Fine-Scale Environmental Heterogeneity Shapes Post-Fire Macrofungal Richness in a Mediterranean Relict Forest
Abstract
1. Introduction
2. Materials and Methods
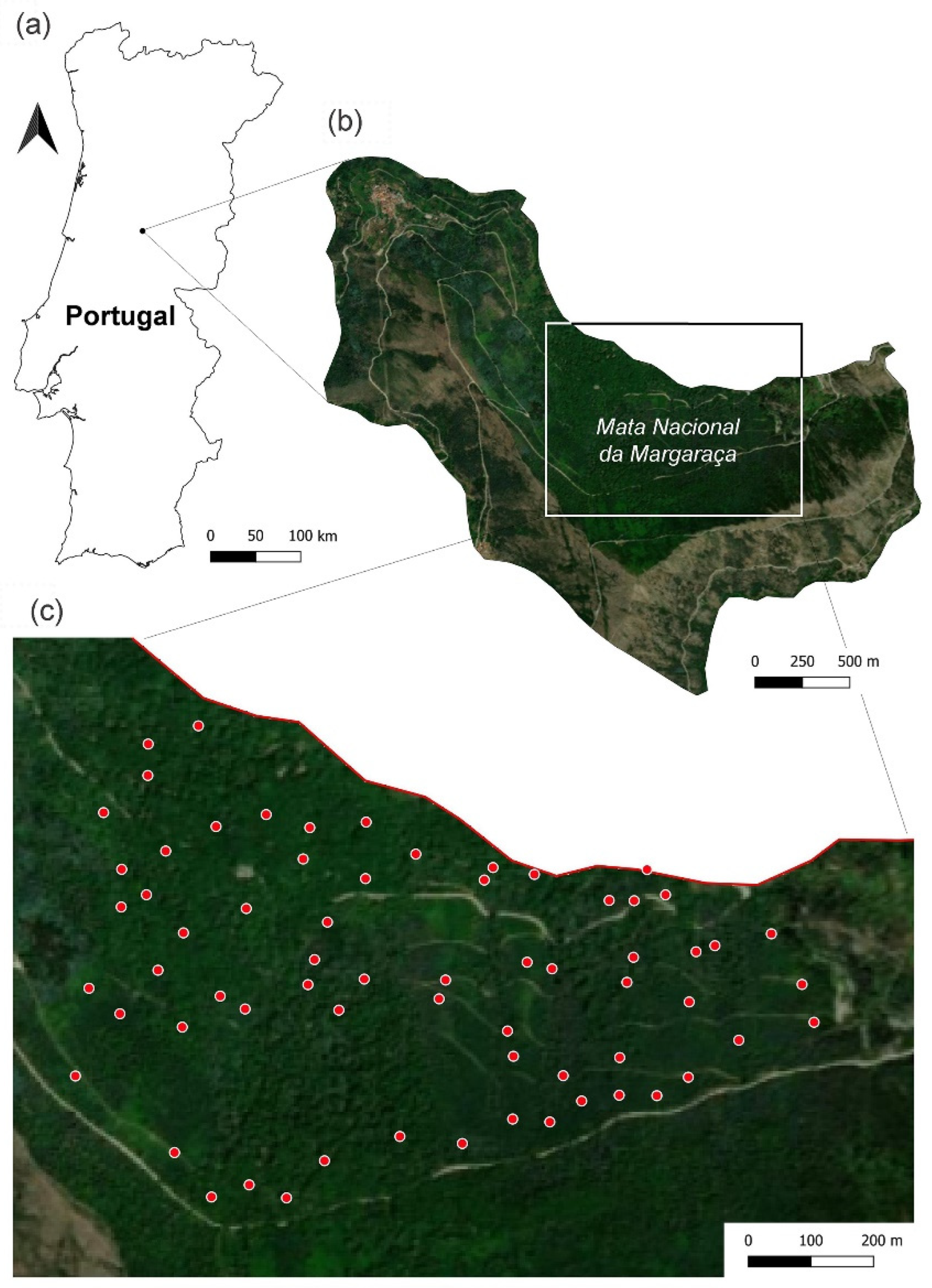
2.1. Study Area
2.2. Macrofungal Surveys
2.3. Environmental Variables
2.4. Data Analyses
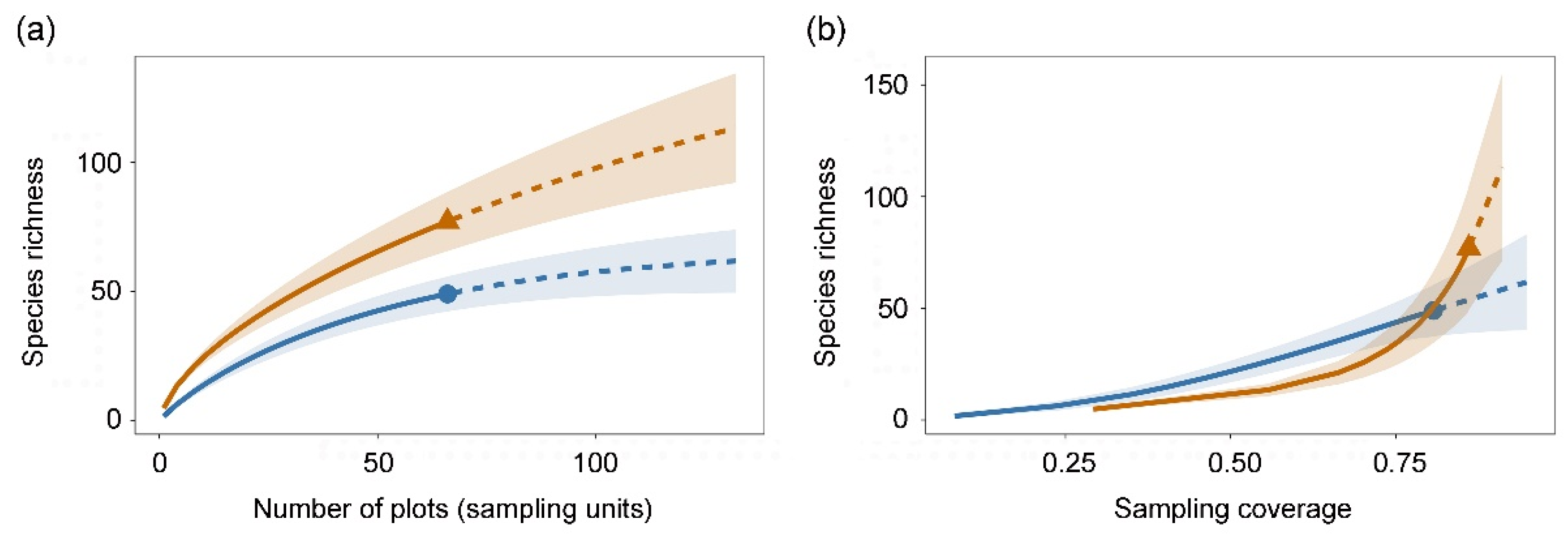
3. Results
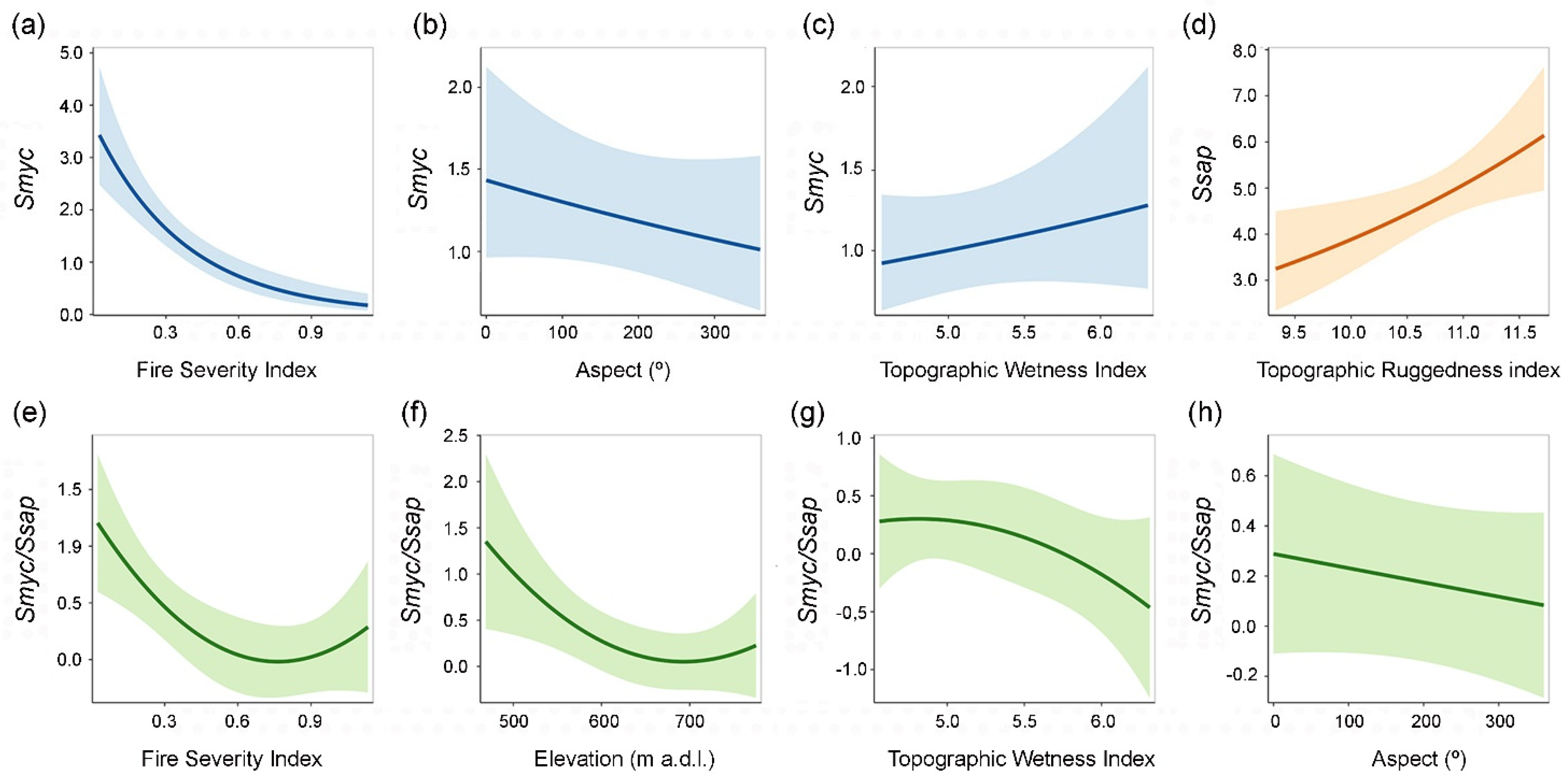
4. Discussion
4.1. Fire Severity and Guild-Specific Responses
4.2. Environmental Drivers of Fungal Richness
4.3. Conservation and Research Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hampe, A.; Jump, A.S. Climate relicts: Past, present, future. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 313–333. [Google Scholar] [CrossRef]
- Pignatti, E.; Pignatti, S.; D’Angeli, D.; De Nicola, C.; Maffei, L.; Testi, A.; Tinelli, A. The laurisilva as cultural heritage: Protection proposal for a laurel forest relict near Ponte Renaro. Rend. Lincei 2015, 26, 643–649. [Google Scholar] [CrossRef]
- Santos-Silva, C. Contribution to the Study of the Protected Landscape of Serra do Açor: Elements on the Bryological Flora and Vegetation Structure; Universidade de Évora: Évora, Portugal, 1985; Available online: http://hdl.handle.net/10174/5368 (accessed on 22 September 2025).
