero-carbon fuel, and it produces no carbon dioxide (CO2) when burned. Compared with hydrogen, ammonia has significant advantages in storage and transportation [1]. Therefore, the ammonia fuel can be used to replace part of fossil fuels in the shipping sector to achieve the goal of reducing carbon emissions.
Due to the relatively high ignition temperature required for ammonia and its slow flame propagation speed after combustion, engines directly using pure ammonia as fuel face problems such as high compression ratios and difficult combustion [2]. For compression-ignition marine engines, when using ammonia as the power fuel, an additional ignition source is required. Injecting a certain amount of diesel into the cylinder and then igniting the ammonia by compressing and igniting the diesel is an effective operation scheme [3,4,5]. Research results show that engines using ammonia as fuel can reduce CO2 emissions. For the design and development of such engines, conducting simulations by coupling flow, chemical reactions, and heat transfer is one of the important ways to save time and material costs. Therefore, for marine engines fueled with ammonia, the chemical reaction mechanisms of ammonia/diesel fuels are required for accurate simulation of the in-cylinder combustion processes.
Currently, diesel reaction mechanisms are mainly divided into two categories: single-component [6,7,8] and multi-component [9,10] mechanisms. Considering the scale and complexity of the mechanisms, as well as the similarity in cetane number between n-heptane and actual diesel, n-heptane (NC7H16) is used as a single-component representative fuel for diesel. Some studies have been conducted on the reaction mechanisms of ammonia and n-heptane [6,11,12,13], and several schemes for their mixed combustion mechanisms have also been proposed [14,15,16,17]. Relevant experiments have been carried out on the ignition delay time (IDT) [17,18], oxidation component concentrations [16,19], and laminar burning velocity (LBV) [20] during the mixed combustion of ammonia and n-heptane. In the study on the ammonia-n-heptane ignition by Fang et al. [17], experimental values of IDT were measured at pressure of 2 atm, temperatures of 1350–1560 K, and equivalence ratios of 1–2 with the ammonia blending ratio ranging from 30% to 95% by molar content. Yu et al. [18] also determined experimentally the IDTs of the ammonia-n-heptane mixtures, with the initial conditions being pressures of 10–15 bar, equivalence ratios of 1–2 within the temperature range of 635–945 K and the ammonia energy content range of 0–40%. For the oxidation of the stoichiometric ammonia-n-heptane mixture, Pan et al. [19] obtained the experimental data for concentrations of the related species such as n-heptane, ammonia, oxygen, hydrogen, water and formaldehyde at the atmospheric pressure and temperatures of 500–1200 K. In the research of Lubrano Lavadera et al. [20] on the ammonia-n-heptane LBV, experimental values were derived using the heat flux method at pressure of 1 atm, temperature of 338 K and equivalence ratios of 0.7–1.3 under different ammonia contents. Such experimental data is basically required to evaluate the existing ammonia-n-heptane reaction mechanisms.
The reaction mechanism of the NH3/NC7H16 mixture is not simply obtained by superimposing the mechanisms of ammonia and n-heptane. During their mixed combustion process, there exists an interaction between C and N, and the reaction between the fuel and NH2 is crucial for the IDT of the mixed fuel [16]. Dong et al. [14] developed a detailed NH3/n-heptane reaction mechanism, which involves 2854 species and 11,790 elementary reactions, by integrating the NUIGMech1.2 mechanism with Glarborg et al.’s NH3 sub-mechanism [21], incorporating C3-C7/N reactions where the hydrogen abstraction rate constants between n-heptane and NH2 were derived from Song et al.’s CH4 + NH2 kinetics [22], while the linear alkene-NH2 radical reaction rates were determined by analogy with Mai et al.’s ethylene-NH2 reaction parameters [23]. Wang et al. [15] developed a reduced NH3/NC7H16 mechanism by modifying Dong’s framework [14], incorporating Chang et al.’s n-heptane sub-mechanism [7] with updated reaction pathways for n-heptane oxidation. Such a mechanism consists of only 74 species and 495 reactions. Both the Dong and Wang mechanisms demonstrate satisfactory predictive capabilities for IDT and LBV of NH3/NC7H16 blends.
Thorsen et al. [16] constructed an NH3/NC7H16 reaction mechanism, which is also a detailed one, and contains 1367 species and 6314 reactions, by augmenting Zhang et al.’s n-heptane model [6] and Glarborg et al.’s NH3 mechanism [21] with additional C3-C7/N reactions, where the rate constants for C-N interactions between n-heptane and ammonia were derived through analogical extrapolation from the studies of Siddique et al. [24,25], Chen et al. [26], Almodovar et al. [27], and Fang et al. [28]. The developed mechanism demonstrates satisfactory predictive capability for both IDT and key oxidized species concentrations in NH3/NC7H16 combustion. Fang et al. [17] developed a detailed NH3/NC7H16 mechanism by modifying Dong et al.’s model [14] through incorporating n-heptane pyrolysis intermediates’ recombination/disproportionation reactions and Meng et al.’s HNNO reactions [29], along with optimizing certain reaction rate coefficients, which demonstrates improved predictions for IDT, LBV and key oxidized species concentrations in NH3/NC7H16 combustion. 2860 species and 11,892 reactions are involved in the mechanism of Fang et al. [17].
In summary, from the perspective of practical engine applications, the in-cylinder combustion involves coupled chemical kinetics, heat transfer, and fluid dynamics. For in-cylinder simulation of marine ammonia/diesel dual-fuel engines, existing chemical reaction mechanisms for ammonia/n-heptane combustion exhibit significant variations in model scale and mechanistic granularity, while the experimental datasets and initial conditions employed for mechanism validation also differ substantially. For the ignition, oxidation, and flame propagation processes of the ammonia-n-heptane mixture, the discrepancies in predictive performances of these mechanisms are not clear enough. Moreover, whether these discrepancies will manifest in engine in-cylinder process simulations also requires further investigation.
Therefore, to reveal the differences in predictive performances among various ammonia-n-heptane reaction mechanisms from points of ignition, oxidation and flame, a comparative analysis of different mechanisms for the ammonia-n-heptane mixture is conducted on the basis of experimental data for ignition delay time, species concentrations for oxidation, and laminar burning velocity, and analyses of sensitivity and reaction pathway are also performed to explain such differences. Furthermore, by incorporating a multi-zone engine model, the prediction accuracy of various mechanisms is evaluated to gain insight into the difference among the mechanisms from a practical application perspective, which will provide a basis for the selection of the NH3/NC7H16 reaction mechanism for simulation of the diesel-ignited ammonia engine.
2. Research Methodology
2.1. Simulation Methods for Ignition, Oxidation and Flame Processes
As introduced previously, the existing NH3/NC7H16 reaction mechanisms mainly include ones developed by Dong et al. [14], Wang et al. [15], Thorsen et al. [16], and Fang et al. [17], respectively, with detailed specifications provided in Table 1. Firstly, this study will systematically compare the accuracy and discrepancies among these four mechanisms across three critical aspects: ignition, oxidation, and laminar flame propagation characteristics. The summary of the experimental data for these three aspects is shown in Table 2.

Table 1.
NH3/NC7H16 reaction mechanisms involved in the present study.

Table 2.
Experimental data of IDT, LBV and species concentration of oxidation used in the present study.
In this study, the CHEMKIN-Pro 15112 software was used to complete calculations of the ignition, oxidation, and flame propagation processes of the ammonia-n-heptane mixture. The ignition process was simulated using a zero-dimensional homogeneous closed reactor model, assuming a constant-volume adiabatic process. The IDT was defined as the time corresponding to the maximum OH concentration. Additionally, for the calculation of the ignition process in a rapid compressor, the variable volume file provided by the experiment was also used. For the prediction of the fuel oxidation process, the perfectly stirred reactor (PSR) module was mainly adopted for calculations, and the end time for the calculation was set to 10 times the residence time to obtain the steady-state modeled values. The laminar burning velocities were calculated using a one-dimensional planar freely propagating premixed laminar flame model. The grid points were adjusted through the gradient and curvature of the numerical solution to ensure the convergence of the calculated laminar burning velocity.
