1. Introduction
Driven by the global pursuit of clean energy and sustainable development, Lithium-ion Batteries (LIBs) have emerged as a cornerstone of modern energy storage systems, prized for their high energy density, long lifespan, and portable design. They are widely used in electric vehicles, electric bicycles and stationary energy storage systems [
1,
2]. Among them, the LFP batteries have attracted considerable attention in high-power and large-capacity applications due to their excellent thermal stability and safety [
3].
However, LIBs often face extreme operational conditions, such as thermal, electrical, and mechanical abuse [
4,
5], which can easily trigger TR events, potentially leading to fire, explosions, and even casualties [
6,
7]. Among the various triggering factors, TR induced by overcharge is notable for its distinct stage characteristics and a certain degree of predictability [
8,
9]. During the overcharging process, the heat inside the battery gradually accumulates, causing the decomposition of organic electrolytes and gas release, which in turn initiates a chain reaction [
10]. Compared to mechanical abuse, such as puncture and extrusion [
11], TR induced by overcharging typically progresses more slowly, follows a clearer reaction pathway, and is accompanied by physical signals that can be used for early detection. In contrast, thermal abuse often originates from the propagation of heat from a single-cell TR to adjacent cells within a battery module [
12], making early warning more challenging. Therefore, the overcharge-to-thermal-runaway process provides an ideal experimental model for research and early warning of TR.