- Silveira, P.C. The flora of the Serra do Açor (Portugal). Guineana 2007, 13, 1–333. [Google Scholar]
- Pausas, J.G.; Keeley, J.E. A burning story: The role of fire in the history of life. BioScience 2009, 59, 593–601. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Wildfires and global change. Front. Ecol. Environ. 2021, 19, 387–395. [Google Scholar] [CrossRef]
- Buscardo, E.; Rodríguez-Echeverría, S.; Freitas, H.; De Angelis, P.; Pereira, J.S.; Muller, L.A. Contrasting soil fungal communities in Mediterranean pine forests subjected to different wildfire frequencies. Fungal Divers. 2015, 70, 85–99. [Google Scholar] [CrossRef]
- Claridge, A.W.; Trappe, J.M.; Hansen, K. Do fungi have a role as soil stabilizers and remediators after forest fire? For. Ecol. Manag. 2009, 257, 1063–1069. [Google Scholar] [CrossRef]
- Gassibe, P.; Fabrero, R.; Hernández-Rodríguez, M.; Oria de Rueda, J.; Martín-Pinto, P. Fungal community succession following wildfire in a Mediterranean vegetation type dominated by Pinus pinaster in NW Spain. For. Ecol. Manag. 2011, 262, 655–662. [Google Scholar] [CrossRef]
- Keeley, J.E.; Bond, W.J.; Bradstock, R.A.; Pausas, J.G.; Rundel, P.W. Fire in Mediterranean Ecosystems: Ecology, Evolution and Management; Cambridge University Press: New York, NY, USA, 2012. [Google Scholar]
- Cairney, J.W.G.; Bastias, B.A. Influences of fire on forest soil fungal communities. Can. J. For. Res. 2007, 37, 207–215. [Google Scholar] [CrossRef]
- Taudière, A.; Richard, F.; Carcaillet, C. Review on fire effects on ectomycorrhizal symbiosis. For. Ecol. Manag. 2017, 391, 446–457. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Tunlid, A. Ectomycorrhizal fungi—Potential organic matter decomposers, yet not saprotrophs. New Phytol. 2015, 205, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.-A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Li, Y.; Wu, X.; Chen, S.; Wang, M.; Li, Y.; Ge, Z.; Zhang, M.; Mao, L. The evolutionary adaptation of wood-decay macrofungi to host gymnosperms differs from that to host angiosperms. Ecol. Evol. 2024, 14, e70019. [Google Scholar] [CrossRef]
- Bahram, M.; Netherway, T. Fungi as mediators linking organisms and ecosystems. FEMS Microbiol. Rev. 2022, 46, fuab058. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Sridhar, K.R.; Deshmukh, S. (Eds.) Advances in Macrofungi: Diversity, Ecology and Biotechnology; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Natário, B.; Louro, R.; Santos-Silva, C. Macrofungi of Mata da Margaraça (Portugal), a relic from the Tertiary Age. Biodivers. Data J. 2019, 7, e38177. [Google Scholar] [CrossRef]
- Deng, X.; Li, M.; Dai, Y.; Zhu, X.; Yan, X.; Wei, Z.; Yuan, Y. The diversity of macrofungi in the forests of Ningxia, western China. Diversity 2024, 16, 725. [Google Scholar] [CrossRef]
- Peay, K.; Baraloto, C.; Fine, P.V.A. Strong coupling of plant and fungal community structure across western Amazonian rainforests. ISME J. 2013, 7, 1852–1861. [Google Scholar] [CrossRef]
- Santos-Silva, C.; Gonçalves, A.; Louro, R. Canopy cover influence on macrofungal richness and sporocarp production in montado ecosystems. Agrofor. Syst. 2011, 82, 149–159. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Bi, S.; Wang, X.; Ye, Y.; Svenning, J.-C. Macrofungal species distributions depend on habitat partitioning of topography, light, and vegetation in a temperate mountain forest. Sci. Rep. 2018, 8, 13589. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Zhang, W.; Zhu, J.; Chen, B.; Jiang, L.; Xu, D.; Li, W.; Liu, J.; He, Z. Soil environments regulate dominant soil fungal communities along an elevational gradient in subtropical forests. Forests 2024, 15, 643. [Google Scholar] [CrossRef]
- Rakić, M.; Marković, M.; Galić, Z.; Galović, V.; Karaman, M. Diversity and distribution of macrofungi in protected mountain forest habitats in Serbia and its relation to abiotic factors. J. Fungi 2022, 8, 1074. [Google Scholar] [CrossRef] [PubMed]
- Dove, N.C.; Hart, S.C. Fire reduces fungal species richness and in situ mycorrhizal colonization: A meta-analysis. Fire Ecol. 2017, 13, 37–65. [Google Scholar] [CrossRef]
- Alday, J.G.; De Aragón, J.M.; de-Miguel, S.; Bonet, J.A. Mushroom biomass and diversity are driven by different spatio-temporal scales along Mediterranean elevation gradients. Sci. Rep. 2017, 7, 45824. [Google Scholar] [CrossRef]
- Yu, H.; Wang, T.; Skidmore, A.; Heurich, M.; Bässler, C.; Kivlin, S. The critical role of tree species and human disturbance in determining macrofungal diversity in Europe. Glob. Ecol. Biogeogr. 2021, 30, 2084–2100. [Google Scholar] [CrossRef]