In this study, the ammonia blending ratio is defined using two methods: energy fraction and molar fraction. The energy fraction of NH3 is the ratio of the energy content of NH3 to the total energy of the blended fuel. The molar fraction of NH3 is the ratio of the number of moles of NH3 to the total number of moles of the blended fuel.
2.2. Methods for Reaction Pathway and Sensitivity Analyses
To compare the differences in the reaction pathways of NH3/NC7H16 blended fuels predicted by different chemical reaction mechanisms, this study analyzed the reaction pathways and consumption characteristics during the ignition process based on the simulation results. The discrepancies among various mechanisms were systematically compared and discussed. To compare the discrepancies in the key elementary reactions governing the consumption of major species among these mechanisms, sensitivity analysis of the mole fractions of NC7H16 and NH3 during the ignition process was performed. The sensitivity coefficients were calculated using Equation (1):
where Cj denotes the mole fraction of the j-th component and ki represents the rate constant of the i-th reaction. A negative sensitivity coefficient indicates that the elementary reaction has a promoting effect on fuel oxidation, while a positive sensitivity coefficient indicates that the elementary reaction has an inhibitory effect on fuel oxidation.
For prediction of the ignition delay time, species concentrations in the oxidation process and laminar burning velocity, the conditions for the modeling were determined according to the corresponding experiments. When conducting reaction pathway analysis, fuel component concentration analysis, and sensitivity analysis, considering the objective of the present study is to evaluate the performances of the ammonia-n-heptane reaction mechanisms in predicting the in-cylinder combustion processes, thus the pressure was elevated to higher values. At the same time, the temperature range was set from 650 K to 1250 K, specifically at 650 K, 950 K, and 1250 K, roughly corresponding to low-, medium-, and high-temperature conditions for the fuel ignition. In addition, given into the nonlinear influences of the ammonia content on combustion processes of the ammonia-n-heptane mixtures, the ammonia blending ratios were set as 10%, 60% and 90%. Comparative analysis of the results under these conditions can elucidate the differences among the studied reaction mechanisms.
2.3. Multi-Zone Engine Simulation Method
In the practical in-cylinder process of an engine, it is a strongly coupled process involving intense flow, heat transfer, and chemistry. To illustrate the influence of chemical kinetic models on engine simulation results, in the present study simulations of the in-cylinder combustion process for a marine diesel-ignited ammonia engine was conducted based on a multi-zone model, which is constructed considering the study of Easley et al. [30], and accordingly compared the predictive performance differences among various NH3/NC7H16 reaction mechanisms. In this study, the multi-zone engine model in CHEMKIN-PRO 15112 software was used. The prototype is a turbocharged four-stroke engine, and its specific parameters are shown in Table 3.

Table 3.
Specific parameters for the prototype engine involved in the present study.
In the process of engine simulation using the multi-zone model, the in-cylinder area of the engine needs to be divided. As shown in Figure 1, the in-cylinder area mainly involves the crevice zone, the boundary layer zone, the inner core zone and the outer zone. Except the inner core zone, which is assumed to be constant mass and adiabatic, the other zones can exchange mass and energy with each other. In the previous research [31], three-dimensional (3D) simulation on the performance and emissions of ammonia/diesel engines under various diesel substitution rates was performed. According to this three-dimensional simulation result, zone 11 corresponds to the mixture of n-heptane, ammonia, and air consumed within the 0–CA10 range; zones 7–10 correspond to the mixture of n-heptane, ammonia, and air consumed within the CA10–CA50 range; the remaining zones are all the remaining ammonia-air mixture. Values of contents of components in each zone were derived from the three-dimensional simulation result. In addition, the calculated starting crank angle is set to −10 °CA ATDC, and the initial pressure is set to 57.83 bar, which is also determined based on the previous three-dimensional simulation result. For the heat transfer model, it is set to no heat transfer loss. The specific area division is shown in Table 4.
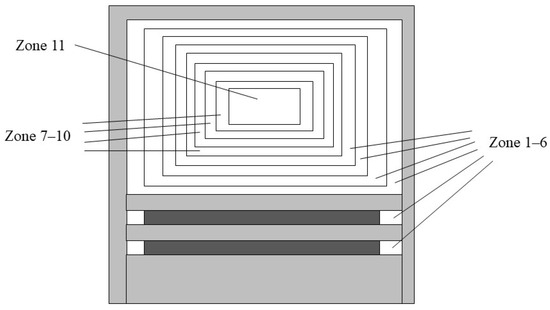
Figure 1.
Schematic diagram of the in-cylinder zone distribution.

Table 4.
Specific setting values for each partition.
3. Results and Discussion
3.1. Comparative Analysis of Ammonia-n-Heptane Reaction Mechanisms from the Points of Ignition, Oxidation and Flame Propagation
3.1.1. Ignition Delay Time
This study first compares the predictive performance differences among existing NH3/NC7H16 chemical reaction mechanisms based on the experimental values of IDT, component concentrations during the oxidation process, and laminar burning velocity.
For the IDT, the differences between the simulated values of four NH3/NC7H16 reaction mechanisms and the experimental data [17,18] were compared under both medium-to-low and high temperatures. The results are presented in Figure 2 and Figure 3, respectively. The conditions include pressures of 15 bar and 10 bar, equivalence ratios of 1 and 2, ammonia blending ratios of 20% and 40%, oxygen concentrations of 10% and 18%, and a temperature range of approximately 650–950 K. In Figure 2, the uncertainty for the presented experimental ignition delay time is less than ±15%. In Figure 3, such uncertainty is no more than ±10%.
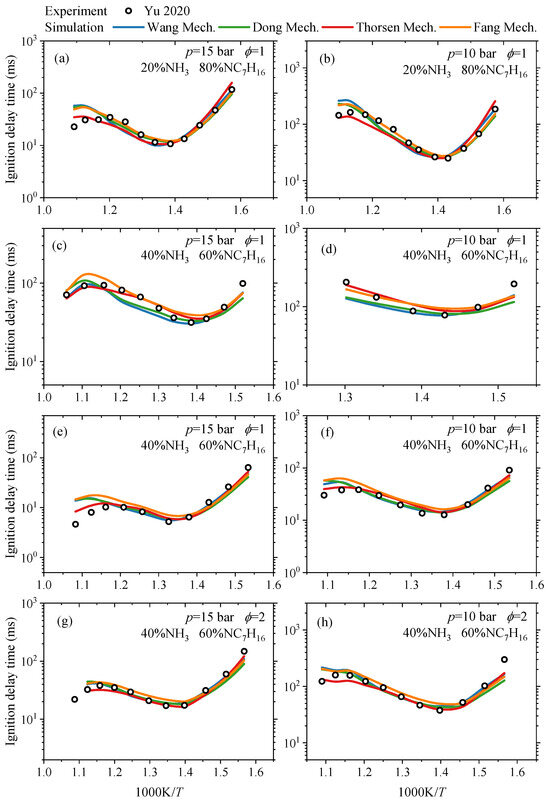
Figure 2.
Comparison of simulated IDTs of Wang mechanism [15], Dong mechanism [14], Thorsen mechanism [16] and Fang mechanism [17] and experimental ones from the study by Yu et al. [18] under medium-low temperatures and various ammonia blending ratios: (a) IDTs at NH3 energy content of 20% under p = 15 bar, ϕ = 1 and O2 mole fraction of 10.15% in the reactant; (b) IDTs at NH3 energy content of 20% under p = 10 bar, ϕ = 1 and O2 mole fraction of 10.15% in the reactant; (c) IDTs at NH3 energy content of 40% under p = 15 bar, ϕ = 1 and O2 mole fraction of 9.90% in the reactant; (d) IDTs at NH3 energy content of 40% under p = 10 bar, ϕ = 1 and O2 mole fraction of 9.90% in the reactant; (e) IDTs at NH3 energy content of 40% under p = 15 bar, ϕ = 1 and O2 mole fraction of 18.73% in the reactant; (f) IDTs at NH3 energy content of 40% under p = 10 bar, ϕ = 1 and O2 mole fraction of 18.73% in the reactant; (g) IDTs at NH3 energy content of 40% under p = 15 bar, ϕ = 2 and O2 mole fraction of 9.37% in the reactant; (h) IDTs at NH3 energy content of 40% under p = 10 bar, ϕ = 2 and O2 mole fraction of 9.37% in the reactant.
