Abstract
Melamine formaldehyde particle boards (MFPBs), commonly utilized as a wooden decorative material in traditional architecture, demonstrate considerable performance deterioration with extended age, with reductions in essential flame retardancy and structural integrity presenting substantial risks to fire safety in structures. This research examines the impact of thermo-oxidative aging on the flame retardancy of MFPBs. The morphological evolution, surface composition, and flame-retardant characteristics of aged MFPBs were examined via scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TG), limiting oxygen index (LOI), and cone calorimeter (CCT). The results indicate that thermo-oxidative aging (60 °C, 1440 h) markedly reduces the activation energy (E, by 17.05%), pre-exponential factor (A, by 68.52%), LOI value (by 4%, from 27.5 to 26.4), and time to ignition (TTI, by 17.1%, from 41 s to 34 s) while augmenting the peak mass loss rate (MHRR, by 4.7%) and peak heat release rate (pHRR, by 20.1%). Subsequent investigation indicates that aging impairs the char layer structure on MFPB surfaces, hastens the migration and degradation of melamine formaldehyde resin (MFR), and alters the dynamic equilibrium between “MFR surface enrichment” and “thermal decomposition”. The identified degradation thresholds and failure mechanisms provide essential parameters for developing aging-resistant fireproof composites, meeting the pressing demands of building safety requirements and sustainable material design.
1. Introduction
In recent years, significant building fires have presented a grave risk to public safety. Global mandatory regulations, including China’s GB 50016 [1], the US ASTM E84 [2], and the EU EN 13501-1 [3], explicitly mandate the utilization of flame-retardant ornamental materials with designated fire ratings in high-rise structures and heavily populated areas. Flame-retardant boards serve as essential flame-retardant elements, effectively inhibiting the spread of fire and providing crucial time for protecting lives and property. Presently, prevalent products include gypsum-based flame-retardant boards, flame-retardant oriented strand boards (OSBs), and rock wool composite boards. MFPBs with MFR as an adhesive have emerged as a fundamental material in furniture and interior design, owing to their superior mechanical qualities and aesthetic versatility. The flame-retardant properties of MFPBs stem from the thermal breakdown features of MFR, which serves as a nitrogen-doped flame-retardant additive. In contrast to main flame retardants (e.g., phosphorus-based systems), MFR predominantly functions as a gas-phase diluent by emitting inert gases, diminishing fuel concentration in the combustion zone. It collaborates with the char development of the wood substrate to inhibit combustion [4].
Nonetheless, flame-retardant panels subjected to natural conditions for extended durations frequently undergo performance deterioration. A 60-month natural weathering test indicates that the flame-retardant efficacy of water-soluble flame-retardant wood markedly diminishes within 3 to 12 months [5]. The heat release during the burning of aged wood increases by more than 30% [6], suggesting that external conditions (temperature [7], humidity [8], and light [9]) significantly contribute to the deterioration of the material’s physicochemical structure, hence impairing flame-retardant ability.
Temperature-induced thermo-oxidative aging is acknowledged as the primary material degradation mechanism among these environmental elements. Thermo-oxidative conditions can induce the hydrolysis of hemicellulose and the loss of hydroxyl groups in wood-based substrates [10], resulting in decreased hygroscopicity [11] and impaired mechanical characteristics [12]. Moreover, the diminished thermal stability of adhesives [13] and the surface migration of flame retardants [14] (including the diffusion of nitrogen-doped flame-retardant additive to the material surface) compromise flame-retardant efficacy [15,16]. Following thermo-oxidative aging at 150 °C, the migration of flame retardants in OMMT/IFR/LGFPP (MCA) composites enhances the porosity of the char layer, resulting in a 21.8% reduction in the LOI [17].
Temperature plays a substantially greater role in the aging process compared to other factors: multiple regression analysis indicates that temperature is responsible for 60–80% of the flame-retardant leaching rate, whereas the direct influences of humidity and light, typically functioning through synergistic mechanisms (e.g., hygrothermal aging), contribute less than 20%. Temperature plays a substantially greater role in the aging process compared to other factors: multiple regression analysis indicates that temperature is responsible for 60–80% of the flame retardant leaching rate [18], whereas the direct influences of humidity and light, typically functioning through synergistic mechanisms (e.g., hygrothermal aging), contribute less than 20% [19]. This establishes a theoretical foundation for simulating intricate environmental aging under single-temperature settings. In the thermo-oxidative life prediction model for polyethylene, the error of the Arrhenius equation derived from single-temperature accelerated aging studies is under 5%, confirming the dependability of this approach [20].
Despite significant advancements in flame-retardant materials [21,22], the long-term performance evolution of MFPBs remains inadequately investigated. Although recent initiatives have concentrated on the formulation of novel flame retardants [23,24,25,26], the alterations in the performance of established MFPBs utilizing MFR as an adhesive in thermo-oxidative conditions-especially the correlations between the nitrogen release kinetics of MFR, modifications in the pore structure of wood substrates, and flame-retardant efficacy-necessitate thorough examination. An extensive examination of these processes is crucial for elucidating the safety application attributes of MFPBs in high-temperature and high-humidity environments.
The progression of flame-retardant performance metrics for MFPBs across various thermo-oxidative aging durations, encompassing the LOI, heat release rate, and char morphology, alongside microstructural attributes elucidated by FTIR, X-ray photoelectron spectroscopy (XPS), and SEM, has been meticulously examined. The interplay between nitrogen-doped flame-retardant additive migration and substrate degradation has been a focal point. The impact of alterations in the internal chemical functional groups, elemental distribution, and surface morphology of MFPBs under thermo-oxidative conditions on flame-retardant efficacy has been scrutinized. This research provides a foundational comprehension of MFPB behavior during thermo-oxidative aging, offers specific theoretical support for prolonging the service life of flame-retardant panels, delineates clear technical pathways for process optimization, and enhances the safety and reliability of MFPBs in practical applications.