- Kelly, L.T.; Giljohann, K.M.; Duane, A.; Aquilué, N.; Archibald, S.; Batllori, E.; Bennett, A.F.; Buckland, S.T.; Canelles, Q.; Clarke, M.F.; et al. Fire and biodiversity in the Anthropocene. Science 2020, 370, eabb0355. [Google Scholar] [CrossRef]
- Silveira, P.C. Contribution to the Knowledge of the Vascular Flora of the Serra do Açor and Its Phytogeographic Interpretation. Ph.D. Thesis, University of Coimbra, Coimbra, Portugal, 2001. [Google Scholar]
- Lourenço, L. Serras de Xisto do Centro de Portugal–Contribuição para o Seu Conhecimento Geomorfológico e Geo-Ecológico. Ph.D. Thesis, Universidade de Coimbra, Coimbra, Portugal, 1996. Available online: https://hdl.handle.net/10316/627 (accessed on 22 September 2025).
- Lodge, D.J.; Ammirati, J.F.; O’Dell, T.E.; Mueller, G.M. Collecting and describing macrofungi. In Biodiversity of Fungi: Inventory and Monitoring Methods; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 128–158. [Google Scholar]
- Buée, M.; Courty, P.E.; Mignot, D.; Garbaye, J. Soil niche effect on species diversity and catabolic activities in an ectomycorrhizal fungal community. Soil Biol. Biochem. 2007, 39, 1947–1955. [Google Scholar] [CrossRef]
- Dickie, I.A.; Dentinger, B.M.; Avis, P.G.; McLaughlin, D.J.; Reich, P.B. Ectomycorrhizal fungal communities of oak savanna are distinct from forest communities. Mycologia 2009, 101, 473–483. [Google Scholar] [CrossRef]
- Durall, D.M.; Gamiet, S.; Simard, S.W.; Kudrna, L.; Sakakibara, S.M. Effects of clearcut logging and tree species composition on diversity and composition of epigeous ectomycorrhizal fruit bodies. Botany 2006, 84, 966–980. [Google Scholar] [CrossRef]
- Kebli, H.; Brais, S.; Kernaghan, G.; Drouin, P. Impact of harvesting intensity on wood-inhabiting fungi in boreal aspen forests of Eastern Canada. For. Ecol. Manag. 2012, 279, 45–54. [Google Scholar] [CrossRef]
- Louro, R.; Santos-Silva, C.; Nobre, T. What is in a name? Terfezia classification revisited. Fungal Biol. 2019, 123, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Robicheau, B.M. Fungal diversity and community structure from coastal and barrier island beaches in the United States Gulf of Mexico. Sci. Rep. 2021, 11, 81688. [Google Scholar] [CrossRef]
- Fernández-Toirán, L.M.; Ágreda, T.; Olano, J.M. Stand age and sampling year effect on the fungal fruit body community in Pinus pinaster forests in central Spain. Botany 2006, 84, 1249–1258. [Google Scholar] [CrossRef]
- Juutilainen, K.; Monkkonen, M.; Kotiranta, H.; Halme, P. The role of novel forest ecosystems in conserving wood-inhabiting fungi in boreal broadleaved forests. Ecol. Evol. 2016, 6, 6943–6954. [Google Scholar] [CrossRef]
- Kranabetter, J.M.; Friesen, J.; Gamiet, S.; Kroeger, P. Ectomycorrhizal mushroom distribution by stand age in western hemlock–lodgepole pine forests of northwestern British Columbia. Can. J. For. Res. 2005, 35, 1527–1539. [Google Scholar] [CrossRef]
- Müller, J.; Engel, H.; Blaschke, M. Assemblages of wood-inhabiting fungi related to silvicultural management intensity in beech forests in southern Germany. Eur. J. For. Res. 2007, 126, 513–527. [Google Scholar] [CrossRef]
- Olsson, J.; Jonsson, B.G.; Hjältén, J.; Ericson, L. Addition of coarse woody debris—Early fungal succession on Picea abies logs in managed forests and reserves. Biol. Conserv. 2011, 144, 1100–1110. [Google Scholar] [CrossRef]
- Oria-de-Rueda, J.A.; Hernández-Rodríguez, M.; Martín-Pinto, P.; Pando, V.; Olaizola, J. Could artificial reforestations provide as much fungal production and diversity as natural forest stands in marginal Mediterranean areas? For. Ecol. Manag. 2010, 260, 171–180. [Google Scholar] [CrossRef]
- Santos-Silva, C.; Louro, R. Assessment of diversity of epigeous Basidiomycota under different soil-management systems in a Montado ecosystem: A case study from Alentejo. Agrofor. Syst. 2016, 90, 117–126. [Google Scholar] [CrossRef]
- Horton, T.R.; Bruns, T.D. The molecular revolution in ectomycorrhizal ecology: Peeking into the black box. Mol. Ecol. 2001, 10, 1855–1871. [Google Scholar] [CrossRef]
- Peay, K.; Kennedy, P.; Bruns, T. Fungal community ecology: A hybrid beast with a molecular master. BioScience 2008, 58, 799–810. [Google Scholar] [CrossRef]
- Nordén, B.; Ryberg, M.; Götmark, F.; Olausson, B. Relative importance of coarse and fine woody debris for wood-inhabiting fungi diversity in temperate broadleaf forests. Biol. Conserv. 2004, 117, 1–10. [Google Scholar] [CrossRef]
- Ascomycete. Ascomycete.org. 2018. Available online: https://ascomycete.org/ (accessed on 22 September 2018).
