Emission of Brominated Flame-Retarding Compounds from Polymeric Textile Materials Used in Firefighter Protective Garment During Thermal Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cone Calorimeter
2.3. Polyurethane Foam (PUT) Disks
2.4. Sample Preparation and Extraction
2.5. Sample Clean-Up
2.6. Gas Chromatography–Mass Spectrometry Analysis
2.7. Quality Assurance
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAH | Polycyclic aromatic hydrocarbon |
| PFA | Per- and polyfluoroalkyl substance |
| IARC | International Agency for Research Cancer |
| FR | Flame retardant |
| BFR | Brominated flame retardant |
| BDE | Brominated diphenyl ether |
| PBDE | Polybrominated diphenyl ether |
| POP | Persistent organic pollutant |
| PUF | Polyurethane foam |
| FTT | Fire Testing Technology |
| SPR | Smoke production rate |
| HRR | Heat release rate |
| MLR | Mass loss rate |
| GC-MS | Gas chromatography–mass spectrometry |
| QA/QC | Quality assurance/quality control |
| LOD | Limit of detection |
| SVOC | Semi-volatile organic compound |
References
- Banks, A.P.; Wang, X.; He, C.; Gallen, M.; Thomas, K.V.; Mueller, J.F. Off-gassing of semi-volatile organic compounds from fire-fighters’ uniforms in private vehicles—A pilot study. Int. J. Environ. Res. Public Health 2021, 18, 3030. [Google Scholar] [CrossRef]
- Cardona, B.; Rodgers, K.M.; Trowbridge, J.; Buren, H.; Rudel, R.A. Breast Cancer-Related Chemical Exposures in Firefighters. Toxics 2024, 12, 707. [Google Scholar] [CrossRef]
- Tian, X.; Cheng, Y.; Chen, S.; Liu, S.; Wang, Y.; Niu, X.; Sun, J. The Emission Characteristics and Health Risks of Firefighter-Accessed Fire: A Review. Toxics 2024, 12, 739. [Google Scholar] [CrossRef]
- Mazumder, N.U.S.; Hossain, M.T.; Jahura, F.T.; Girase, A.; Hall, A.S.; Lu, J.; Ormond, R.B. Firefighters’ exposure to per-and polyfluoroalkyl substances (PFAS) as an occupational hazard: A review. Front. Mater. 2023, 10, 1143411. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.; Almeida, L.; Dias, I.; Baptista, J.S.; Santos, J.; Vaz, M.; Guedes, J. Occupational chemical exposure and health status of wildland firefighters at the firefront: A systematic review. Safety 2024, 10, 60. [Google Scholar] [CrossRef]
- Mokoana, V.; Asante, J.; Okonkwo, J. Brominated flame-retardant composition in firefighter bunker gear and its thermal performance analysis. J. Fire Sci. 2021, 39, 207–223. [Google Scholar] [CrossRef]
- Boorady, L.M. Bunker Gear for Fire Fighters: Does it fit today’s fire fighters? J. Text. Appar. Technol. Manag. 2015, 9, 1–15. [Google Scholar]
- Boorady, L.M.; Barker, J.; Lin, S.H.; Lee, Y.A.; Cho, E.; Ashdown, S.P. Exploration of firefighter bunker gear part 2: Assessing the needs of the female firefighter. J. Text. Appar. Technol. Manag. 2013, 8, 1–12. [Google Scholar]
- Conley, R. Flammable Firefighter Training Gear. U.S. Patent 11,826,595, 28 November 2023. [Google Scholar]
