Investigation of Air Foam and Heptafluoropropane Foam Fire Extinguishment for Storage Tanks Containing Low-Boiling-Point Flammable Liquids
Abstract
1. Introduction
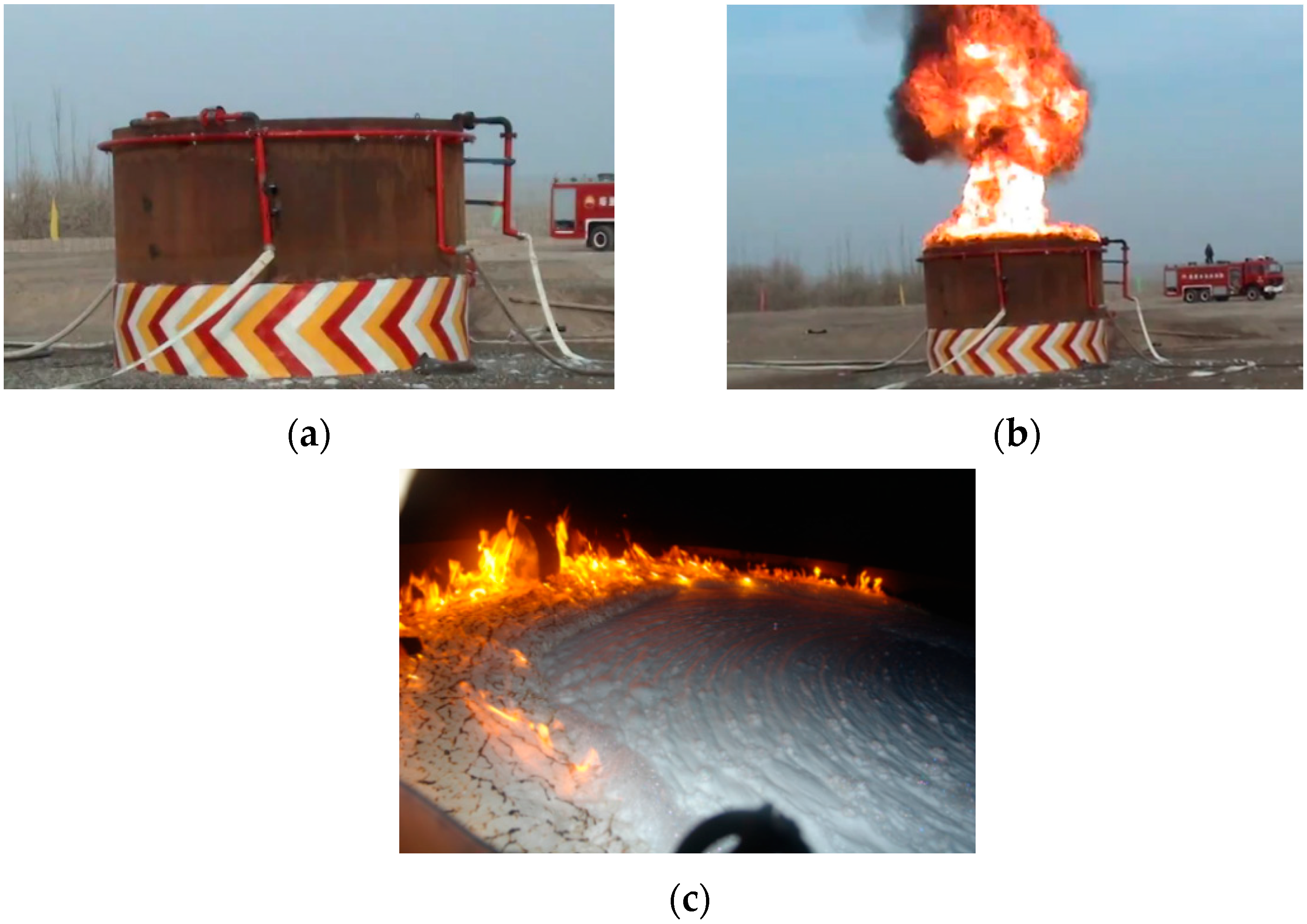
2. Air Foam Fire Extinguishment Experiments
2.1. Fire Extinguishing Results for Propylene Oxide
2.2. Fire Extinguishing Results for n-Pentane
