3.3. The Results of Micro-Turbojet Engine Experiments
The following section presents the variation of the main parameters recorded during the operation of the micro-turbojet engine.
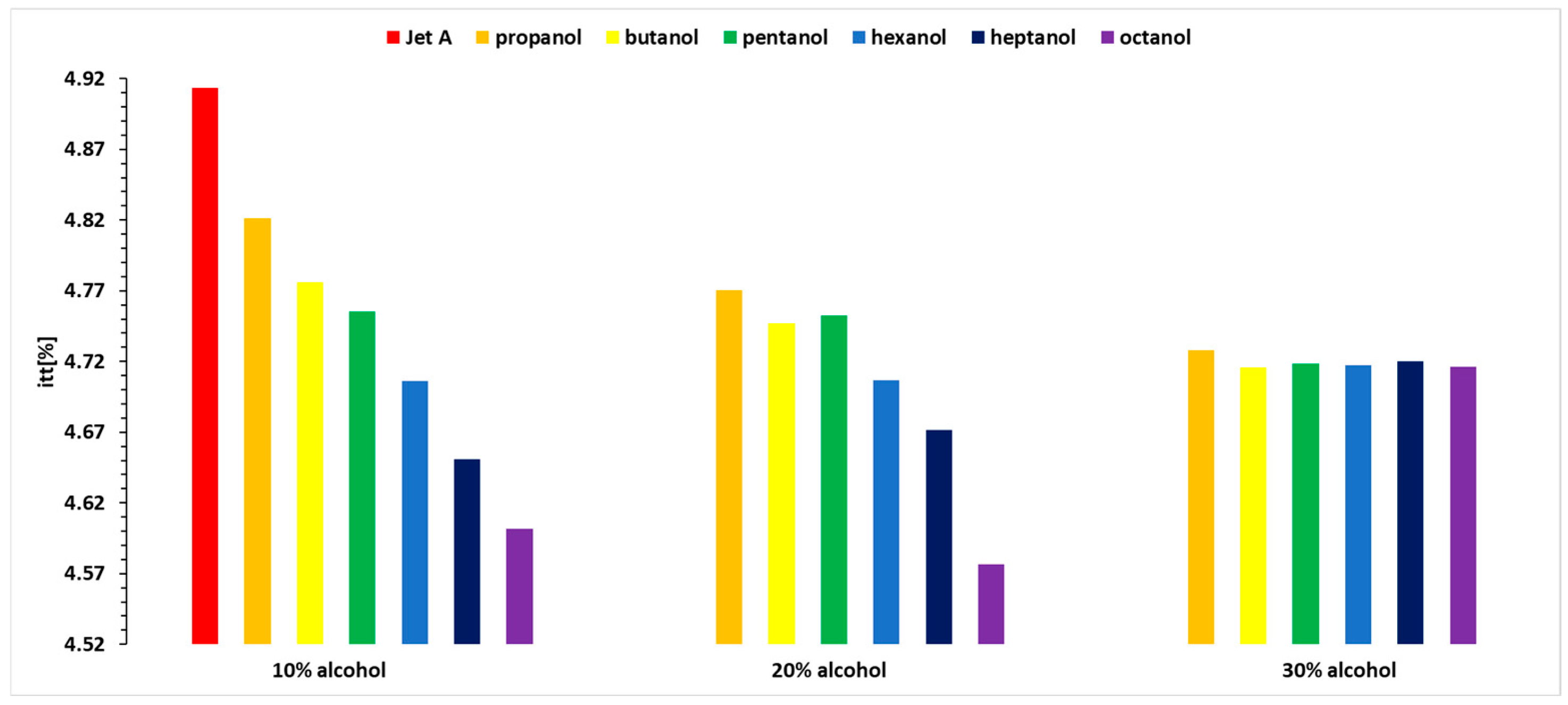
Figure 3,
Figure 4 and
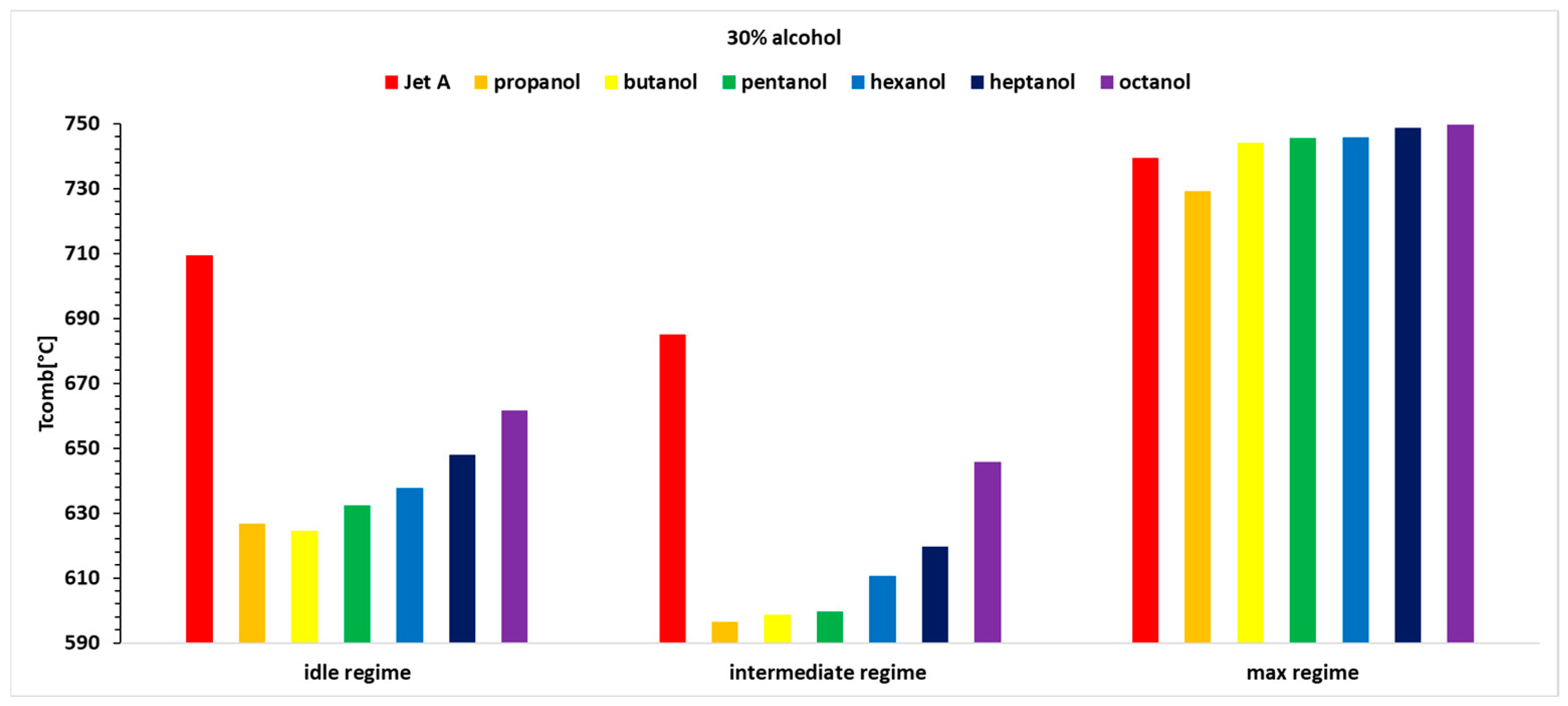
Figure 5 show the variations in exhaust gas temperature before the turbine for the three operating regimes of the micro-turbojet engine. Thus,
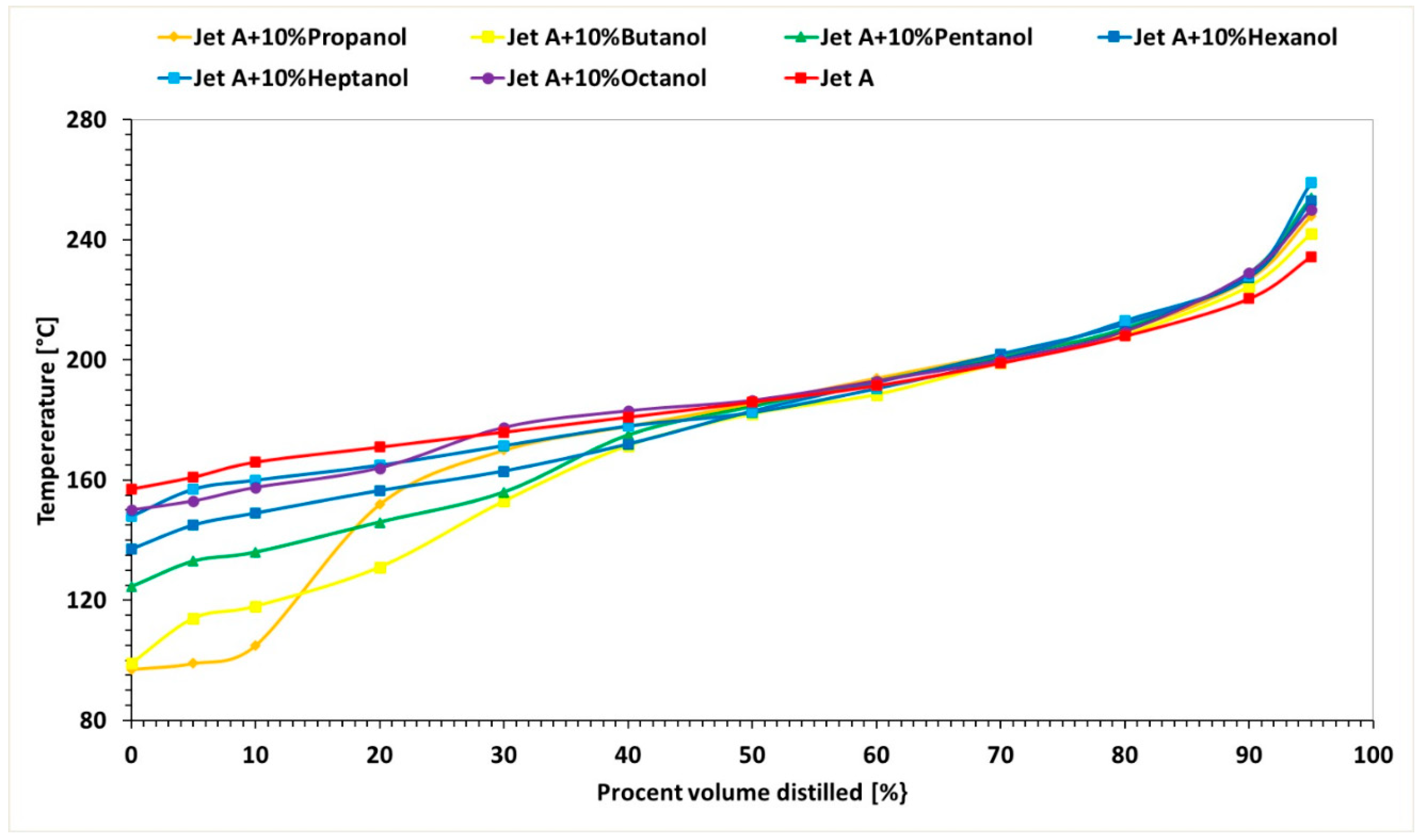
Figure 3 illustrates the variation of the exhaust gas temperature before the turbine.
Analyzing
Figure 3,
Figure 4 and
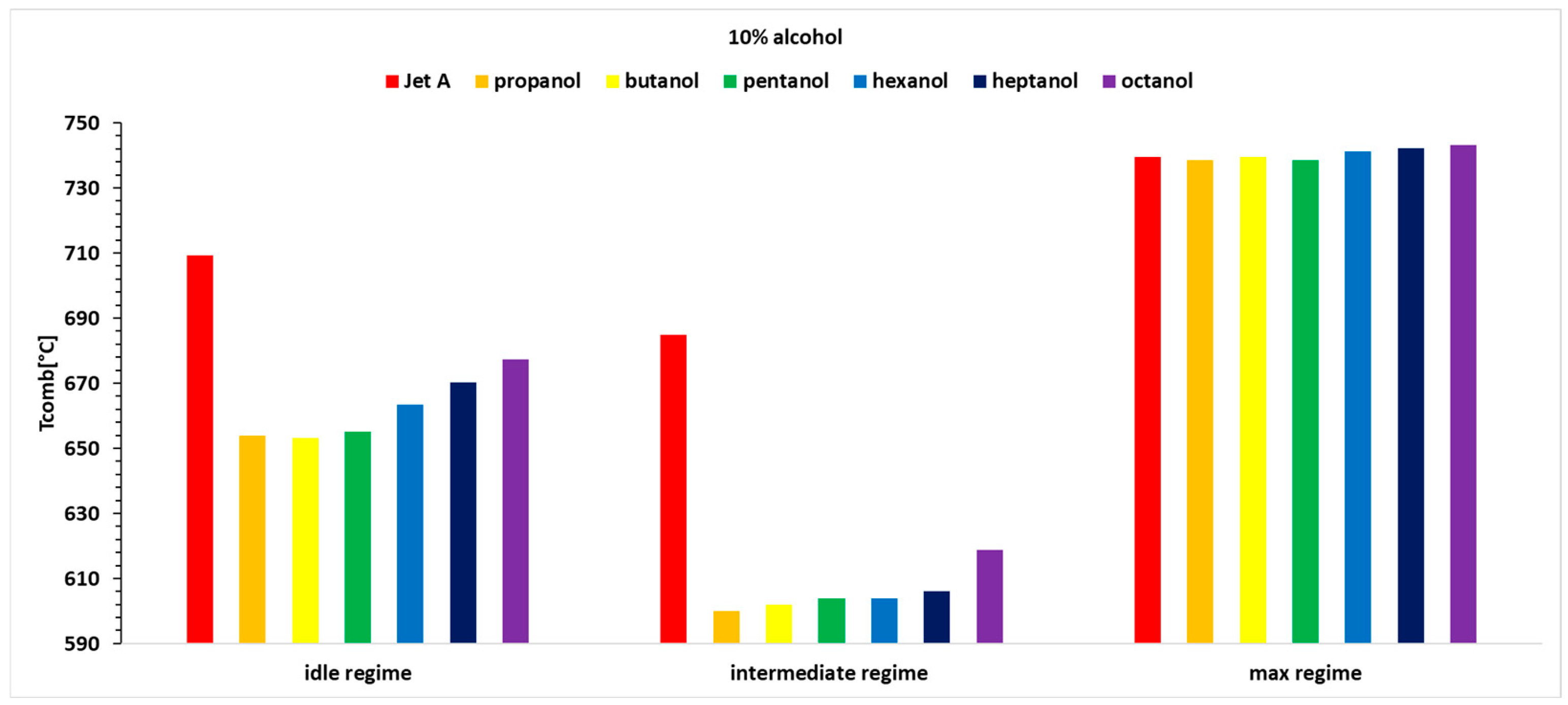
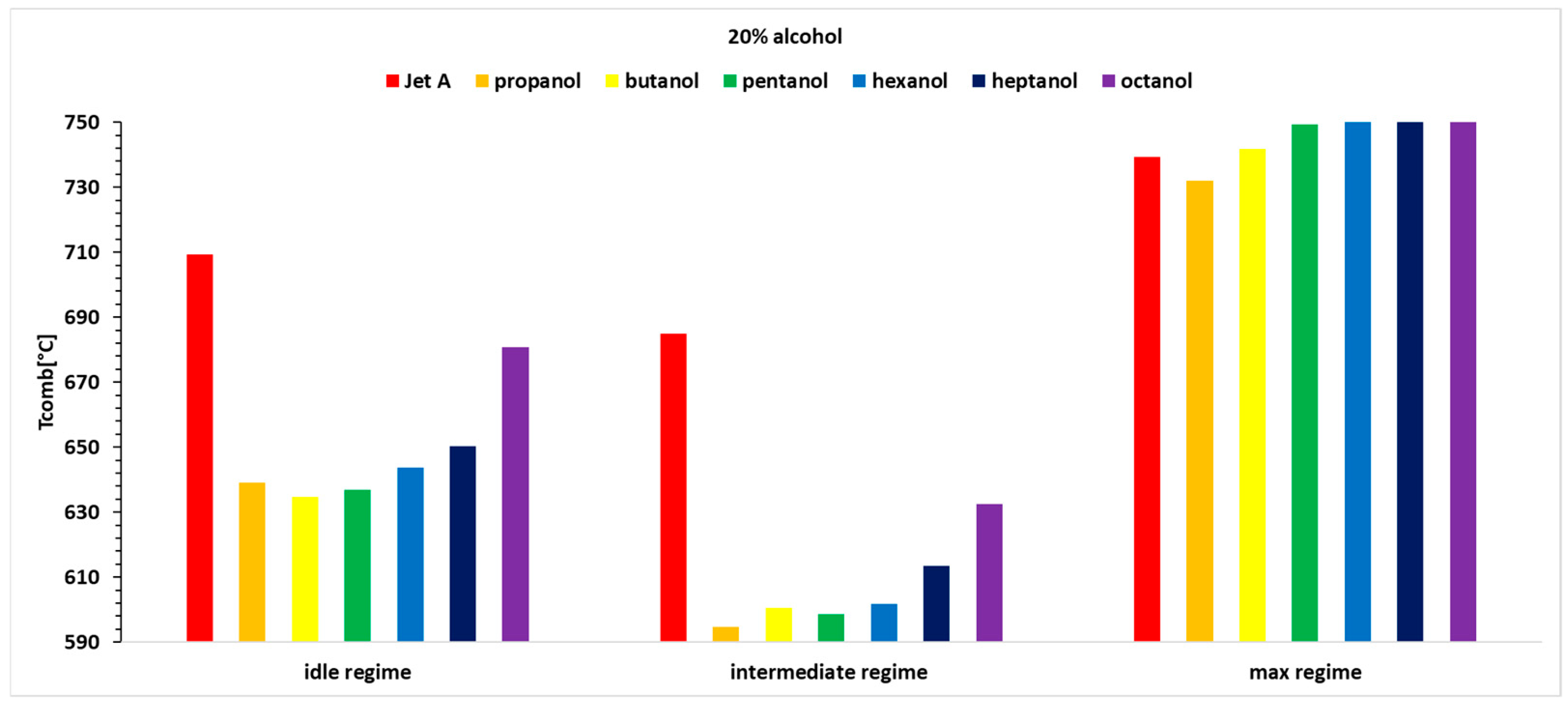
Figure 5, it can be observed that the exhaust gas temperature for the first two regimes and for all three alcohol concentrations is lower than the exhaust gas temperature when using the base fuel Jet A. This can be