In recent years, overcharging-induced TR has attracted widespread attention as a major cause of lithium-ion battery accidents. Related research has primarily focused on three key areas: phenomenological characteristics, underlying mechanisms, and early warning and monitoring strategies [
13].
First, in terms of phenomenological and characteristic research, Wilson [
14] analyzed the internal heat distribution of LiCoO
2 batteries under overcharging and short-circuit conditions in the early stage; Wang [
15] demonstrated that high C rate will lead to more violent gas eruption and higher explosion risk; Meng [
16] investigated the influence of ambient temperature on TR behavior; Jia [
17] reported that the toxicity of gas under overcharge conditions lasted up to 1211 s, which was significantly higher than that under heating conditions; Kang [
18] pointed out that batteries with larger capacities tend to exhibit more destructive TR behavior; Yang [
19] and Larsson [
20], respectively, studied the flammability and toxicity of gases generated during TR; Deng [
21] systematically discussed the combustion characteristics and dangers of LFP batteries under various TR-triggering conditions.
Secondly, in the area of mechanistic and theoretical studies, Aiello [
22] examined the propagation path of TR in soft-pack batteries from the perspective of mechanical constraints; Santhanagopalan [
23] developed an electrochemical model to analyze internal short-circuit behavior; Ouyang [
24] summarized the evolution mechanism of LIB TR and proposed intervention strategies; Feng [