2.3. Fire Extinguishing Results for Condensate Oil
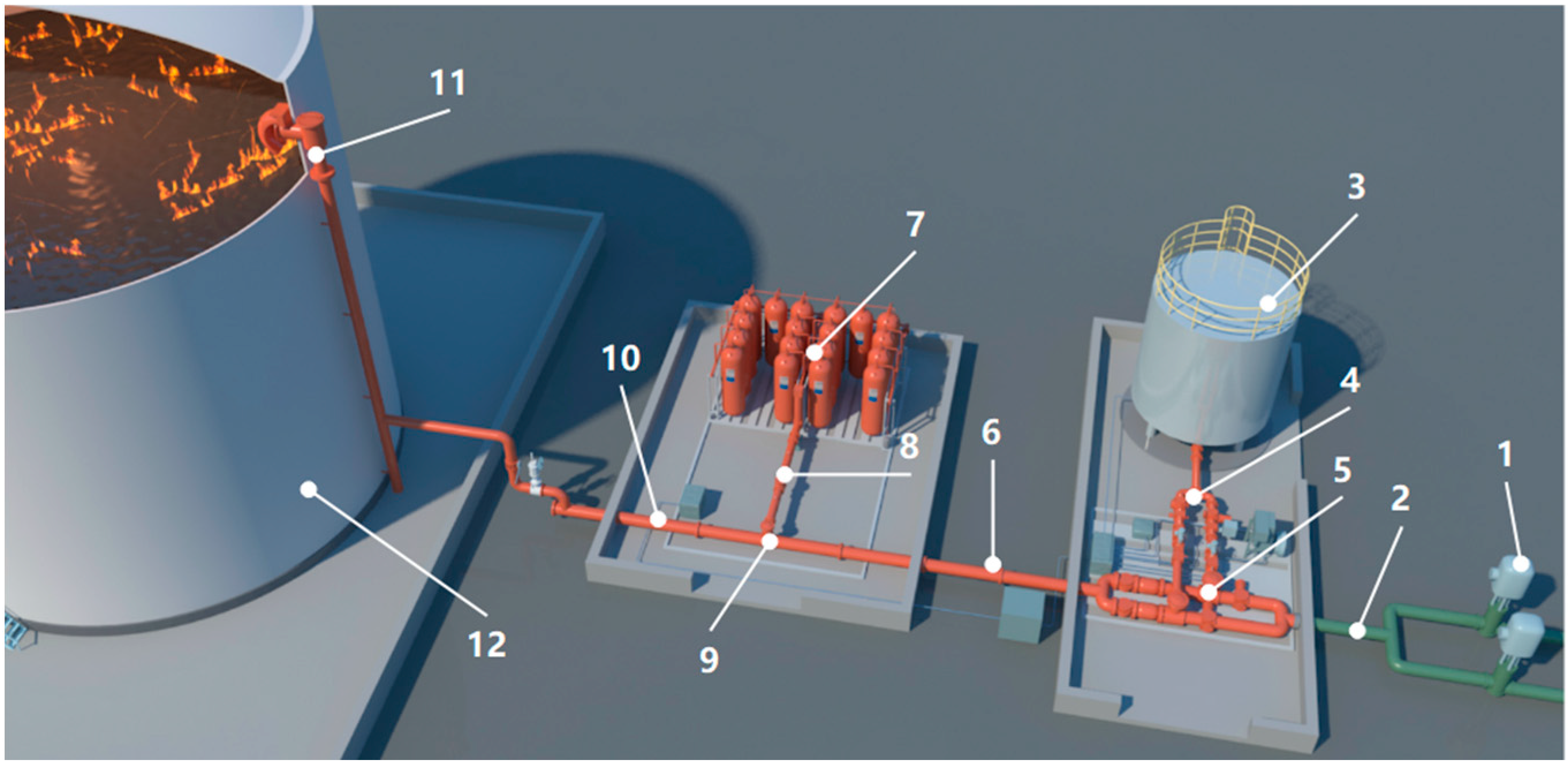
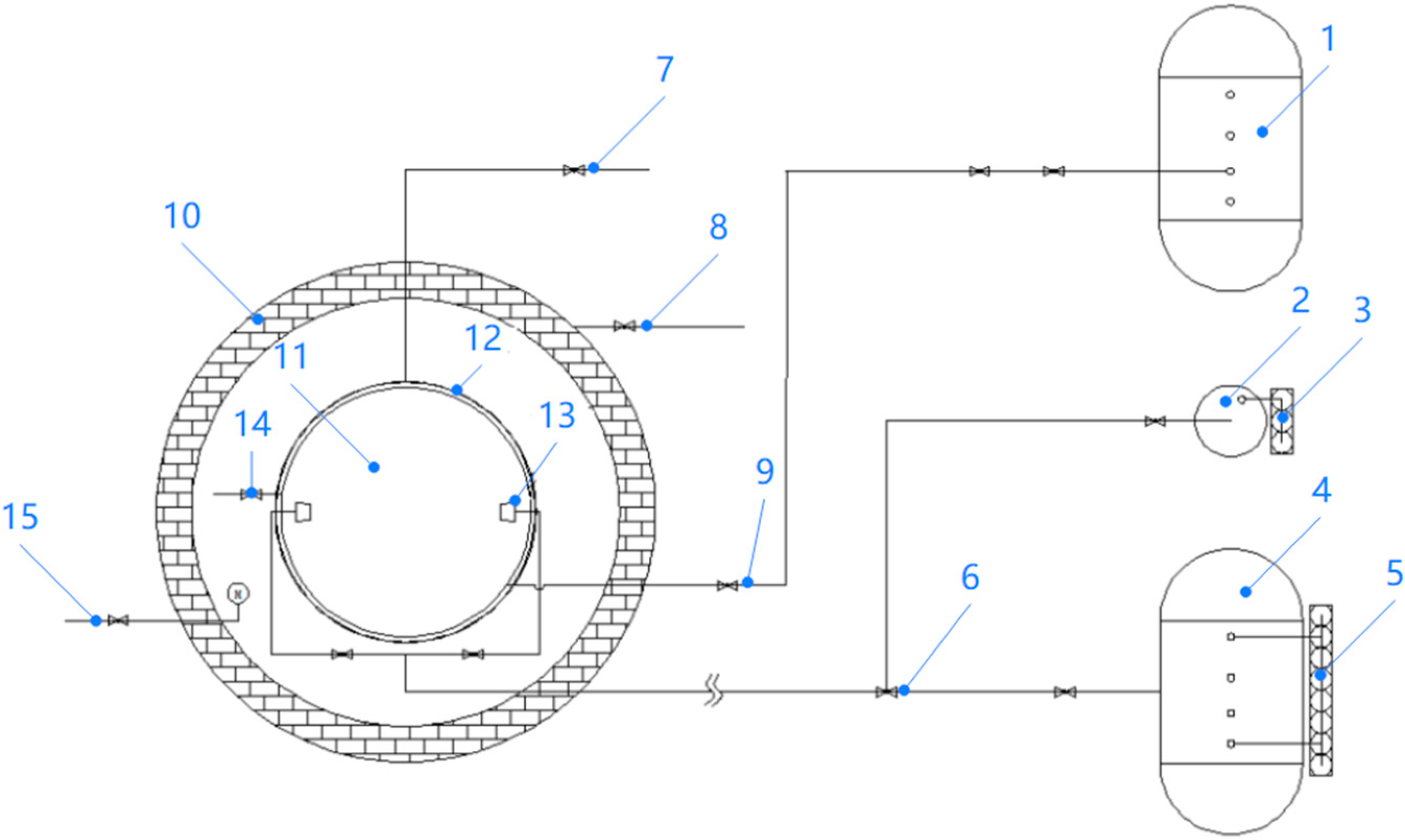
3. Development of HFC227ea Foam Extinguishing Technology and the Fire Extinguishing Experiments
3.1. HFC227ea Foam Extinguishing System
3.2. HFC227ea Foam Extinguishing Results
3.3. Limitations of HFC227ea Foam Extinguishing Technology
4. Conclusions and Future Perspectives
- (1)
- The air foam method was used to evaluate the fire-extinguishment performance of storage tanks with LBPF liquids in detail for the first time, and the results show that it is not effective for extinguishing fires.