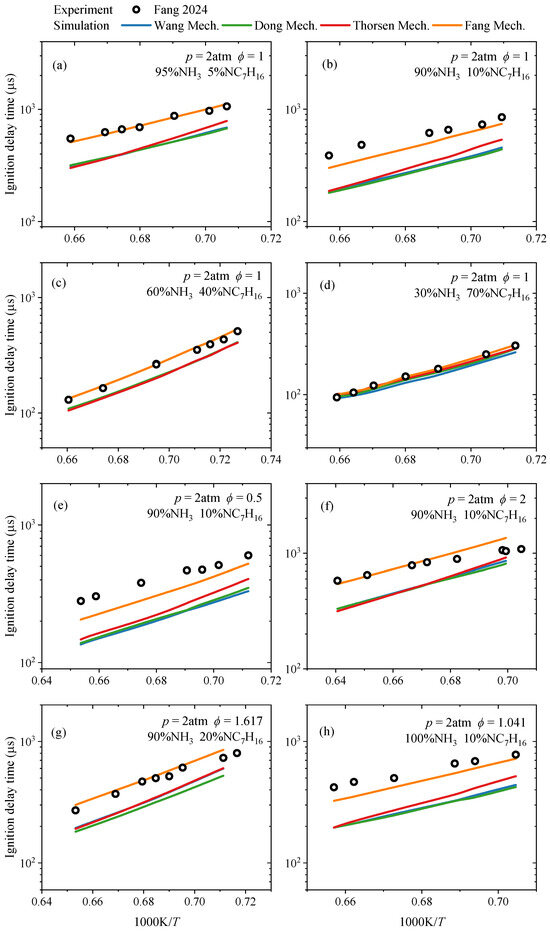
Figure 3.
Comparison of modeled values of IDTs from Wang mechanism [15], Dong mechanism [14], Thorsen mechanism [16] and Fang mechanism [17] and corresponding experimental ones from the study by Fang et al. [17] under high temperatures and various ammonia blending ratios (molar content): (a) IDTs at NH3 and NC7H16 contents of 95% and 5%, respectively, with p = 2 atm and ϕ = 1; (b) IDTs at NH3 and NC7H16 contents of 90% and 10%, respectively, with p = 2 atm and ϕ = 1; (c) IDTs at NH3 and NC7H16 contents of 60% and 40%, respectively, with p = 2 atm and ϕ = 1; (d) IDTs at NH3 and NC7H16 contents of 30% and 70%, respectively, with p = 2 atm and ϕ = 1; (e) IDTs at NH3 and NC7H16 contents of 90% and 10%, respectively, with p = 2 atm and ϕ = 0.5; (f) IDTs at NH3 and NC7H16 contents of 90% and 10%, respectively, with p = 2 atm and ϕ = 2; (g) IDTs at NH3 and NC7H16 contents of 90% and 20%, respectively, with p = 2 atm and ϕ = 1.617; (h) IDTs at NH3 and NC7H16 contents of 100% and 10%, respectively, with p = 2 atm and ϕ = 1.041.
From Figure 2, it can be seen that in general, under medium-to-low temperature conditions, the predicted IDT of the NH3/NC7H16 fuel by the four mechanisms are relatively consistent, and the differences from the experimental values are also small. Furthermore, there are certain discrepancies between the mechanism predictions and experimental data under specific temperature conditions. For instance, as shown in Figure 2a,b, when the ammonia blending ratio is 20% and the temperature ranges from 800 to 850 K, the simulated IDT by the Dong, Wang, and Fang mechanisms are higher than the corresponding experimental data. This phenomenon also occurs in Figure 2e,f, where the ammonia blending ratio is 40% with the equivalence ratio of 1 and the oxygen concentration of 18%. Meanwhile, Figure 2d indicates that the simulated IDT by the Dong and Wang mechanisms are lower in the vicinity of 650–750 K. Overall, within the temperature range shown in Figure 2, the discrepancies between the predicted IDT by the mechanisms and the experimental data mainly occur at relatively high temperatures.
Figure 3 presents a comparison between the simulated IDTs of four NH3/NC7H16 reaction mechanisms and experimental data under high-temperature conditions (1350–1600 K), with ammonia blending ratios of 30%, 60%, 90%, and 95%, equivalence ratios of 0.5, 1, and 2, and a pressure of 2 atm. It can be seen that all the investigated reaction mechanisms can capture the trend of IDT with the temperature under the given conditions. However, in terms of specific values, when the ammonia blending ratio is 30% and the equivalence ratio is 1, the predicted IDT of the four mechanisms is in good agreement with the experimental data. Under other conditions, the Fang mechanism shows higher prediction accuracy for the IDT, while the predictions of the remaining mechanisms, although relatively consistent, are all lower than the corresponding experimental values. According to the study by Fang et al. [17], this is caused by the lack of interaction reactions between n-heptane decomposition products and ammonia under high-temperature conditions. Decomposition products of n-heptane, such as C2H4, C3H6, and C4H6 undergo disproportionation, dehydrogenation and recombination reactions with NH2, which significantly affect the IDT of NH3/NC7H16 under high-temperature conditions.
Based on the comprehensive analysis of Figure 2 and Figure 3, it can be concluded that the NH3/NC7H16 reaction mechanisms involved in this study can predict the variation trend of IDT for NH3/NC7H16 mixtures within a wide range of ammonia blending ratios. In detail, under low ammonia blending ratios (≤40%), the simulated IDT of each mechanism at both medium-to-low and high temperatures shows good consistency with the experimental data. However, under high ammonia blending ratios and high-temperature conditions, the predicted IDTs of the Thorsen, Dong, and Wang mechanisms for NH3/NC7H16 mixtures are lower than the experimental values, while the Fang mechanism exhibits relatively higher prediction accuracy.
3.1.2. Oxidation
In terms of predicting the oxidation process of NH3/NC7H16 mixtures, the predicted species concentrations during oxidation by four NH3/NC7H16 reaction mechanisms and their comparison with experimental data are shown in Figure 4. The experimental data are obtained using a JSR setup by Pan et al. [19], with the conditions being a pressure of 1 atm, an equivalence ratio of 1, a mixture composition of 0.5% NH3, 0.5% NC7H16, 87.25% Ar, and 11.75% O2, and a residence time of 3 s. The data points represent values of species concentrations during the oxidation process at given temperatures after a predetermined reaction period (i.e., the residence time). In Figure 4, the uncertainty for the experimental species concentration in the oxidation depends on the type of species, which is within ±20%.
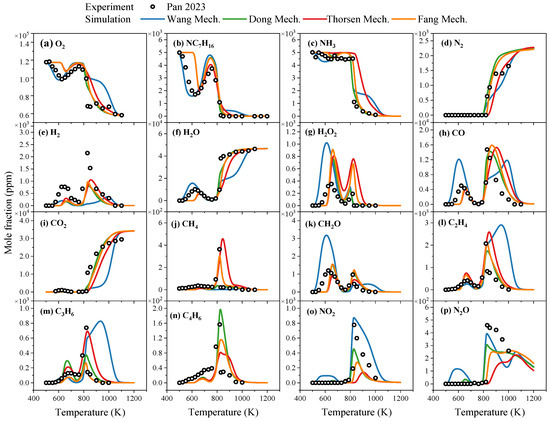
Figure 4.