2. Materials and Methods
2.1. Sample Preparation
The MFPBs were sourced from Jiangsu Xinsheng Steel-Wood Furniture Co., Ltd. (production batch XSP-2022-MF01, Suzhou, China). Samples measuring 100 mm × 100 mm × 15 mm were fabricated by ISO 291:2008 [27], encompassing seven aging periods (0, 240, 480, 720, 960, 1200, and 1440 h) with five replicates per group (total n = 35). Before aging, the samples received thermo-oxidative treatment in a model 101-3B forced-air drying oven (Alwhales Electronic Technology (Shanghai) Co., Ltd., Shanghai, China). The chamber temperature was meticulously regulated throughout the aging process at 60 ± 1 °C (seen by four-zone thermocouples at a sampling frequency of 1 Hz). At the same time, the air velocity was adjusted to 0.5 ± 0.1 m/s utilizing an ANEMOMASTER 6242 anemometer. The samples were selected in the center for each aging interval to mitigate edge effects. After aging, the samples were secondary-machined to dimensions specified in Table 1 according to the respective testing requirements.

Table 1.
Dimensional requirements for test samples in various tests.
2.2. Structural Characterization Techniques
2.2.1. SEM
Microstructural characterization of the MFPB samples subjected to varying aging durations was performed using a MIRA LMS field-emission scanning electron microscope (FE-SEM, TESCAN, Brno, Czech Republic), coupled with energy-dispersive X-ray spectroscopy (EDS) for elemental analysis. The accelerating voltage was maintained at 20 eV with a working distance optimized for 1 mm thick samples. Before analysis, all the samples were sputter-coated with a 10 nm gold layer to enhance surface conductivity.
2.2.2. FTIR Spectroscopy
The chemical structures of the MFPB samples under different aging durations were characterized via a Nicolet iS10 FTIR spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The spectrometer exhibits a spectral range of 4000 cm−1 to 400 cm−1, with a resolution superior to 0.5 cm−1 and wavenumber precision exceeding 0.01 cm−1. Before measurement, the samples were pulverized and sieved, and fine powders of approximately 320 mesh were selected for sample preparation to ensure homogeneity of the test samples.
2.3. Flame Retardancy Evaluation
2.3.1. TG
TG was performed utilizing a TA-Q50 analyzer (TA Instruments, Newcastle, DE, USA). Approximately 10 mg of the powdered MFPB samples, subjected to different aging periods, were compacted into thin films and placed in alumina crucibles. The studies were conducted with a 50 mL/min airflow and 5 K/min heating rate, ranging from 30 °C to 800 °C, to evaluate thermal breakdown behavior.
2.3.2. LOI
The LOI of the MFPB samples exposed to different durations of thermo-oxidative aging was measured using a JF-3 oxygen index meter (JN Analysis Instruments Co., Ltd., Nanjing, China), in strict compliance with the Chinese National Standard GB/T 2406.2-2009 (Plastics-Determination of burning behavior by oxygen index-Part 2: Ambient-temperature test) [28]. Each experimental group comprised three separately prepared samples, and the average oxygen index was recorded as the final result.
2.3.3. CCT
The combustion behavior of the MFPB samples with varying thermo-oxidative aging durations was investigated using a cone calorimeter (Motis Combustion Technology (China) Co., Ltd., Suzhou, China) under horizontal configuration without grid fixtures following the ISO 5660-1 standard [29] at a controlled heat flux of 50 kW/m2. Each group of experiments included three independently prepared samples, and the average values of key parameters (such as peak heat release rate and total heat release) were taken as the final results.
3. Results and Discussion
3.1. Morphological Characteristics and Surface Composition
3.1.1. Macro-/Microscopic Morphological Evolution Analysis
The macroscopic morphological evolution analysis primarily focused on investigating the influence of thermo-oxidative aging duration on the surface and subsurface microstructure of MFPBs. Thus, it provided experimental evidence for elucidating the processes through which thermo-oxidative aging affects the flame retardancy of MFPBs.
Figure 1 displays the macroscopic morphologies of the MFPB samples subjected to varying thermo-oxidative aging durations. Prolonged aging induced a progressive chromatic transition from pale yellow to dark brown (ΔELab = 32.09361, measured according to the DIN 6176 [30]). This phenomenon is attributed to the formation of chromophoric carbonyl groups generated through the cooperative thermo-oxidative decomposition of MFPB constituents.

Figure 1.
Macro-morphological evolution of MFPBs under thermo-oxidative aging durations.
Furthermore, the tactile evaluation revealed significant powder accumulation on the MFPB surface after extended aging (1440 h), with the visual assessment scale increasing from Grade 0 [no powdering] to Grade 3.5 [near-complete coverage] on a 4-point classification system. The powder formation processes can be primarily attributed to the following:
(1) Thermally driven migration of MFR to the material surface;
(2) Partial disintegration of hemicellulose, cellulose, lignin, and MFR components under thermo-oxidative conditions, resulting in lower molecular weight and the subsequent exudation of low-molecular-weight samples.
Figure 2 illustrates the microstructural history of MFPB surfaces before and after thermo-oxidative aging, examined at magnifications of 200× and 500× to elucidate surface topography and intricate features.
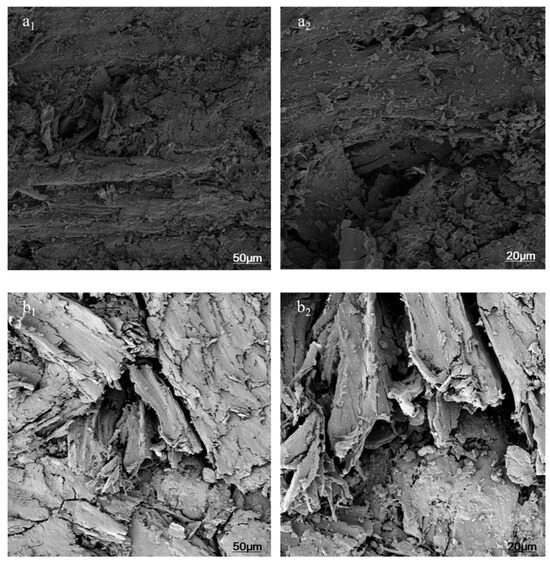
Figure 2.
Comparative SEM micrographs of MFPB surfaces before and after thermo-oxidative aging: (a1) pristine sample at 200×; (a2) pristine sample at 500×. (b1) aged 1440 h samples by thermo-oxidative aging at 200×; (b2) aged 1440 h samples by thermo-oxidative aging at 500×.