explained by the lower calorific value of the alcohols compared with Jet A. In the third regime, the exhaust gas temperature before the turbine increases slightly as the carbon content in the alcohols increases.
A detailed analysis is presented in
Table 4, which shows the percentage variation of the exhaust gas temperature before the turbine when using 10%, 20%, and 30% blends of the six alcohols for the three analyzed regimes. All of the tabulated data use as a reference baseline the temperatures obtained while using Jet A alone. The results have been obtained using Equation (8).
Analyzing the variation in exhaust gas temperature before the turbine, as shown in
Figure 3,
Figure 4 and
Figure 5 and
Table 4, it is observed that temperature variations reach up to 13%, indicating that the combustion temperature decreases by up to 13%.
For Regime 1, the exhaust gas temperature before the turbine displays a decreasing trend for all three concentrations of 10%, 20%, and 30% alcohol added to Jet A, with the highest values for butanol, followed by propanol, pentanol, hexanol, heptanol, and octanol.
In Regime 2, a decreasing trend is observed for all three concentrations of alcohol added to Jet A, resulting in a lower exhaust gas temperature before the turbine compared with Jet A. This temperature decreases as the carbon content in the alcohol decreases, with the most significant drop observed for propanol and the smallest for octanol.
In Regime 3 (maximum regime), higher alcohols lead to an increase in exhaust gas temperature before the turbine compared with Jet A. For the 10% alcohol blend, temperature variations are minimal (below 1%). With a 20% alcohol blend, the temperature variations are positive, except for propanol, and increase with the carbon