- (2)
- The HFC227ea foam extinguishing technology was proposed in this work, and the corresponding experimental system was established. The fire-extinguishing performance of the system was validated. The results show that the fire-extinguishing efficiency is significantly superior to that of air foam, and the fire can be controlled and extinguished in a shorter time with a smaller supply intensity. HFC227ea foam extinguishing technology is very promising for the fire extinguishment of storage tanks containing low-boiling-point flammable liquids.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, C.; Wang, X.; Li, H. Studied of flammable liquid boil over at low pressure and high altitude. Chem. Technol. Fuels Oils 2019, 55, 310–318. [Google Scholar] [CrossRef]
- Saso, Y.; Liao, C.; Naito, H. A technological consideration of the foam discharge during the full-surface tank fire in Tomakomai in September, 2003. Rep. Natl. Res. Inst. Fire Disaster 2007, 103, 19–24. [Google Scholar]
- Kim, A.K.; Crampton, G.P. Evaluation of the fire suppression effectiveness of manually applied compressed-air-foam (CAF) system. Fire Technol. 2012, 48, 549–564. [Google Scholar] [CrossRef]
- Kim, T.S.; Park, T.H.; Park, J.H.; Yang, J.H.; Han, D.H.; Lee, B.C.; Kwon, J.S. Thermal characteristics of fire extinguishing agents in compartment fire suppression. Sci. Prog. 2024, 107, 00368504241263435. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Wang, K.; Fang, J.; Shah, H.R.; Lang, X.; Mu, S.; Wang, J.; Wang, J. Suppression behavior difference between compressed air/nitrogen foam over liquid fuel surface under constant radiation heat flux. Fire Technol. 2024, 60, 1225–1243. [Google Scholar] [CrossRef]
- NFPA 11: Standard for Low-, Medium-, and High-Expansion Foam; NFPA: Quincy, MA, USA, 2024.
- BS EN 13565-2; Fixed Firefighting Systems—Foam Systems Part 2: Design, Construction and Maintenance. British Standards Institution: London, UK, 2018.
- GB 50151; Code of Design for Foam Extinguishing Systems. Standards Press of China: Beijing, China, 2011. (In Chinese)
- Carson, P.A.; Mumford, C.J. Hazardous Chemicals Handbook; Butterworth-Heinemann: Oxford, UK, 2002. [Google Scholar]
- Lemmon, E.W.; Bell, I.H.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10.0; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2019.
- Zhi, H.; Bao, Y.; Wang, L.; Mi, Y. Extinguishing performance of alcohol-resistant firefighting foams on polar flammable liquid fires. J. Fire Sci. 2020, 38, 53–74. [Google Scholar] [CrossRef]
- ISO 7203-3; Fire Extinguishing Media—Foam Concentrates—Part 3: Specification for Low-Expansion Foam Concentrates for Top Application to Water-Miscible Liquids. ISO: Geneva, Switzerland, 2019.
- GB 50074; Code for Design of Oil Depot. Standards Press of China: Beijing, China, 2014. (In Chinese)
- ISO 7203-1; Fire Extinguishing Media—Foam Concentrates—Part 2: Specification for Low-Expansion Foam Concentrates for Top Application to Water-Immiscible Liquids. ISO: Geneva, Switzerland, 2019.