Comparison of predicted oxidation concentrations of key components from Wang mechanism [15], Dong mechanism [14], Thorsen mechanism [16] and Fang mechanism [17] and experimental data from the study by Pan et al. [19] for NH3/NC7H16 mixtures under pressure of 1 atm and equivalence ratio of 1: (a) O2 concentrations; (b) NC7H16 concentrations; (c) NH3 concentrations; (d) N2 concentrations; (e) H2 concentrations; (f) H2O concentrations; (g) H2O2 concentrations; (h) CO concentrations; (i) CO2 concentrations; (j) CH4 concentrations; (k) CH2O concentrations; (l) C2H4 concentrations; (m) C3H6 concentrations; (n) C4H6 concentrations; (o) NO2 concentrations; (p) N2O concentrations.
As can be seen from Figure 4, for reactants NH3, NC7H16, O2 and complete combustion end products H2O, CO2, N2, the four existing mechanisms exhibit basically consistent predictive performance. For other end products such as CO, NO2, N2O and intermediate species like H2, H2O2, CH4, CH2O, C2H4, etc., the consistency of predictive performance among mechanisms varies depending on the type of component. Specifically, for NO2 and N2O concentrations, the prediction results of each mechanism differ significantly, with the Wang mechanism showing better agreement with experimental values. For H2 and H2O2, except for the Wang mechanism, other mechanisms demonstrate similar prediction trends but all have certain discrepancies with experimental data. For CO and CH2O, the predictions of the Dong, Thorsen, and Fang mechanisms are consistent and relatively close to experimental values. For C2H4, when the temperature exceeds 800 K, the prediction results of the Wang mechanism start to differ from those of other reaction mechanisms, while for CH4, C3H6, and C4H6, the prediction results of each mechanism are inconsistent with each other.
In summary, the studied four NH3/NC7H16 reaction mechanisms can basically predict the concentrations of reactants NH3, NC7H16 and complete combustion products N2, H2O and CO2, but for other products and intermediate species, the differences among the prediction results of each mechanism will vary depending on the type of species and reaction conditions.
3.1.3. Laminar Flame Propagation
In terms of premixed laminar burning velocities, the prediction results of Dong, Fang, and Wang mechanisms and their comparison with experimental data are shown in Figure 5. For the laminar burning velocity shown in Figure 5, the experimental uncertainty is usually less than 1 cm/s. The experimental data are derived from the study by Lubrano Lavadera et al. [20], under the conditions of 1 atm pressure, an initial temperature of 338 K, and ammonia blending ratios (molar fractions) of 25% and 50%. Since the Thorsen mechanism does not provide transport data, the predicted laminar burning velocities can not obtained from this mechanism. Thus, only results from the other three mechanisms are shown in the figure. As can be seen from Figure 5, under the investigated conditions, the Dong, Fang, and Wang mechanisms can all predict the variation trend of laminar burning velocity of NH3/NC7H16 mixtures with the equivalence ratio. The differences between the simulated values and experimental data are relatively small at both low and high equivalence ratios, where the results derived from the Fang mechanism are between those from Dong and Wang mechanisms. Additionally, the differences in laminar burning velocity prediction results among the mechanisms depend on the equivalence ratio, and the differences among the mechanisms at low and high equivalence ratios are lower than those near the stoichiometric equivalence ratio.
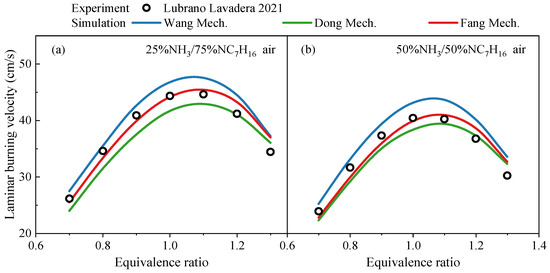
Figure 5.
Comparison of simulated laminar burning velocities by Wang mechanism [15], Dong mechanism [14] and Fang mechanism [17] and experimental data from the study by Lubrano Lavadera et al. [20] at pressure of 1 atm and temperature of 338 K under 25% and 50% ammonia blending ratios (molar content): (a) LBVs at NH3 content of 25% with various equivalence ratios; (b) LBVs at NH3 content of 50% with various equivalence ratios.
In summary, the existing NH3/NC7H16 reaction mechanisms can predict the variation trend of laminar burning velocities of NH3/NC7H16 mixtures with equivalence ratio under different ammonia blending ratios, and the predicted values show small differences from the experimental data at both low and high equivalence ratios.
3.2. Analyses of Reaction Pathways and Consumption Processes for Ammonia-n-Heptane Mixtures
3.2.1. Reaction Pathway Analysis
Previous analyses have revealed the differences in the predictive performance of existing NH3/NC7H16 reaction mechanisms for IDT, oxidation process component concentrations, and laminar burning velocity. To explore the mechanism underlying these discrepancies, this study investigates the reaction pathways of ammonia and n-heptane predicted by different mechanisms and compares the differences in key reactions affecting the consumption of ammonia and n-heptane.
Based on the ignition processes predicted by different NH3/NC7H16 reaction mechanisms, this study analyzes the reaction pathways of ammonia and n-heptane, as shown in Figure 6 and Figure 7 and Figures S1 and S2 in the Supplementary Material. The reactants include NC7H16, NH3, O2, and N2, with an equivalence ratio of 1, a pressure of 25 atm, a temperature range of 650 K to 1250 K, and an ammonia blending ratio of 90%. The analysis focuses on the pathways when 20% of n-heptane is consumed. Since the reaction pathways of n-heptane are already partially understood, this study only presents the main reaction pathways.
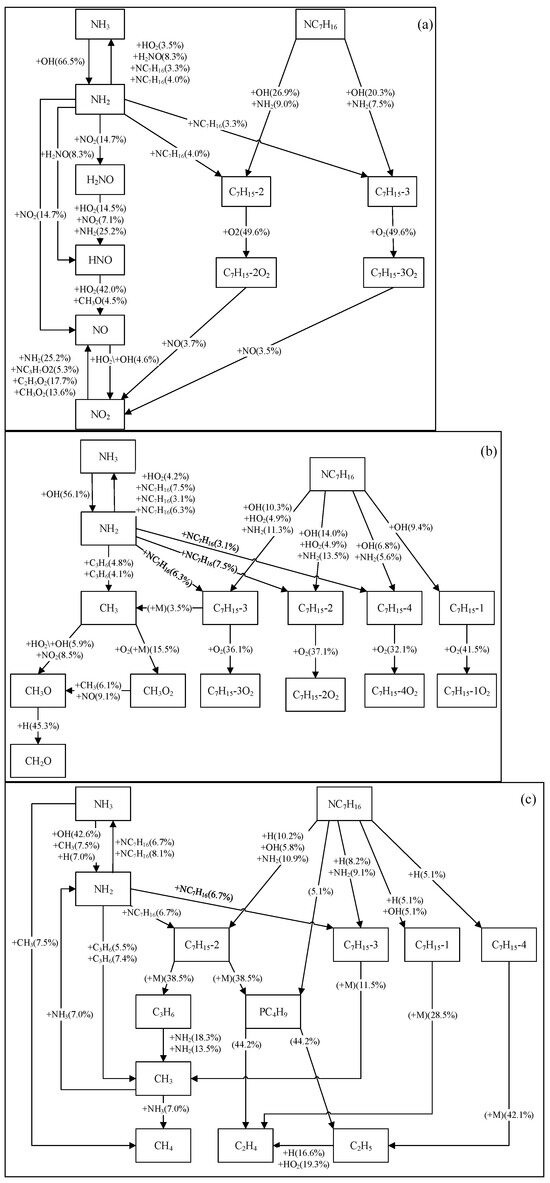
Figure 6.
Reaction pathway analyses of NH3 and NC7H16 based on Fang mechanism at 25 atm, 90% doped with ammonia (molar content), and different temperatures: (a) 650 K, (b) 950 K, (c) 1250 K.