25] proposed a three-level protection strategy for electric vehicle batteries, which significantly enhanced the system safety.
Third, in terms of early warning and monitoring methods, Shah [
26] proposed to distinguish overheating state based on heat generation rate and temperature rise gradient, offering a thermal-source-based approach to predicting TR; Kong [
27] used CFD and heat transfer simulation to reveal the relationship between the SOC (State of Charge) state of the battery cell and the jet flame, and predicted the start time of TR; Feng presented a coupled electrochemical-TR model [
2] and an automatic identification method of thermophysical parameters based on optimization algorithm [
12] to predict TR behavior; Cui [
28] proposed an early warning mechanism based on gas production behavior, and introduced TRD (Thermal Runaway Degree) to quantify the severity of TR; Jin [
10] developed an H
2 gas capture method that provided an effective warning 639 s prior to the onset of TR in experiments. Furthermore, the combination of multi-physics simulation and deep learning has emerged as a promising avenue. For instance, Li [
29] proposed an integration framework based on CFD and deep learning (CNN-LSTM) for early TR detection in battery packs, achieving high accuracy. Similarly, Pang [
30] developed reliable, multi-physics data from 3D models for training deep learning prognostics models, emphasizing the importance of high-fidelity data for hot spot prediction.
Additionally, the integration of artificial intelligence has significantly enriched the methods for TR early warning, with the combination of machine learning and data-driven approaches leading to new breakthroughs in TR prediction [