content, reaching a positive variation of 1.7% for octanol. For the 30% alcohol blend, the temperature variation is positive for all alcohols except propanol.
The exhaust gas temperature before the turbine is correlated with the fuel flow rate supplied by the fuel pump to the combustion chamber, as shown in
Figure 6,
Figure 7 and
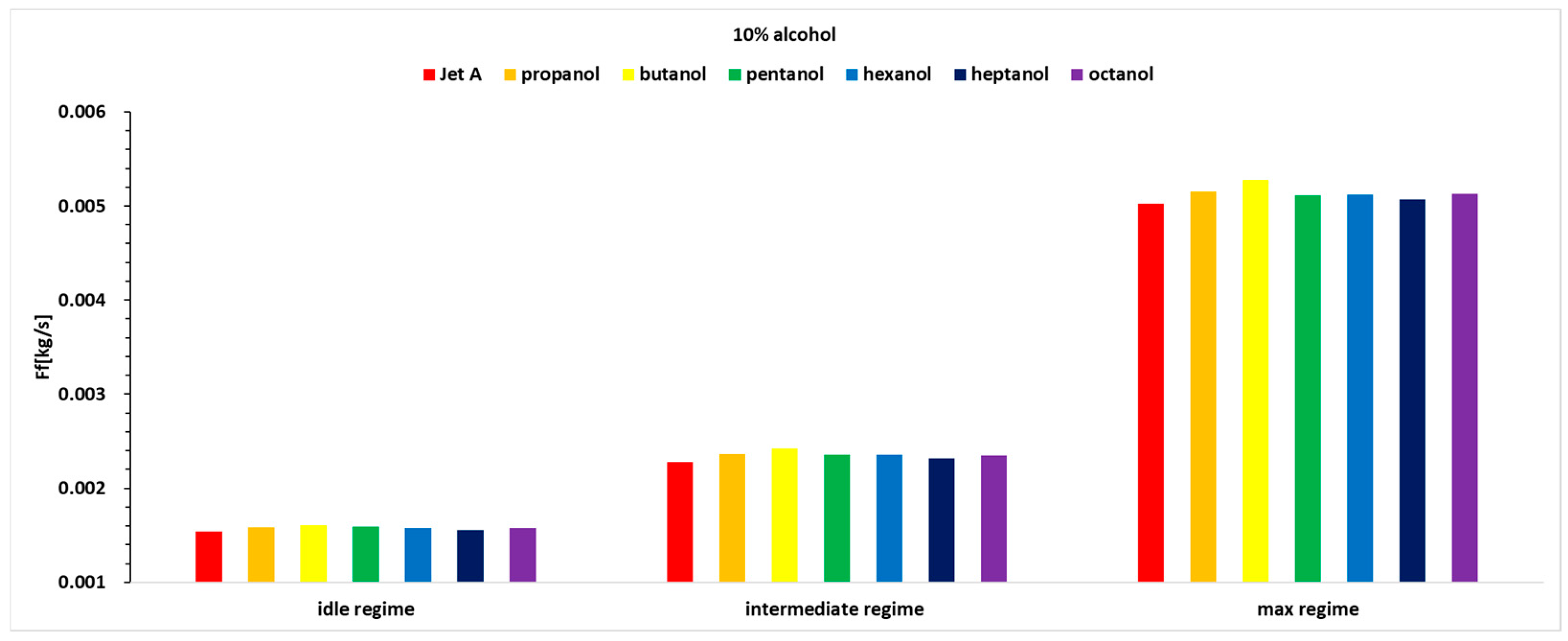
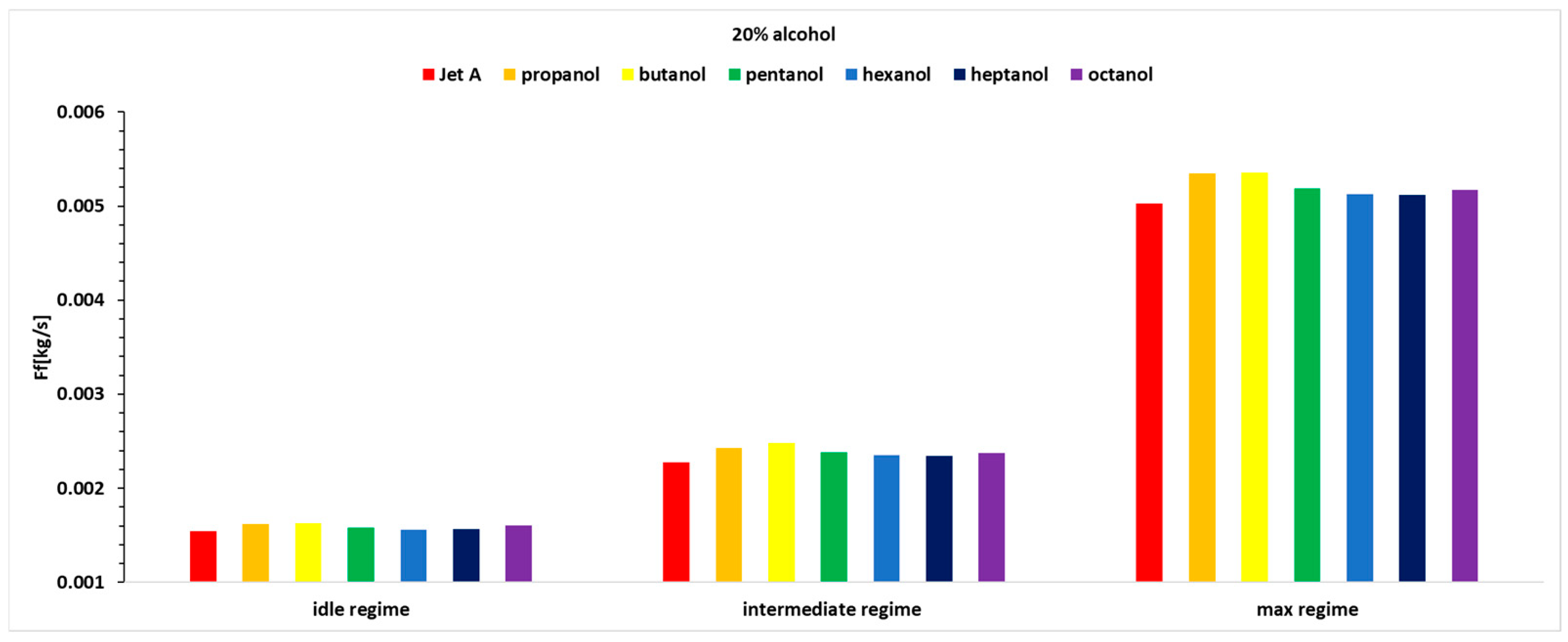
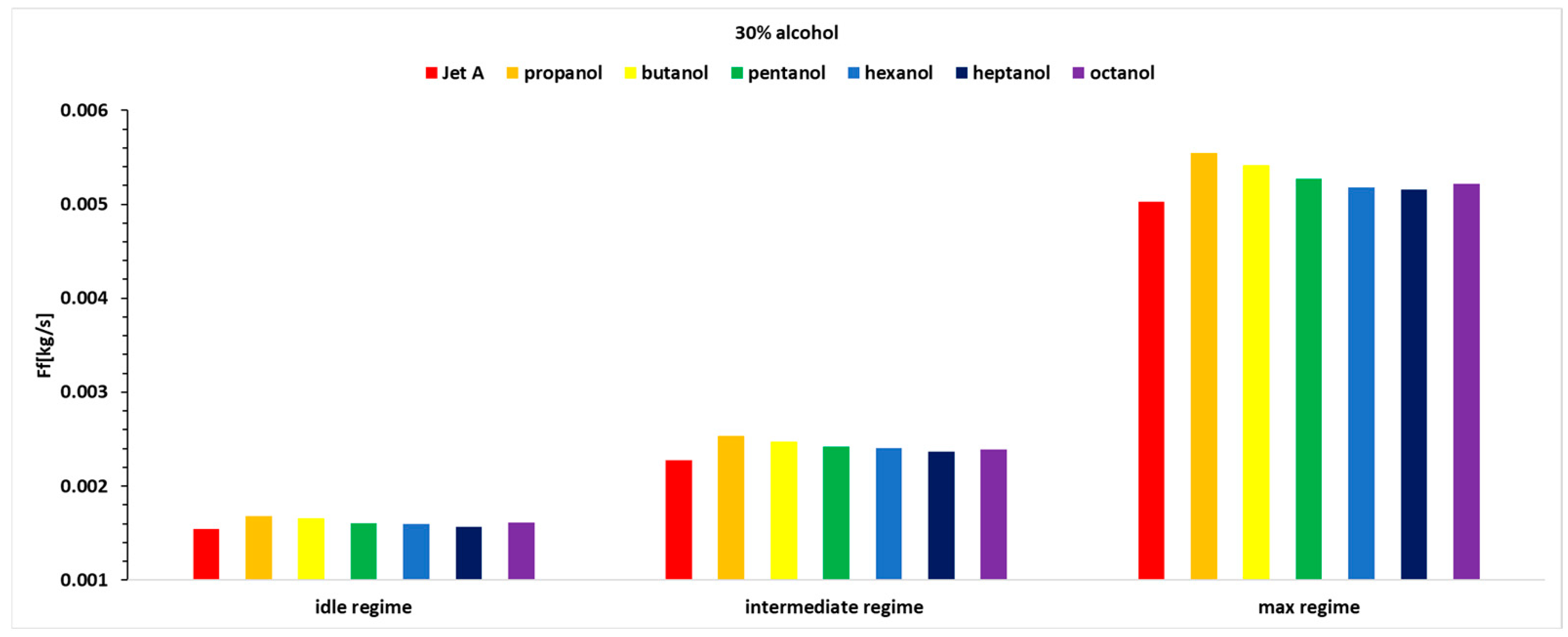
Figure 8. The analysis is based on mass flow rate, even though the engine instrumentation records volumetric flow rate. Using the determined densities for all tested fuels, the conversion from liters to kilograms was performed accurately.
Analyzing
Figure 6,
Figure 7 and
Figure 8, it can be observed that the fuel flow rate is higher in all cases where alcohol is added at the three analyzed concentrations of 10%, 20%, and 30%.
