Research on Vaporization and Sudden Cooling Performance of Heptafluoropropane in Prefabricated Fire-Extinguishing Devices Based on Numerical Method
Abstract
1. Introduction
2. Theoretical Analyses and Modeling of the Vaporization Processes of Heptafluoropropane
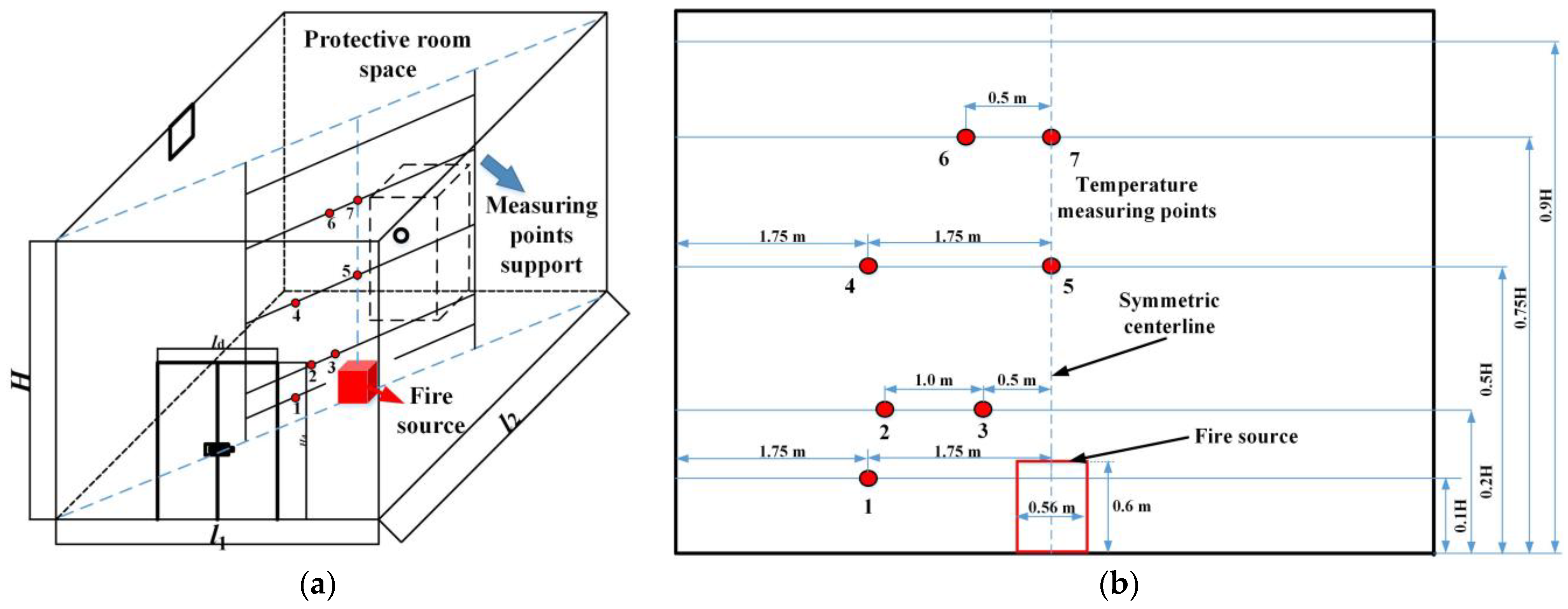
2.1. The Geometry of the Prefabricated Fire-Extinguishing Device and Protective Room
2.2. The Numerical Model of the Vaporization Processes
3. The Numerical Simulation Method and Experimental Validation
3.1. The Physical Model and Numerical Parameter Settings
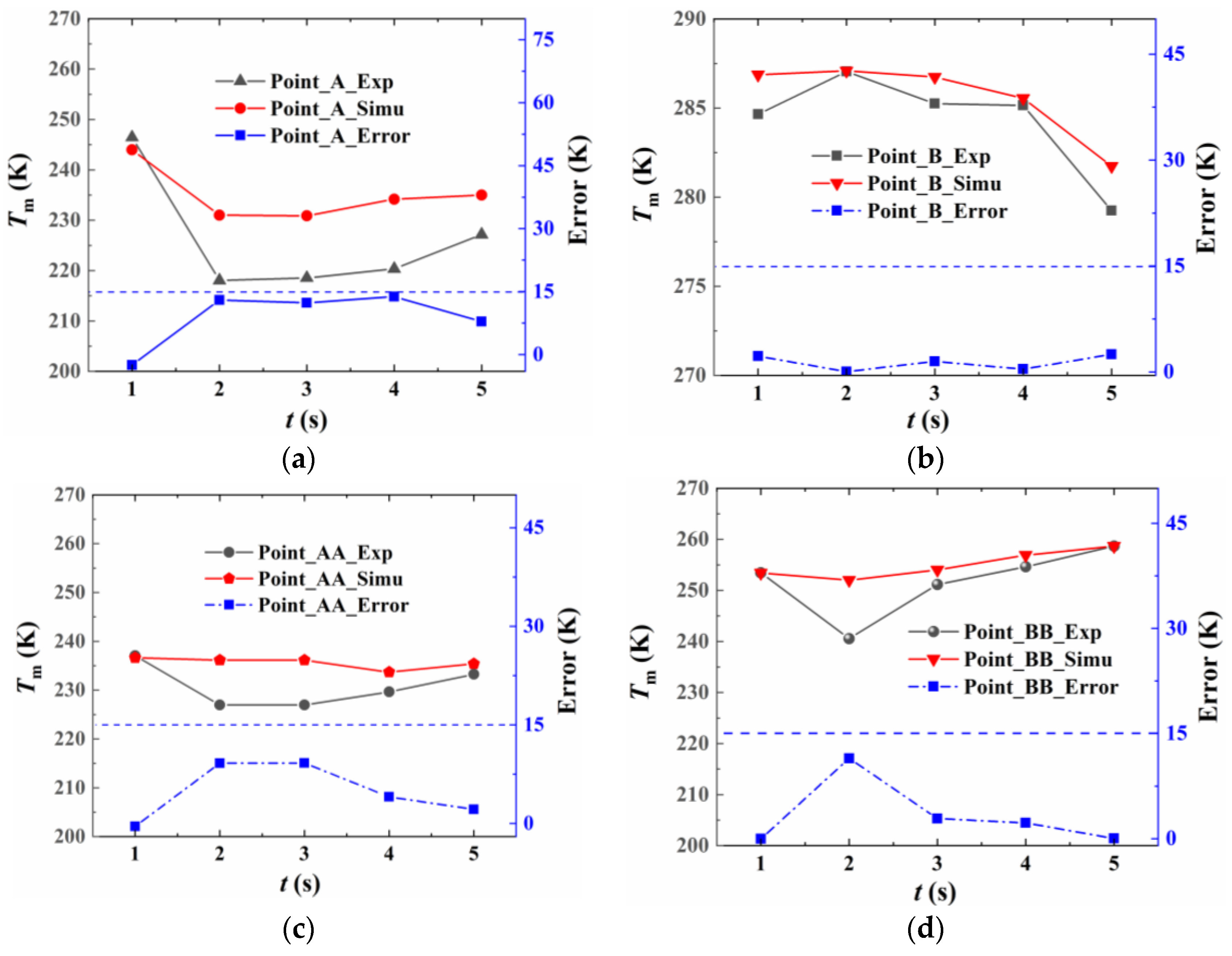
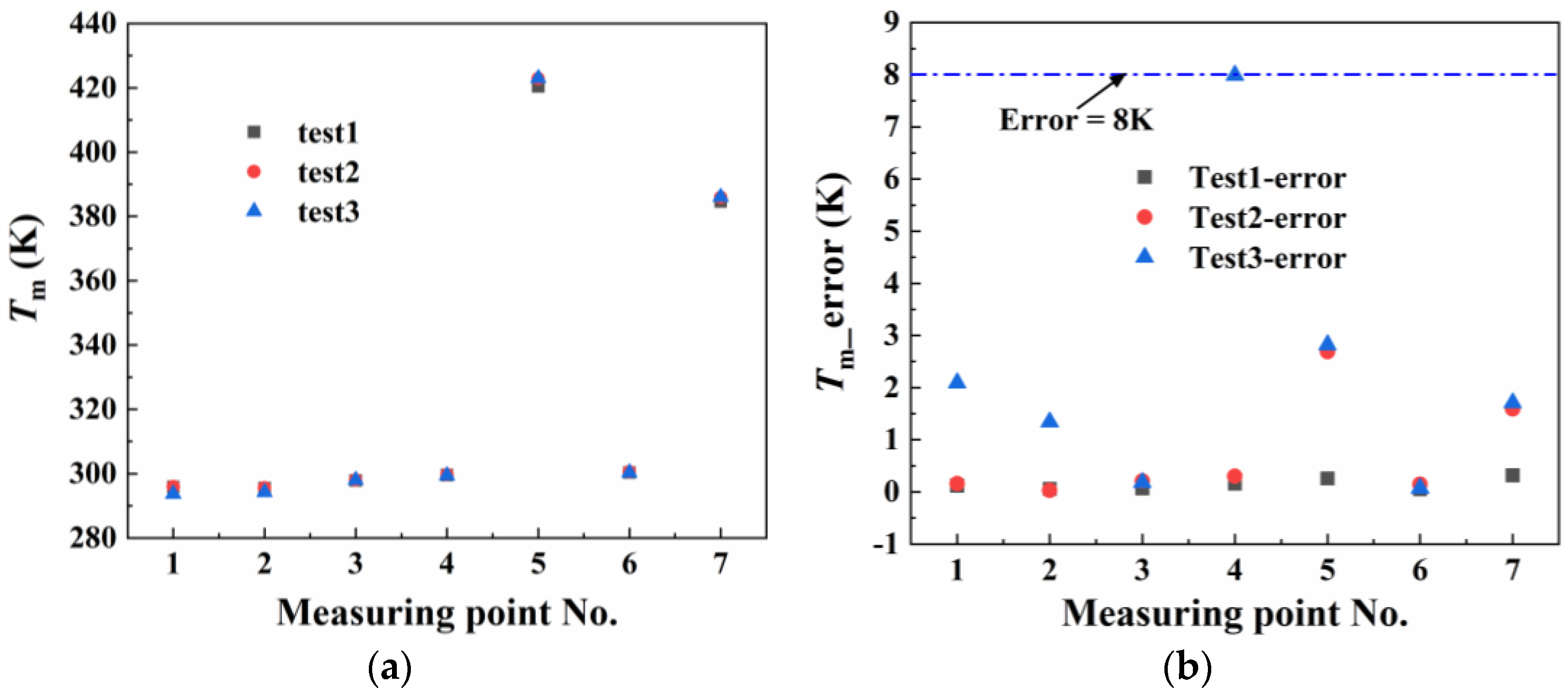
3.2. The Experimental Measurements and Numerical Method Validation