31]. Jia [
32] proposed a fast classification model based on single-cycle voltage-temperature data to achieve efficient TR state identification; Zhu [
33] used ResNet-CNN combined with transfer learning to accurately identify internal short circuits; Das Goswami [
34] combined deep learning with multi-physics models to establish a high-precision TR prediction framework, successfully forecasting the conditions under which cylindrical cells undergo TR. These results show that the fusion of multimodal data and intelligent algorithms can significantly improve the accuracy, timeliness and adaptability of early warning systems, validating the great potential of combining multimodal information with AI techniques.
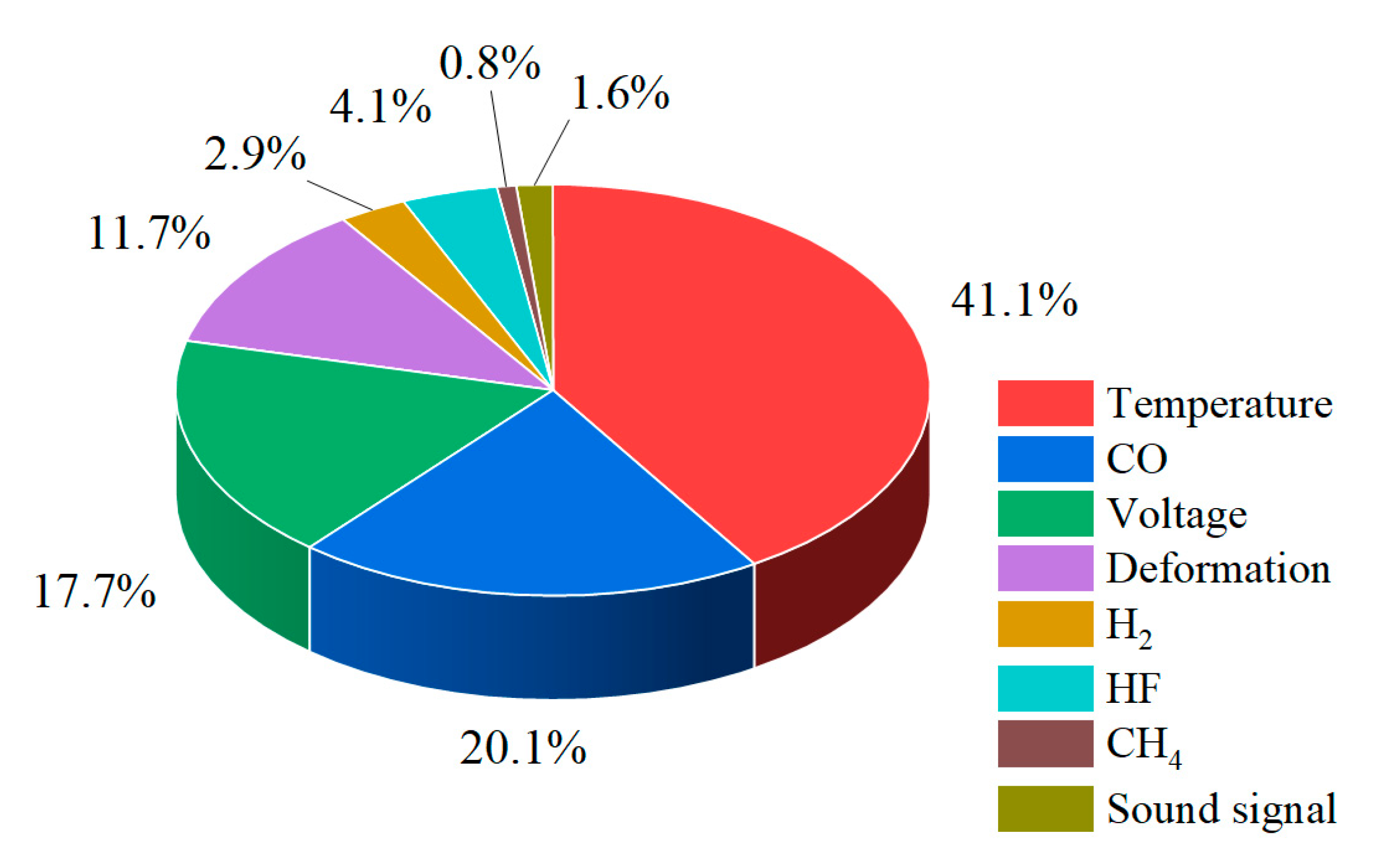
Although the aforementioned studies have laid a solid foundation for understanding the mechanisms of TR and for developing early warning methods, several challenges remain in the context of systematic research tailored to real-world engineering applications: (1) Existing early warning methods often rely on single-modal signals, leading to poor robustness under complex operating conditions and a lack of feature redundancy design. (2) For the overcharge-to-thermal-runaway process, intervention and processing at different time points may lead to varying types of damage and risks. Current approaches to precise early warning and real-time monitoring primarily focus on identifying critical points during the TR phase, with limited attention to the early stages of overcharging, where accurate forecasting