- Bon, M. The Mushrooms and Toadstools of Britain and North-Western Europe; Hodder & Stoughton: London, UK, 1987. [Google Scholar]
- Breitenbach, J.; Kränzlin, F. Fungi of Switzerland; Edition Mykologia: Lucerne, Switzerland, 2000; Volume I–V. [Google Scholar]
- Courtecuisse, R.; Duhem, B. Collins Field Guide–Mushrooms and Toadstools of Britain and Europe, 1st ed.; HarperCollins: London, UK, 1995. [Google Scholar]
- Champignons de Charente-Maritime, Charente et Deux-Sèvres. Species Index. 2018. Available online: https://www.mycocharentes.fr/index.php?page=Alpha (accessed on 22 September 2018).
- Moser, M. Keys to Agarics and Boleti, 4th ed.; Roger Phillips: London, UK, 1983. [Google Scholar]
- Sarnari, G. Monografia Illustrata del Genere Russula in Europa; Associazione Micologica Bresadola, Fondazione Centro Studi Micologici: Trento, Italy, 2005; Volume 1–2. [Google Scholar]
- Bánki, O.; Roskov, Y.; Döring, M.; Ower, G.; Hernández Robles, D.R.; Plata Corredor, C.A.; Stjernegaard Jeppesen, T.; Örn, A.; Pape, T.; Hobern, D.; et al. Catalogue of Life (Version 2025-09-11); Catalogue of Life Foundation: Amsterdam, The Netherlands, 2025. [Google Scholar] [CrossRef]
- Kirk, P.M. Species Fungorum. In Species 2000 & ITIS Catalogue of Life, 2019 Annual Checklist; Roskov, Y., Ower, G., Orrell, T., Nicolson, D., Bailly, N., Kirk, P.M., Bourgoin, T., DeWalt, R.E., Decock, W., van Nieukerken, E., et al., Eds.; Species 2000; Naturalis: Leiden, The Netherlands, 2019; Available online: https://www.catalogueoflife.org/annual-checklist/2019 (accessed on 22 September 2025).
- Dix, N.J.; Webster, J. Phoenicoid fungi. In Fungal Ecology; Dix, N.J., Webster, J., Eds.; Chapman and Hall: London, UK, 1995; pp. 302–321. [Google Scholar] [CrossRef]
- Robinson, R.M.; Mellican, A.E.; Smith, R.H. Epigeous macrofungal succession in the first five years following a wildfire in karri (Eucalyptus diversicolor) regrowth forest in Western Australia. Austral Ecology 2008, 33, 807–820. [Google Scholar] [CrossRef]
- Sumorok, B. Post-fire macrofungi in the burnt area in the Jelonka reserve (Bialowieza region). Acta Mycol. 2001, 36, 149–158. [Google Scholar] [CrossRef][Green Version]
- ESRI. ArcGIS Desktop: Release 10.5.1; Environmental Systems Research Institute: Redlands, CA, USA, 2017. [Google Scholar][Green Version]
- Gupta, P.; Shukla, A.K.; Shukla, D.P. Sentinel-2 based burn severity mapping and post-fire impact assessment in Mizoram. Remote Sens. Appl. Soc. Environ. 2024, 36, 101279. [Google Scholar] [CrossRef]
- Miller, J.D.; Thode, A.E. Quantifying burn severity with a relative version of the dNBR. Remote Sens. Environ. 2007, 109, 66–80. [Google Scholar] [CrossRef]
- Sørensen, R.; Zinko, U.; Seibert, J. On the Calculation of the Topographic Wetness Index: Evaluation of Different Methods Based on Field Observations. Hydrol. Earth Syst. Sci. 2006, 10, 101–112. [Google Scholar] [CrossRef]
- Evans, J.S.; Oakleaf, J.; Cushman, S.A.; Theobald, D. Geomorphometry and Gradient Metrics Toolbox: A Toolbox for Surface Gradient Modelling. 2013. Available online: https://evansmurphy.wixsite.com/evansspatial (accessed on 22 September 2018).
- Jenness, J.S. DEM Surface Tools: An ArcGIS Extension for Analysing Raster Elevation Datasets. 2011. Available online: http://www.jennessent.com/arcgis/surface_area.htm (accessed on 22 September 2019).