4. Results of the Vaporization and Fluid Flow Performance of Heptafluoropropane Spraying
4.1. Heptafluoropropane Release in the Fire Cabinet
4.2. The Vaporization of Heptafluoropropane from the Nozzle to Protective Room
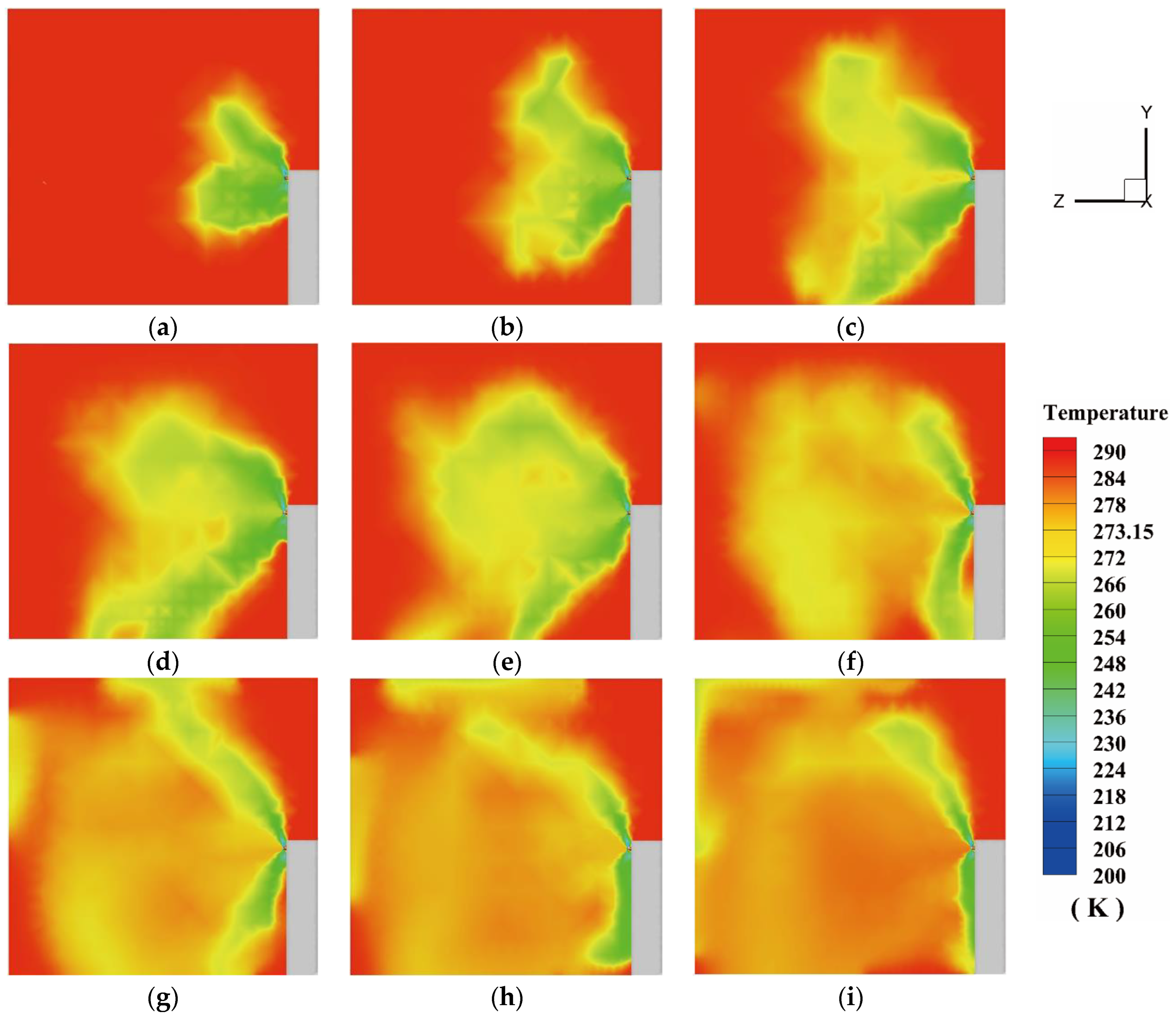
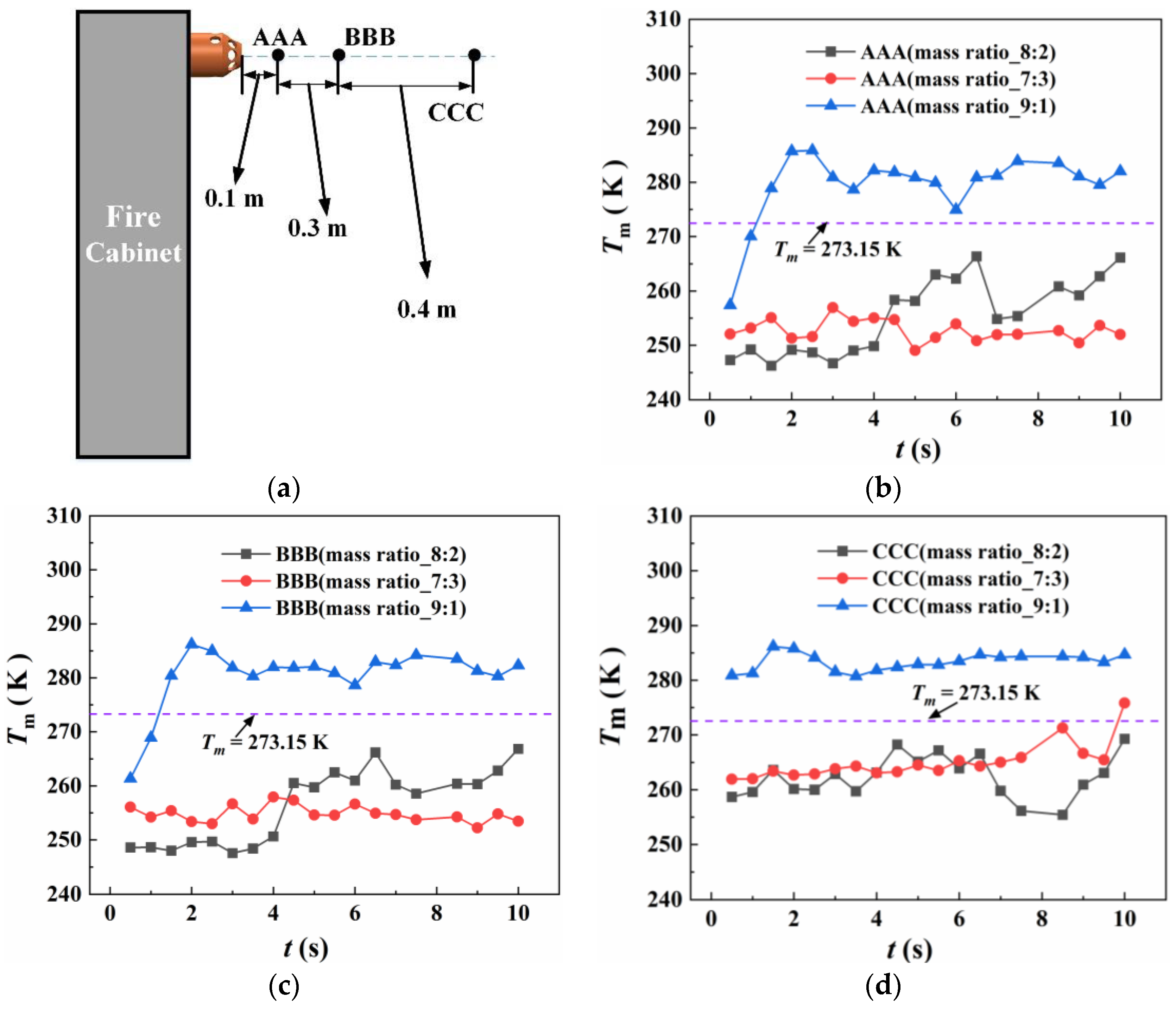
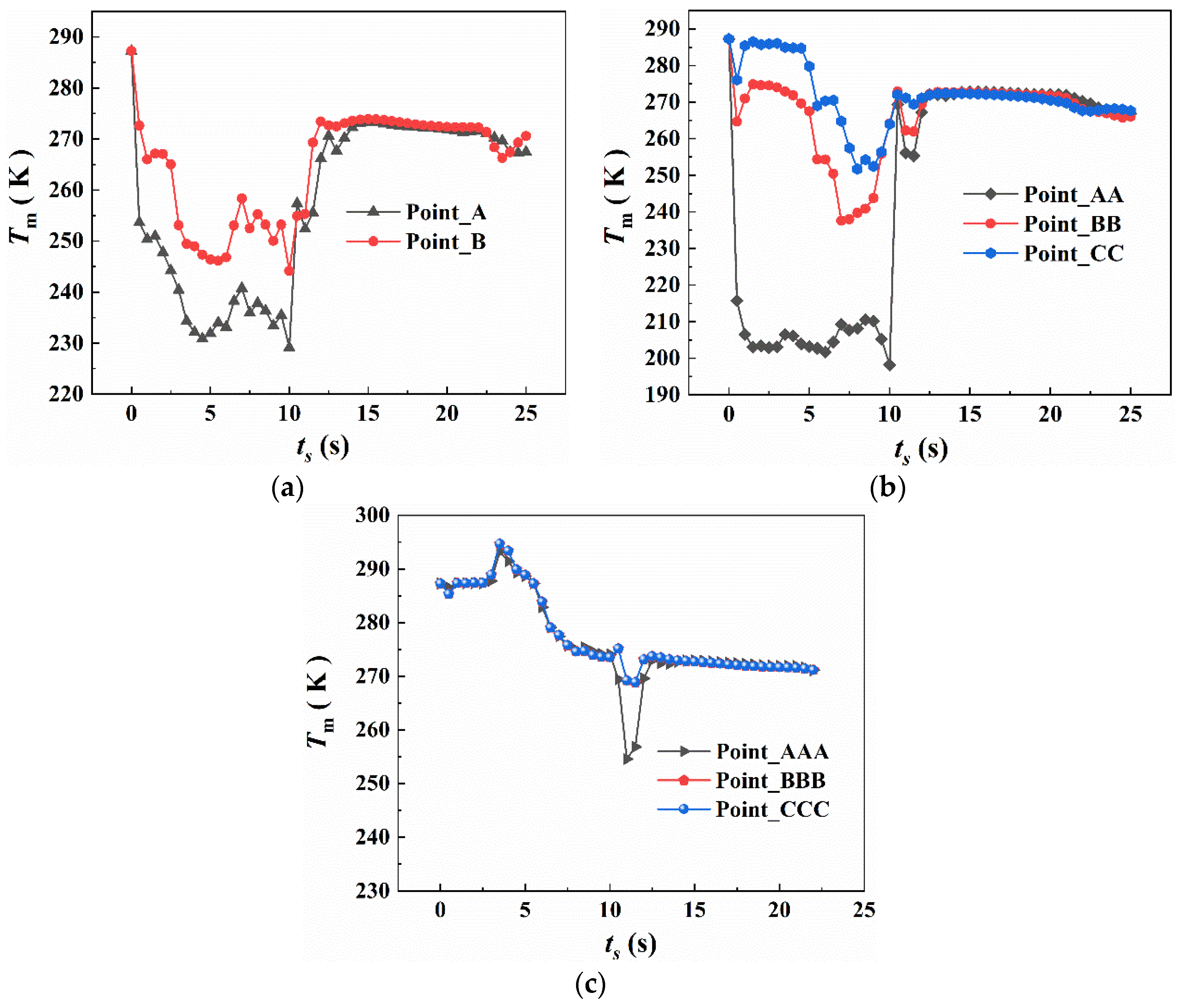
4.2.1. The Temperature Variation of the Protective Room
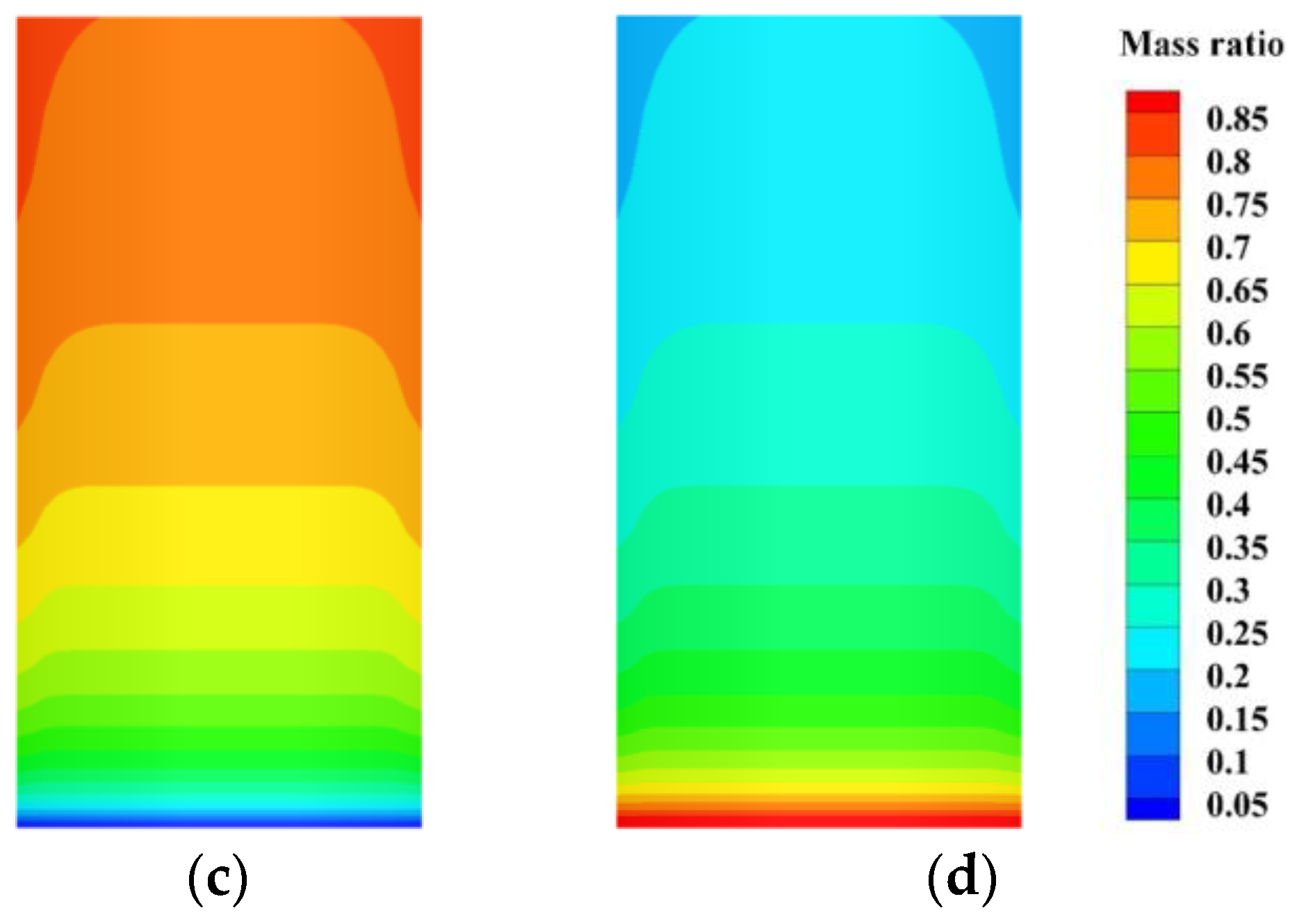
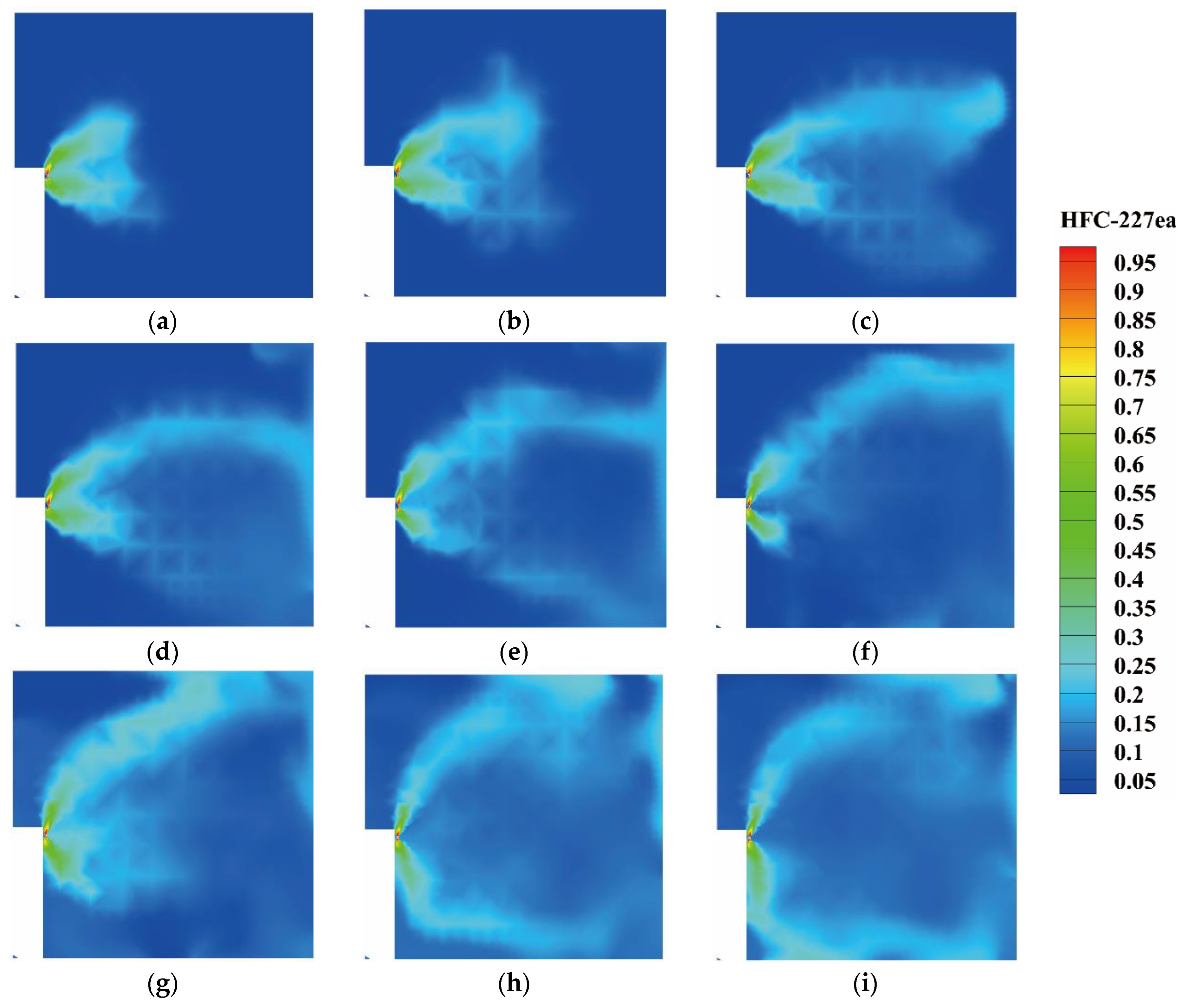
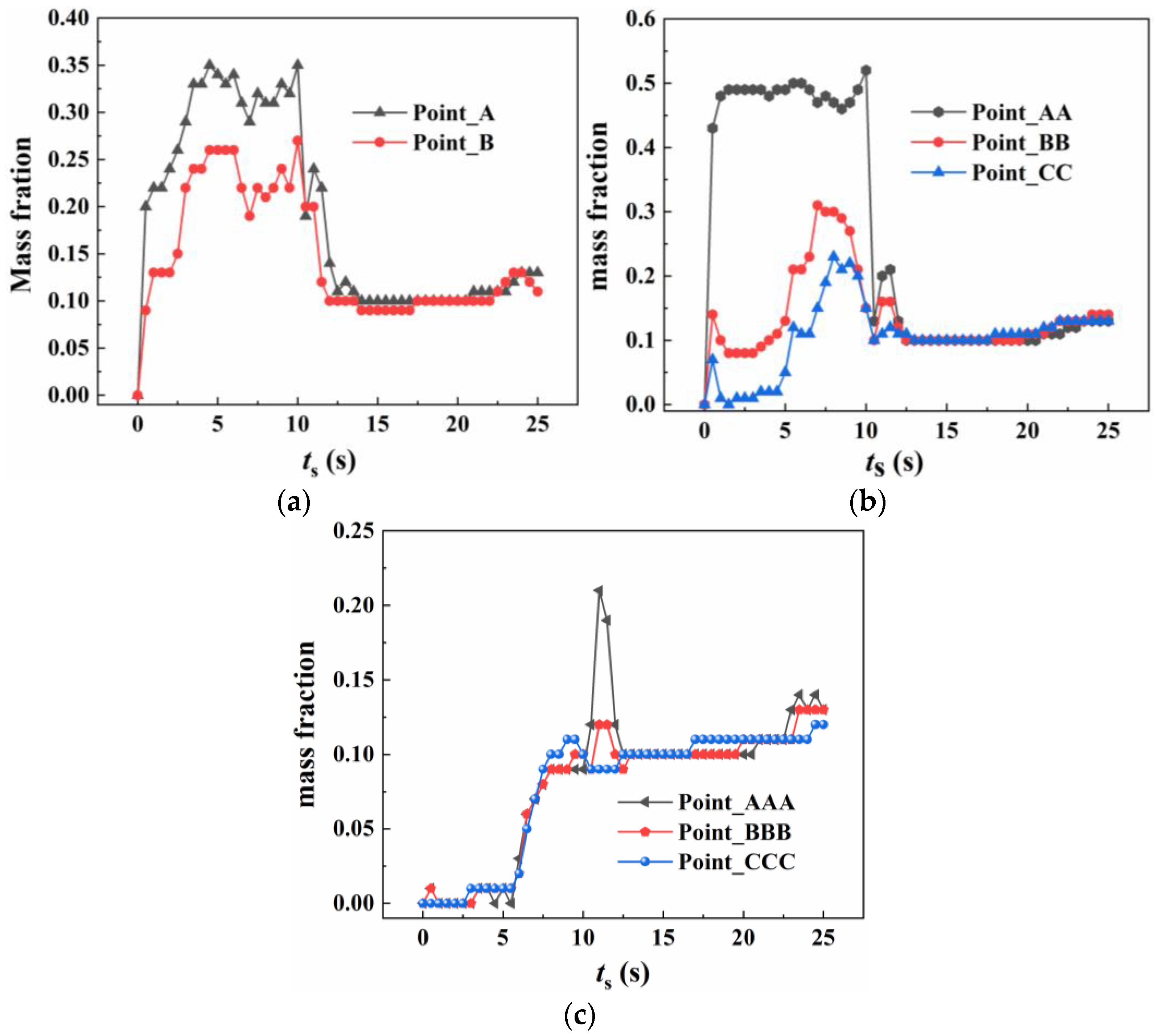
4.2.2. The Concentration Variation of Heptafluoropropane in the Protective Room
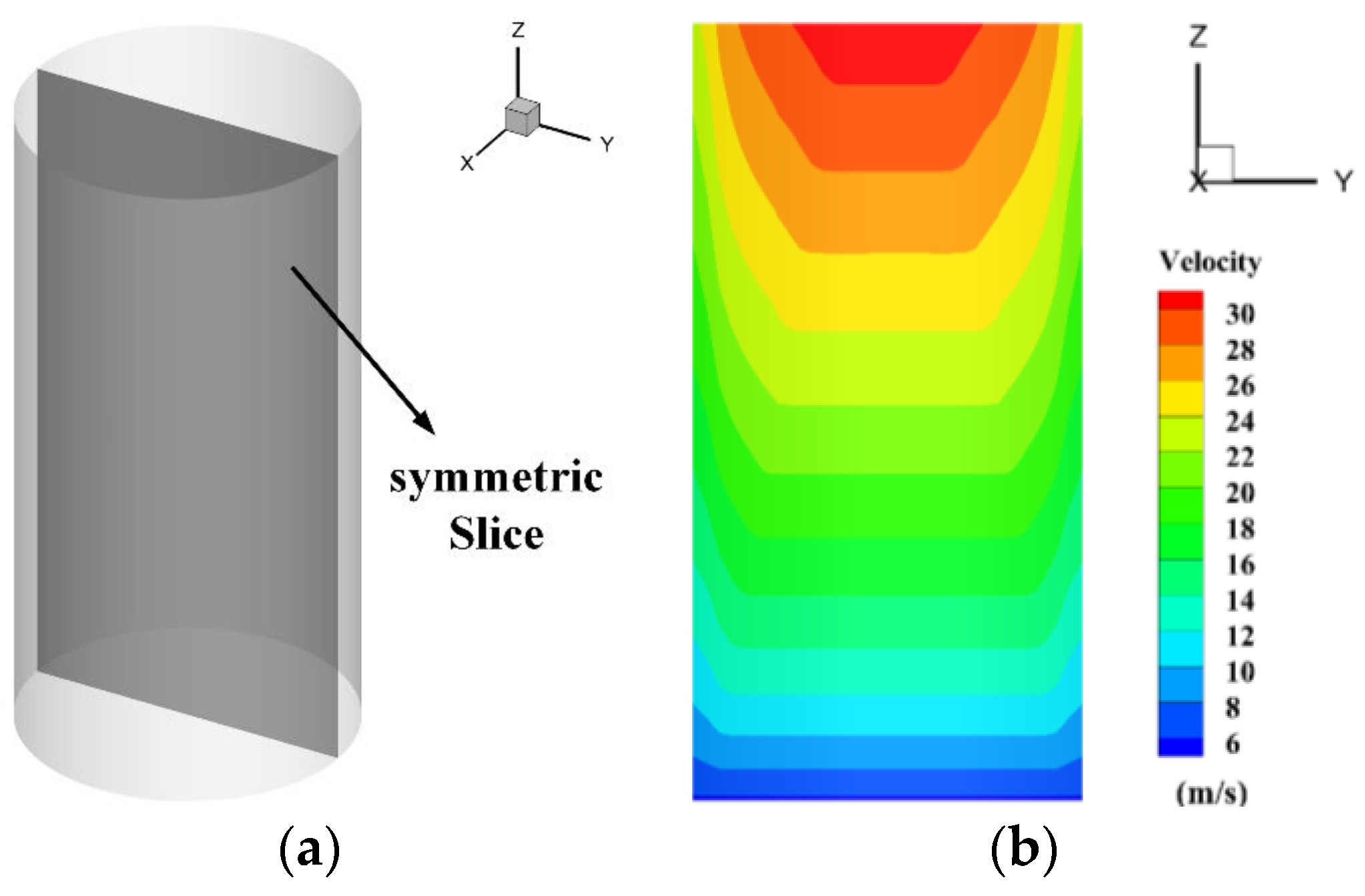
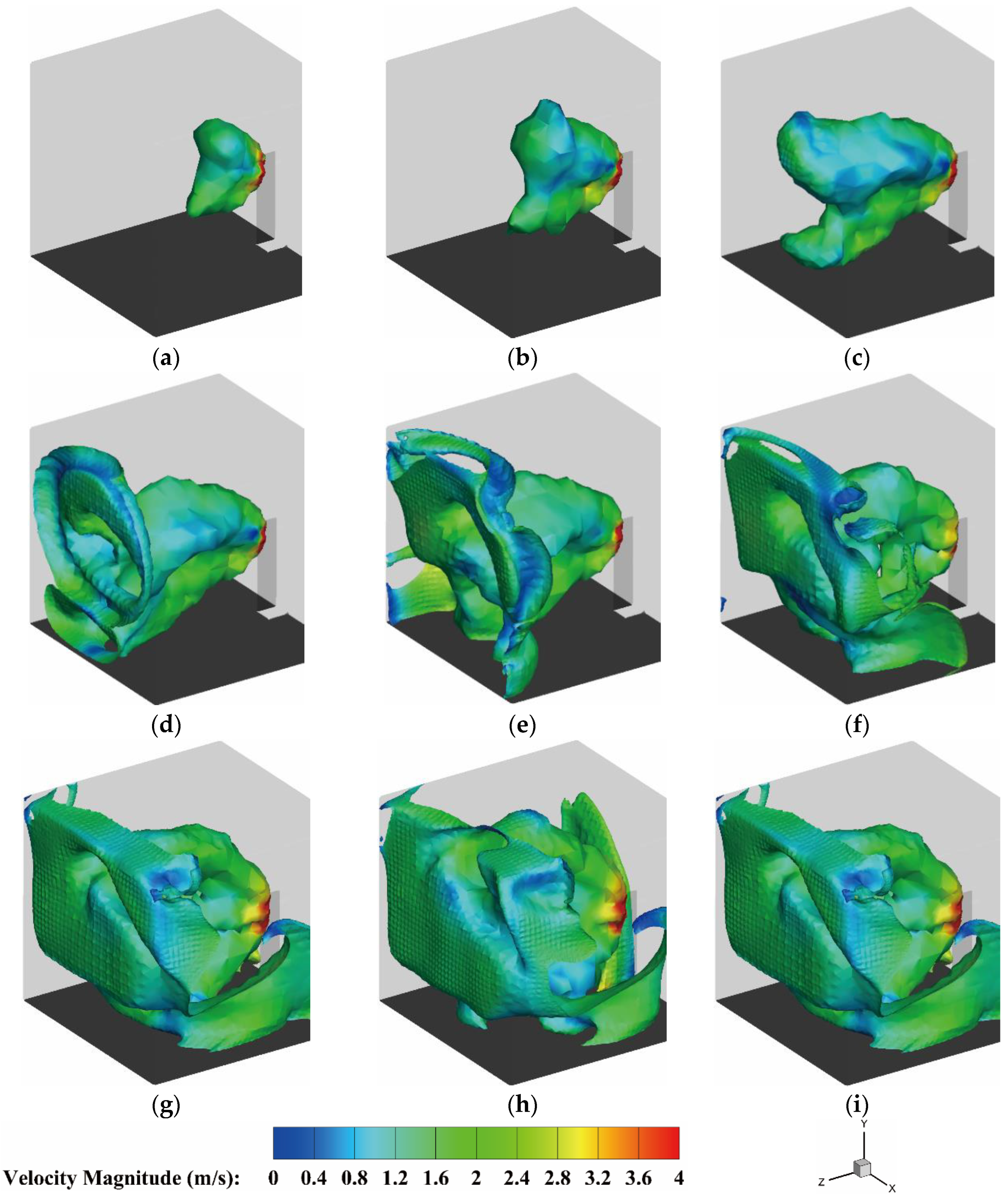