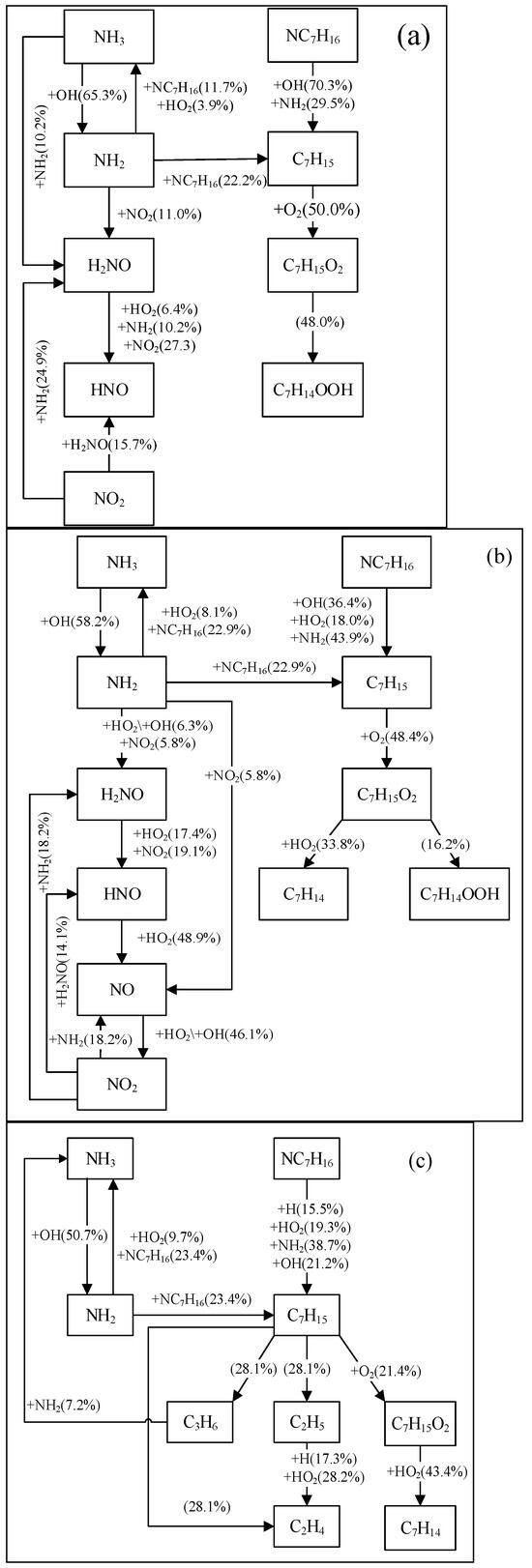
Figure 7.
Reaction pathway analyses of NH3 and NC7H16 based on Wang mechanism at 25 atm, 90% doped with ammonia (molar content), and different temperatures: (a) 650 K, (b) 950 K, (c) 1250 K.
Combining Figure 6 and Figure 7 and Figures S1 and S2, it can be seen that under the conditions of ammonia blending and 650 K, the simulation results of each NH3/NC7H16 reaction mechanism show that in addition to the dehydrogenation reaction of NC7H16 with OH radicals to form heptyl radicals, it can also undergo dehydrogenation by reacting with NH2 radicals. These heptyl radicals then undergo oxygen addition reactions with O2 to form RO2 radicals. The differences in the reaction pathways predicted by each mechanism are as follows: for the Fang mechanism, NO can react with C7H15-2O2 and C7H15-3O2 to form NO2. Since the Wang mechanism employs a simplified n-heptane mechanism, its predicted reaction pathway shows that NC7H16 forms C7H15 through reactions with NH2 and OH radicals. Additionally, NH2 is converted to H2NO via NO2, and subsequently, H2NO reacts with NO2 to generate HNO.
At 950 K, for the Fang, Dong, and Thorsen mechanisms, in addition to the similar reaction pathways shown in the figures, the Fang mechanism’s predicted pathway indicates that NH2 not only participates in the dehydrogenation of NC7H16 but also reacts with C3H6 to form NH3 and CH3. Additionally, in the Thorsen mechanism’s predicted pathway, C7H15-2 undergoes not only oxygen addition but also a cleavage reaction (i.e., C7H15-2 (+M) = C3H6 + PC4H9). For the Wang mechanism, its predicted pathway shows an increased importance of NO, and C7H15O2 not only isomerizes to form C7H14OOH but also generates C7H14.
At high temperatures of 1250 K, the differences in reaction pathways predicted by each mechanism are as follows. For the reaction pathway predicted by the Fang mechanism, C7H15-1 and C7H15-4 generate C2H4 and C2H5, respectively. The reaction pathway derived from the Dong mechanism shows that NC7H16 not only forms heptyl radicals through reactions with NH2, H, and OH radicals but also reacts with HO2 radicals to form C7H15-2 and C7H15-3. Subsequently, C7H15-3 reacts to form C4H8-1, n-C3H7, and CH3; C7H15-4 continues to react to produce C5H10-1. In the Thorsen mechanism, the predicted reaction pathway shows a reduction in the path flux of NC7H16 reacting to form C7H15-4. In the reaction pathway predicted by the Wang mechanism, the proportion of C7H15 cleavage to form C3H6 and C2H5 increases significantly.
In summary, the main reaction pathways of ammonia and n-heptane predicted by the Dong, Thorsen, and Fang mechanisms are basically consistent, but there are certain differences in the reaction flux of each pathway, which are mainly reflected in the NC7H16-NH2 reaction pathway. Since the Wang mechanism is a simplified mechanism, it can predict the main pathways of ammonia and n-heptane reactions, but there are significant differences in specific pathways compared with the prediction results of the other three mechanisms.
3.2.2. Consumption Process Analysis
On the basis of the above reaction pathway analysis, to further illustrate the consumption timing characteristics of ammonia and n-heptane fuels during the combustion process, this study analyzed the changes in the molar fraction distribution of ammonia and n-heptane over time during the ignition process based on each NH3/NC7H16 reaction mechanism. The conditions included pressures of 10–40 atm, temperatures of 650–1250 K, an equivalence ratio of 1, and ammonia blending ratios of 10%, 60%, and 90%, with the results shown in Figure 8 and Figures S3 and S4 in the Supplementary Material.
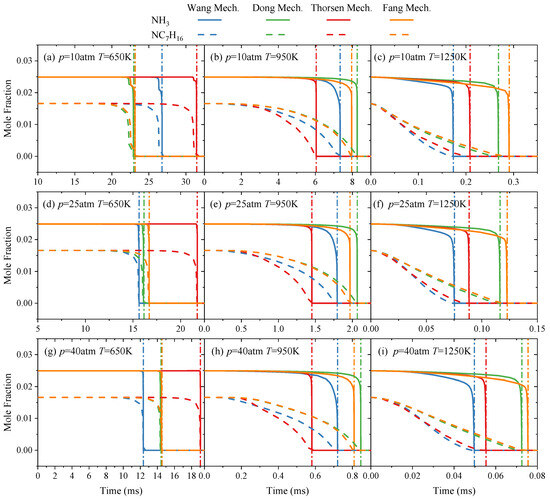
Figure 8.
Variations of NC7H16 and NH3 concentrations over time predicted by Wang mechanism [15], Dong mechanism [14], Thorsen mechanism [16] and Fang mechanism [17] at 60% ammonia blending ratio (molar content): (a) NC7H16 and NH3 concentrations at p = 10 atm and T = 650 K; (b) NC7H16 and NH3 concentrations at p = 10 atm and T = 950 K; (c) NC7H16 and NH3 concentrations at p = 10 atm and T = 1250 K; (d) NC7H16 and NH3 concentrations at p = 25 atm and T = 650 K; (e) NC7H16 and NH3 concentrations at p = 25 atm and T = 950 K; (f) NC7H16 and NH3 concentrations at p = 25 atm and T = 1250 K; (g) NC7H16 and NH3 concentrations at p = 40 atm and T = 650 K; (h) NC7H16 and NH3 concentrations at p = 40 atm and T = 950 K; (i) NC7H16 and NH3 concentrations at p = 40 atm and T = 1250 K. The Dot-dashed line indicates the position of ignition.