(a) Pristine Sample
a1 (200×): The pristine sample has a consistently smooth surface with interstices occupied by MFR, creating a continuous and cohesive barrier layer. This structure effectively obstructs the transfer of external oxygen and internal pyrolysis gases, thereby improving flame retardancy.
a2 (500×): Higher magnification supports the original structure’s integrity, exhibiting a well-bonded interface between MFR and the matrix without apparent fissures.
(b) Aged 1440 h Samples by Thermo-Oxidative Aging
b1 (200×): The aged sample exhibits markedly heightened surface roughness accompanied by numerous irregular fissures, signifying structural deterioration caused by thermo-oxidative influences.
b2 (500×): Detailed examination uncovers “fish-scale” cracking patterns and voids resulting from decomposing MFR, which inadequately emits inert gases (e.g., N2 or NH3) to occupy the interstices. These structural defects significantly impair the material’s capacity to impede heat and flammable gas transfer, reducing flame retardancy.
A comparative investigation at various magnifications demonstrates that thermo-oxidative aging facilitates the degradation of cellulose, hemicellulose, and MFR, altering the MFPB surface from a “thick insulating covering” to a “porous cracked structure”. The microstructural evolution directly relates to the degradation processes of the MFPB’s flame-retardant performance.
3.1.2. EDS Analysis
The primary components identified in the MFPBs investigated in this study include hemicellulose, cellulose, lignin, and MFR, all containing predominant C and O elements. Notably, the MFR component exhibited trace N content compared to the other constituents. Additionally, minor inorganic elements such as Na, Mg, Al, and Si were detected in the MFPB matrix.
To elucidate the flame-retardant processes of MFPBs under thermo-oxidative aging conditions, comparative EDS analyses were conducted on the surface elemental composition of MFPB samples before and after aging treatment.
Figure 3 and Table 2 present the EDS spectra and corresponding compositional data of the MFPB samples in pristine and thermally oxidized states (aged 1440 h), respectively. The EDS analysis revealed a marked increase in the N mass fraction from 12.35% (pristine) to 17.24% (aged 1440 h), accompanied by an elevated N/C atomic ratio from 0.18 to 0.26. These quantitative changes indicate the surface-directed migration of MFR components under thermo-oxidative aging conditions.
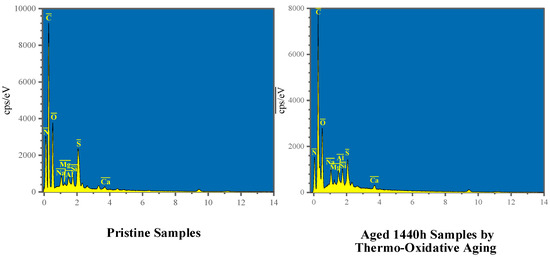
Figure 3.
Comparative EDS spectra of MFPB surfaces before and after thermo-oxidative aging.

Table 2.
Surface EDS data of MFPB samples before and after thermo-oxidative aging.
Concurrently, the C content decreased from 58.07% to 55.61%, while the O content declined from 20.71% to 19.88%. This inverse correlation suggests oxidative degradation processes, whereby hemicellulose, cellulose, and MFR undergo chain scission into low-molecular-weight samples. These fragmented compounds combine with oxygen and volatilize as CO2, as evidenced by the diminished surface oxygen retention.
The observed elemental redistribution provides a mechanistic explanation for the surface pulverization phenomenon, which arises from the partial decomposition of surface constituents. Such microstructural evolution aligns with thermo-oxidative cleavage of glycosidic bonds in polysaccharides and β-O-4 aryl ether linkages in lignin, consistent with established polymer aging paradigms.
3.1.3. FTIR Spectroscopy Analysis
FTIR spectroscopy can identify the characteristic functional groups of plant fibers qualitatively and MFR in MFPBs and analyze the destructive effects of thermo-oxidative aging on their chemical structure. Figure 4 shows the FTIR spectra of MFPBs under different thermo-oxidative aging times. Table 3 presents the assignments of the prominent absorption peaks of MFPBs.
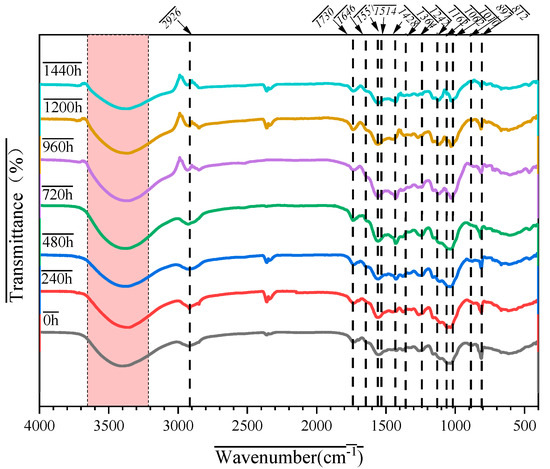
Figure 4.
Time-dependent FTIR spectra of MFPBs during thermo-oxidative aging.

Table 3.
Assignment of characteristic FTIR absorption bands for MFPBs (400–4000 cm−1).
Based on Figure 4 and Table 3, the functional groups corresponding to the following characteristic peaks can be clearly defined:
(1) Cellulose: 2926 cm−1 (CH stretching), 1162 cm−1 (asymmetric stretching of C-O-C), and 897 cm−1 (β-glycosidic bond);
(2) Hemicellulose: 1730 cm−1 (C=O stretching, acetyl/carboxyl group) and 1428 cm−1 (C-H vibration);
(3) Lignin: 1646 cm−1 (conjugated C=O) and 1514 cm−1 (aromatic ring of phenylpropane);
(4) MFR: 1557 cm−1 (N–C=N of triazine ring) and 812 cm−1 (out-of-plane vibration of triazine ring).
By comparing the FTIR spectra of different aging times (Figure 4), the following qualitative changes can be observed:
(1) Cellulose structure destruction:
The characteristic peak at 897 cm−1 (β-glycosidic bond) gradually disappears with aging, which proves the fracture of cellulose molecular chains.
The peak position of 2926 cm−1 (CH stretching) shifts to a lower wavenumber, reaching 2918 cm−1. This is attributed to the early-stage oxidation of -CH2- groups or the destruction of the hydrogen-bond network induced by thermo-oxidative conditions.