| Components | Mole Fraction (%) | Components | Mole Fraction (%) |
|---|---|---|---|
| C3 | 0.03 | C6 | 27.64 |
| iC4 | 0.08 | C7 | 26.43 |
| C4 | 6.38 | C8 | 12.22 |
| iC5 | 8.97 | C9 | 3.21 |
| C5 | 14.02 | C10 | 1.03 |
| Type of the Foam Concentrate | Expansion | 25% Drainage Time (min) | Wall Cooling | Foam Solution Supply Rate (L/min·m2) | Results |
|---|---|---|---|---|---|
| 6% S/AR | ~7.5 | ~8.8 | with water jacket | 6.6 | Fire controlled at 50 s, extinguished at 185 s |
| without water jacket | 6.6 | Fire controlled at 60 s, fire in the edge of the oil pan still existed at 300 s | |||
| 6% AFFF/AR | ~6.9 | ~9.5 | with water jacket | 6.6 | Fire controlled at 150 s, flame existed above the foam coating at 300 s |
| 3% AFFF/AR | ~7.4 | ~7.5 | with water jacket | 6.6 | Fire controlled at 240 s, flame existed above the foam coating at 300 s |
| Type of the Foam Concentrate | Expansion | 25% Drainage Time (min) | Cooling Water Supply Rate (L/min·m²) | Foam Solution Supply Rate (L/min·m²) | Results |
|---|---|---|---|---|---|
| 3% FP | ~5.4 | ~4.8 | 2.5 | 5.04 | flame existed above the foam coating at 15 min |
| 6% AFFF | ~7.4 | ~3.3 | 2.5 | 5.04 | flame existed above the foam coating at 24 min |
| Type of the Foam Concentrate | Expansion | 25% Drainage Time (min) | Cooling Water Supply Rate (L/min·m²) | Foam Solution Supply Rate (L/min·m²) | Results |
|---|---|---|---|---|---|
| 6% FFFP | ~5.3 | ~3.6 | 2.5 | 12.0/24.0 | The fire was almost controlled at 2 min using one foam generator, and the fire still existed at the edge of the tank. Then, the second foam generator was used, the fire in the edge of the tank still existed until the foam filled the tank. |
| 6% AFFF | ~6.3 | ~2.6 | 2.5 | 12.0/24.0 | The results are similar to the above measures. |
| Category | Boiling Point (°C) | Density (kg/m3) | pC (MPa) | TC (°C) | Vapor Pressure (20 °C) (MPa) |
|---|---|---|---|---|---|
| N2 | −195.6 | 810 (−196 °C) | 3.40 | −147.0 | 1.03 (−173 °C) |
| CO2 | −78.5 | 1101 (−37 °C) | 7.29 | 31.2 | 5.73 |
| HFC-23 | −82.0 | 806 | 4.84 | 25.9 | 4.27 |
| HFC236fa | −1.4 | 1337 | 3.20 | 124.9 | 0.23 |
| HFC227ea | −16.4 | 1407 | 2.91 | 101.7 | 0.40 |
| Test Liquid | Type of the Foam Concentrate | Expansion | 25% Drainage Time (min) | Foam Solution Supply Rate (L/min·m2) | HFC227ea Foam Mixing Ratio | Results |
|---|---|---|---|---|---|---|
| Propylene oxide | 6% S/AR | ~8.2 | ~13.2 | 11.7 | 5.20% | Fire controlled at 60 s, extinguished at 110 s |
| ~11.5 | ~14.6 | 11.8 | 8.23% | Fire controlled at 56 s, extinguished at 134 s | ||
| n-pentane | 3% AFFF | ~10.3 | ~6.9 | 6.7 | 6.04% | Fire controlled at 55 s, extinguished at 390 s |
| ~8.5 | ~5.8 | 13.1 | 5.71% | Fire controlled at 30 s, extinguished at 120 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Y.; Wang, L.; Zhi, H.; Lu, S.; Wang, J.; Du, X.; Huang, Y.; Xu, K.; Shu, Q.; Wang, X. Investigation of Air Foam and Heptafluoropropane Foam Fire Extinguishment for Storage Tanks Containing Low-Boiling-Point Flammable Liquids. Fire 2025, 8, 152. https://doi.org/10.3390/fire8040152
Bao Y, Wang L, Zhi H, Lu S, Wang J, Du X, Huang Y, Xu K, Shu Q, Wang X. Investigation of Air Foam and Heptafluoropropane Foam Fire Extinguishment for Storage Tanks Containing Low-Boiling-Point Flammable Liquids. Fire. 2025; 8(4):152. https://doi.org/10.3390/fire8040152
Chicago/Turabian StyleBao, Youquan, Lu Wang, Huiqiang Zhi, Shichang Lu, Junyang Wang, Xia Du, Yiliang Huang, Kanghui Xu, Qiyang Shu, and Xiaopo Wang. 2025. "Investigation of Air Foam and Heptafluoropropane Foam Fire Extinguishment for Storage Tanks Containing Low-Boiling-Point Flammable Liquids" Fire 8, no. 4: 152. https://doi.org/10.3390/fire8040152
APA StyleBao, Y., Wang, L., Zhi, H., Lu, S., Wang, J., Du, X., Huang, Y., Xu, K., Shu, Q., & Wang, X. (2025). Investigation of Air Foam and Heptafluoropropane Foam Fire Extinguishment for Storage Tanks Containing Low-Boiling-Point Flammable Liquids. Fire, 8(4), 152. https://doi.org/10.3390/fire8040152