Taking the case at the 60% ammonia blending ratio as an example, it can be seen from Figure 8 that overall, the molar fraction of n-heptane starts to decrease earlier than that of ammonia, indicating that n-heptane is consumed first during the combustion process, and ammonia is rapidly consumed near the ignition timing. The higher the temperature, the earlier n-heptane starts to be consumed. Such phenomenon is also observed in the methane-n-heptane ignition process [32]. In fact, similar to methane, ammonia also has relatively weaker reactivity than n-heptane. Then when the auto-ignition conditions are reached, n-heptane with high reactivity will react firstly, leading to the establishment of the radical pool. After enough radicals are formed, ammonia with low reactivity starts to be consumed, occurring near the ignition time point. Additionally, the figure shows that under different temperatures and pressures, the fuel consumption rates predicted by each NH3/NC7H16 reaction mechanism vary to some extent. Specifically, at 650 K, within the studied pressure range, the Thorsen mechanism predicts the slowest fuel consumption rate. When the pressure increases, the fuel consumption rate predicted by the Wang mechanism accelerates, and at 25 atm, this mechanism corresponds to the fastest fuel consumption rate. At 950 K and 1250 K, increasing the pressure does not change the ranking of fuel consumption rates among the mechanisms. At the fixed pressure, with the temperature rising from 650 K to 950 K, the fuel consumption rate predicted by the Thorsen mechanism increases, while when the temperature rises to 1250 K, the Wang mechanism predicts the fastest fuel consumption rate.
Based on the above analyses of fuel reaction pathways and consumption processes, it is evident that different NH3/NC7H16 reaction mechanisms predict varying fuel consumption patterns. To further explain these discrepancies, this study comparatively analyzes the results of fuel consumption sensitivity analyses based on different NH3/NC7H16 reaction mechanisms, as shown in Figure 9 and Figure 10, and Figures S5 and S6 in the Supplementary Material. The sensitivity analysis is carried out based on the ignition process, with a pressure of 25 atm, temperatures of 650, 950 and 1250 K, an equivalence ratio of 1, and an ammonia blending ratio of 60%. This study mainly presents the sensitivity results when 20% of NH3 and NC7H16 are consumed.
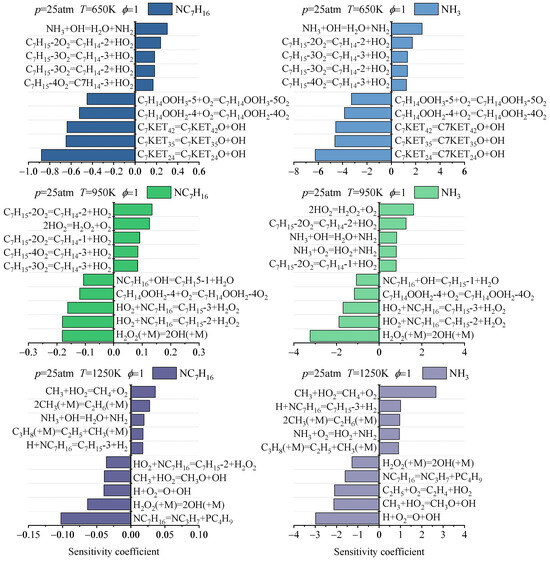
Figure 9.
Sensitivity analyses of NC7H16 and NH3 consumption based on Fang mechanism under 60% ammonia blending ratio (molar content).
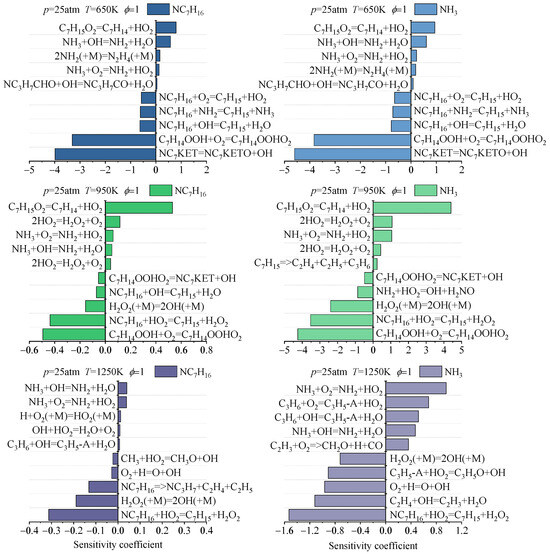
Figure 10.
Sensitivity analyses of NC7H16 and NH3 consumption based on Wang mechanism under 60% ammonia blending ratio (molar content).
As can be seen from Figure 9 and Figure 10, and Figures S5 and S6, reactions significantly influencing the consumption of NH3 and NC7H16 are primarily related to carbon-containing species reactions, illustrating the crucial role of NC7H16 in promoting NH3 consumption. More specifically, at T = 650 K, except for the Wang mechanism, the NH3/NC7H16 reaction mechanisms predict that the decomposition reactions of C7 (C7KET24 = C7KET24O + OH, C7KET35 = C7KET35O + OH, C7KET42 = C7KET42O + OH) and the secondary oxygen addition reactions of C7 molecules (C7H14OOH + O2) significantly promote the consumption of NH3 and NC7H16, while the reaction forming C7H14 and the reaction between NH3 and OH inhibit the consumption of NH3 and NC7H16. At this point, for the Wang mechanism, these reaction categories also have a significant impact on the consumption of NH3 and NC7H16. However, due to the lumped treatment adopted by this mechanism, the number of specific elementary reactions included is reduced. At 950 K, compared with the results at 650 K, for the Fang, Dong, and Thorsen mechanisms, the differences in sensitivity analysis results mainly manifest in the enhanced role of HO2 radicals in the fuel consumption process. The reaction of H2O2 decomposition to form OH has the greatest impact on promoting fuel consumption. For the sensitivity analysis results of the Wang mechanism, HO2 radicals also play a significant role, but at this time, the secondary oxygen addition reaction of C7 molecules still has the greatest impact on fuel consumption. When the temperature is increased to 1250 K, for the sensitivity analysis results of these four mechanisms, it can be seen that the influences of small-molecule-related reactions and the cracking reaction of n-heptane molecules on fuel consumption are significantly enhanced. However, for the Wang mechanism, the difference from the sensitivity analysis results of other mechanisms is that the reaction with the most important influences on the consumption of NC7H16 and NH3 is NC7H16 + HO2 = C7H15 + H2O2.
In summary, the sensitivity analyses of ammonia and n-heptane consumption processes indicate that, from the perspective of reaction categories, the reactions significantly influencing the consumption of ammonia and n-heptane in the four mechanisms exhibit certain similarities. However, in terms of specific reactions and their sensitivity coefficients, the fuel consumption sensitivity analysis results based on the four mechanisms show certain differences.
3.3. Comparisons of Ammonia-n-Heptane Reaction Mechanisms Based on Engine Simulation
The above analysis illustrates the differences in the prediction results of the Thorsen, Dong, Fang, and Wang mechanisms for ignition, oxidation and flame processes. Given into the multifactor coupling characteristics within the engine cylinder, this study also compares the predictive performance of the four mechanisms based on an engine simulation model, with the results shown in Figure 11 and Figure 12. The engine operating conditions are a diesel substitution rate of 60%, a rotational speed of 1500 RPM, and a 75% load. In previous studies, bench tests and three-dimensional simulation studies of diesel-ignited ammonia fuel engines were also carried out based on the engine with parameters shown in Table 2 [31]. For comparison, Figure 11 shows the experimental and simulated values of in-cylinder pressure under the corresponding conditions, and Figure 12 shows the in-cylinder temperature curves obtained from three-dimensional simulations, in which the Wang mechanism [15] was used for calculations in the three-dimensional simulation studies.

Figure 11.
Comparison of in-cylinder pressures predicted by Wang mechanism [15], Dong mechanism [14], Thorsen mechanism [16] and Fang mechanism [17] based on multi-zone model with experimental and 3D simulated values from the study by Huang et al. [31].