(2) Hemicellulose Degradation:
The peak position of 1730 cm−1 (C=O) shifts to around 1710 cm−1 in the later aging stage, and the characteristic peak at 1428 cm−1 (C-H) appears intermittently. This indicates that the acetyl groups and polysaccharide structures in hemicellulose are decomposed by thermo-oxidative conditions, and a carboxylic C=O vibration peak appears, suggesting the partial oxidation of hemicellulose to form carboxylic acid groups.
(3) Aromatic Ring Opening of Lignin:
The intensities of the characteristic peaks at 1514 cm−1 (aromatic ring of phenylpropane) and 1266 cm−1 (C-O of guaiacyl group) decrease significantly, indicating the decomposition of the aromatic structure of lignin.
(4) Decomposition of Triazine Ring in MFR:
The characteristic peaks at 1557 cm−1 (N-C=N) and 812 cm−1 (vibration of the triazine ring) gradually disappear after 1440 h of aging, confirming the destruction of the triazine ring structure.
(5) Disintegration of Hydrogen-Bond Network:
The broad peak at 3500 cm−1 (O-H/N-H stretching) becomes narrower and shifts to 3550 cm−1, indicating that the hydrogen-bond network composed of -OH in cellulose, lignin, and hemicellulose, as well as -NH in MFR, is destroyed.
The FTIR results revealed pathways of thermo-oxidative aging weakening MFPB flame-retardant performance:
(1) Reduced Gas-Barrier Ability: The decomposition of the triazine ring in MFR leads to a decrease in the release of N2/NH3 at high temperatures, reducing the dilution effect on oxygen and combustible gases.
(2) Deteriorated Char Layer Structure: The fracture of β-glycosidic bonds in cellulose/hemicellulose hinders the formation of an ordered char layer during the pyrolysis process, reducing the heat-barrier efficiency of the char layer.
(3) Damaged Physical Structure: The disintegration of the hydrogen-bond network and the generation of new carboxylic acid groups weaken the bonding force between fibers, leading to an increase in the internal porosity of the material (consistent with the SEM results) and accelerating the diffusion of heat and gases.
3.2. Flame Retardancy Properties
3.2.1. TG Analysis
Figure 5 displays the TG and DTG curves of MFPBs subjected to various thermo-oxidative aging durations, while Figure 6 systematically illustrates the corresponding combustion phase divisions and characteristic temperature positions. As outlined in Figure 6, the combustion processes of the MFPB samples across different aging periods can be categorized into four distinct phases: (1) Moisture Evaporation and Thermodynamic Equilibrium Phase, (2) Thermal Decomposition Phase, (3) Combustion Phase, and (4) Burnout Phase. Key parameters, including phase-specific temperature ranges, mass loss rates, and predominant reaction processes, are thoroughly summarized in Table 4.
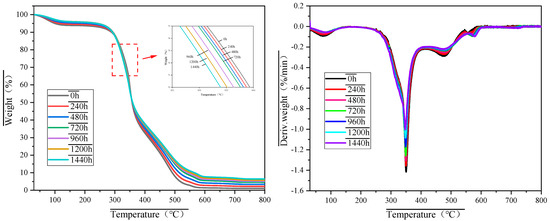
Figure 5.
TG and DTG profiles of MFPB samples subjected to varied thermo-oxidative aging durations.
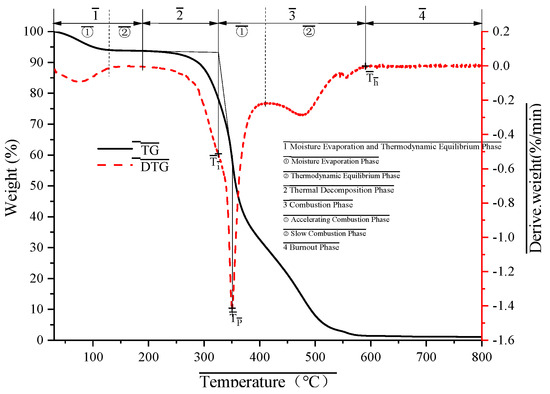
Figure 6.
Phase demarcation and characteristic temperature markers in thermogravimetric analysis of MFPBs under thermo-oxidative aging conditions.

Table 4.
Combustion phase progression of MFPBs: mechanistic elucidation of thermo-oxidative degradation effects.
To systematically evaluate the effects of thermo-oxidative aging on the combustion characteristics of MFPBs, five essential parameters were identified: ignition temperature (Ti), burnout temperature (Tf), maximum combustion rate (VP), temperature at maximum combustion rate (TP), and mean combustion rate (VM). The operational definitions and measurement methodologies are delineated as follows:
(1) Ignition Temperature (Ti)
Ti is a critical quantity that defines the minimum temperature necessary to commence sustained combustion of a sample in an oxidative environment. A thermogravimetric–derivative thermogravimetric (TG-DTG) study reveals that Ti has an inverse relationship with fuel ignitability; reduced Ti values facilitate increased volatile release during pyrolysis, augmenting flammability.
(2) Burnout Temperature (Tf)
Characterized as the temperature at which 99% of the total mass loss occurs during the combustion of MFPBs. Reduced Tf values indicate enhanced combustion efficiency and more thorough oxidation of carbonaceous residues.
(3) Maximum Combustion Rate (VP) and Temperature at Maximum Combustion Rate (TP)
VP signifies the maximum mass loss rate (absolute peak of the DTG curve) during the combustion phase, whereas TP indicates the temperature at which this maximum transpires. These characteristics collectively indicate post-ignition combustion intensity, with increased VP magnitudes and reduced TP values associated with improved flame propagation velocity and combustion stability.
(4) Mean Combustion Rate (VM)
Calculated according to Equation (1):
where -heating rate (5 °C/min, controlled parameter); -residual mass percentage at Ti (%); and -residual mass percentage at Tf (%).