is still lacking. (3) Once TR enters the irreversible stage, the high-concentration release of toxic gases such as CO and HF will significantly threaten the safety of personnel, and the visibility will drop sharply to below 0.5m due to smoke obstruction. For assessing such hazardous scenarios, traditional manual interpretation-based responses are evidently incompatible with the characteristics of lithium battery fires—namely, rapid onset and difficulty to extinguish. The traditional manual judgment and conventional response process fail to match the characteristics of battery fires, which are high temperature, high toxicity, and rapid development, and lack real-time data to assist subsequent emergency command and decision-making
Therefore, there is an urgent need for a systematic framework that integrates real-time multimodal data collection, stage-wise identification, intelligent early warning, and emergency response decision support to achieve closed-loop management from pre-incident detection to the rescue phase.
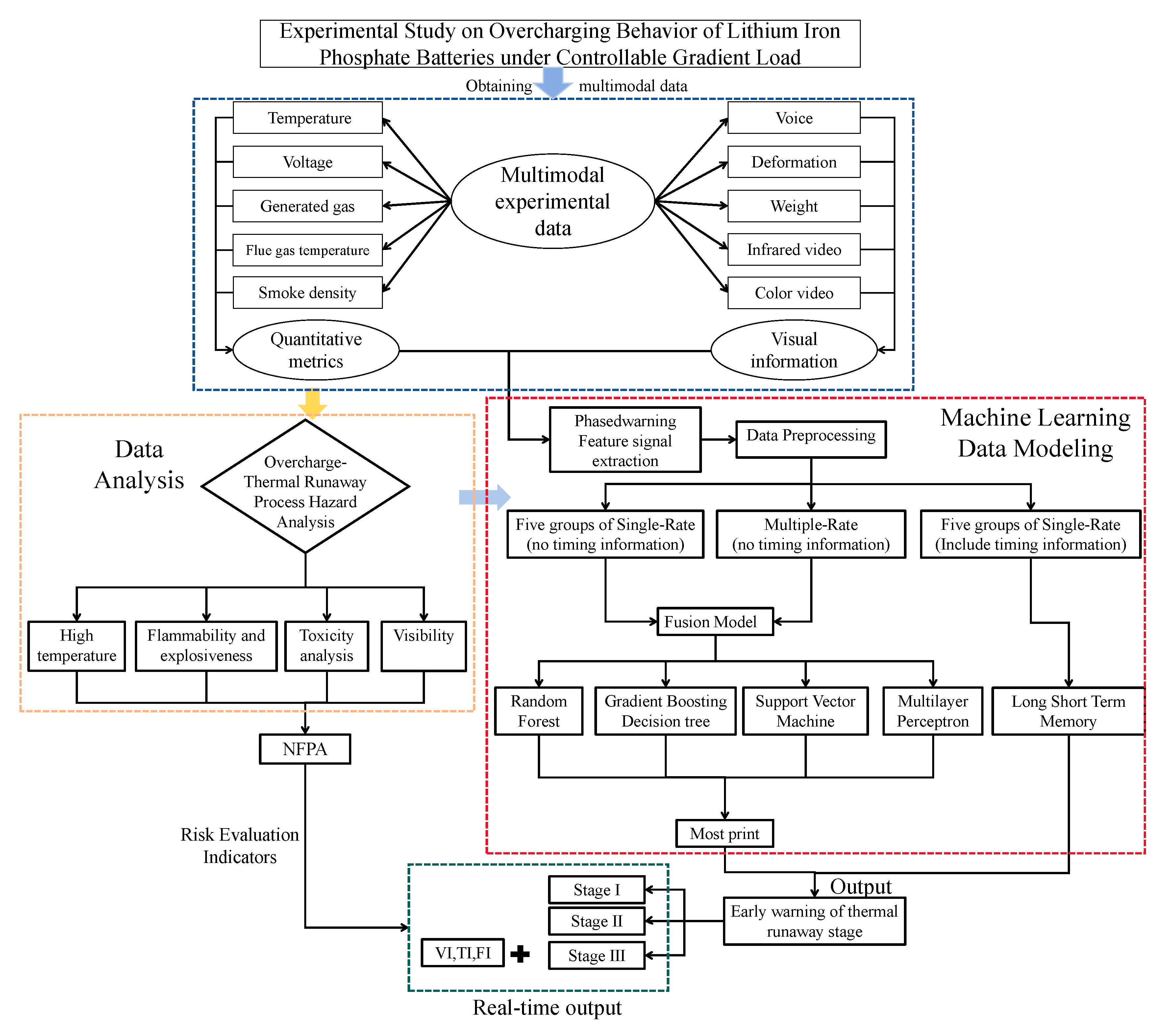
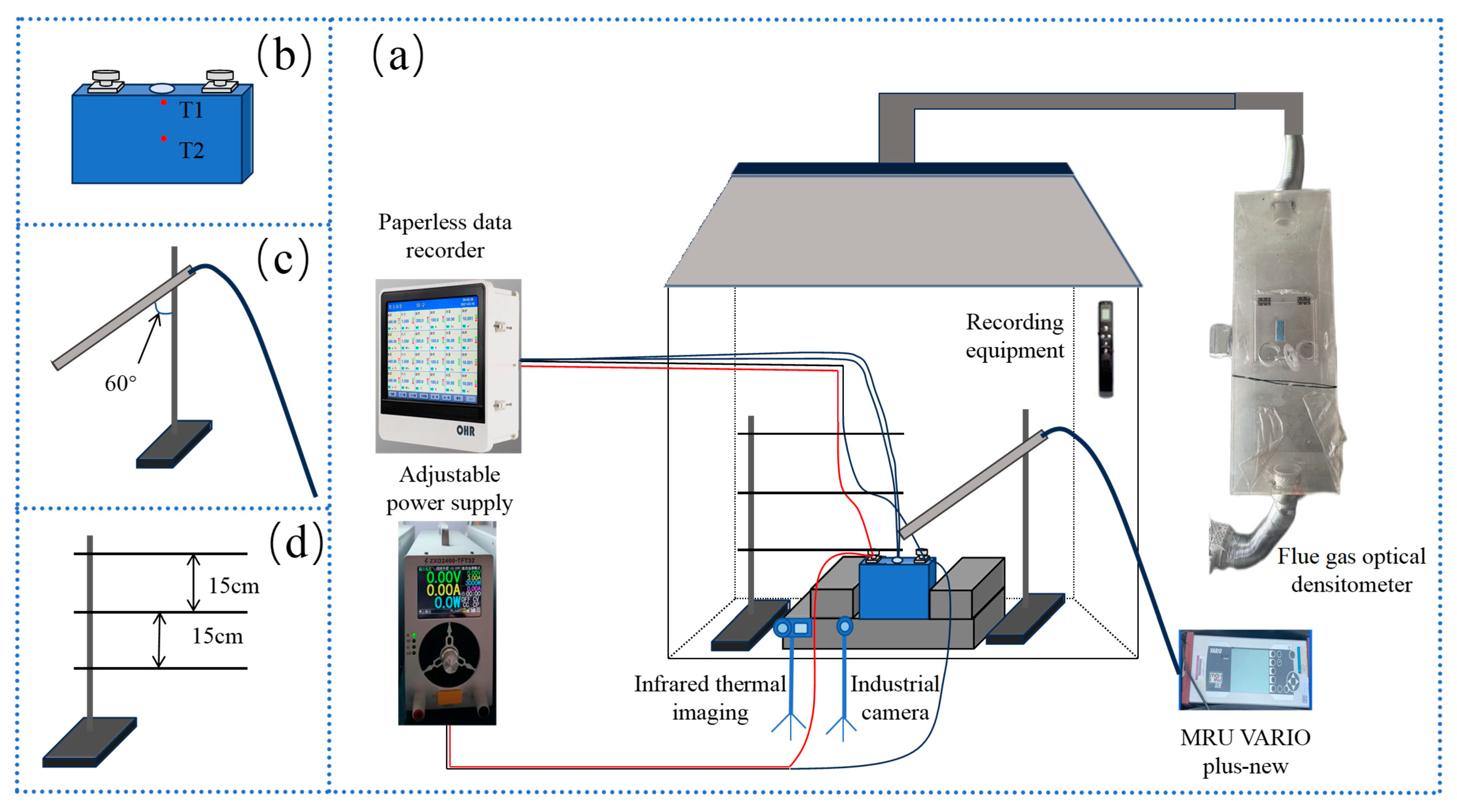
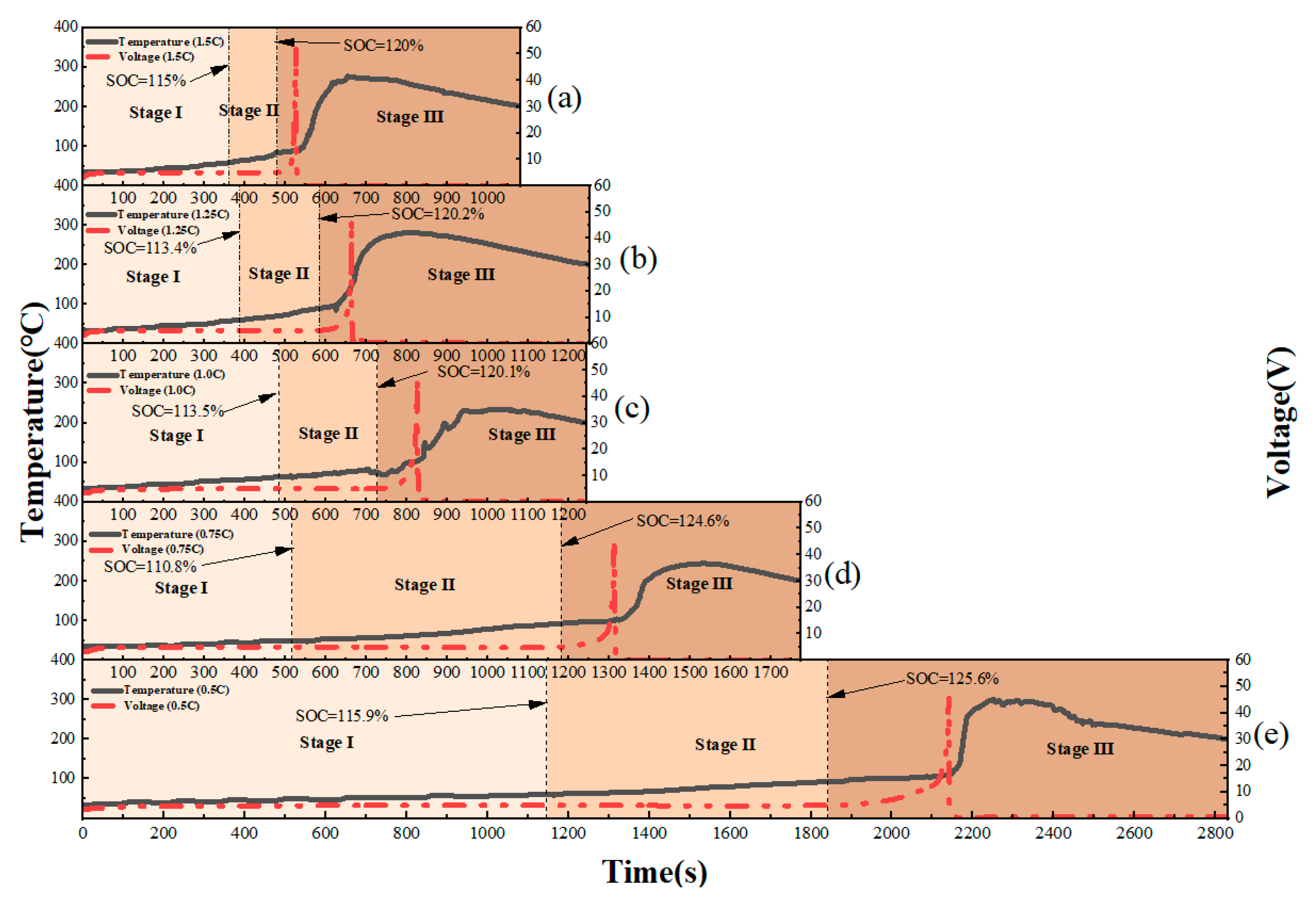
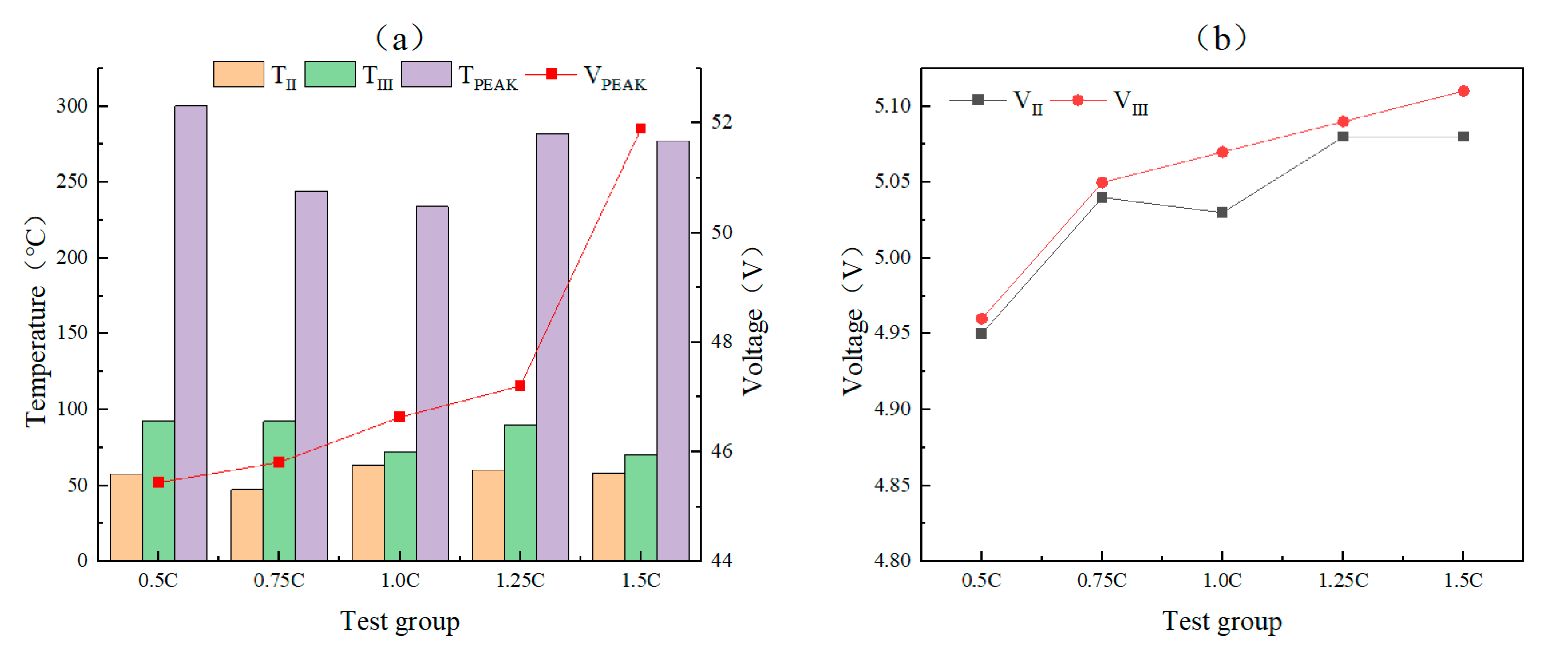
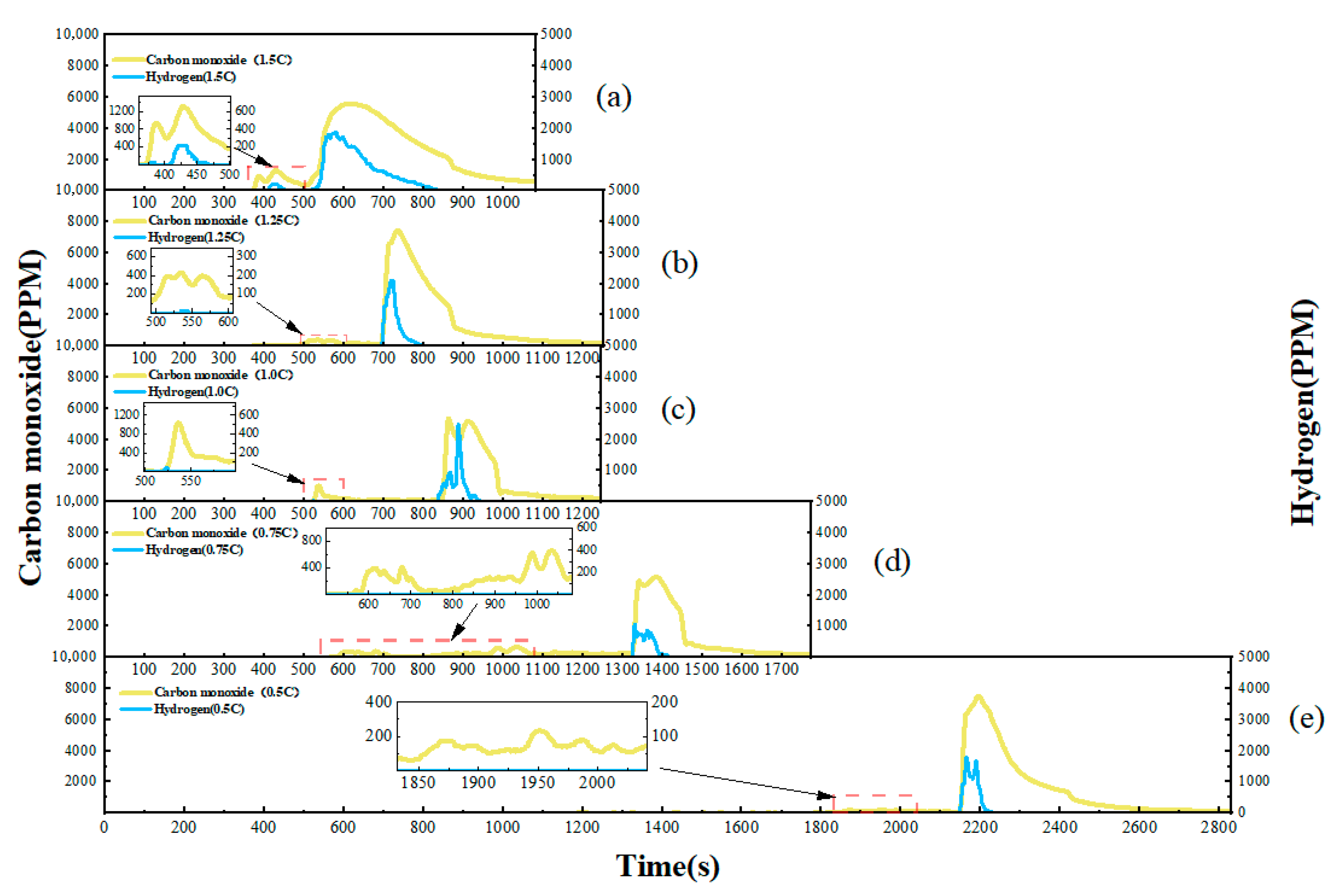
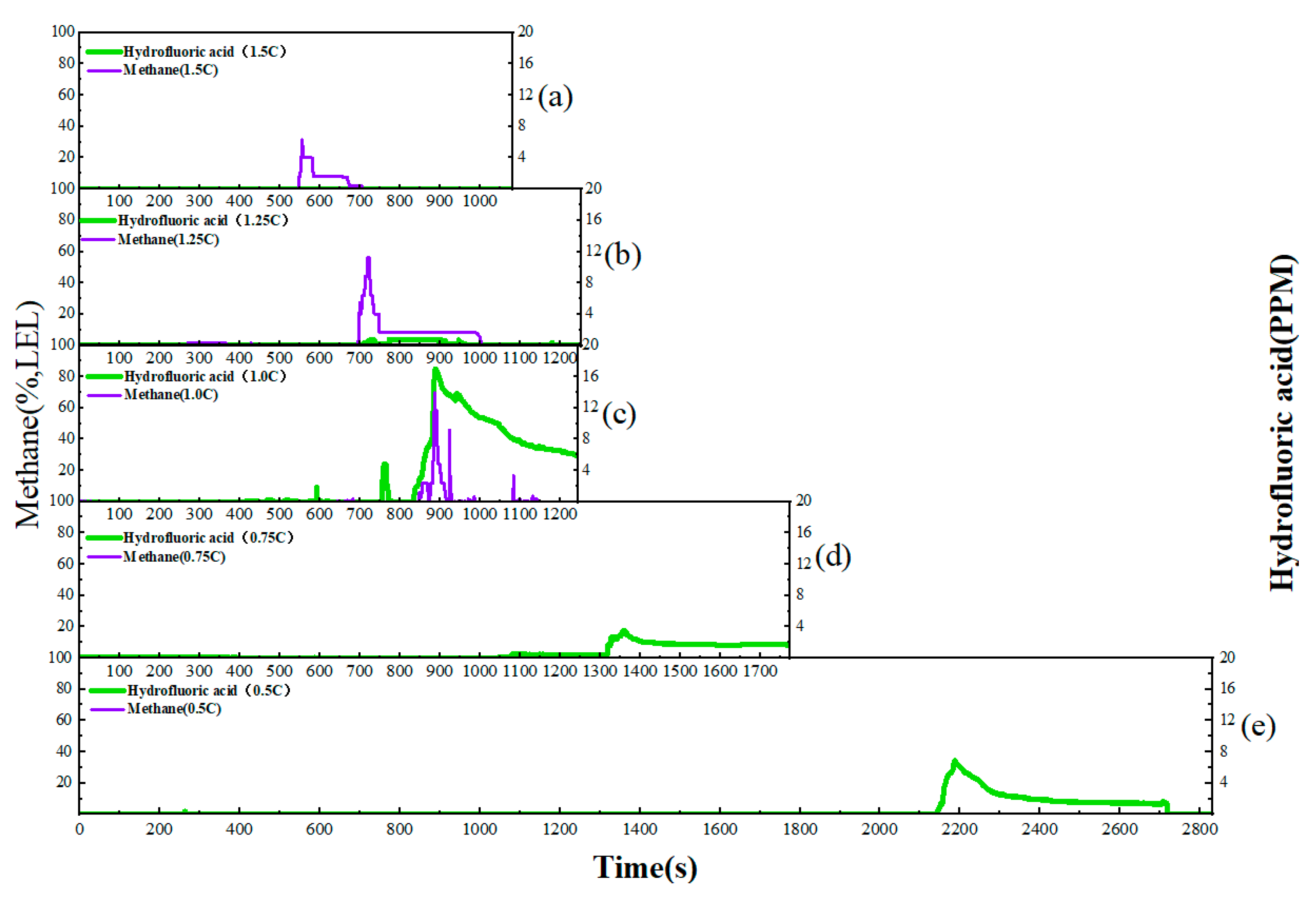
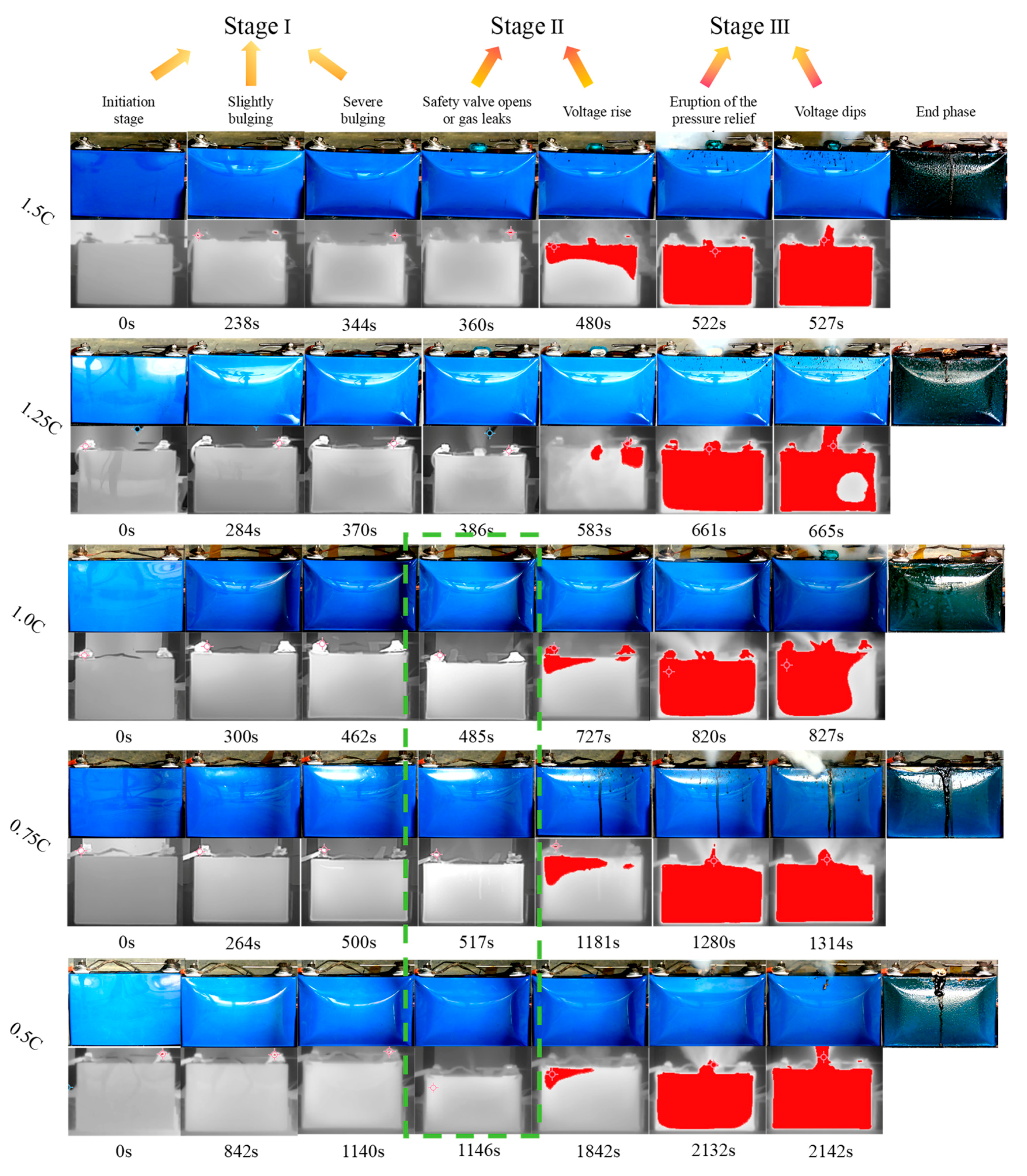
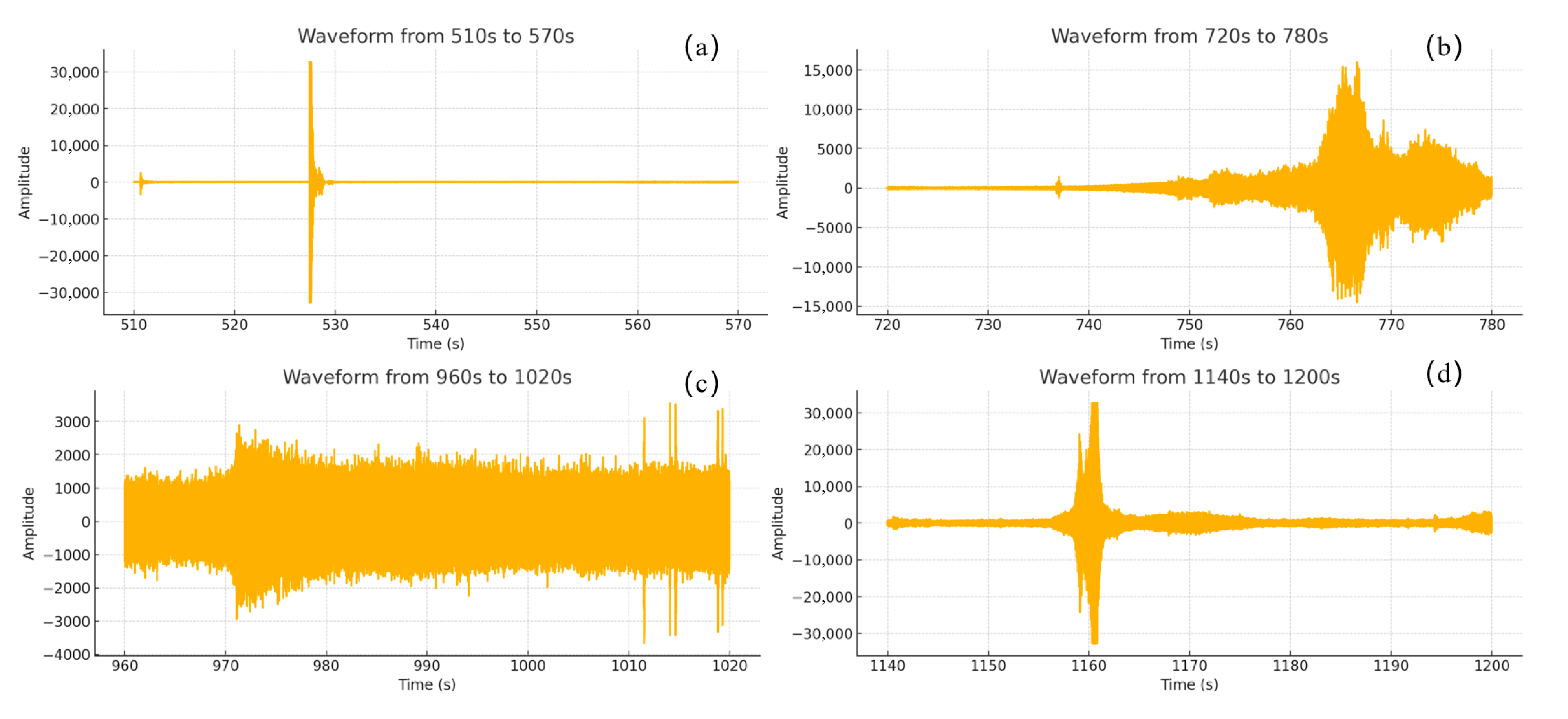
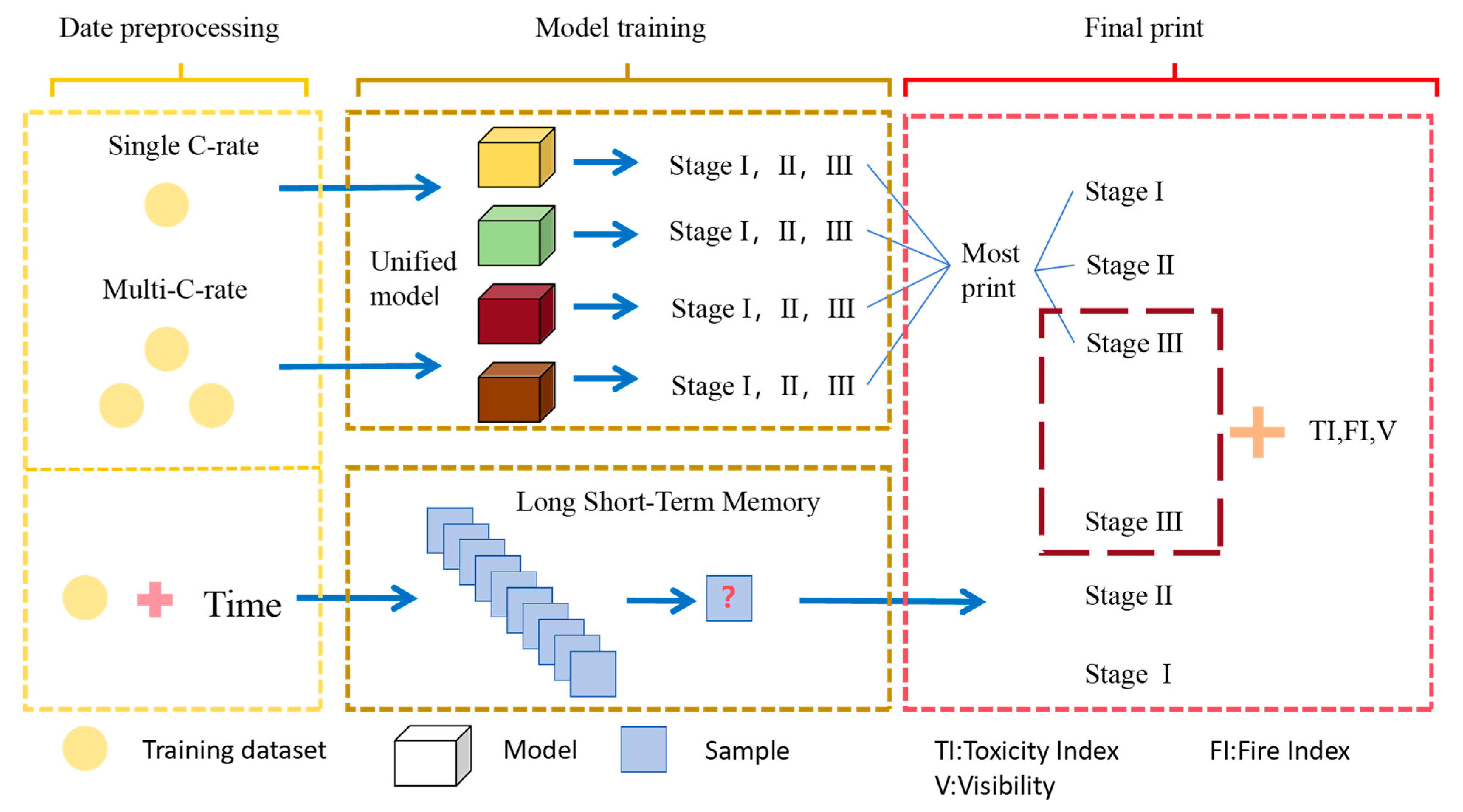