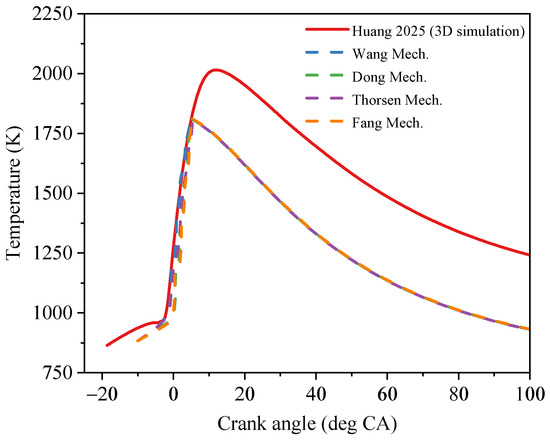
Figure 12.
Comparison of in-cylinder temperatures predicted by Wang mechanism [15], Dong mechanism [14], Thorsen mechanism [16] and Fang mechanism [17] based on multi-zone model with 3D simulated values from the study by Huang et al. [31].
As can be seen from Figure 11, for each of the studied NH3/NC7H16 reaction mechanisms, the curves of pressure versus crankshaft angle during the combustion stage before the in-cylinder pressure peak predicted by the multi-zone model are coincident, and show good agreement with the 3D simulation values and experimental values. In the expansion stage after the in-cylinder pressure peak, the in-cylinder pressure simulation values of each mechanism are lower than the experimental values and 3D simulation values. This is mainly because the used multi-zone model simplifies the in-cylinder process. Specifically, in the multi-zone engine simulation, n-heptane and ammonia have entirely entered the cylinder at the start of the in-cylinder combustion reaction, whereas in the 3D simulation and experiment, the diesel injection duration is longer and can extend to the top dead center, thereby leading to the in-cylinder pressure values in the expansion stage from the experiment and 3D simulation being higher than those predicted by the multi-zone model.
Additionally, at the in-cylinder pressure peak, the simulated value of the Thorsen mechanism is on the low side, while the simulated values of the remaining mechanisms are basically consistent. Furthermore, in Figure 12, it can be seen that the curves of in-cylinder temperature versus crankshaft angle predicted by each NH3/NC7H16 reaction mechanism are basically consistent. Compared with the in-cylinder temperature curves obtained from the 3D simulation, the in-cylinder temperature based on the multi-zone model of the engine is overall lower, which is also related to the differences in the way diesel enters the cylinder between the 3D simulation model and the multi-zone model, as well as the influence of strong turbulence in the actual engine cylinder.
In summary, although the Thorsen, Dong, Fang, and Wang mechanisms exhibit certain differences in the predictions of IDT, component concentrations during the oxidation process, and laminar burning velocities, from the perspective of engine simulations, each reaction mechanism shows no significant differences in the simulation of in-cylinder pressure and temperature.
4. Conclusions
This study comparatively analyzed the differences in predictive performance of existing ammonia-n-heptane reaction mechanisms from the perspectives of fundamental combustion characteristics and engine simulations. Specifically, based on experimental data for ignition delay time, species concentration during oxidation, and laminar burning velocity for ammonia-n-heptane mixtures, the predictive discrepancies among the Thorsen, Dong, Fang, and Wang mechanisms were compared. Reaction pathway analysis and sensitivity analysis were conducted to explore the underlying mechanisms of these differences. Finally, using a multi-zone engine model and comparing with experimental and three-dimensional simulation results, the study analyzed the differences among different ammonia-n-heptane reaction mechanisms from the perspective of in-cylinder process simulation. The main conclusions of this study are as follows:
- (1)
- In terms of ignition delay time, under conditions of low ammonia blending ratios (≤40%), the simulated values of each mechanism show good agreement with experimental data. Under high ammonia blending ratios and high-temperature conditions, the Fang mechanism exhibits relatively higher prediction accuracy, while the predictions of other mechanisms are lower than experimental values. In the oxidation process, each reaction mechanism can generally predict the concentrations of reactants and complete combustion end products. However, for other end products and intermediate species, the discrepancies in the prediction results of each mechanism vary, depending on the type of substance and reaction conditions. Regarding laminar burning velocities, existing reaction mechanisms can predict the variation trend of laminar burning velocities with the equivalence ratio, and the predictions at low and high equivalence ratios show small differences from the experimental values.
- (2)
- The main reaction pathways of ammonia and n-heptane predicted by the Dong, Thorsen, and Fang mechanisms are basically consistent, but there are certain differences in the reaction fluxes of each pathway. The Wang mechanism can predict the main pathways of ammonia and n-heptane reactions, but there are significant differences in specific pathways compared with the prediction results of the other three mechanisms. Under different temperatures and pressures, the fuel consumption rates corresponding to each ammonia-n-heptane reaction mechanism are somewhat different. From the perspective of reaction classes, the reactions significantly affecting the consumption of ammonia and n-heptane in each mechanism have certain similarities, but in terms of specific reactions and the sensitivity coefficients, the sensitivity analysis results of each mechanism present certain differences.
- (3)
- As a consequence, the Fang mechanism can be selected to understand more accurately ignition, oxidation and flame processes of ammonia-n-heptane mixtures, while to reduce the engineering computational cost of the engine simulation, the Wang mechanism tends to be a good choice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fire8090357/s1, Figure S1: Reaction pathway analyses of NH3 and NC7H16 based on Dong mechanism at 25 atm, 90% doped with ammonia (molar content), and different temperatures: (a) 650 K, (b) 950 K, (c) 1250 K; Figure S2: Reaction pathway analyses of NH3 and NC7H16 based on Thorsen mechanism at 25 atm, 90% doped with ammonia (molar content), and different temperatures: (a) 650 K, (b) 950 K, (c) 1250 K; Figure S3: Variations of NC7H16 and NH3 concentrations over time predicted by Wang mechanism [15], Dong mechanism [14], Thorsen mechanism [16] and Fang mechanism [17] at 10% ammonia blending ratio (molar content); Figure S4: Variations of NC7H16 and NH3 concentrations over time predicted by Wang mechanism [15], Dong mechanism [14], Thorsen mechanism [16] and Fang mechanism [17] at 90% ammonia blending ratio (molar content); Figure S5: Sensitivity analyses of NC7H16 and NH3 consumption based on Dong mechanism under 60% ammonia blending ratio (molar content); Figure S6: Sensitivity analyses of NC7H16 and NH3 consumption based on Thorsen mechanism under 60% ammonia blending ratio (molar content).