The influence of thermo-oxidative aging on the combustion properties of MFPBs resulted in notable alterations in combustion parameters during the experiment. The material’s Ti consistently diminished with the extension of aging time (ΔTi = −15.25 °C). The fundamental cause of this phenomenon is the pyrolysis of organic materials throughout the aging process. The degradation of organic matter facilitates the early release of volatiles, increasing reaction activity during the initial combustion phase and reducing Ti. The Tf experienced a substantial increase (ΔTf = +92.09 °C). This may result from the secondary cross-linking of aged products, which creates a thermally stable structure in high-temperature conditions, impedes mass transfer, extends the burnout duration of combustion residues, and consequently elevates Tf. VP and TP diminished by 0.4112 mg·min−1 and 3.75 °C, respectively, while VM concurrently fell by 0.4925 mg·min−1. This parameter combination signifies that while the maximum combustion intensity diminished, the temperature range of the combustion response (Tf − Ti) markedly increased, from Δ237.25 °C to Δ344.59 °C. The decrease rate of Ti (0.0106 °C/h) and the increase rate of Tf (0.0640 °C/h) demonstrate asymmetric progression, imparting the material with the composite attribute of “low ignition threshold-high sustained combustion”. This distinctive alteration renders MFPB more readily ignitable in a fire, while extending the combustion duration and substantially elevating the danger of re-ignition. The alteration in the association among metrics, particularly the diminishing strong correlation between VP and TP, further substantiates the fundamental transformation in the material combustion mode, transitioning from quick, volatile-dominated combustion to multi-stage composite combustion.
In summary, thermo-oxidative aging forms an exceptional “easy to ignite-difficult to terminate” combustion mode by changing the thermal stability of MFPB components and the characteristics of combustion residues. This dual effect significantly affects materials’ fire hazard assessment and provides an important experimental basis for the durability study of flame-retardant materials.
Combustion kinetic parameters serve as core indicators for evaluating the oxidative reactivity of materials. Studies have shown that the activation energy (E) and frequency factor (A), key variables in the Arrhenius equation, dominate the kinetic process of combustion reactions. Here, E is defined as the minimum energy threshold required for effective collisions of reactant molecules. A decrease in E significantly enhances the probability of molecular thermal motion overcoming the energy barrier, accelerating the ignition process and reinforcing the system’s tendency for self-sustained combustion. A characterizes the statistical base of effective collisions of activated molecules per unit time; an increase in A exponentially amplifies the effective collision frequency, leading to a simultaneous rise in reaction severity and heat release rate. Together, these parameters determine the critical conditions of the material combustion reaction pathway and the intensity of energy release.
According to Arrhenius’s theory, the decomposition rate of a sample with initial mass under controlled heating can be expressed as follows [31,32]:
where ; ; .
From Equations (2) and (3), it follows that
By integrating Equation (4), and letting , it follows that
where -initial mass of sample, mg; -instantaneous mass at time t, mg; -final residual mass, mg; -mass loss percentage at t, %; -frequency factor, min−1; -frequency factor, kJ·mol−1; -universal gas constant, 8.314 × 10−3 kJ· (mol·K)−1; -heating rate (experimental setting) 5 °C·min−1; and -absolute temperature, K.
Adopting the single heating rate non-isothermal method, we approximate Equation (5) using the Coats–Redfern integral method:
From Equation (6), plotting against ,. the slope is and the intercept are . Thus, E and A can be derived from the slope and intercept terms in the plot. The common forms of g(α) used in the kinetic studies of typical solid-state reactions are presented in Table 5 below.

Table 5.
Combustion performance parameters of MFPBs under varied thermo-oxidative aging durations.
According to Ref. [33], the TG curves of the combustion stage of MFPBs with different thermo-oxidative aging times were fitted. This paper considers that the combustion stage of MFPBs in an air atmosphere conforms to the second-order reaction model, so is adopted. As seen in Figure 7, the fitting of this mechanism function shows linearity, indicating that the selection of is correct. The kinetic parameters of the combustion stage of MFPBs under different thermo-oxidative aging times calculated by the established model are shown in Table 6:
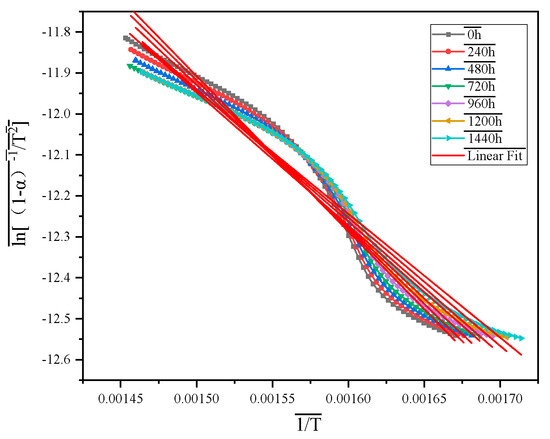
Figure 7.
Linear fitting curves of MFPBs combustion phase mechanism pyrolysis function under varying thermo-oxidative aging durations.

Table 6.
Common functional forms of for solid-state reaction kinetics.
As shown in Table 7, the E and A of MFPBs in the combustion stage gradually decrease with the extension of thermo-oxidative aging time. After 1440 h of thermo-oxidative aging, its E value and A value are reduced by 5.38 kJ/mol and 25.94 min−1, respectively, compared with the original sample, indicating that thermo-oxidative aging significantly lowers the energy barrier for the oxidation reaction of MFPBs, thus enhancing its flammability. The dual decreasing trend of the kinetic parameters reveals the deterioration processes of the flame-retardant performance of the material. It reflects the important characteristic of the extended combustion duration of aged samples.

Table 7.
Mechanistic function fitting parameters and kinetic parameters for the combustion phase of MFPBs under varying thermo-oxidative aging durations.
3.2.2. LOI Analysis
The LOI, a widely adopted quantitative method for evaluating flame-retardant performance, was employed to preliminarily assess the impact of thermo-oxidative aging durations on the flame retardancy of MFPBs. Figure 8 illustrates the LOI values of MFPBs under varying aging times.
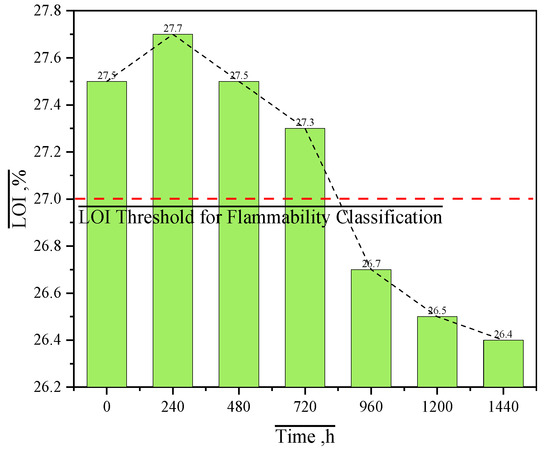
Figure 8.