- Nayak, R.; Houshyar, S.; Padhye, R. Recent trends and future scope in the protection and comfort of fire-fighters’ personal protective clothing. Fire Sci. Rev. 2014, 3, 4. [Google Scholar] [CrossRef]
- Huston, T.N. Identification of Soils on Firefighter Turnout Gear from the Philadelphia Fire Department. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2014. [Google Scholar]
- Dolez, P.I.; Tomer, N.S.; Malajati, Y. A quantitative method to compare the effect of thermal aging on the mechanical performance of fire protective fabrics. J. Appl. Polym. Sci. 2019, 136, 47045. [Google Scholar] [CrossRef]
- Avci, H.; Hassanin, A.; Hamouda, T.; Kılıç, A. High performance fibers: A review on current state of art and future challenges. Eskişehir Osman. Üniversitesi Mühendislik Mimar. Fakültesi Dergisi. 2019, 27, 130–155. [Google Scholar] [CrossRef]
- Papachlimitzou, A.; Barber, J.L.; Losada, S.; Bersuder, P.; Law, R.J. A review of the analysis of novel brominated flame retardants. J. Chroma. A 2012, 1219, 15–28. [Google Scholar] [CrossRef]
- Ali, N.; Eqani, S.A.M.A.S.; Ismail, I.M.I.; Malarvannan, G.; Kadi, M.W.; Albar, H.M.S.; Rehan, M.; Covaci, A. Brominated and organophosphate flame retardants in indoor dust of Jeddah, Kingdom of Saudi Arabia: Implications for human exposure. Sci. Total Environ. 2016, 569, 269–277. [Google Scholar] [CrossRef]
- Grand, A.F.; Wilkie, C.A. Fire Retardancy of Polymeric Materials; Taylor & Francis: Abingdon, UK, 2000; pp. 81–113. [Google Scholar] [CrossRef]
- Olukunle, O.I. Gas Chromatography-Mass Spectrometry Detection, Identification, and Quantification of Polybrominated Diphenyl Ethers, Novel Brominated Flame Retardants and Hexabromocyclododecane in Selected Environmental Samples from Nigeria and South Africa. Doctoral Dissertation, Tshwane University of Technology, Pretoria, South Africa, 2016. [Google Scholar]
- Shaw, S. Halogenated flame retardants: Do the fire safety benefits justify the risks? Rev. Environ. Health 2010, 25, 261–306. [Google Scholar] [CrossRef] [PubMed]
- De Wit, C.A. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Abdallah, M.A.E.; Drage, D.S.; Sharkey, M.; Berresheim, H.; Harrad, S. A rapid method for the determination of brominated flame retardant concentrations in plastics and textiles entering the waste stream. J. Sep. Sci. 2017, 40, 3873–3881. [Google Scholar] [CrossRef] [PubMed]
- Alcock, R.E.; MacGillivray, B.H.; Busby, J.S. Understanding the mismatch between the demands of risk assessment and practice of scientists—The case of Deca-BDE. Environ. Int. 2011, 37, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Horn, G.P.; Kerber, S.; Andrews, J.; Kesler, R.M.; Newman, H.; Stewart, J.W.; Fent, K.W.; Smith, D.L. Impact of repeated exposure and cleaning on protective properties of structural firefighting turnout gear. Fire Technol. 2021, 57, 791–813. [Google Scholar] [CrossRef]
- Nkabinde, S.N.; Okonkwo, J.O.; Olukunle, O.I.; Daso, A.P. Determination of legacy and novel brominated flame retardants in dust from end of life office equipment and furniture from Pretoria, South Africa. Sci. Total Environ. 2018, 622, 275–281. [Google Scholar] [CrossRef]