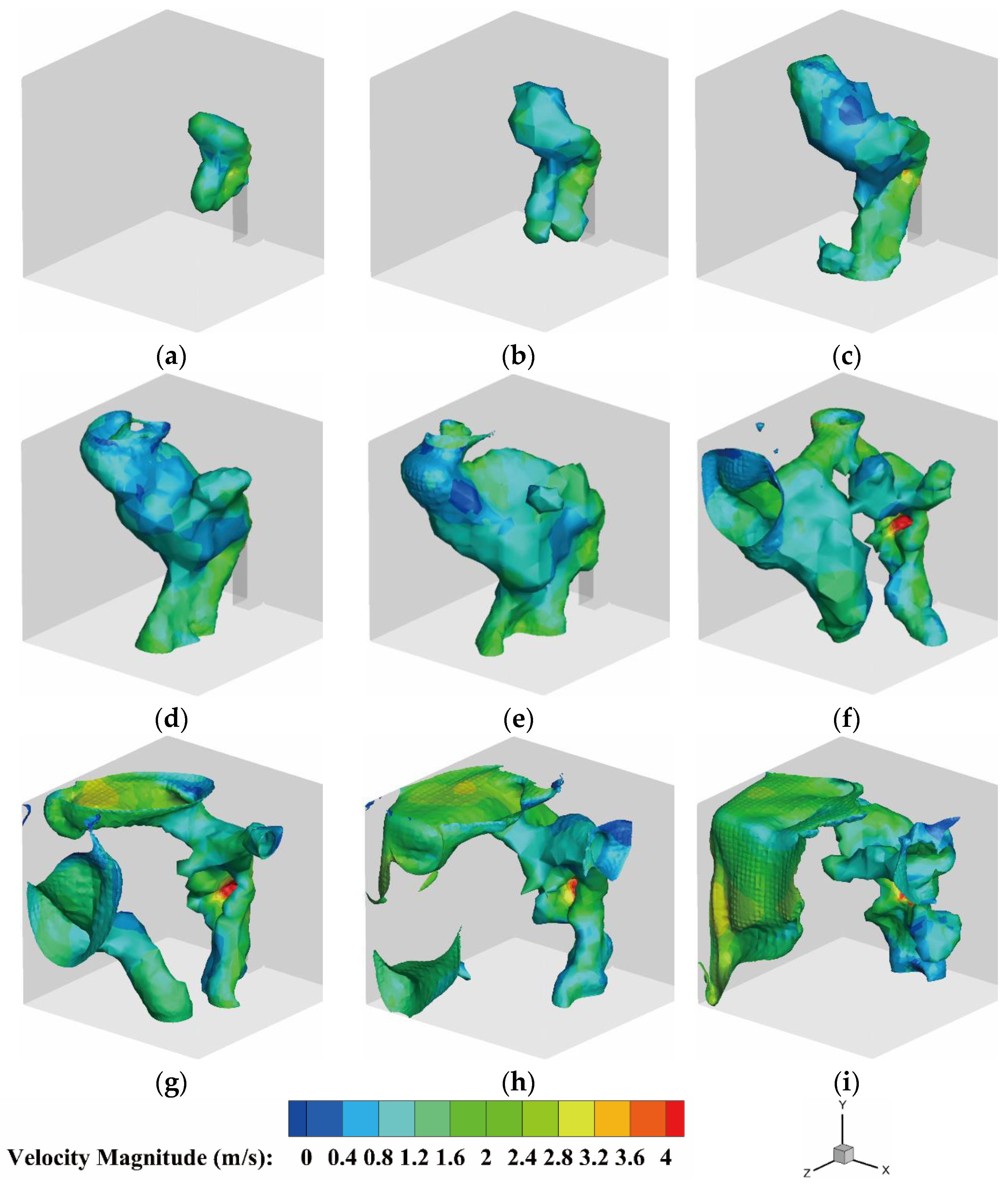
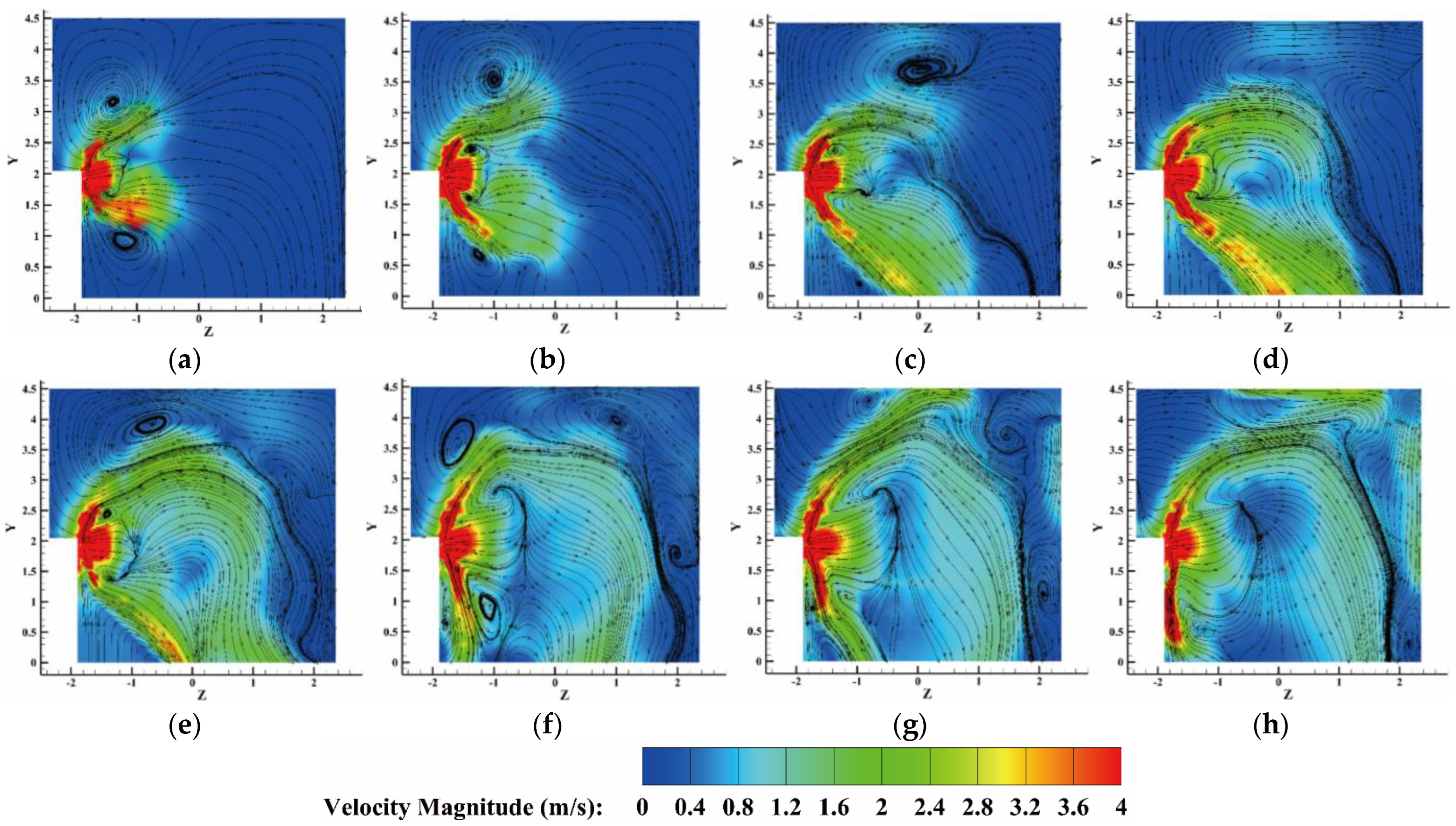
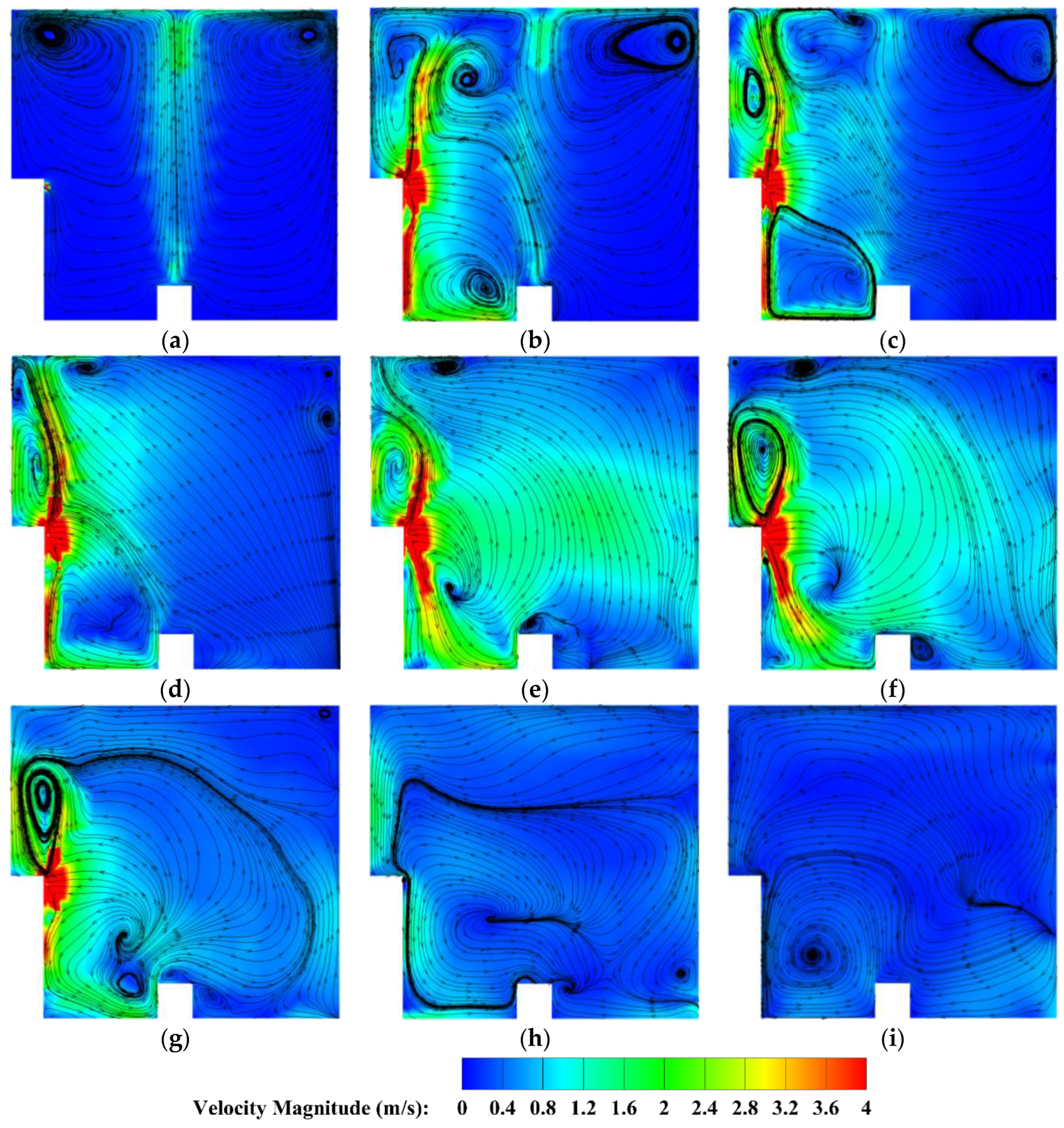
4.2.3. The Velocity Distribution of the Heptafluoropropane in the Protective Room
4.2.4. The Cooling Effect and Safe Distance of the Heptafluoropropane Spraying
5. Discussions on the Cooling Effect of Prefabricated Fire-Extinguishing Device in the Fire Suppression Process
5.1. The Heat Transfer Rate of the Fire Source and Experimental Validation
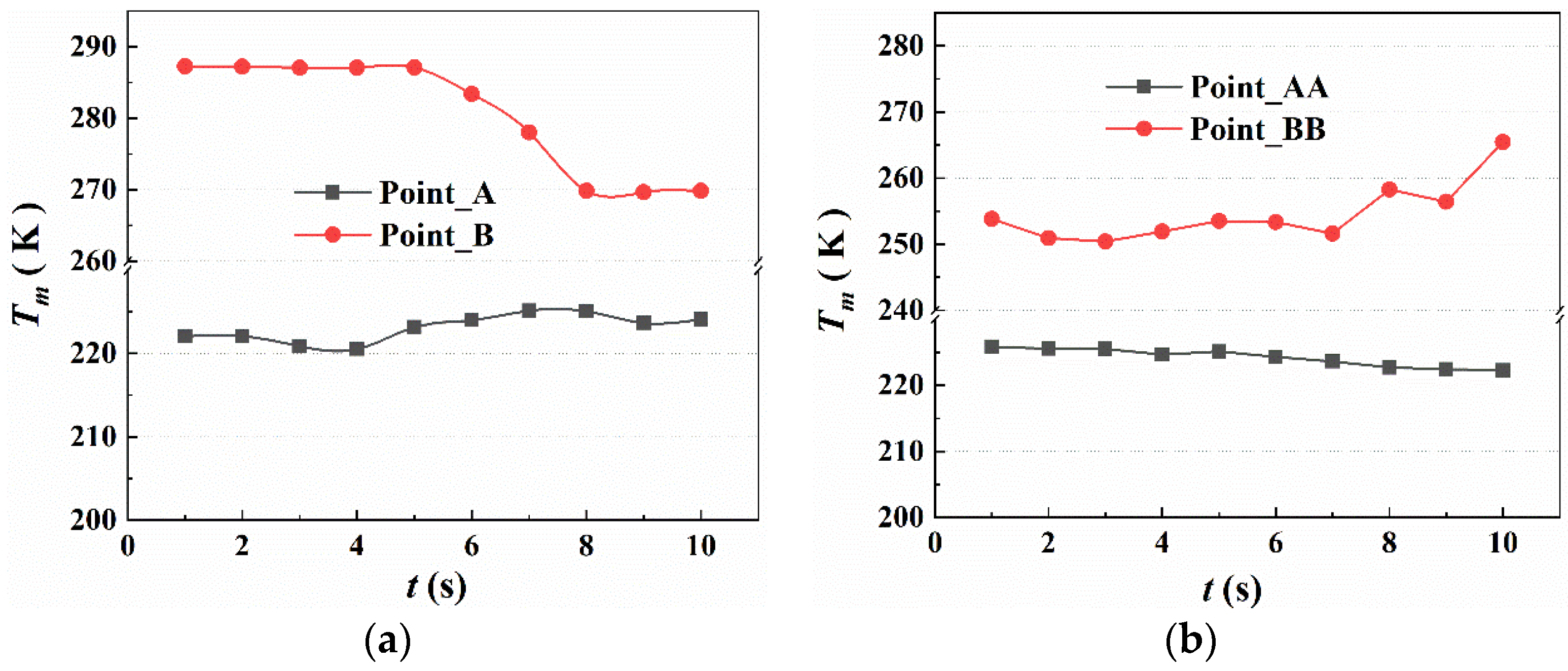
5.2. The Temperature Variation in the Fire Suppression
5.3. The Velocity and Concentration Distribution in the Fire Suppression Process
6. Conclusions
- (1)
- A numerical simulation model was established based on the phase change and fluid flow process. The release of heptafluoropropane from the cabinet and its spraying from the nozzle were analyzed based on Eulerian and DPM models, respectively. The results indicated that the vaporization of heptafluoropropane in the cabinet presents certain differences with that relating to its spraying from the nozzle. The numerical method was validated through experimental temperature comparisons and can help to improve the vaporization performance of heptafluoropropane.
- (2)
- For heptafluoropropane spraying without a fire source, analysis of its release in the fire cabinet (step I) illustrated that the velocity, liquid, and gas phases of heptafluoropropane are 29.0 m/s, 20.2%, and 79.8%, respectively. These results reflect the fact that the heptafluoropropane is a mixture of gas and liquid phases during the spraying process, although its boiling point is 256.75 K. The sudden cooling effect will occur with the lowest temperature of 220 K, which can contribute to its concentration and velocity distribution in the protective room. Analysis of the vaporization process indicated that a distance of 0.8 m in front of the prefabricated fire cabinet can serve as a reference value for the safe distance. The heptafluoropropane was shown to gradually fill the entire room, with part of the gas phase settling at the bottom.