LOI curves of MFPBs under varying thermo-oxidative aging durations.
Thermo-oxidative aging significantly affects the flame-retardant performance of MFPBs, with its LOI exhibiting a non-monotonic evolution of first increasing and then decreasing (Figure 8). The pristine sample has an LOI of 27.5, meeting the GB/T 2406.2-2009 standard for flame-retardant materials (LOI > 27%) [34], primarily through gas-phase inhibition. During combustion, surface MFR decomposes to release non-flammable gases such as N2 and NH3, diluting the oxygen concentration on the material surface to hinder the combustion reaction. On the other hand, deep-layer MFR promotes char layer expansion, physically blocking heat and mass transfer.
After 240 h of aging, the LOI slightly increases to 27.7, attributed to the thermal-driven migration of MFR to the material surface (verified by EDS observation of increased surface N element content in aged MFPBs). The surface-enriched MFR accelerates the early release of flame-retardant gases (N2/NH3), leading to faster char layer formation. The char layer becomes thinner but denser, temporarily enhancing flame retardancy by optimizing gas-phase inhibition.
When the aging duration extends to 840 h, the LOI drops to 27.0 (the threshold for flammable materials). This decrease is due to the excessive thermo-oxidative decomposition of surface-enriched MFR, reducing the amount of MFR available for gas release. MFR decomposition causes an increase in gaps within the char layer, further damaging its heat-insulating and oxygen-barrier capabilities and accelerating heat and oxygen penetration.
At 1440 h of aging, the LOI decreases by 4% compared to the initial value. Long-term thermo-oxidative effects disrupt the dynamic balance between MFR migration and decomposition: surface MFR is almost completely decomposed, leading to the failure of gas-phase inhibition. Insufficient MFR content makes the char layer porous (observed as enlarged pores by SEM), and the porous structure cannot block heat transfer, resulting in accelerated internal pyrolysis and sustained combustion.
The evolution of LOI indicates that thermo-oxidative aging dynamically regulates the flame-retardant processes through two interrelated processes: short-term migration enhances gas-phase inhibition. At the same time, long-term decomposition depletes surface flame-retardant components. The decomposition of MFR simultaneously weakens both gas-phase inhibition and char layer protection. This dynamic interaction transforms the material’s flame retardancy from “cooperative gas-phase/condensed-phase protection” (pristine) to “dual-phase inhibition failure” (long-term aged), providing a mechanistic explanation for the non-monotonic variation trend of LOI.
3.2.3. CCT Analysis
CCT was employed to evaluate the combustion behavior of MFPBs under thermo-oxidative aging (0–1440 h). Compared to the LOI, CCT provides a more realistic reflection of material fire behavior via parameters like time to ignition (TTI), heat release rate (HRR), and total heat release (THR).
The experimental data (Figure 9 and Figure 10 and Table 8) show that with prolonged aging, the integrity of MFPBs combustion residues decreased, and crack density increased. The pristine sample retained a dense char layer; after 1440 h aging, the char layer disintegrated into loose ash.
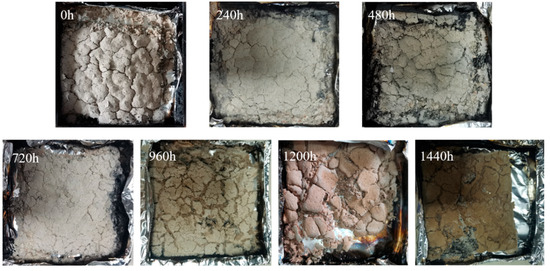
Figure 9.
Residue morphologies of MFPBs after cone calorimeter testing under varying thermo-oxidative aging durations.
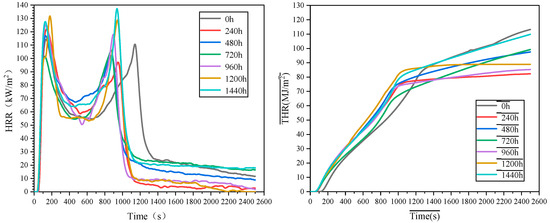
Figure 10.
HRR and THR curves of MFPBs under varying thermo-oxidative aging durations.

Table 8.
CCT data of MFPBs under varying thermo-oxidative aging durations.
Time to ignition (TTI) exhibited non-linear variation: 41 s (pristine) → 46 s (240 h, +12.2%) → 35 s (1440 h, −14.6%), showing a non-monotonic trend of “first prolonged then shortened”.
Heat release rate (HRR) curves displayed typical double-peak characteristics (peak interval: 600–1000 s). The first peak intensity followed an “increase–decrease–surge” trend: 114.3 kW/m2 (pristine) → 121.5 kW/m2 (240 h, +6.3%) → 107.8 kW/m2 (720 h, −5.7%) → 137.3 kW/m2 (1440 h, +20.1%). The second peak occurrence time shortened from 480 s to 320 s (33.3% decrease), indicating a significantly accelerated combustion process. The mean heat release rate (MHRR) revealed stage-dependent flame retardancy: a 14.5% decrease during 0–240 h (temporary enhancement), an 8.2% increase during 240–720 h (fluctuating heat release), and a sharp rise to peak value after 720 h (26.8% higher than pristine), signifying drastically enhanced combustion hazard.
Thermo-oxidative aging-induced changes in combustion behavior were governed by MFR migration and decomposition: ① Initial aging (<240 h): Thermal-driven MFR migration to the surface formed a dense char layer and released N2/NH3, diluting oxygen to delay ignition (TTI prolonged to 46 s). The char layer blocked heat transfer, reducing HRR. ② Mid-term aging (240–720 h): Sustained deep-layer MFR migration optimized char layer structure (reduced porosity), enhancing thermal insulation and causing the temporary decline of the first HRR peak. The dynamic balance between internal pyrolysis and surface protection triggered fluctuating MHRR. ③ Long-term aging (>720 h): The thermo-oxidative decomposition of surface MFR caused char layer cracking and pore formation, compromising gas-phase flame retardancy. This increased flammable gas release, surging HRR to 137.3 kW/m2. Concurrently, the defective char layer accelerated internal pyrolysis, reducing TTI to 35 s and decomposing residues into loose ash.