Author Contributions
Conceptualization, L.L. and Y.C.; methodology, J.L.; investigation, Y.H. and Q.C.; validation and data curation, J.L. and H.Y.; writing—original draft preparation, Y.H. and Q.C.; writing—review and editing, Y.C., J.L. and N.Z.; visualization, Y.H. and Q.C.; supervision, L.L. and Y.C.; project administration and funding acquisition, L.L. and N.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Guangxi Yuchai Marine and Genset Power Co., Ltd., and National Natural Science Foundation of China (No. 52301382).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Yue Chen was employed by the company Guangxi Yuchai Marine and Genset Power Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for Power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A Review of Ammonia as a Compression Ignition Engine Fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Niki, Y. Reductions in Unburned Ammonia and Nitrous Oxide Emissions From an Ammonia-Assisted Diesel Engine With Early Timing Diesel Pilot Injection. J. Eng. Gas Turbines Power 2021, 143, 91014. [Google Scholar] [CrossRef]
- Yousefi, A.; Guo, H.; Dev, S.; Lafrance, S.; Liko, B. A Study on Split Diesel Injection on Thermal Efficiency and Emissions of an Ammonia/Diesel Dual-Fuel Engine. Fuel 2022, 316, 123412. [Google Scholar] [CrossRef]
- Jin, S.; Wu, B.; Zi, Z.; Yang, P.; Shi, T.; Zhang, J. Effects of Fuel Injection Strategy and Ammonia Energy Ratio on Combustion and Emissions of Ammonia-Diesel Dual-Fuel Engine. Fuel 2023, 341, 127668. [Google Scholar] [CrossRef]
- Zhang, K.; Banyon, C.; Bugler, J.; Curran, H.J.; Rodriguez, A.; Herbinet, O.; Battin-Leclerc, F.; B’Chir, C.; Heufer, K.A. An Updated Experimental and Kinetic Modeling Study of N-Heptane Oxidation. Combust. Flame 2016, 172, 116–135. [Google Scholar] [CrossRef]
- Chang, Y.; Jia, M.; Wang, P.; Niu, B.; Liu, J. Construction and Derivation of a Series of Skeletal Chemical Mechanisms for N-Alkanes with Uniform and Decoupling Structure Based on Reaction Rate Rules. Combust. Flame 2022, 236, 111785. [Google Scholar] [CrossRef]
- Frassoldati, A.; Cuoci, A.; Stagni, A.; Faravelli, T.; Ranzi, E. Skeletal Kinetic Mechanism for Diesel Combustion. Combust. Theory Model. 2017, 21, 79–92. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y.; Wang, X.; Wang, P. Development of a Skeletal Mechanism for Tri-Component Diesel Surrogate Fuel: N-Hexadecane/Iso-Cetane/1-Methylnaphthalene. Fuel 2020, 259, 116217. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, D.; Yu, L.; Qian, Y.; Lu, X. Construction of a Skeletal Multi-Component Diesel Surrogate Model by Integrating Chemical Lumping and Genetic Algorithm. Fuel 2022, 313, 122711. [Google Scholar] [CrossRef]
- Otomo, J.; Koshi, M.; Mitsumori, T.; Iwasaki, H.; Yamada, K. Chemical Kinetic Modeling of Ammonia Oxidation with Improved Reaction Mechanism for Ammonia/Air and Ammonia/Hydrogen/Air Combustion. Int. J. Hydrogen Energy 2018, 43, 3004–3014. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Lhuillier, C.; Barbosa, A.A.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C.; Seidel, L.; Mauss, F. An Experimental and Modeling Study of Ammonia with Enriched Oxygen Content and Ammonia/Hydrogen Laminar Flame Speed at Elevated Pressure and Temperature. Proc. Combust. Inst. 2021, 38, 2163–2174. [Google Scholar] [CrossRef]
- Zhou, S.; Cui, B.; Yang, W.; Tan, H.; Wang, J.; Dai, H.; Li, L.; Rahman, Z.U.; Wang, X.; Deng, S.; et al. An Experimental and Kinetic Modeling Study on NH3/Air, NH3/H2/Air, NH3/CO/Air, and NH3/CH4/Air Premixed Laminar Flames at Elevated Temperature. Combust. Flame 2023, 248, 112536. [Google Scholar] [CrossRef]
- Dong, S.; Wang, B.; Jiang, Z.; Li, Y.; Gao, W.; Wang, Z.; Cheng, X.; Curran, H.J. An Experimental and Kinetic Modeling Study of Ammonia/n-Heptane Blends. Combust. Flame 2022, 246, 112428. [Google Scholar] [CrossRef]
- Wang, B.; Dong, S.; Jiang, Z.; Gao, W.; Wang, Z.; Li, J.; Yang, C.; Wang, Z.; Cheng, X. Development of a Reduced Chemical Mechanism for Ammonia/n-Heptane Blends. Fuel 2023, 338, 127358. [Google Scholar] [CrossRef]
- Thorsen, L.S.; Jensen, M.S.T.; Pullich, M.S.; Christensen, J.M.; Hashemi, H.; Glarborg, P.; Alekseev, V.A.; Nilsson, E.J.K.; Wang, Z.; Mei, B.; et al. High Pressure Oxidation of NH3/n-Heptane Mixtures. Combust. Flame 2023, 254, 112785. [Google Scholar] [CrossRef]
- Fang, Y.; Qu, W.; Feng, L. Experimental and Kinetic Modeling Study on Auto-Ignition of Ammonia/n-Heptane Mixtures at Intermediate Temperatures. Combust. Flame 2024, 265, 113488. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, W.; Feng, Y.; Wang, W.; Zhu, J.; Qian, Y.; Lu, X. The Effect of Ammonia Addition on the Low-Temperature Autoignition of n-Heptane: An Experimental and Modeling Study. Combust. Flame 2020, 217, 4–11. [Google Scholar] [CrossRef]
- Pan, J.; Tang, R.; Wang, Z.; Gao, J.; Xu, Q.; Shu, G.; Wei, H. An Experimental and Modeling Study on the Oxidation of Ammonia and N-Heptane with JSR. Proc. Combust. Inst. 2023, 39, 477–485. [Google Scholar] [CrossRef]
- Lubrano Lavadera, M.; Han, X.; Konnov, A.A. Comparative Effect of Ammonia Addition on the Laminar Burning Velocities of Methane, n -Heptane, and Iso-Octane. Energy Fuels 2021, 35, 7156–7168. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling Nitrogen Chemistry in Combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Song, S.; Golden, D.; Hanson, R.; Bowman, C.; Senosiain, J.; Musgrave, C.; Friedrichs, G. A Shock Tube Study of the Reaction NH2 + CH4 → NH3 + CH3 and Comparison with Transition State Theory. Int. J. Chem. Kinet. 2003, 35, 304–309. [Google Scholar] [CrossRef]
- Mai, T.V.T.; Duong, M.v.; Huynh, L.K. Theoretical Kinetics of the C2H4 + NH2 Reaction. Combust. Flame 2020, 215, 193–202. [Google Scholar] [CrossRef]
- Siddique, K.; Altarawneh, M.; Gore, J.; Westmoreland, P.R.; Dlugogorski, B.Z. Hydrogen Abstraction from Hydrocarbons by NH2. J. Phys. Chem. A 2017, 121, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Siddique, K.; Altarawneh, M.; Saeed, A.; Zeng, Z.; Gore, J.; Dlugogorski, B.Z. Interaction of NH2 Radical with Alkylbenzenes. Combust. Flame 2019, 200, 85–96. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Yang, Y.; Brear, M.J.; He, X.; Wang, Z. Impact of Nitric Oxide (NO) on n-Heptane Autoignition in a Rapid Compression Machine. Combust. Flame 2017, 186, 94–104. [Google Scholar] [CrossRef]
- Almodovar, C.A.; Goldsmith, C.F. Laser Schlieren Study of the Thermal Decomposition of 2-Ethylhexyl-Nitrate. Proc. Combust. Inst. 2021, 38, 997–1005. [Google Scholar] [CrossRef]
- Fang, R.; Saggese, C.; Wagnon, S.W.; Sahu, A.B.; Curran, H.J.; Pitz, W.J.; Sung, C.-J. Effect of Nitric Oxide and Exhaust Gases on Gasoline Surrogate Autoignition: Iso-Octane Experiments and Modeling. Combust. Flame 2022, 236, 111807. [Google Scholar] [CrossRef]
- Meng, Q.; Lei, L.; Lee, J.; Burke, M.P. On the Role of HNNO in NOx Formation. Proc. Combust. Inst. 2023, 39, 551–560. [Google Scholar] [CrossRef]
- Easley, W.; Agarwal, A.; Lavoie, G. Modeling of HCCI Combustion and Emissions Using Detailed Chemistry. SAE Tech. Pap. 2001, 110, 1045–1061. [Google Scholar] [CrossRef]
- Huang, Y.; Lyu, L.; Liang, J.; Yang, H.; Zhu, N.; Sang, H.; Zhang, X. Performance and Emission Characteristics of Marine Ammonia/Diesel Dual-Fuel Engines at Different Diesel Substitution Rates. Fuel 2025, 379, 132967. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Z.; Li, G.; Wan, Q.; Xu, L.; Fan, S. Experimental and Kinetic Studies of Ignition Processes of the Methane–n-heptane Mixtures. Fuel 2019, 235, 522–529. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).