- (3)
- For heptafluoropropane spraying with a fire source, an actual fire-extinguishing scenario with a wood stack fire was assessed through experiments and simulations. A large vortex was formed, helping to achieve a sudden temperature drop, due to the high speed of heptafluoropropane spraying. The concentration at a distance of 0.8 m from the nozzle remained at a mass fraction of 0.1. Furthermore, the uniform distribution of heptafluoropropane is beneficial in terms of fire-extinguishing performance as well as the protection of electrical equipment. The results obtained offer guidance for the design of prefabricated fire-extinguishing systems using various mass ratios regarding heptafluoropropane vaporization.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanli, E.A.; Brown, R.; Simmons, D. A Pilot Study to Assess the Feasibility of Comparing Ultra-High Pressure to Low-Pressure Fire Suppression Systems for a Simulated Indirect Exterior Attack. Fire 2023, 6, 278. [Google Scholar] [CrossRef]
- Coulombe, R.G.; Rao, A. Fires and local labor markets. J. Environ. Econ. Manag. 2025, 130, 103109. [Google Scholar] [CrossRef]
- Gaidukova, O.; Misyura, S.; Donskoy, I.; Morozov, V.; Volkov, R. Pool Fire Suppression Using CO2 Hydrate. Energies 2022, 15, 9585. [Google Scholar] [CrossRef]
- Rohilla, M.; Saxena, A.; Tyagi, Y.K.; Singh, I.; Tanwar, R.K.; Narang, R. Condensed Aerosol Based Fire Extinguishing System Covering Versatile Applications: A Review. Fire Technol. 2021, 58, 327–351. [Google Scholar] [CrossRef]
- Marco, S.; Nicolo, T.; Luca, T.; Paolo, E. Experimental Assessment of the Acoustic Performance of Nozzles Designed for Clean Agent Fire Suppression. Appl. Sci. 2022, 13, 186. [Google Scholar] [CrossRef]
- Rocha, H.; Campos, T.; Gamboa, P. Design and analysis of air launched fire-extinguishing devices. CEAS Aeronaut. J. 2024, 15, 849–865. [Google Scholar] [CrossRef]
- Yang, K.; Miao, H.; Ji, H.; Chen, S.; Xing, Z.; Jiang, J.; Zheng, K.; Liu, G. Experimental study on the coupling effect of heptafluoropropane and different arrangement of obstacles on methane-air explosion. Fuel 2024, 358, 130204. [Google Scholar] [CrossRef]
- Higashi, Y.; Shima, K.; Suzuki, M.; Fujishiro, M.; Kawai, T.; Morimoto, T. Synthetic Utilization of 2H-Heptafluoropropane: Ionic 1, 4-Addition to Electron-Deficient Carbon–Carbon Unsaturated Bonds. J. Org. Chem. 2024, 89, 3962–3969. [Google Scholar] [CrossRef]
- Papas, P.; Cao, C.; Kim, W.; Baldwin, E.; Chattaway, A. Fire suppression using trifluoroiodomethane (CF3I)-carbon dioxide (CO2) mixtures. Proc. Combust. Inst. 2023, 39, 3765–3773. [Google Scholar] [CrossRef]
- Gaidukova, O.; Donskoy, I.; Misyura, S.; Morozov, V.; Volkov, R. The Interaction between a Liquid Combustion Front and a Fire Barrier Made of CO2 Hydrate. Fire 2023, 6, 124. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Li, R.; Tao, C.; Chen, Z.; Liu, T.; Li, Y. The experimental investigation of thermal runaway characteristics of lithium battery under different concentrations of heptafluoropropane and air. J. Energy Storage 2024, 84, 110828. [Google Scholar] [CrossRef]
- García-Anteportalatina, V.M.; Martín, M. Process synthesis for the valorisation of low-grade heat: Geothermal brines and industrial waste streams. Renew. Energy 2022, 198, 733–748. [Google Scholar] [CrossRef]
- Cao, X.; Lu, Y.; Jiang, J.; Wang, Z.; Wei, H.; Li, Y.; Li, Y.; Lin, C. Experimental study on explosion inhibition by heptafluoropropane and its synergy with inert gas. J. Loss Prev. Process Ind. 2021, 71, 104440. [Google Scholar] [CrossRef]
- Ji, H.; Lu, R.; Yang, K.; Jiang, J.; Xing, Z.; Guo, J. Experimental study on methane explosion suppression by heptafluoropropane drived modified ABC powder. Process Saf. Environ. Prot. 2023, 170, 623–635. [Google Scholar] [CrossRef]
- Robin, M.L. Suppression of class a fires with HFC-227ea. Process Saf. Prog. 1998, 17, 209–212. [Google Scholar] [CrossRef]
- Roy, A.; Chawhan, R.S.; Patel, R.; Varadharajan, S.; Tiwari, L.M.; Chakraborty, A.L.; Ghoroi, C.; Srivastava, G. Quantifying the CO and CO2 Mole Fraction in the Plume of an Aerosol-Based Fire Extinguishing Agent Using 4560 nm and 4320 nm QCLs. IEEE Sens. J. 2019, 19, 9728–9735. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, X.; Wang, Z.; Jin, H.; Zhao, Y.; Wang, C.; Shen, Z.; Liu, Y.; Wang, W. The flame mitigation effect of N2 and CO2 on the hydrogen jet fire. Process Saf. Environ. Prot. 2022, 165, 658–670. [Google Scholar] [CrossRef]
- Aydin, M. An analysis of human error and reliability in the operation of fixed CO2 systems on cargo ships using HEART Dempster-Shafer evidence theory approach. Ocean. Eng. 2023, 286, 115686. [Google Scholar] [CrossRef]
- Tanaka, F.; Kato, D. Experimental and numerical study on fire extinguishing by water spray. Fire Saf. J. 2023, 141, 103984. [Google Scholar] [CrossRef]
- Xiao, T.; Gupta, V.; Dunn, M.J.; Masri, A.R. Joint OH-PLIF and Mie scattering imaging of enhanced water mist suppression of buoyant fires. Proc. Combust. Inst. 2024, 40, 105331. [Google Scholar] [CrossRef]
- Zhdanova, A.O.; Volkov, R.S.; Voytkov, I.S.; Osipov, K.Y.; Kuznetsov, G.V. Suppression of forest fuel thermolysis by water mist. Int. J. Heat Mass Transf. 2018, 126, 703–714. [Google Scholar] [CrossRef]
- Ma, L.; Fan, J.; Guo, R.Z.; Zhang, P.Y.; Li, C.H. Characteristics of fires in coal mine roadways and comparative analysis of control effectiveness between longitudinal ventilation and cross-section sealing. Case Stud. Therm. Eng. 2024, 53, 103878. [Google Scholar] [CrossRef]
- Nikam, P.C.; Rao, A.R.; Shertukde, V.V. Effect of polyethylene terephthalate fiber reinforced with non-hydrophilic nano-silica on the mechanical, thermic, and chemical shielding characteristics of saturated polyurethane composite. J. Appl. Polym. Sci. 2022, 140, e53334. [Google Scholar] [CrossRef]
- Baalisampang, T.; Saliba, E.; Salehi, F.; Garaniya, V.; Chen, L. Optimisation of smoke extraction system in fire scenarios using CFD modelling. Process Saf. Environ. Prot. 2021, 149, 508–517. [Google Scholar] [CrossRef]
- Bolshova, T.A.; Shvartsberg, V.M.; Shmakov, A.G. Synergism of trimethylphosphate and carbon dioxide in extinguishing premixed flames. Fire Saf. J. 2021, 125, 103406. [Google Scholar] [CrossRef]
- Kim, T.S.; Park, T.H.; Park, J.H.; Yang, J.H.; Han, D.H.; Lee, B.C.; Kwon, J.S. Thermal characteristics of fire extinguishing agents in compartment fire suppression. Sci. Prog. 2024, 107, 1–16. [Google Scholar] [CrossRef]
- Cagli, E.; Liu, H.; Khokhar, V.; Klemm, A.; Gurkan, B.E. Thermal and Physical Properties of CO2-Reactive Binary Mixtures. J. Chem. Eng. Data 2024, 69, 2676–2687. [Google Scholar] [CrossRef]
- Dong, A.; Chen, Y.; Zhan, T.; Hou, K.; He, M.; Zhang, Y. Optimization of crossover SRK equation of state for thermodynamic properties calculation of CO2. Fluid Phase Equilibria 2025, 587, 114200. [Google Scholar] [CrossRef]
- Yuan, Y.; Lu, Q.; Xie, Q. Study of injection characteristics of high-pressure fluoropropane and local damage by sudden cooling in enclosed room. Fire Sci. Technol. 2022, 41, 1440–1444. [Google Scholar]
- Leedham Elvidge, E.C.; Bönisch, H.; Brenninkmeijer, C.A.; Engel, A.; Fraser, P.J.; Gallacher, E.; Langenfelds, R.; Mühle, J.; Oram, D.E.; Ray, E.A.; et al. Evaluation of stratospheric age of air from CF4, C2F6, C3F8, CHF3, HFC-125, HFC-227ea and SF6; implications for the calculations of halocarbon lifetimes, fractional release factors and ozone depleti. J. Atmos. Chem. Phys. 2018, 18, 3369–3385. [Google Scholar]
- Han, J.; Lee, J.; Burla, S.K.; Chung, S.; Lee, J.D. Exploring the Potential of HFC-125a Hydrate for Innovative Fire Suppression: A Gas Hydrate Technology Approach. Energy Fuels 2024, 38, 6238–6250. [Google Scholar] [CrossRef]
- Wang, Z.; He, C.; Geng, Z.; Li, G.; Zhang, Y.; Shi, X.; Yao, B. Experimental study of thermal runaway propagation suppression of lithium-ion battery module in electric vehicle power packs. Process Saf. Environ. Prot. 2024, 182, 692–702. [Google Scholar] [CrossRef]
- Bellas, R.; Gómez, M.A.; González-Gil, A.; Porteiro, J.; Míguez, J.L. Assessment of the Fire Dynamics Simulator for Modeling Fire Suppression in Engine Rooms of Ships with Low-Pressure Water Mist. Fire Technol. 2020, 56, 1315–1352. [Google Scholar] [CrossRef]
- Kanyama, T.; Fukuda, N.; Uezu, K.; Kawahara, T. Field Experimental Investigations on the Performance of an Environmentally Friendly Soap-Based Firefighting Agent on Indonesian Peat Fire. Fire Technol. 2023, 59, 1007–1025. [Google Scholar] [CrossRef]
- Majeed, F.; Jamal, H.; Kamran, U.; Noman, M.; Ali, M.M.; Shahzad, T.; Baig, M.M.; Akhtar, F. Review–Recent Advances in Fire-Suppressing Agents for Mitigating Lithium-Ion Battery Fires. J. Electrochem. Soc. 2024, 171, 060522. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, L.; Chu, Y.; Su, Z.; Cai, C.; Chen, X.; Huang, C. Explosion suppression range and the minimum amount for complete suppression on methane-air explosion by heptafluoropropane. Fuel 2022, 328, 125331. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, G.; Liu, C.; Gao, Y. Comparison of fire extinguishing performance of four halon substitutes and Halon 1301. J. Fire Sci. 2021, 39, 370–399. [Google Scholar] [CrossRef]
- Ke, W.; Wang, K.; Zhou, B.; Wang, Z.; Wang, W.; Sun, X.; Qiu, B.; Han, Y. The cooling performance of halogenated alkane fire extinguishing agent and its quantitative prediction model. Therm. Sci. Eng. Prog. 2021, 26, 101093. [Google Scholar] [CrossRef]
- Yao, J.; Yang, Y.; Huang, Z.; Sun, J.; Wang, J.; Yang, Y. Impact of viscosity model on simulation of condensed particle flow by Euler multiphase flow model. Huagong Xuebao/CIESC J. 2020, 71, 4945–4956. [Google Scholar] [CrossRef]
- Wang, Z.; He, Y.; Huang, J. Numerical Simulation of Atomization Characteristics of Perfluorohexanone under Low-Pressure Environment. J. Phys. Conf. Ser. 2023, 2562, 012088. [Google Scholar] [CrossRef]
- Adio, S.A.; Muritala, A.O.; Binuyo, A.S.; Oketola, T.; Veeredhi, V.R. Eulerian multiphase technique for detailed investigation on hydro-thermal enhancement in a cooling microchannel using pulsating alumina nanofluid: A numerical simulation. Case Stud. Therm. Eng. 2024, 59, 104482. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, C.; Liu, M.; Wang, S.; Liu, Y.; Feng, G. Numerical Simulations on the Extinguishing Effect of Water Mist System with Different Parameters of Longitudinal Ventilation in Curve Tunnel Fire. Adv. Civ. Eng. 2021, 2021, 7373685. [Google Scholar] [CrossRef]
- Hu, P.; Chen, L.-X.; Zhu, W.-B.; Jia, L.; Chen, Z.-S. Vapor-liquid equilibria for the binary system of 2,3,3,3-tetrafluoroprop-1-ene (HFO-1234yf) + 1,1,1,2,3,3,3-heptafluoropropane (HFC-227ea). Fluid Phase Equilibria 2014, 379, 59–61. [Google Scholar] [CrossRef]
- Khubaib, S.M.; Luo, Y.; Wang, X. Experimental Investigation on Solubility and Viscosity of 1,1,1,2,3,3,3-Heptafluoropropane (R227ea) and Polyol Ester Oil (POE 22) Mixtures. J. Chem. Eng. Data 2021, 67, 104–112. [Google Scholar] [CrossRef]
- Soave, G. Equilibrium constants from a modified Redlich-Kwong equation of state. Chem. Eng. Sci. 1972, 27, 1197–1203. [Google Scholar]
- GB25972-2010; Gas Fire Extinguish System and Components. China Standardization Administration: Beijing, China, 2011.