The CCT and LOI results corroborate that the dynamic balance of MFR migration–decomposition governs combustion behavior: short-term migration delays combustion via cooperative “gas-phase inhibition-condensed-phase protection”, while long-term decomposition depletes surface flame retardants and destroys char layer, escalating fire hazard. This process not only reveals the essence of flame-retardant failure under thermo-oxidative conditions but also provides a quantifiable theoretical basis for extending service life-by regulating MFR migration–decomposition kinetics (e.g., optimizing flame-retardant distribution gradients or adjusting thermo-oxidative protective coatings), the safety and reliability of MFPBs in practical applications can be enhanced.
4. Conclusions
This study elucidates the thermo-oxidative aging-induced degradation processes and quantitative performance decline of MFPBs. The experimental results demonstrate that prolonged thermo-oxidative aging at 60 °C for 1440 h causes a 17.05% decrease in E (from 31.54 to 26.16 kJ·mol−1) and A 68.52% reduction in the pre-exponential factor (from 37.86 to 11.92 min−1), reflecting significantly degraded thermal stability. In terms of flame retardancy, the LOI decreases by 4% (from 27.5 to 26.4), the TTI shortens by 17.1% (from 41 to 34 s), and the pHRR surges by 20.1% (from 114.3 to 137.3 kW·m−2), indicating enhanced flammability and combustion intensity. The microstructural analysis reveals that aging disrupts the dense char layer into a “porous cracked structure” with fish-scale patterns observed by SEM. At the same time, FTIR confirms the decomposition of MFR triazine rings (disappearance of 1557 cm−1 and 812 cm−1 peaks), breaking the dynamic equilibrium between MFR surface enrichment and thermal decomposition. This degradation invalidates the cooperative flame-retardant processes of gas-phase inhibition (N2/NH3 release) and condensed-phase char layer protection. These findings establish critical degradation thresholds (e.g., LOI < 27 as a replacement indicator) and highlight the need for nano-clay reinforcement or phosphorus–nitrogen modification to enhance MFR thermal stability. Future research should integrate accelerated aging models with real-time monitoring to predict flame retardancy evolution, facilitating the development of aging-resistant fireproof composites for architectural applications.
Author Contributions
Conceptualization, S.L.; Methodology, S.L.; Validation, S.L.; Investigation, S.L. and D.Y.; Resources, Y.Z. (Yanni Zhang); Data curation, S.L. and Y.Z. (Yuchen Zhang); Writing—original draft, S.L.; Writing—review & editing, Y.Z. (Yanni Zhang) and L.H.; Visualization, D.Y.; Supervision, Y.Z. (Yanni Zhang) and L.H.; Funding acquisition, Y.Z. (Yanni Zhang). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Research and Development Projects of Shaanxi Province grant number 2024GX-YBXM-444 and the S&T Program of Energy Shaanxi Laboratory grant number ESLB202427. And the APC was funded by Shiyue Ling.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the particularity of the experimental data, the team may continue to study other data involved in the following work.
Acknowledgments
We are grateful for the support of laboratories and assistants who provide experimental conditions for this work. During the preparation of this work the authors used Deepseek R1 and Grammarly Pro in order to Language Refinement. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- GB 50016-2014; Code for Fire Protection Design of Buildings. China Plans Publishing House: Beijing, China, 2014.
- ASTM E84; Standard Test Method for Surface Burning Characteristics of Building Materials. ASTM International: West Conshohocken, PA, USA, 2023.
- EN 13501-1; Fire Classification of Construction Products and Building Elements-Part 1: Classification Using Data from Reaction to Fire Tests. European Committee for Standardization: Brussels, Belgium, 2019.
- Luo, S.Y.; Gao, M.Y.; Pan, X.L.; Wang, Y.; He, Y.P.; Zhu, L.H.; Si, T.; Sun, Y.L. Fragrance oil microcapsules with low content of formaldehyde: Preparation and characterization. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 1165–1173. [Google Scholar] [CrossRef]
- Kawarasaki, M.; Hiradate, R.; Hirabayashi, Y.; Kikuchi, S. Fire retardancy of fire-retardant-impregnated wood after natural weathering I: Effects of chemical types and coatings at up to 60-months of exposure. Mokuzai Gakkaishi. 2018, 6, 105–114. [Google Scholar] [CrossRef]
- Song, J.J.; Deng, J.; Zhao, J.Y.; Lu, S.P.; Ming, H.Q.; Shu, C.M. Comparative analysis of exothermic behaviour of fresh and aged pine wood. Therm. Anal. Calorim. 2022, 147, 14393–14406. [Google Scholar] [CrossRef]
- Sjökvist, T.; Blom, Å.R.; Ahmed, S.A. Liquid water absorption in coated Norway spruce: Impact of heartwood, sapwood, density and weather exposure. Maderas Cienc. Tecnol. 2020, 22, 335–346. [Google Scholar] [CrossRef]
- Rostom, L.; Courtier-murias, D.; Rodts, S.; Care, S. Investigation of the effect of aging on wood hygroscopicity by 2D 1H NMR relaxometry. Holzforschung 2020, 74, 400–411. [Google Scholar] [CrossRef]
- Todar, L.; D’auri, M.; Langerame, F.; Salvi, A.M. Surface characterization of untreated and hydro-thermally pre-treated Turkey oak woods after UV-C irradiation. Surf. Interface Anal. 2015, 47, 206–215. [Google Scholar] [CrossRef]
- Esteves, B.; Pereira, H. Wood modification by heat treatment: A review. BioResources 2008, 4, 370–404. [Google Scholar] [CrossRef]