The fuel flow rate is higher for alcohols with lower carbon content, decreasing slightly when higher alcohols are used. As the exhaust gas temperature before the turbine decreases, the injected fuel flow rate increases.
A detailed analysis is presented in
Table 5, showing the percentage variation of the fuel mass flow rate when using 10%, 20%, and 30% blends of the six alcohols for the three operating regimes analyzed. All of the tabulated data use as a reference baseline the fuel mass flow obtained while using Jet A alone. The results have been obtained by using an equation similar to Equation (8).
Analyzing the variation in fuel mass flow rate, it can be observed from
Figure 6,
Figure 7 and
Figure 8 and
Table 5 that there are variations of up to 11.3%, meaning the fuel mass flow rate increases by up to 11.3%.
For Regime 1, the fuel mass flow rate shows a decreasing curve for 10% and 20% alcohol concentrations added to Jet A, with maximum values observed for butanol. However, for the 30% alcohol concentration in regime 1, butanol no longer exhibits a maximum value.
In Regime 2, a similar trend to Regime 1 is observed.
In Regime 3, the maximum regime, the same trend as in Regimes 1 and 2 is observed. As the concentration of alcohol added to Jet A increases, the fuel flow rate percentage also increases.
The exhaust gas temperature and the mass flow rate of fluid expelled through the reaction nozzle dictate the thrust produced.
Even in cases where the exhaust gas temperature is lower than that of Jet A, the thrust is compensated by the fuel flow rate, as the higher density of the alcohols contributes to an increase in the total fluid flow rate.
A detailed analysis is provided in
Table 6, presenting the percentage variation of thrust when using 10%, 20%, and 30% blends of the six alcohols with Jet A for the three operating regimes analyzed. All the tabulated data use as a reference baseline the thrust obtained while using Jet A alone. The results have been obtained by using an equation similar to Equation (8).
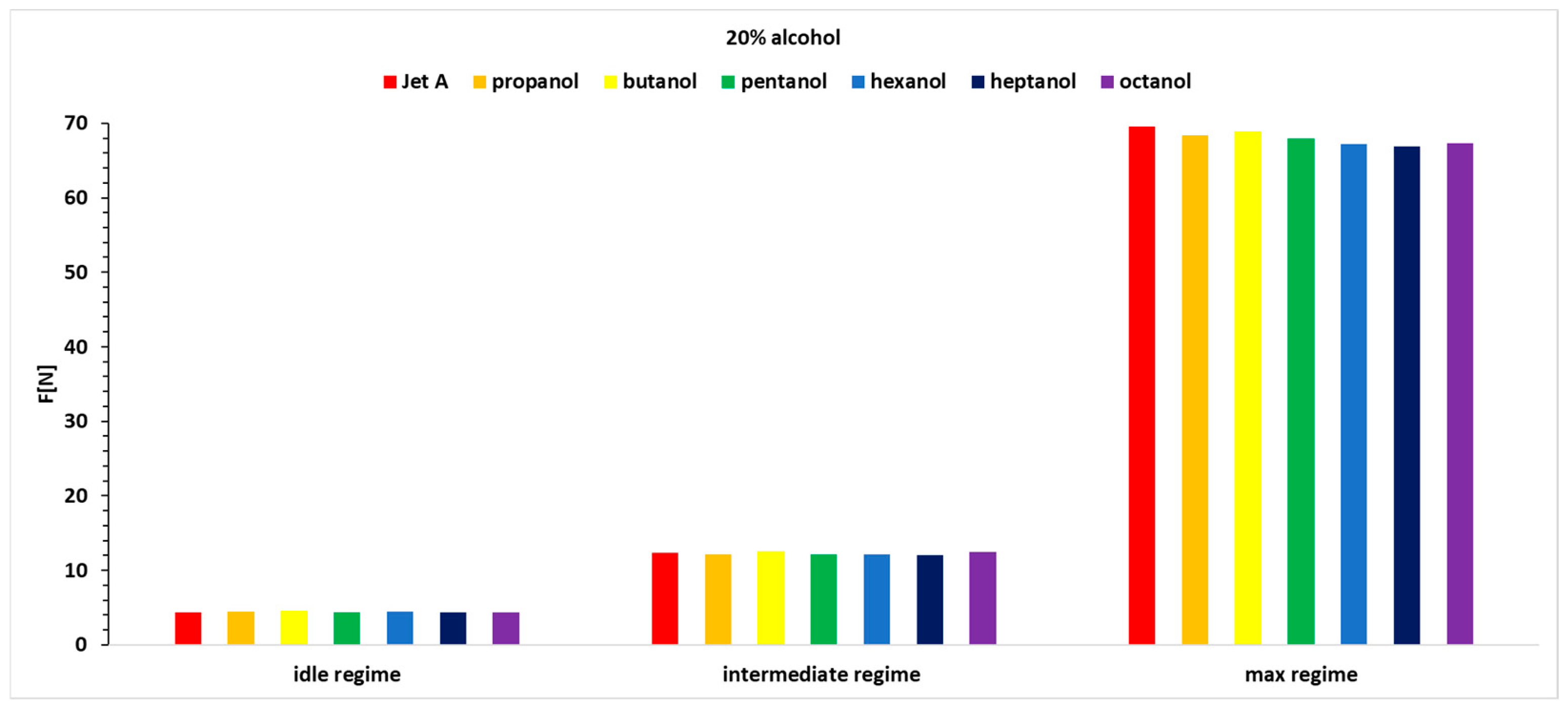
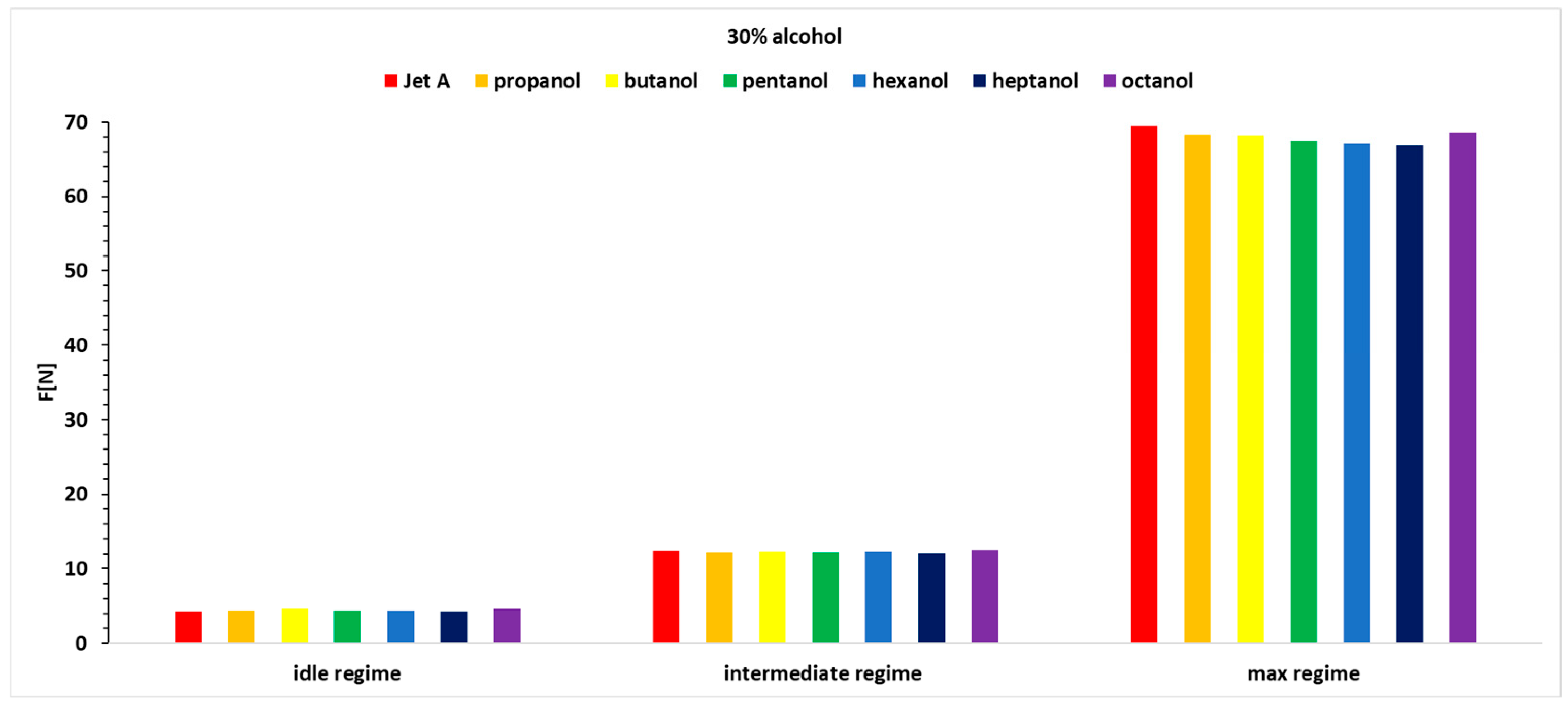
Analyzing the variation in thrust, as shown in
Figure 9,
Figure 10 and
Figure 11 and
Table 6, reveals positive variations of up to 7.4% and negative variations of up to −4.92%.