This study focuses on the overcharge-TR behavior of LFP batteries under different charging rates, and constructs a three-stage TR evolution model: Stage I (from the onset of overcharging to the activation of the safety valve), Stage II (from safety valve activation to voltage rebound) and Stage III (from sharp fluctuations in voltage and temperature to thermal stabilization). Based on five sets of C-rate gradient experiments, the system collects multi-modal data including voltage, temperature, gas emission, deformation and acoustic signals, extracting key indicators at each stage to assess their hazard potential.
Leveraging the staged TR model and multimodal data, we build a fusion early warning model based on traditional machine learning and an LSTM model based on deep learning. A multi-level TR state recognition network was constructed to enable automatic stage classification and precise early warning throughout the entire TR process. At the same time, for high-risk emergency scenarios following severe TR in Stage III, we propose a Multi-Dimensional Dynamic Emergency Decision Matrix. This framework integrates key parameters, including Toxicity Index (TI), Flammability Index (FI) and smoke visibility into the response framework to support the tiered formulation of rescue strategies and improve the accuracy, safety and intelligence of emergency management during critical incidents.
The objective of this research is to establish a comprehensive technical closed-loop system by integrating mechanism modeling, signal recognition, intelligent early warning, and emergency response. This study proposes a comprehensive framework that aims to support both theoretical understanding and practical implementation of safety management across the full lifecycle of lithium-ion batteries.
The research framework of this paper is shown in the
Figure 1 below.