- Jenness, J.S.; Brost, B.; Beier, P. Land Facet Corridor Designer—Extension for ArcGIS. 2013. Available online: http://www.jennessent.com/arcgis/land_facets.htm (accessed on 22 September 2019).
- Fleming, M.D.; Hoffer, R.M. Machine processing of Landsat MSS data and DMA topographic data for forest cover type mapping. In Proceedings of the Symposium on Machine Processing of Remotely Sensed Data, West Lafayette, IN, USA, 27–29 June 1979; p. 062879. Available online: http://docs.lib.purdue.edu/lars_symp/302 (accessed on 22 September 2025).
- Jones, K.H. A comparison of algorithms used to compute hill slope as a property of the DEM. Comput. Geosci. 1998, 24, 315–323. [Google Scholar] [CrossRef]
- Stage, A.R. An expression for the effect of aspect, slope, and habitat type on tree growth. For. Sci. 1976, 22, 457–460. [Google Scholar] [CrossRef]
- Riley, S.J.; DeGloria, S.D.; Elliot, R. A terrain ruggedness index that quantifies topographic heterogeneity. Intermt. J. Sci. 1999, 5, 23–27. [Google Scholar]
- Beven, K.J.; Kirkby, M.J. A physically based, variable contributing area model of basin hydrology. Hydrol. Sci. J. 1979, 24, 43–69. [Google Scholar] [CrossRef]
- Schmidt, F.; Persson, A. Comparison of DEM data capture and topographic wetness indices. Precis. Agric. 2003, 4, 179–192. [Google Scholar] [CrossRef]
- Conrad, O.; Bechtel, B.; Bock, M.; Dietrich, H.; Fischer, E.; Gerlitz, L.; Wehberg, J.; Wichmann, V.; Böhner, J. System for Automated Geoscientific Analyses (SAGA) v.2.1.4. Geosci. Model Dev. 2015, 8, 1991–2007. [Google Scholar] [CrossRef]
- Dobrowski, S.Z. A Climatic Basis for Microrefugia: The Influence of Terrain on Climate. Glob. Change Biol. 2011, 17, 1022–1035. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the Earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.; Loppi, S.; De Dominicis, V. Environmental factors and proportions of fungal trophic groups in Mediterranean forests. For. Ecol. Manag. 1999, 124, 145–151. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Lindén, A.; Mäntyniemi, S. Using the negative binomial distribution to model overdispersion in ecological count data. Ecology 2011, 92, 1414–1421. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analysis in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol. Model. 2002, 153, 51–68. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. vegan: Community Ecology Package, R Package Version 2.6-4. 2022. Available online: https://cran.r-project.org/web/packages/vegan (accessed on 22 September 2025).
- Marshall, L.A.E.; Fornwalt, P.J.; Stevens-Rumann, C.S.; Rodman, K.C.; Rhoades, C.C.; Zimlinghaus, K.; Chapman, T.B.; Schloegel, C.A. North-facing aspects, shade objects, and microtopographic depressions promote the survival and growth of tree seedlings planted after wildfire. Fire Ecol. 2023, 19, 26. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 22 September 2025).
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D.; Ripley, M.B. MASS, R Package. 2013. Available online: https://cran.r-project.org/web/packages/MASS/ (accessed on 22 September 2025).
- Barton, K.; Barton, M.K. MuMIn: Multi-Model Inference, R Package Version 1.46.0. 2015. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 22 September 2025).
- Raimbault, A.; Brin, A.; Manzi, S.; Savoie, J.M.; Gandois, L.; Oliva, P.; Fogliani, O.; Roy-Camille, C.; Gratacap, L.; Roy, M. Influence of habitat fragmentation and habitat amount on soil fungi communities in ancient forests. Landsc. Ecol. 2024, 39, 19. [Google Scholar] [CrossRef]
- Dahlberg, A. Effects of fire on ectomycorrhizal fungi in Fennoscandian boreal forests. Silva Fenn. 2002, 36, 69–80. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; Oria-de-Rueda, J.A.; Martín-Pinto, P. Post-fire fungal succession in a Mediterranean ecosystem dominated by Cistus ladanifer L. For. Ecol. Manag. 2013, 289, 48–57. [Google Scholar] [CrossRef]
- Fox, S.; Sikes, B.A.; Brown, S.P.; Cripps, C.L.; Glassman, S.I.; Hughes, K.; Semenova-Nelsen, T.; Jumpponen, A. Fire as a driver of fungal diversity—A synthesis of current knowledge. Mycologia 2022, 114, 215–241. [Google Scholar] [CrossRef]
- Semenova-Nelsen, T.A.; Platt, W.J.; Patterson, T.R.; Huffman, J.; Sikes, B.A. Frequent fire reorganizes fungal communities and slows decomposition across a heterogeneous pine savanna landscape. New Phytol. 2019, 224, 916–927. [Google Scholar] [CrossRef]