In the first regime, which is more unstable, the percentage variation in thrust fluctuates for all three alcohol concentrations. However, octanol and butanol stand out with more significant increases in thrust.
Butanol and octanol exhibit the best performance across the three operating regimes and all three alcohol concentrations added to Jet A.
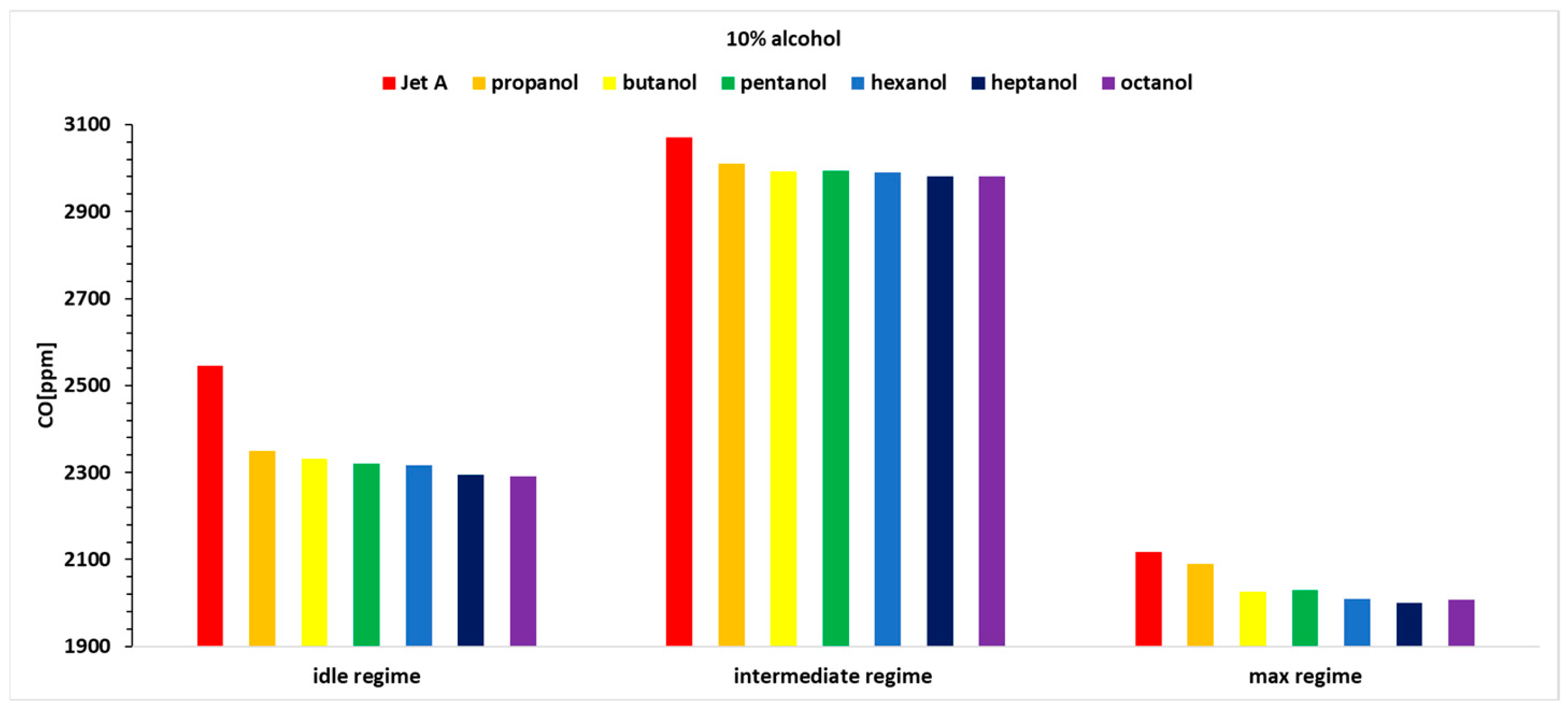
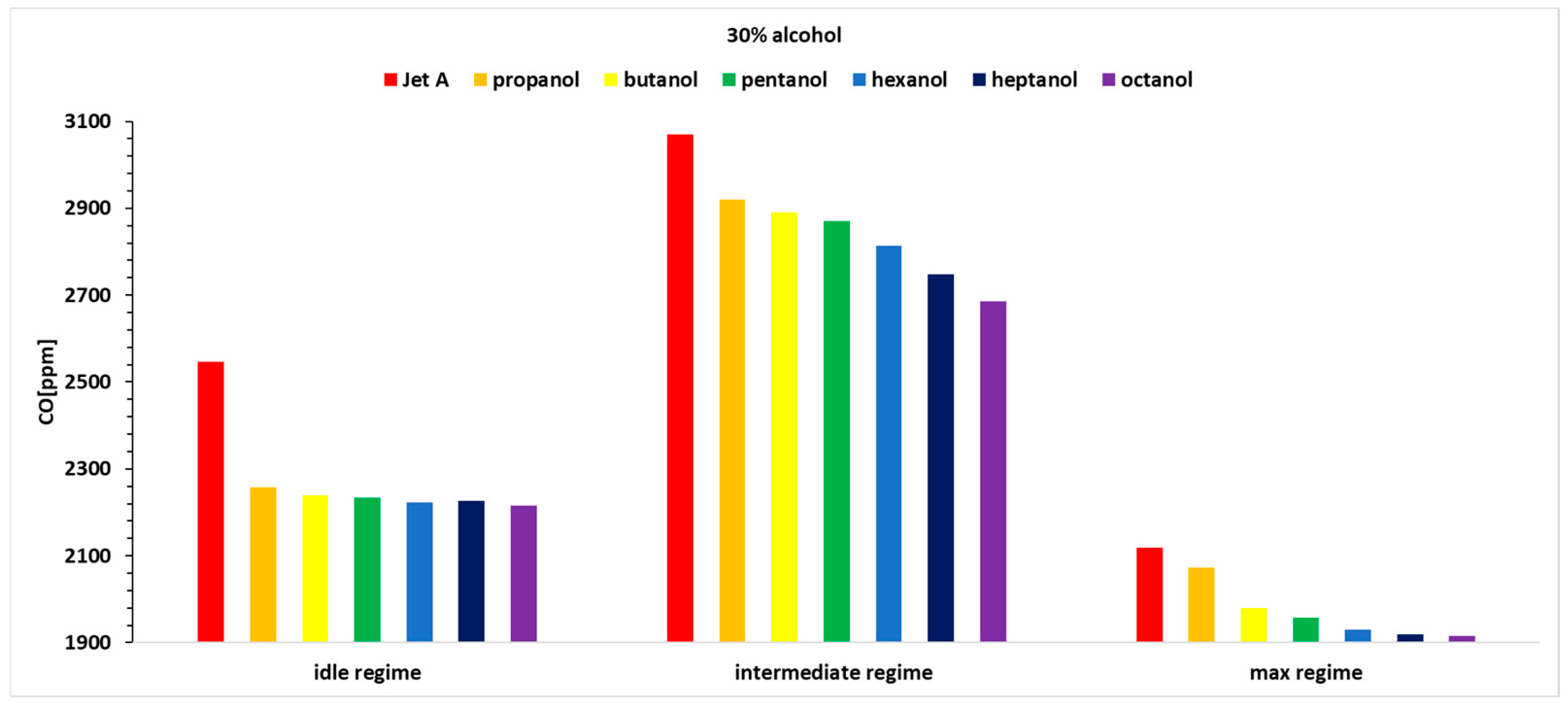
Hence, only the variation in CO concentration for the three operating regimes and the seven fuels is presented in
Figure 12,
Figure 13 and
Figure 14. The CO sensor used on the analyzer is specially designed to eliminate the effects of hydrogen cross sensitivity (hydrogen compensated sensor). The sensor contains an extra electrode that allows the effect of sensitivity to hydrogen to be counteracted within the electronics. Thus, as well as the sensor being calibrated using a certified carbon monoxide test gas it is also calibrated using 1000 ppm of hydrogen. This ensures improved accuracy of the CO reading under all normal circumstances.