- ISO 14520-9; Gaseous Fire-Extinguishing Systems—Physical Properties and System Design. International Organization for Standardizatio: Geneva, Switzerland, 2016.
- Boonaert, E.; Valtz, A.; Brocus, J.; Coquelet, C.; Beucher, Y.; De Carlan, F.; Fourmigué, J.-M. Vapor-liquid equilibrium measurements for 5 binary mixtures involving HFO-1336mzz(E) at temperatures from 313 to 353 K and pressures up to 2.735 MPa. Int. J. Refrig. 2020, 114, 210–220. [Google Scholar] [CrossRef]
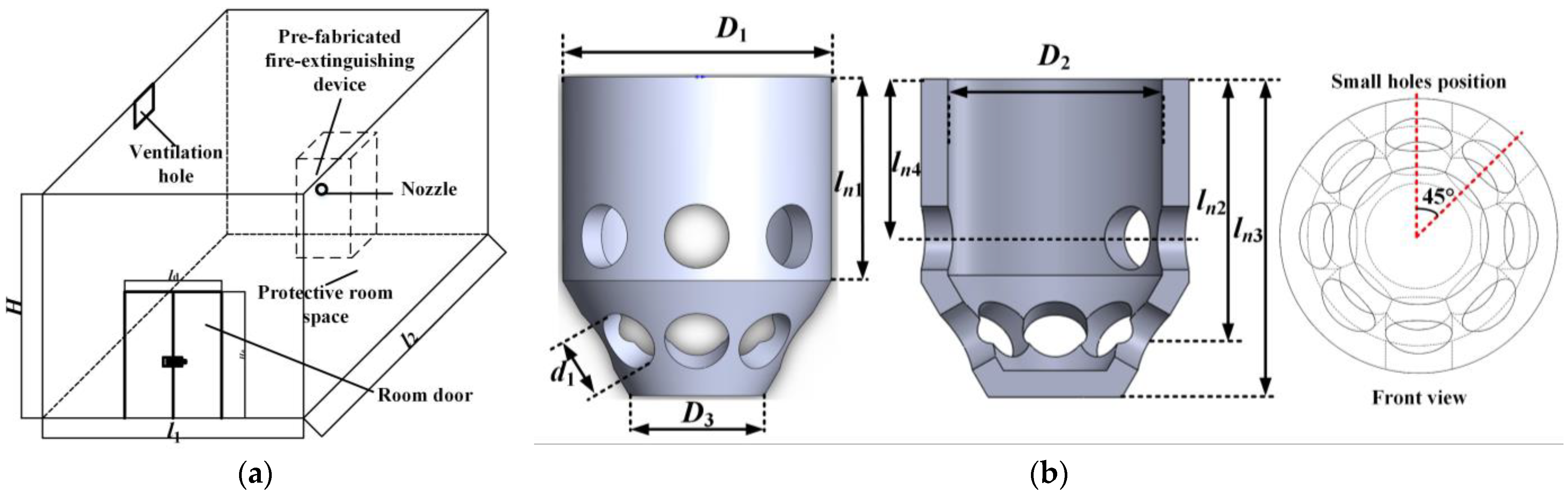
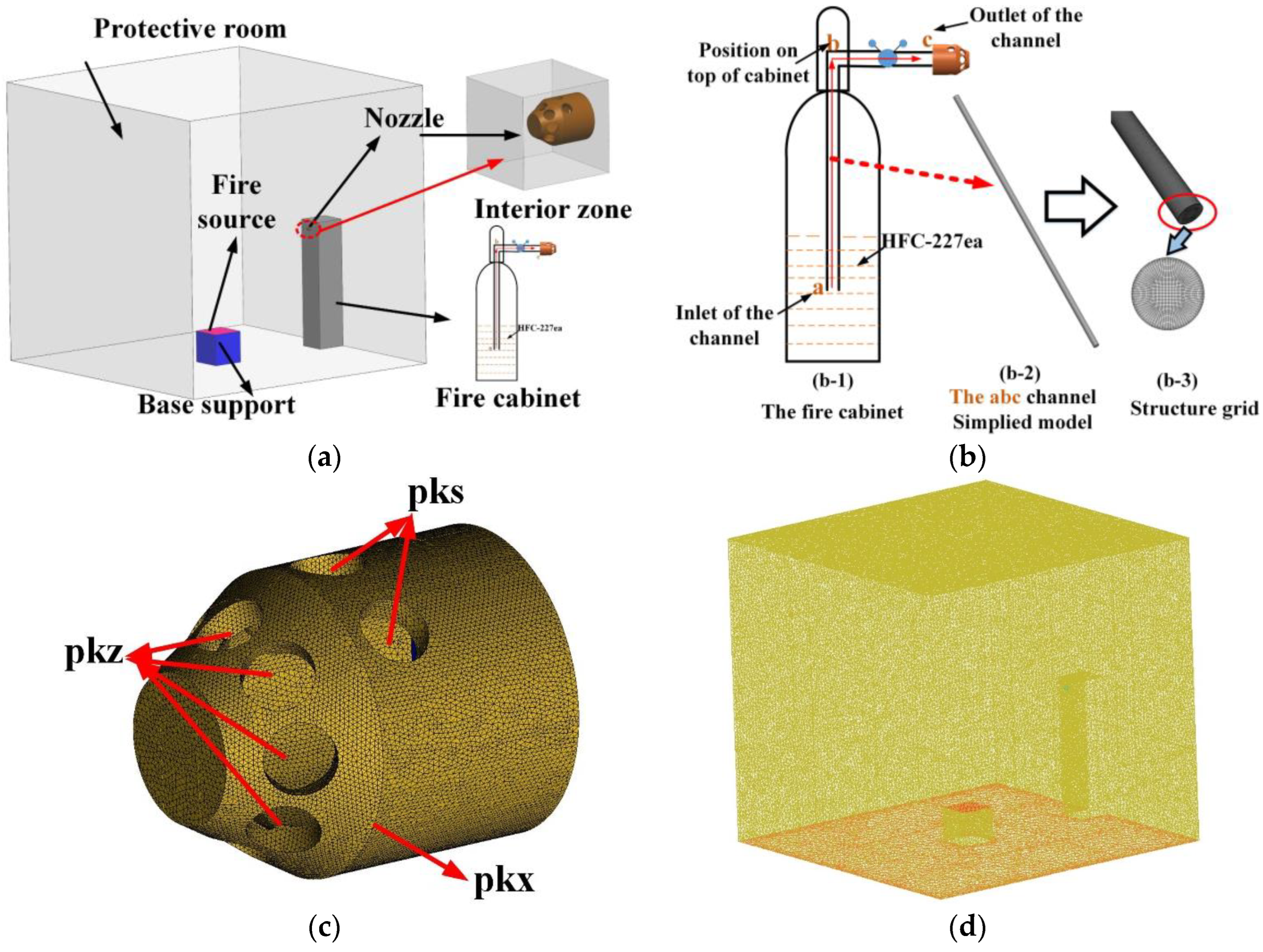
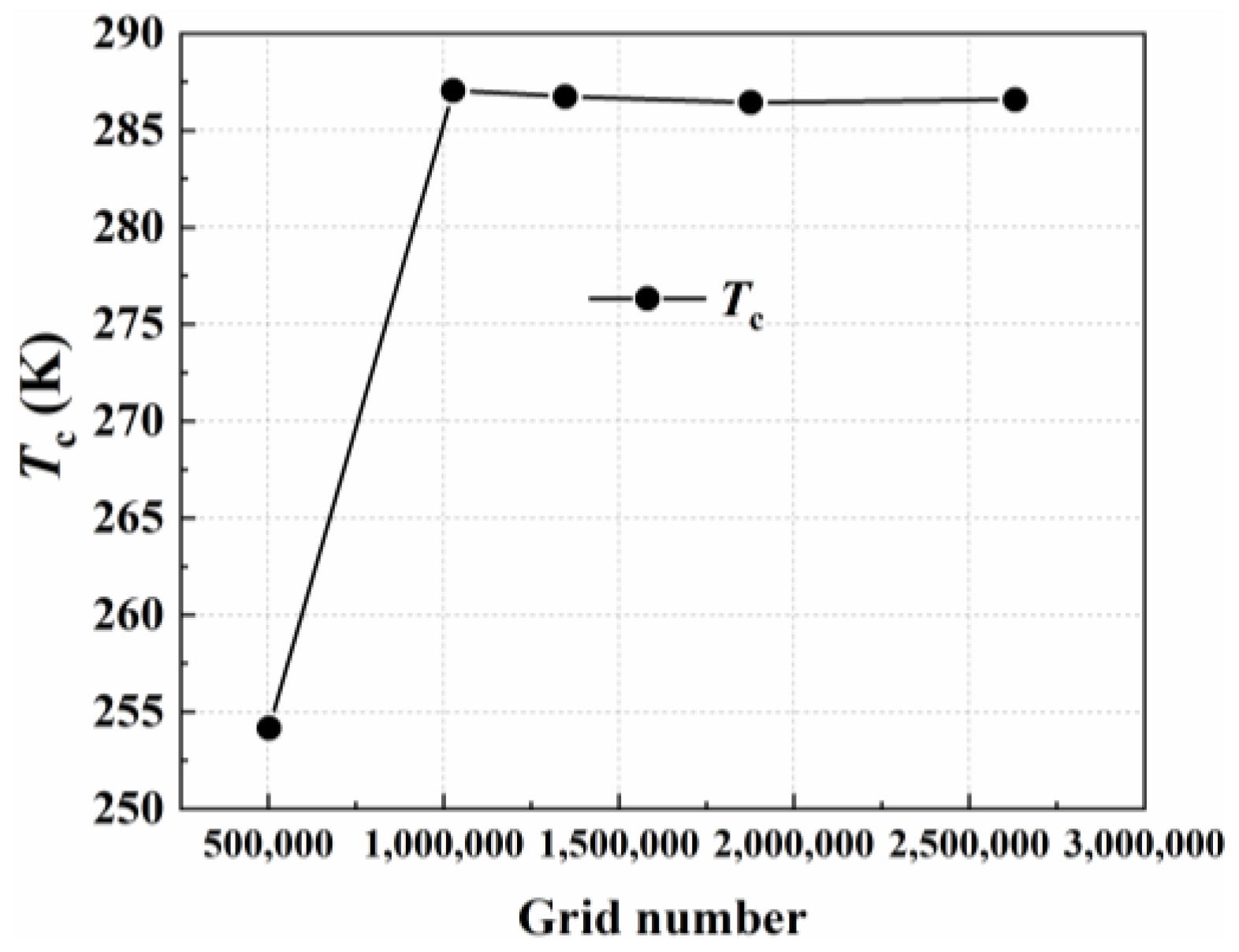

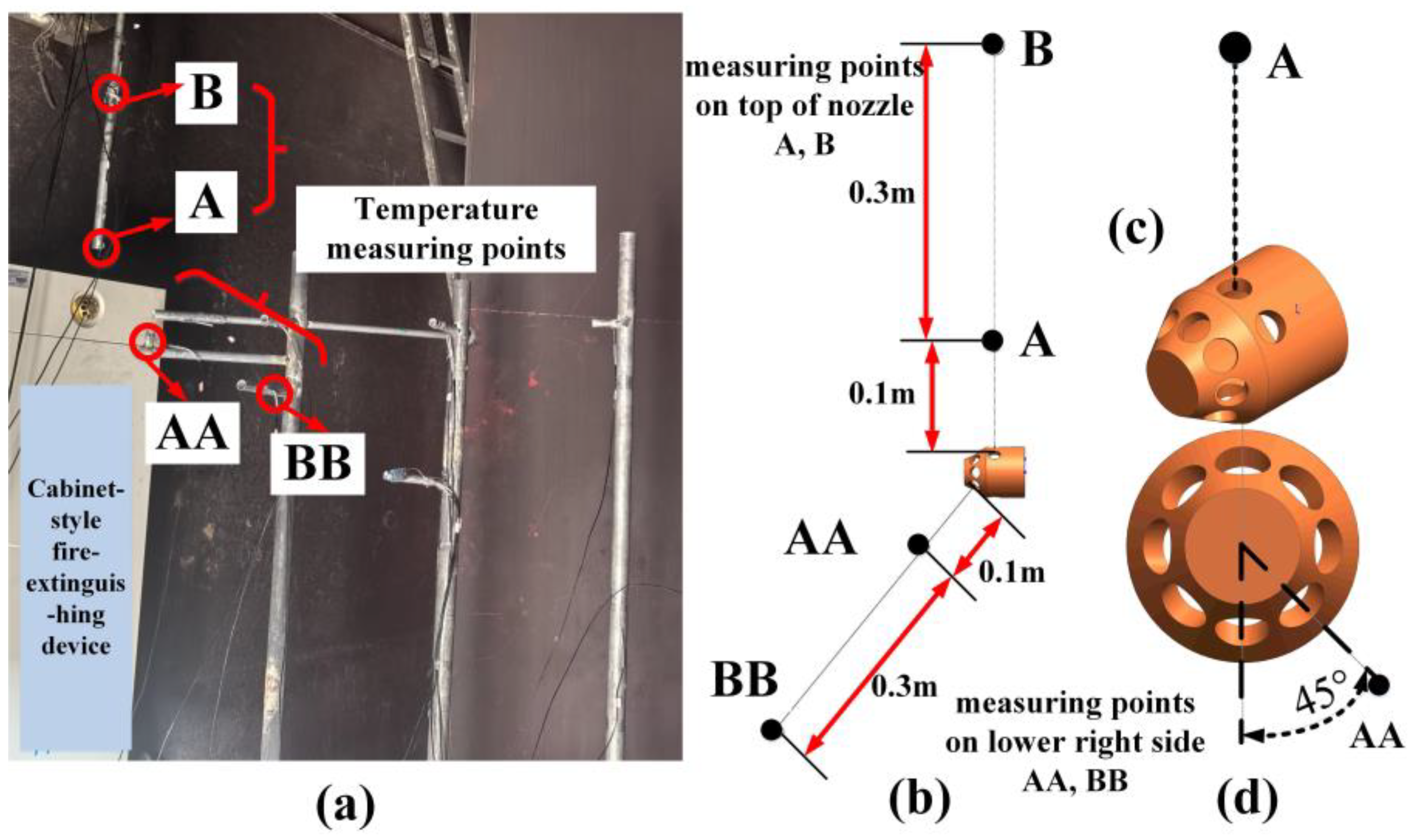

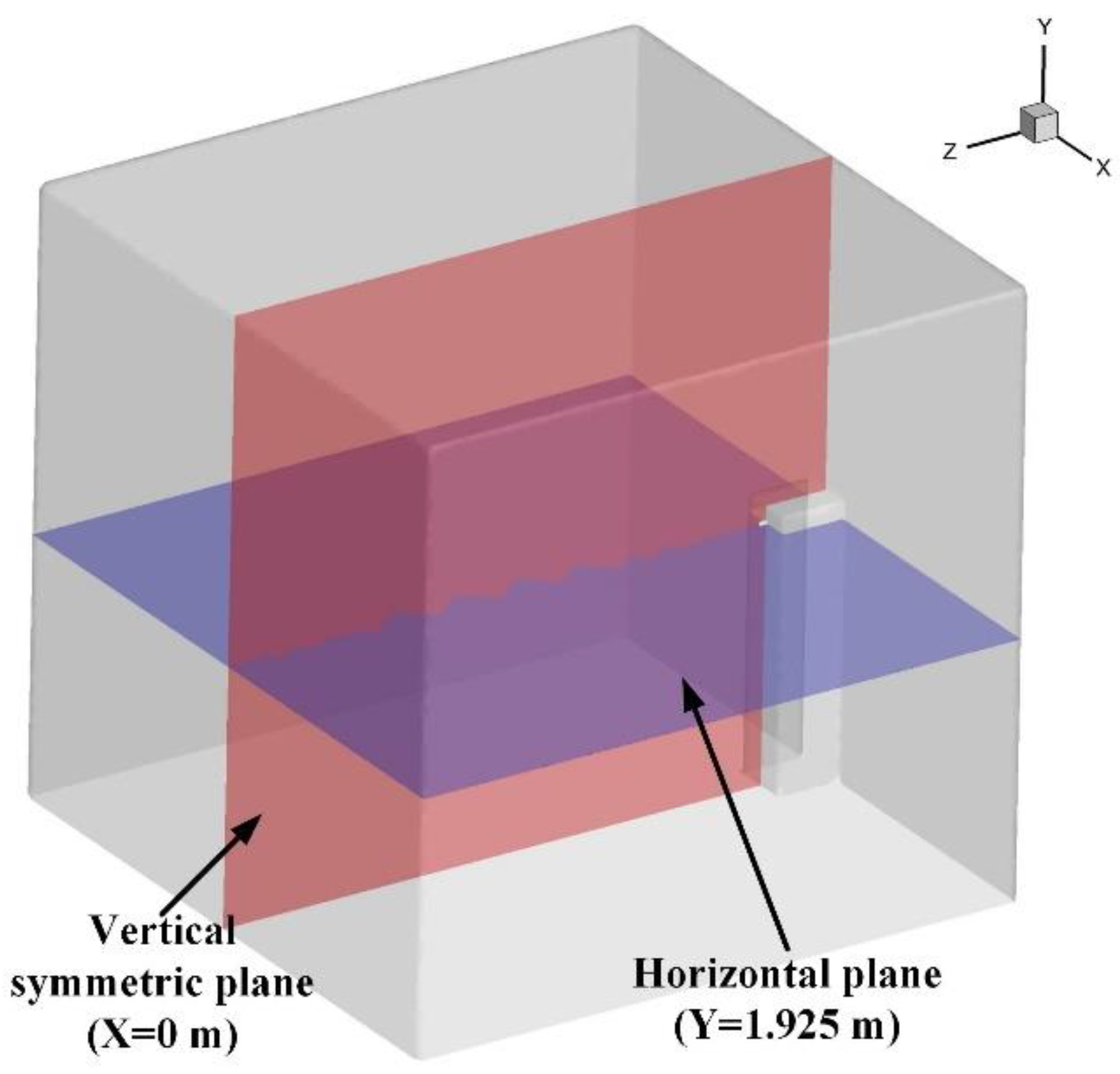



| Parameter | Value |
|---|---|
| H (m) | 4.5 |
| l1 (m) | 4.72 |
| l2 (m) | 4.72 |
| ld (m) | 1.9 |
| Hd (m) | 2.61 |
| D1 (m) | 0.050 |
| D2 (m) | 0.040 |
| D3 (m) | 0.025 |
| d1 (m) | 0.012 |
| ln1 (m) | 0.0384 |
| ln2 (m) | 0.0492 |
| ln3 (m) | 0.060 |
| ln4 (m) | 0.030 |
| Region | Protective Room | Interior Zone | Inlet | Nozzle |
|---|---|---|---|---|
| Mesh size (m) | 0.064 | 0.001 | 0.001 | 0.001 |
| HFC-227ea | Liquid Phase | Gas Phase |
|---|---|---|
| Molecular weight | 170 | 170 |
| ρ (kg·m−3) | 1410 | 5.56 |
| λ (W·m−1·K−1) | 0.053 | 0.013 |
| Cp (J·kg−1·K−1) | 1.247 | 0.8136 |
| Latent heat (J·mol−1) | 132,600 | — |
| Boiling point (K) | 256.75 | — |
| Critical temperature (K) | — | 375.95 |
| Critical pressure (Pa) | — | 2,987,740 |
| Parameter | Mass Ratio of Liquid Phase (%) | Mass Ratio of Gas Phase (%) | Outlet Velocity (m/s) |
|---|---|---|---|