- Owen, S.M.; Patterson, A.M.; Gehring, C.A.; Sieg, C.H.; Baggett, L.S.; Fulé, P.Z. Large, high-severity burn patches limit fungal recovery 13 years after wildfire in a ponderosa pine forest. Soil Biol. Biochem. 2019, 139, 107616. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Egerton-Warburton, L.M.; Allen, M.F. Topographic position modulates the mycorrhizal response of oak trees to interannual rainfall variability. Ecology 2009, 90, 649–662. [Google Scholar] [CrossRef]
- Claridge, A.W.; Barry, S.C.; Cork, S.J.; Trappe, J.M. Diversity and habitat relationships of hypogeous fungi. II. Factors influencing the occurrence and number of taxa. Biodivers. Conserv. 2000, 9, 175–199. [Google Scholar] [CrossRef]
- Dove, N.C.; Keeton, W.S. Structural complexity enhancement increases fungal species richness in northern hardwood forests. Fungal Ecol. 2015, 13, 181–192. [Google Scholar] [CrossRef]
- Fernández-Ruiz, A.; Vicente-Villardón, J.L.; Sánchez-Sánchez, J.; García-Jiménez, P.; Sánchez-Durán, S.; Rodríguez-de la Cruz, D. A statistical approach to macrofungal diversity in a Mediterranean ecosystem dominated by the holm oak. Forests 2023, 14, 1662. [Google Scholar] [CrossRef]
- Tsujino, R.; Sato, H.; Imamura, A.; Yumoto, T. Topography-specific emergence of fungal fruiting bodies in warm temperate evergreen broad-leaved forests on Yakushima Island, Japan. Mycoscience 2009, 50, 388–399. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Lang, X.; Huang, X.; Su, J. Effects of microtopography on soil fungal community diversity, composition and assembly in a subtropical monsoon evergreen broadleaf forest of Southwest China. Catena 2022, 211, 106025. [Google Scholar] [CrossRef]
- Lumley, T.C.; Gignac, L.D.; Currah, R.S. Microfungus communities of spruce and aspen logs at different decay stages in disturbed/undisturbed sites. Can. J. Bot. 2001, 79, 76–92. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Hynson, N.A.; Bruns, T.D. Stayin’ alive: Survival of mycorrhizal fungal propagules from 6-yr-old forest soil. Fungal Ecol. 2012, 5, 741–746. [Google Scholar] [CrossRef]
- Pepe, A.; Giovannetti, M.; Sbrana, C. Lifespan and functionality of mycorrhizal fungal mycelium are uncoupled from host plant lifespan. Sci. Rep. 2018, 8, 10235. [Google Scholar] [CrossRef]
- Siknia, M.; Skiada, V.; Ipsilantis, I.; Vasileiadis, S.; Kavroulakis, N.; Genitsaris, S.; Papadopoulou, K.K.; Hart, M.; Klironomos, J.; Karpouzas, D.G.; et al. Strong host-specific selection and over-dominance characterize arbuscular mycorrhizal fungal root colonizers of coastal sand dune plants of the Mediterranean region. FEMS Microbiol. Ecol. 2021, 97, fiab109. [Google Scholar] [CrossRef]
- Yang, T.; Tedersoo, L.; Lin, X.; Fitzpatrick, M.C.; Jia, Y.; Liu, X.; Shen, Z. Distinct fungal successional trajectories following wildfire between soil horizons in a cold-temperate forest. New Phytol. 2020, 227, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Orumaa, A.; Köster, K.; Tullus, A.; Tullus, T.; Metslaid, M. Forest Fires Have Long-Term Effects on the Composition of Vascular Plants and Bryophytes in Scots Pine Forests of Hemiboreal Estonia. Silva Fenn. 2022, 56, 10598. [Google Scholar] [CrossRef]
- Giordani, P.; Calderisi, G.; Cogoni, D.; Fenu, G. Asynchronous Postfire Recovery Dynamics between Epilithic Lichens and Vascular Plants in Mediterranean Ecosystems. J. Environ. Manag. 2025, 394, 127645. [Google Scholar] [CrossRef] [PubMed]
- Ruffault, J.; Curt, T.; Moron, V.; Trigo, R.M.; Mouillot, F.; Koutsias, N.; Pimont, F.; Martin-StPaul, N.; Barbero, R.; Dupuy, J.-L.; et al. Increased likelihood of heat-induced large wildfires in the Mediterranean Basin. Sci. Rep. 2020, 10, 13790. [Google Scholar] [CrossRef]
- Mansourian, S.; Rossi, M.; Vallauri, D. Ancient Forests in the Northern Mediterranean: Neglected High Conservation Value Areas; WWF: Marseille, France, 2013; 80p. [Google Scholar]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Hyde, K.; Mortimer, P. Reviewing the contributions of macrofungi to forest ecosystem processes and services. Fungal Biol. Rev. 2022, 44, 100294. [Google Scholar] [CrossRef]
- Mediavilla, O.; Oria-de-Rueda, J.A.; Martín-Pinto, P. Changes in sporocarp production and vegetation following wildfire in a Pinus nigra forest in northern Spain. For. Ecol. Manag. 2014, 331, 85–92. [Google Scholar] [CrossRef]