Analyzing the above figures, it can be observed that the CO concentration decreases across all three operating regimes as the carbon content in the alcohols increases.
A detailed analysis is presented in
Table 7, showing the percentage variation of CO concentration when using 10%, 20%, and 30% blends of the six alcohols for the three analyzed regimes. All the tabulated data use as a reference baseline the CO emissions obtained while using Jet A alone. The results have been obtained by using an equation similar to Equation (8).
It can be observed that the percentage variation of CO concentration, relative to the baseline case for Jet A, shows values lower by up to nearly 13%.
A trend of decreasing concentration can be noticed as the carbon content in the alcohol molecule increases. Thus, the best alcohol in terms of CO concentration is octanol.
3.4. Micro-Turbojet Performance Results
Based on the measurements recorded by the micro-turbojet instrumentation, several important engine performance parameters can be calculated.
One of the most important parameters for turbomachinery is the specific fuel consumption, according to Formula (5), which combines fuel consumption and the thrust produced by the micro-turbojet into a single value.
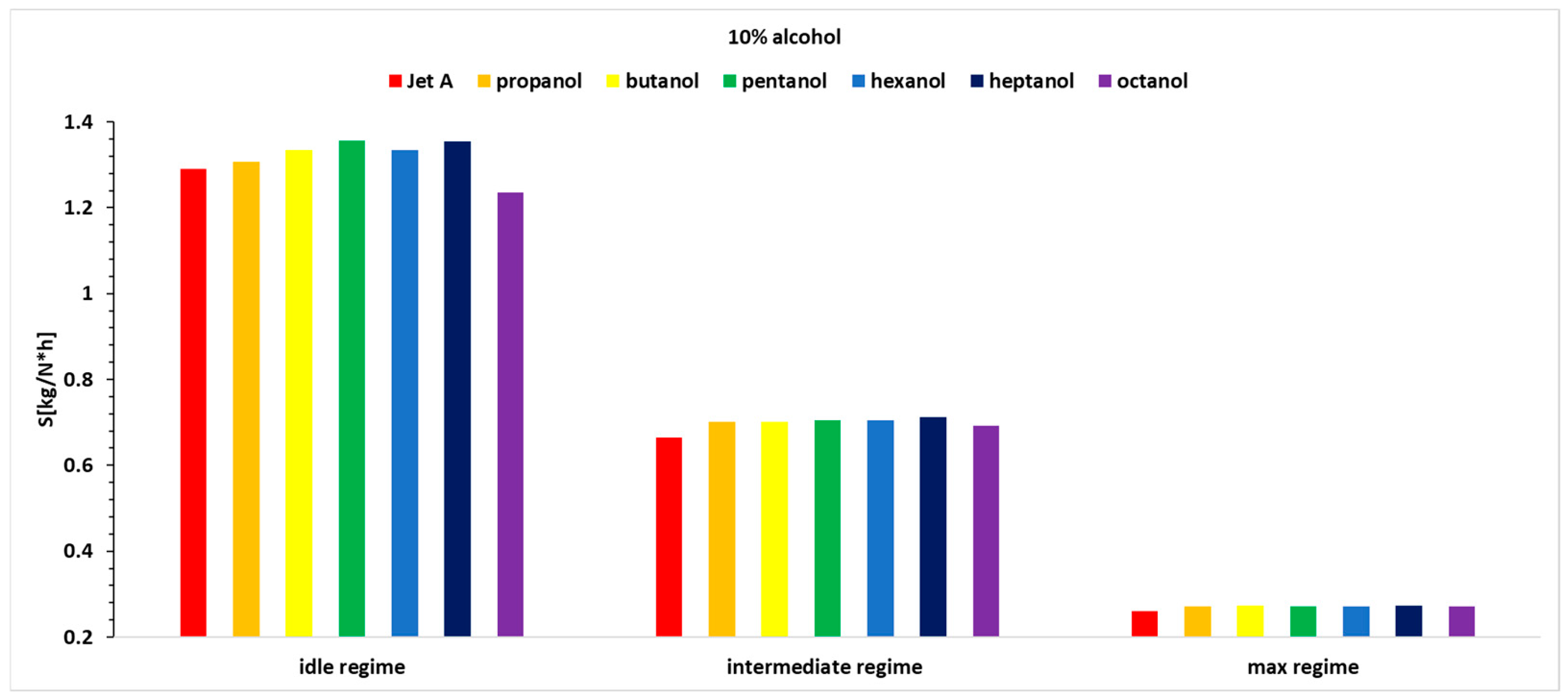
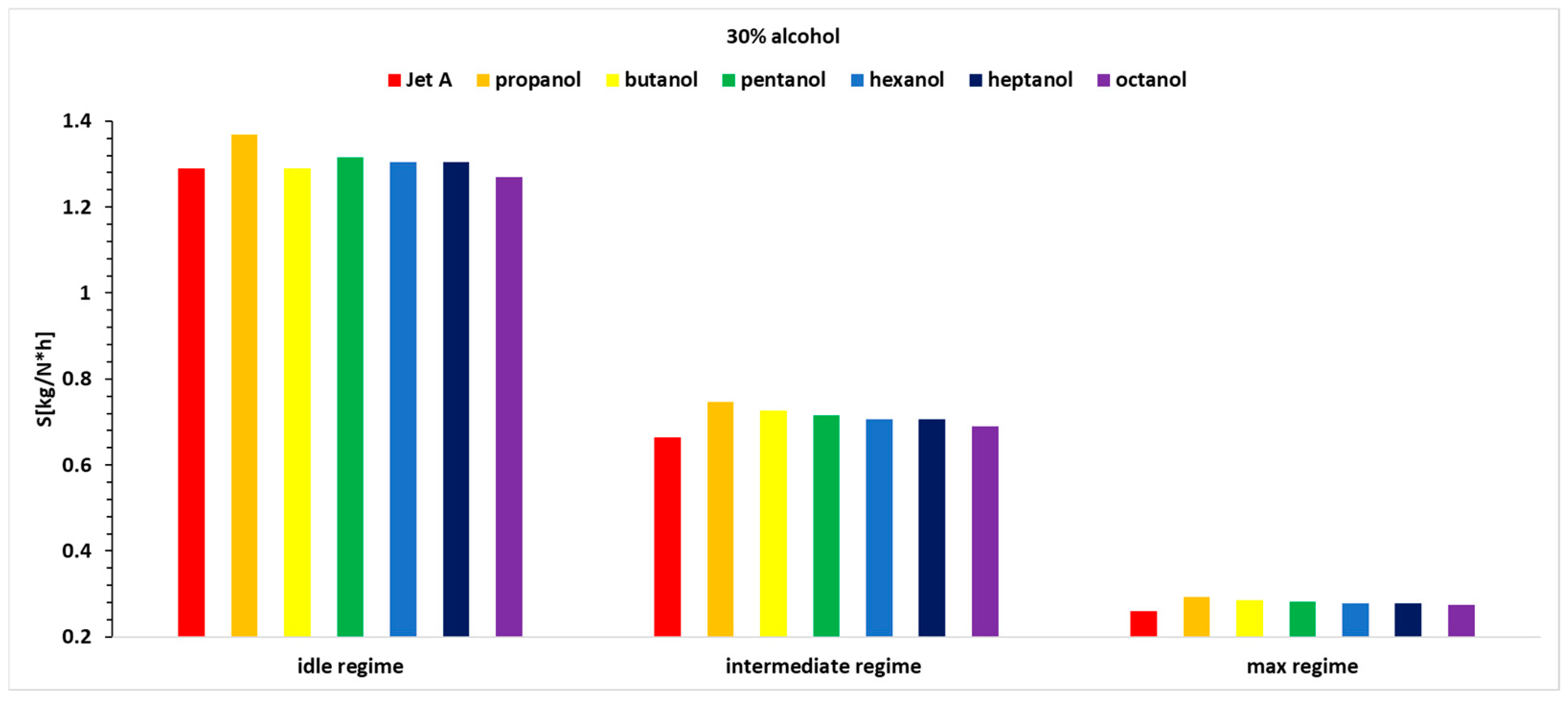
Figure 15,
Figure 16 and
Figure 17 present the variation in specific fuel consumption for the three studied regimes (1 being idle, 2 being intermediate and 3 being max), for the seven fuels at the three alcohol concentrations used.
Analyzing
Figure 15,
Figure 16 and
Figure 17, it can be observed that the idle regime, as known, is a more unstable regime compared with the other operating regimes. It does not show a clear trend of increase or decrease as the carbon concentration in the alcohols increases, but the variations are not significant. In the second regime, a higher specific fuel consumption is observed when using alcohol blends, and this increases with the alcohol concentration, which is expected because the calorific value of alcohols is lower than that of Jet A. The specific fuel consumption is highest for propanol, gradually decreasing to octanol, which shows the smallest increase compared with the specific fuel consumption when using Jet A. A detailed analysis is presented in
Table 8, showing the percentage variation of specific fuel consumption when using 10%, 20%, and 30% blends of the six alcohols for the three regimes studied. All of the tabulated data use as a reference baseline the specific fuel consumption obtained while using Jet A alone. The results have been obtained by using an equation similar to Equation (8).
Regarding the specific fuel consumption, it can be observed that for regime 1, which is a more unstable regime, no specific variation trend is apparent. However, octanol shows advantages at a 10% concentration, while butanol shows advantages at a 20% concentration, and both butanol and octanol show advantages at a 30% concentration.
For regime 2, a decreasing trend in specific fuel consumption can be observed from propanol to octanol when using 20% and 30% alcohol in the blend. In the case of regime 2 and 10% alcohol, the trend is increasing from propanol to heptanol.
For Regime 3, a similar decreasing trend in specific fuel consumption is observed from propanol to octanol when using 20% and 30% alcohol in the blend. In the case of Regime 3 and 10% alcohol, the variations do not present any specific trend.
Therefore, adding 10% alcohol does not present a clear picture in terms of specific fuel consumption variations. When using 20% and 30% alcohol, in Regimes 2 and 3, the trends are clear, showing a decreasing variation from propanol to octanol.
In
Table 9, the compressor outlet temperature and the air mass flow rate recorded by the micro-turbine instrumentation at Regime 3 are presented, as these are the parameters involved in Equations (7) and (8), which are used to generate the data shown in