| Value | 20.2 | 79.8 | 29.0 |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Experiment (K) | 295.18 | 295.15 | 297.97 | 299.55 | 422.08 | 300.40 | 385.53 |
| Simulation (K) | 296.24 | 296.3 | 296.73 | 296.28 | 415.36 | 298.15 | 381.71 |
| Absolute Error (K) | 1.04 | 1.15 | 1.24 | 3.27 | 6.72 | 2.25 | 3.82 |
| Parameter | Heat Release Rate (W/m2) | Fire Source Area (m2) | Heat Flux (W) |
|---|---|---|---|
| Value | 30,000 | 0.125 | 3750 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.-B.; Yin, Q.; Liu, M.-R.; Li, C.-Q.; Wang, Z.-C.; Hu, Z.-M. Research on Vaporization and Sudden Cooling Performance of Heptafluoropropane in Prefabricated Fire-Extinguishing Devices Based on Numerical Method. Fire 2025, 8, 124. https://doi.org/10.3390/fire8040124
Zhang W-B, Yin Q, Liu M-R, Li C-Q, Wang Z-C, Hu Z-M. Research on Vaporization and Sudden Cooling Performance of Heptafluoropropane in Prefabricated Fire-Extinguishing Devices Based on Numerical Method. Fire. 2025; 8(4):124. https://doi.org/10.3390/fire8040124
Chicago/Turabian StyleZhang, Wen-Bin, Qian Yin, Ming-Rui Liu, Chun-Qiang Li, Zong-Cun Wang, and Zhang-Mao Hu. 2025. "Research on Vaporization and Sudden Cooling Performance of Heptafluoropropane in Prefabricated Fire-Extinguishing Devices Based on Numerical Method" Fire 8, no. 4: 124. https://doi.org/10.3390/fire8040124
APA StyleZhang, W.-B., Yin, Q., Liu, M.-R., Li, C.-Q., Wang, Z.-C., & Hu, Z.-M. (2025). Research on Vaporization and Sudden Cooling Performance of Heptafluoropropane in Prefabricated Fire-Extinguishing Devices Based on Numerical Method. Fire, 8(4), 124. https://doi.org/10.3390/fire8040124