4. Conclusions
This study focused on the TR behavior of LFP batteries induced by overcharging at different charging rates. Through comprehensive experimental observation, signal feature analysis, intelligent identification modeling and emergency response strategy construction, the following conclusions and innovative contributions have been achieved:
1. A three-stage TR evolution model was constructed based on C-rate conditions. By integrating experimental results, the process was clearly divided into Stage I (from the onset of overcharging to the activation of the safety valve), Stage II (from safety valve activation to voltage rebound) and Stage III (from sharp fluctuations in voltage and temperature to thermal stabilization). This provides a structured framework for stage-specific signal identification and mechanisti analysis throughout the entire TR process.
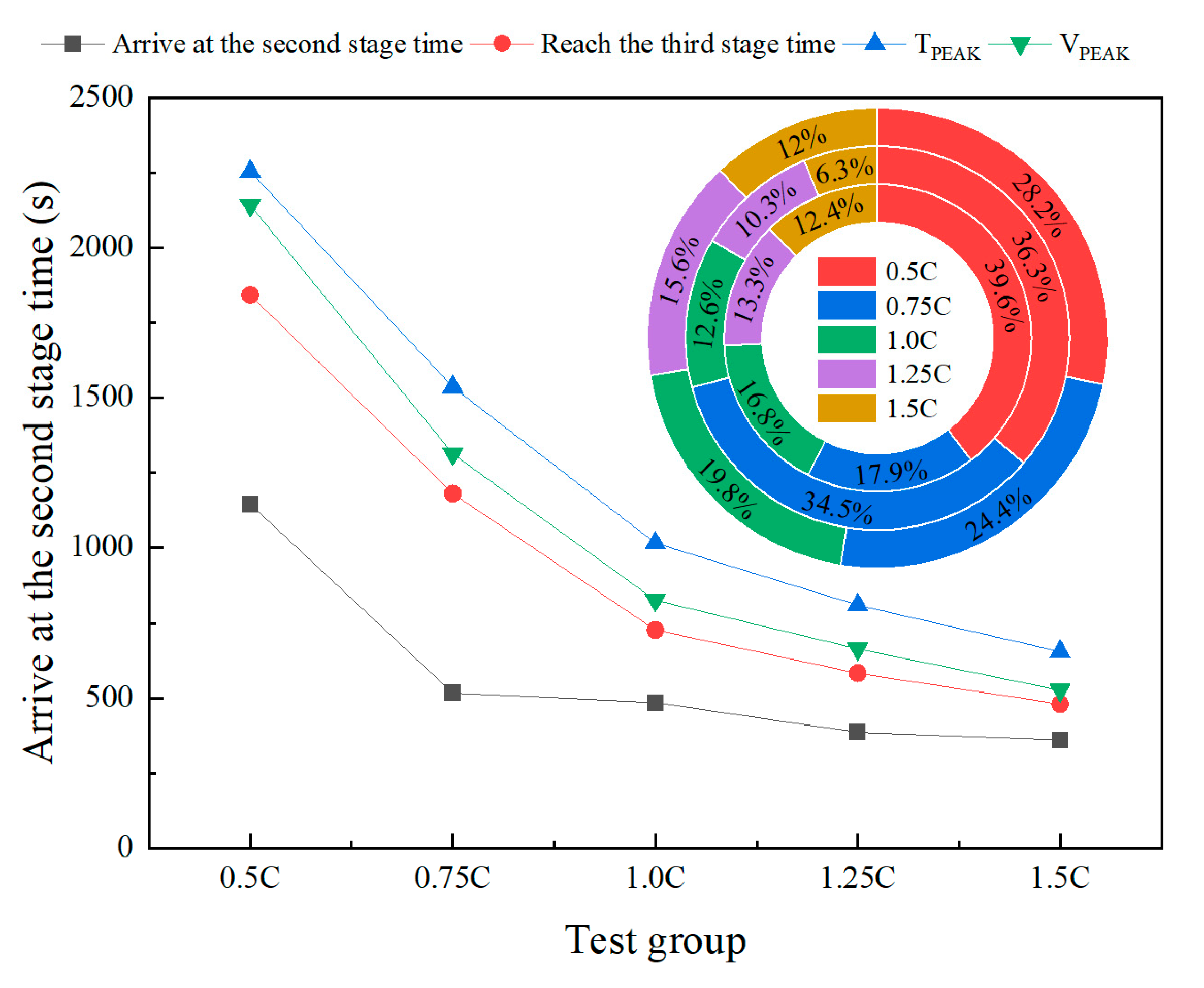
2. The study reveals the synergistic effects of charge rate on gas generation behavior and thermal response characteristics. Multimodal data show that with increased of charging rate, gas release is advanced and the FED index increases. The coordinated interactions among individual cells in a closed environment pose tangible risks of toxicity and explosion, which be incorporated into the safety assessment framework for battery packs.
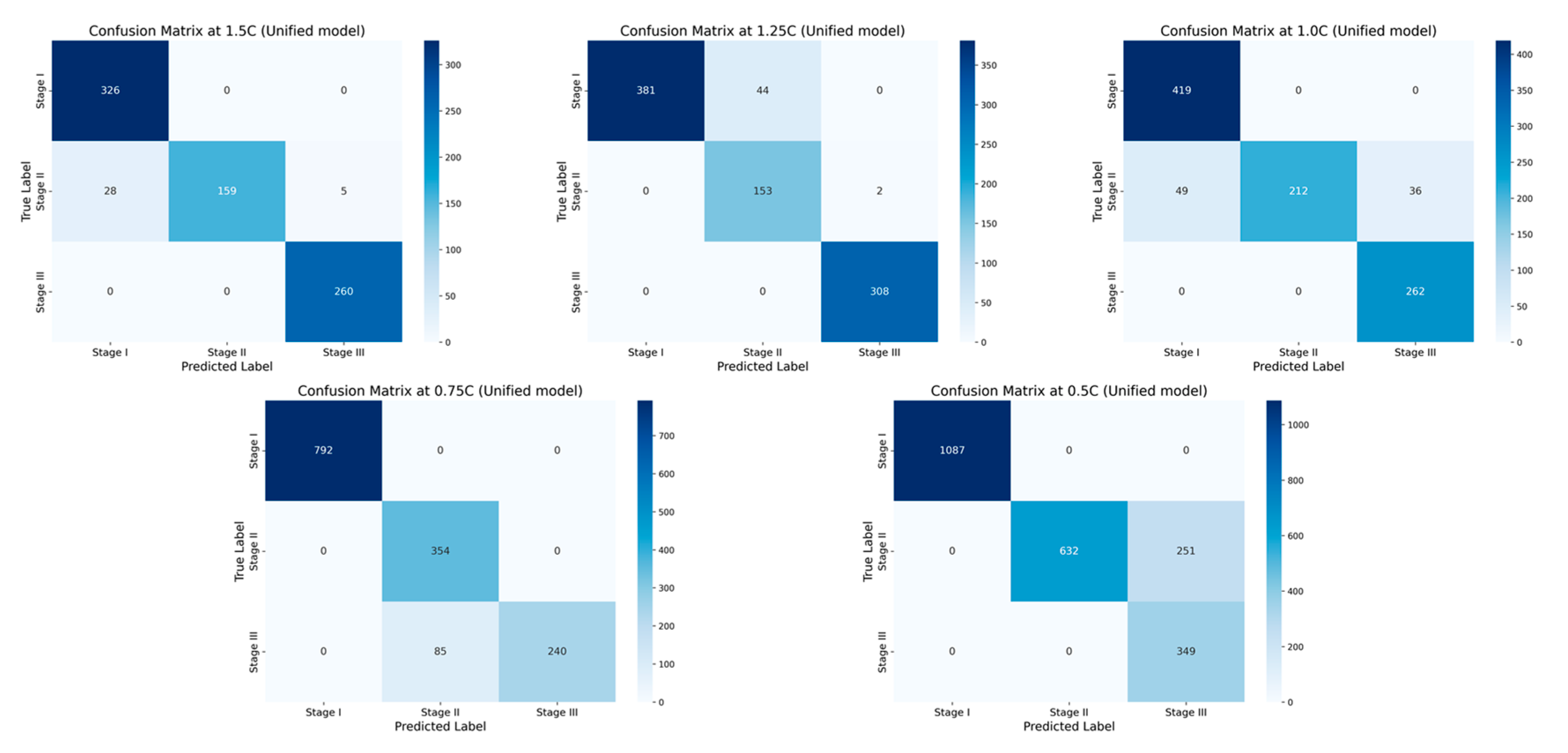
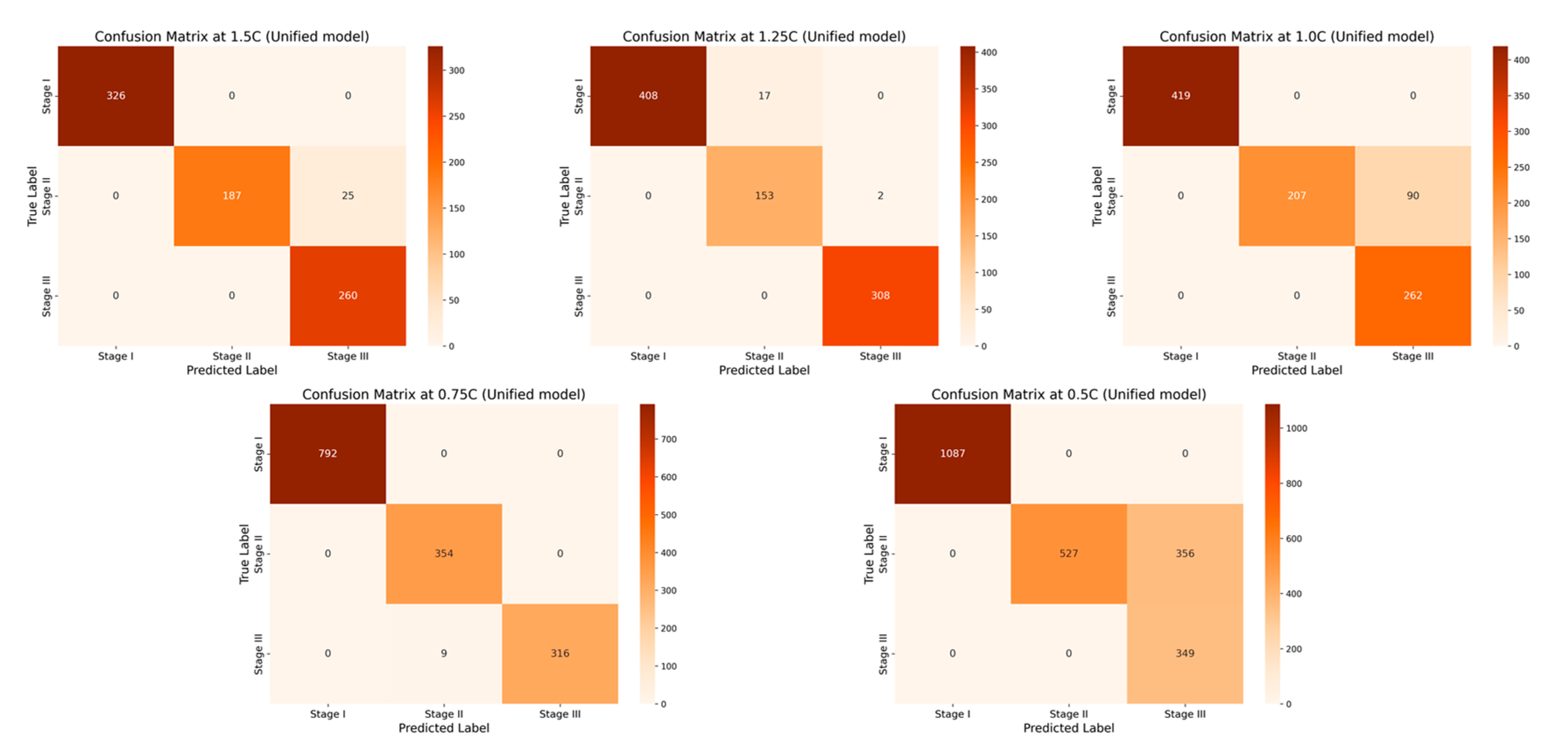
3. A fusion-based early warning model strategy is proposed integrating four types of traditional base learners (random forest, support vector machine, neural network and boosting tree). Compared to individual models, this fusion strategy shows stronger adaptability and robustness under various working conditions and complex scenarios. The prediction results of each base learner are integrated through the majority voting mechanism, the model outputs the class label with the highest proportion, thereby enhancing overall recognition stability and generalization capability. The experimental results show that the accuracy of the single-rate fusion model in all test groups is more than 90%, while the accuracy of the multi-rate fusion model reaches an optimal accuracy of 99.39%, which verifies the engineering practicality and model reliability of fusion modeling in complex and variable TR scenarios.
4. LSTM was introduced for time-series modeling. This approach addresses the limitations of traditional models in capturing continuous signal dynamics. The model achieves over 95% accuracy across various C-rates, making it suitable for real-time continuous monitoring and early warning deployment. At the same time, the high generalization ability and computational efficiency of the fusion model (especially the multi-rate) complement LSTM’s superior temporal precision and early warning potential.