Figure 18 and
Table 9.
It can be observed that the variations in temperature for the blends are relatively small (ranging from 117.9 °C to 121.6 °C) and fall within the error margin of the type K thermocouples used (±1.1 °C), indicating good thermal stability of the system. In the case of certain blends, such as 10% propanol or 20–30% octanol, a slight decrease in the compressor outlet temperature is observed, without affecting the proper functioning of the engine. This decrease may be attributed to changes in the air–fuel mixture density but does not significantly influence the compressor efficiency. Regarding the airflow rate, the values obtained (between 0.222 and 0.225 kg/s) indicate stable operation of the compressor–combustion chamber assembly, with no major deviations induced by the type of fuel used. This consistency confirms the good compatibility of the blends with the tested engine and supports their use without the need to recalibrate the air supply system.
Another important parameter, according to Equation (7), is the engine’s thermal efficiency.
Figure 18 shows the variation in thermal efficiency for the maximum regime when using 10%, 20%, and 30% alcohol in the fuel blends.
By analyzing
Figure 18, it can be observed that the thermal efficiency in the maximum regime decreases with the increase in alcohol concentration. According to Equation (7), this combination of propulsive force, exhaust gas flow, and calorific power causes the thermal efficiency to exhibit small variations.
Another parameter that can be analyzed, according to Equation (6), is the combustion efficiency in the combustion chamber. Thus, the most indicative results are for the maximum regime.
By analyzing
Table 10, it can be observed that the combustion efficiency shows small variations around the value obtained for Jet A, which is 85.28%, with variations of up to ±2%.
The errors associated with the parameters in Equation (6) must be considered in order to evaluate the significance of the variations observed between the different blends. The type K thermocouples used for temperature measurements have a typical uncertainty of ±1.1 °C, which introduces a possible variation in the calculation of the enthalpy difference (the terms involving T_comb and T_comp). Regarding the mass flow rates, the flow sensors have an estimated uncertainty of ±1–2%, directly affecting the and terms. The specific heat capacities (cp) are determined based on temperature and may introduce an additional error of ±1% in the calculation. Moreover, the lower calorific value (LCP) of each fuel blend naturally varies depending on the alcohol type and concentration; however, these values were considered constant and were taken from bibliographic sources. Therefore, the overall propagated error in the calculation of ηb can be estimated at approximately ±1.5%. This value must be considered when interpreting the differences in combustion efficiencies among the blends—for example, differences below 1% between propanol and Jet A fall within this uncertainty range and cannot be considered statistically significant. In contrast, the larger differences (over 2%) observed for blends with pentanol, heptanol, or octanol suggest a possible real thermodynamic advantage, but one that requires confirmation through repeated testing and further experimental validation.
Similar values to those presented in this study were also obtained in [
13,
14], with some differences that are mainly due to varying atmospheric conditions, as the experiments were conducted on different days. It is well known that ambient temperature and pressure significantly influence the values recorded during testing. Other bibliographic sources [
16,
17,
18,
19] also report similar findings regarding the variation of key parameters, consistent with those observed in this study. However, the tests in those studies were performed on different types of gas turbine engines.