- Orumaa, A.; Agan, A.; Anslan, S.; Drenkhan, T.; Drenkhan, R.; Kauer, K.; Tedersoo, L.; Metslaid, M. Long-term effects of forest fires on fungal community and soil properties along a Scots pine fire chronosequence. Sci. Total Environ. 2022, 851, 158173. [Google Scholar] [CrossRef]
- Hewitt, R.E.; Day, N.J.; DeVan, M.R.; Taylor, D.L. Wildfire Impacts on Root-Associated Fungi and Predicted Plant–Soil Feedbacks in the Boreal Forest: Research Progress and Recommendations. Funct. Ecol. 2023, 37, 2110–2125. [Google Scholar] [CrossRef]
- Morman, K.E.; Buckley, H.L.; Higgins, C.M.; Tosi, M.; Dunfield, K.E.; Day, N.J. Simulated fire and plant–soil feedback effects on mycorrhizal fungi and invasive plants. iScience 2024, 27, 111193. [Google Scholar] [CrossRef]
- Zak, J.C.; Wildman, H.G. Fungi in stressful environments. In Biodiversity of Fungi: Inventory and Monitoring Methods; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Elsevier: London, UK, 2004; pp. 303–331. [Google Scholar]

| Category | Variable | Units | Mean ± SE | Range |
|---|---|---|---|---|
| Fire | Severity index (dNBR) | Numeric | 0.471 ± 0.041 | 0.025–1.133 |
| Vegetation | Plant richness | Integer | 11.546 ± 0516 | 3–21 |
| Plant cover | Proportion | 0.320 ± 0.038 | 0.010–1.090 | |
| Topography | Elevation | m a.s.l. | 623.848 ± 9.513 | 469–775 |
| Slope | Degrees | 23.801 ± 0.883 | 3.931–37.793 | |
| Aspect | Degrees | 165.204 ± 19.358 | 0.000–357.879 | |
| Sine slope/aspect transformation | Numeric | 0.029 ± 0.021 | −0.292–0.392 | |
| Cosine slope/aspect transformation | Numeric | 0.403 ± 0.022 | −0.138–0.763 | |
| Topographic ruggedness index (TRI) | Numeric | 10.731 ± 0.081 | 9.392–11.720 | |
| Topographic wetness index (TWI) | Numeric | 5.192 ± 0.061 | 4.562–6.312 |
| Response | Candidate Model | Code | AICc | ΔAICc | AICc-Weights | R2 |
|---|---|---|---|---|---|---|
| Smyc | Fire severity + pcnmmyc | M-1 | 183.61 | 0.00 | 0.31 | 0.637 |
| Fire severity + Aspect + pcnmmyc | M-2 | 184.65 | 1.05 | 0.18 | 0.644 | |
| Fire severity + TWI + pcnmmyc | M-3 | 185.06 | 1.45 | 0.15 | 0.642 | |
| Ssap | TRI + pcnmsap | S-1 | 278.44 | 0.00 | 0.47 | 0.248 |
| Smyc/Ssap | Fire severity 2 + Elevation 2 + pcnmrms | MSr-1 | 148.99 | 0.00 | 0.29 | 0.619 |
| Fire severity 2 + Elevation 2 + TWI 2 + pcnmrms | MSr-2 | 150.50 | 1.51 | 0.14 | 0.644 | |
| Fire severity 2 + Elevation 2 + Aspect + pcnmrms | MSr-3 | 150.63 | 1.65 | 0.13 | 0.626 | |
| Fire severity 2 + pcnmrms | MSr-4 | 150.81 | 1.82 | 0.12 | 0.572 |
| Response | Inference | Predictor | Mean ± SE Effect Size | 95% CI | Variable Importance |
|---|---|---|---|---|---|
| Smyc | Model averaging | Fire severity | −0.856 ± 0.167 | −1.130–−0.582 | 1 |
| Aspect | −0.148 ± 0.134 | −0.369–0.073 | 0.285 | ||
| TWI | 0.093 ± 0.099 | −0.070–0.255 | 0.234 | ||
| pcnmmyc | 0.499 ± 0.091 | 0.349–0.649 | 1 | ||
| Ssap | Single model | TRI | 0.176 ± 0.069 | 0.062–0.290 | - |
| pcnmsap | 0.111 ± 0.066 | 0.002–0.220 | - | ||
| Smyc/Ssap | Model averaging | Fire severity | −0.447 ± 0.167 | −0.721–−0.173 | 1 |
| Fire severity 2 | 0.247 ± 0.131 | 0.031–0.462 | |||
| Elevation | −0.315 ± 0.151 | −0.563–−0.067 | 0.826 | ||
| Elevation 2 | 0.171 ± 0.100 | 0.006–0.335 | |||
| TWI | −0.129 ± 0.160 | −0.392–0.134 | 0.204 | ||
| TWI 2 | −0.085 ± 0.118 | −0.280–0.109 | |||
| Aspect | −0.090 ± 0.099 | −0.254–0.073 | 0.19 | ||
| pcnmrms | 0.459 ± 0.093 | 0.306–0.612 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Silva, C.; Natário, B.; Pita, R. Fine-Scale Environmental Heterogeneity Shapes Post-Fire Macrofungal Richness in a Mediterranean Relict Forest. Fire 2025, 8, 438. https://doi.org/10.3390/fire8110438
Santos-Silva C, Natário B, Pita R. Fine-Scale Environmental Heterogeneity Shapes Post-Fire Macrofungal Richness in a Mediterranean Relict Forest. Fire. 2025; 8(11):438. https://doi.org/10.3390/fire8110438
Chicago/Turabian StyleSantos-Silva, Celeste, Bruno Natário, and Ricardo Pita. 2025. "Fine-Scale Environmental Heterogeneity Shapes Post-Fire Macrofungal Richness in a Mediterranean Relict Forest" Fire 8, no. 11: 438. https://doi.org/10.3390/fire8110438
APA StyleSantos-Silva, C., Natário, B., & Pita, R. (2025). Fine-Scale Environmental Heterogeneity Shapes Post-Fire Macrofungal Richness in a Mediterranean Relict Forest. Fire, 8(11), 438. https://doi.org/10.3390/fire8110438