5. An innovative multidimensional emergency decision matrix was constructed based on three core dimensions: Toxicity Index, Flammability Index, and visibility. This matrix implements a dynamic, graded response mechanism to establish a closed-loop rescue strategy encompassing detection, assessment, and response during TR incidents, thereby enhancing the scientific basis and safety of emergency management.
6. A closed-loop system linking early warning modeling to response decision-making was realized through the coordinated integration of multimodal perception, staged modeling, and emergency response. This approach bridges the entire process from pre-incident warning to post-incident emergency management. It overcomes the limitations of traditional single-point identification and static thresholds, and having good engineering practicality and promotion prospects.
Innovation and Contribution: The study’s most significant contribution is the establishment of a comprehensive, closed-loop safety management framework that advances the state-of-the-art in three key aspects: (1) utilizing a complementary dual-model strategy (Fusion for robustness, LSTM for temporal precision) to achieve high accuracy across variable C-rates; (2) identifying multimodal, stage-specific warning signals, moving beyond single-modal thresholds; and (3) pioneering a Multi-Dimensional Dynamic Emergency Decision Matrix (TI, FI, V) for quantified, action-oriented rescue response, bridging the gap from warning to action.
Limitations and Future Work: While the framework demonstrates strong performance and robustness within the experimental scope, the study has three primary limitations that define the direction for future research. Firstly, the experiments were conducted only at a single ambient temperature of 35 °C and focused on the maximum initial SOC (100%). Future work is required to validate the framework’s generalizability by conducting comprehensive comparative experiments across a range of lower and higher ambient temperatures (e.g., 25 °C, 45 °C) and various initial SOC levels (e.g., 50%, 80%). Secondly, this study was limited to overcharge-induced TR and did not explore other abuse scenarios (e.g., external heating or crushing). Finally, the current study focused exclusively on single-cell TR. Future efforts will extend the framework to the battery pack level, incorporating cell-to-cell TR propagation dynamics and scale-up effects to develop a truly robust system for real-world engineering applications.