- Altgen, M.; Rautkari, L. Humidity-dependence of the hydroxyl accessibility in Norway spruce wood. Cellulose 2020, 28, 45–58. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Bajwa, D.S. Degradation in the mechanical and thermo-mechanical properties of natural fiber filled polymer composites due to recycling. Constr. Build. Mater. 2018, 172, 1–9. [Google Scholar] [CrossRef]
- Kujawa, M.; Paczos, P.; Smakosz, L.; Piasecki, A.; Jan, F.; Winkelmann, K.; Konopińska-Zmysłowska, V.; Eremeyev, V. Impact of thermal and humidity conditions on structural epoxy adhesives during medim-term exposure. Int. J. Adhes. Adhes. 2025, 139, 234–243. [Google Scholar] [CrossRef]
- Lu, Y.X.; Feng, J.B.; Yi, D.Q.; Xie, H.Y.; Xu, Z.G.; Cao, C.F.; Huo, S.Q.; Wang, H.; Song, P.A. Strong synergistic effects between P/N-containing supramolecular microplates and aluminum diethylphosphinate for fire-retardant PA6. Compos. Part A Appl. Sci. Manuf. 2024, 176, 107834. [Google Scholar] [CrossRef]
- Wang, C.L.; Li, J.; Ding, P. Roles of supermolecule structure of melamine phosphomolybdate in intumescent flame retardant polypropylene composites. Anal. Appl. Pyrolysis 2016, 119, 139–146. [Google Scholar] [CrossRef]
- Hua, Y.F.; Liu, J.; Zhang, J.Y.; Liu, Z.S.; Hu, G.T.; Yang, Y.; Sui, Y.F.; Sun, J.; Gu, X.Y.; Zhang, S. A compound with boron and phosphorus towards epoxy resin with excellent flame retardancy, smoke suppression, transparency, and dielectric properties. Chem. Eng. J. 2024, 483, 149212. [Google Scholar] [CrossRef]
- Zhou, Y.; He, W.D.; Wu, Y.F.; Xu, D.H.; Chen, X.L.; He, M.; Guo, J.B. Influence of thermo-oxidative aging on flame retardancy, thermal stability, and mechanical properties of long glass fiber–reinforced polypropylene composites filled with organic montmorillonite and intumescent flame retardant. Fire Sci. 2019, 37, 176–189. [Google Scholar] [CrossRef]
- Yao, X.L.; Peng, R.; Du, C.G.; Hua, Y.T.; Zhang, J.J.; Huang, Q.L.; Liu, H.Z. A two-step method for fabricating bamboo culm coated with MgAl-LDHs and its fire resistance properties. Bio Resour. 2019, 14, 5150–5161. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Du, C.G.; Yu, H.L.; Yao, X.L.; Huang, Q.L. Promotion effect of nano-SiO2 on hygroscopicity, leaching resistance and thermal stability of bamboo strips treated by nitrogen-phosphorus-boron fire retardants. Wood Res. 2020, 65, 693–704. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Huang, Z.A.; Liu, L.L. The aging property and life forecast of the silicone rubber. China Plast. Ind. 2015, 43, 61–64. (In Chinese) [Google Scholar]
- Yu, Q.L.; Brouwers, H.J.H. Thermal properties and microstructure of gypsum board and its dehydration products: A theoretical and experimental investigation. Fire Mater. 2011, 36, 575–589. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Yang, F.; Du, C.; Zhu, J.; Ran, Y.; Bao, Q.; Shan, Y.; Zhang, W. Sodium silicate/urea/melamine ternary synergistic waterborne acrylic acid flame-retardant coating and its flame-retardant mechanism. Molecules 2024, 29, 1472. [Google Scholar] [CrossRef]
- He, Y.; Jin, X.; Li, J.; Qin, D. Mechanical and fire properties of flame-retardant laminated bamboo lumber glued with phenol formaldehyde and melamine urea formaldehyde adhesives. Polymer 2024, 16, 781. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Chen, X.; Zhang, B.; Lu, Z.; Jiang, W.; Fang, X.; Li, J.; Liu, B.; Ding, T.; Xu, Y. Synergistic modification of polyformaldehyde by biobased calcium magnesium bi-ionic melamine phytate with intumescent flame retardant. Polymer 2024, 16, 614. [Google Scholar] [CrossRef] [PubMed]
- Çekiç, Y.; Duyar, H.; Hacıvelioğlu, F. Preparation and characterization of melamine–benzoguanamine–formaldehyde resins and their flame-retardant properties in impregnated paper for low pressure laminates. Coatings 2024, 14, 873. [Google Scholar] [CrossRef]
- ISO 291:2008; Plastics-Standard Atmospheres for Conditioning and Testing. International Organi-zation for Standardization: Geneva, Switzerland, 2008.
- GB/T 2406.2-2009; Plastics-Determination of Burning Behaviour by Oxygen Index-Part 2: Ambient-Temperature Test. Standards Press of China: Beijing, China, 2009.
- ISO 5660-1; Reaction-to-Fire Tests -Heat Release, Smoke Production and Mass Loss Rate Part 1: Heat Release Rate (cone Calo-Rimeter Method) and Smoke Production Rate (Dynamic Measurement). International Organization for Standardization: Geneva, Switzerland, 2015.
- DN6176: 2018-10; Colorimetric Determination of Colour Differences of Object Colours According to the DIN99o Formula. Deutsches Institut für Normung: Berlin, Germany, 2018.
- Zhang, X.Q.; Yang, H.Y.; Guo, Y.X.; Zhou, J.; Liu, H.; He, S.Q.; Huang, M.M.; Xu, W.L.; Zhu, C.S.; Liu, W.T. Pyrolysis kinetics and flame retardant enhancement of bio-based polyamide 56/6. Thermochim. Acta 2024, 741, 179869. [Google Scholar] [CrossRef]
- Deng, Z.; Shi, M.; Liang, Y.; Yang, X.; Huang, Z. Phosphorus-nitrogen synergistic flame retardant (PNFR) towards epoxy resin with excellent flame retardancy and satisfactory mechanical strength: An insight into pyrolysis and flame retardant mechanism. Polym. Test. 2024, 13, 108352. [Google Scholar] [CrossRef]
- Deng, J.; Liu, T.S.; Yao, M.; Yi, X.; Bai, G.X.; Huang, Q.R.; Li, Z. Comparative study of the combustion and kinetic characteristics of fresh and naturally aged pine wood. Fuel 2023, 343, 127962. [Google Scholar] [CrossRef]
- He, W.T.; Song, P.G.; Yu, B.; Fang, Z.P.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Prog. Mater. Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).