- Kirk, K.M.; Logan, M.B. Structural fire fighting ensembles: Accumulation and off-gassing of combustion products. J. Occup. Environ. Hyg. 2015, 12, 376–383. [Google Scholar] [CrossRef]
- ISO 5660-1; Reaction-to-Fire Tests, Heat Release, Smoke Production, and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). ISO: Geneva, Switzerland, 2015.
- Mokoana, V.N.; Asante, J.K.; Okonkwo, O.J. A review on volatilization of flame retarding compounds from polymeric textile materials used in firefighter protective garment. J. Fire Sci. 2023, 41, 107–121. [Google Scholar] [CrossRef]
- Tata, J.; Alongi, J.; Carosio, F.; Frache, A. Optimization of the procedure to burn textile fabrics by cone calorimeter: Part I. Combustion behavior of polyester. Fire Mater. 2011, 35, 397–409. [Google Scholar] [CrossRef]
- Santiago, E.C.; Cayetano, M.G. Polycyclic aromatic hydrocarbons in ambient air in the Philippines derived from passive sampler with polyurethane foam disk. Atmos. Environ. 2007, 41, 4138–4147. [Google Scholar] [CrossRef]
- Alexander, B.M.; Baxter, C.S. Flame-retardant contamination of firefighter personal protective clothing–a potential health risk for firefighters. J. Occup. Environ. Hyg. 2016, 13, D148–D155. [Google Scholar] [CrossRef]
- Shin, J.H.; Baek, Y.J. Analysis of polybrominated diphenyl ethers in textiles treated by brominated flame retardants. Text. Res. J. 2012, 82, 1307–1316. [Google Scholar] [CrossRef]
- Ionas, A.C.; Gómez, A.B.; Uchida, N.; Suzuki, G.; Kajiwara, N.; Takata, K.; Takigami, H.; Leonards, P.E.; Covaci, A. Comprehensive characterisation of flame retardants in textile furnishings by ambient high resolution mass spectrometry, gas chromatography-mass spectrometry and environmental forensic microscopy. Environ. Res. 2015, 142, 712–719. [Google Scholar] [CrossRef]
- Kajiwara, N.; Sueoka, M.; Ohiwa, T.; Takigami, H. Determination of flame-retardant hexabromocyclododecane diastereomers in textiles. Chemosphere 2009, 74, 1485–1489. [Google Scholar] [CrossRef]
- Keller, A.S.; Raju, N.P.; Webster, T.F.; Stapleton, H.M. Flame retardant applications in camping tents and potential exposure. Environ. Sci. Technol. Lett. 2014, 1, 152–155. [Google Scholar] [CrossRef]
- Kajiwara, N.; Noma, Y.; Takigami, H. Brominated and organophosphate flame retardants in selected consumer products on the Japanese market in 2008. J. Hazard. Mater. 2011, 192, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Kemmlein, S.; Hahn, O.; Jann, O. Emissions of organophosphate and brominated flame retardants from selected consumer products and building materials. Atmos. Environ. 2003, 37, 5485–5493. [Google Scholar] [CrossRef]
- Stubbings, W.A.; Harrad, S. Extent and mechanisms of brominated flame retardant emissions from waste soft furnishings and fabrics: A critical review. Environ. Int. 2014, 71, 164–175. [Google Scholar] [CrossRef] [PubMed]
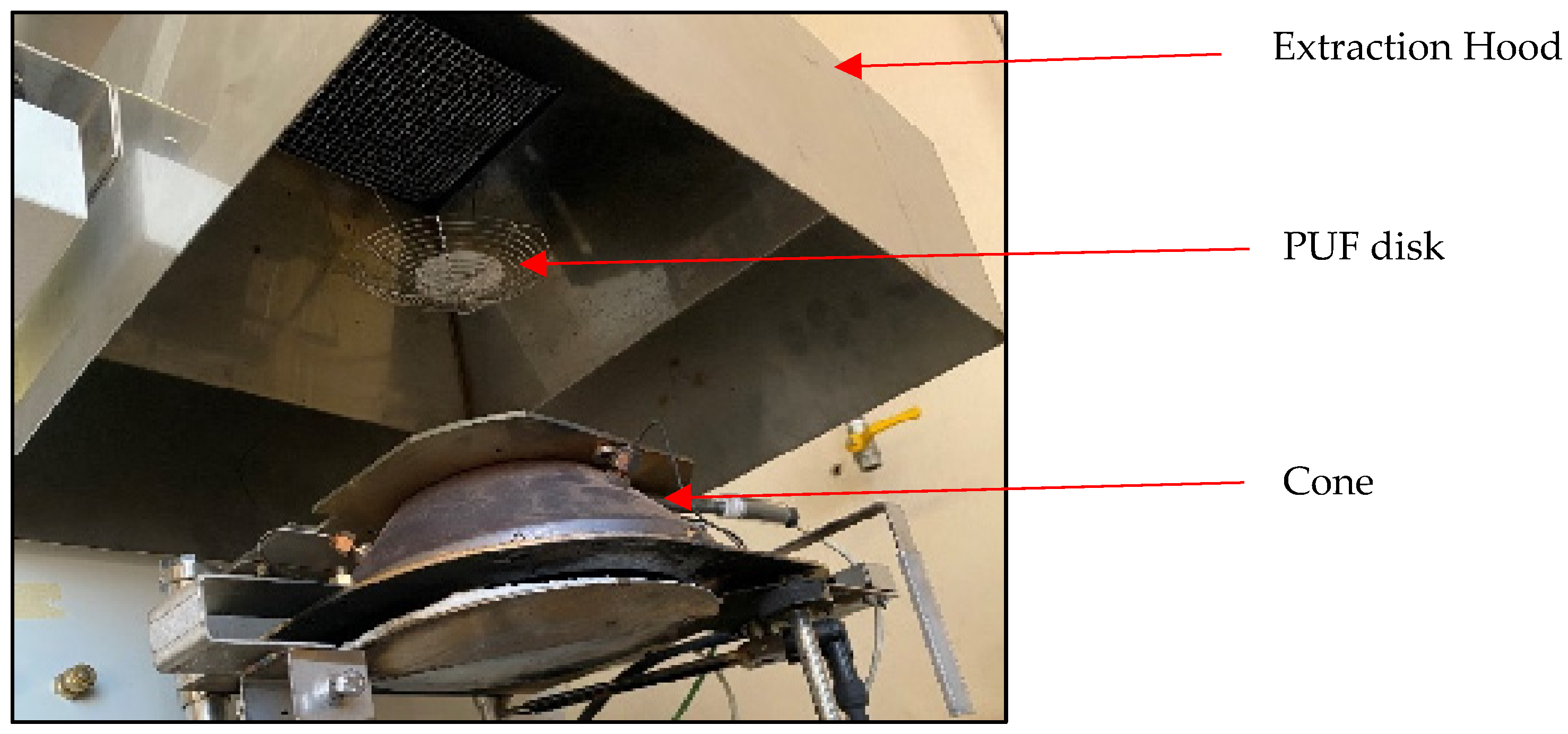
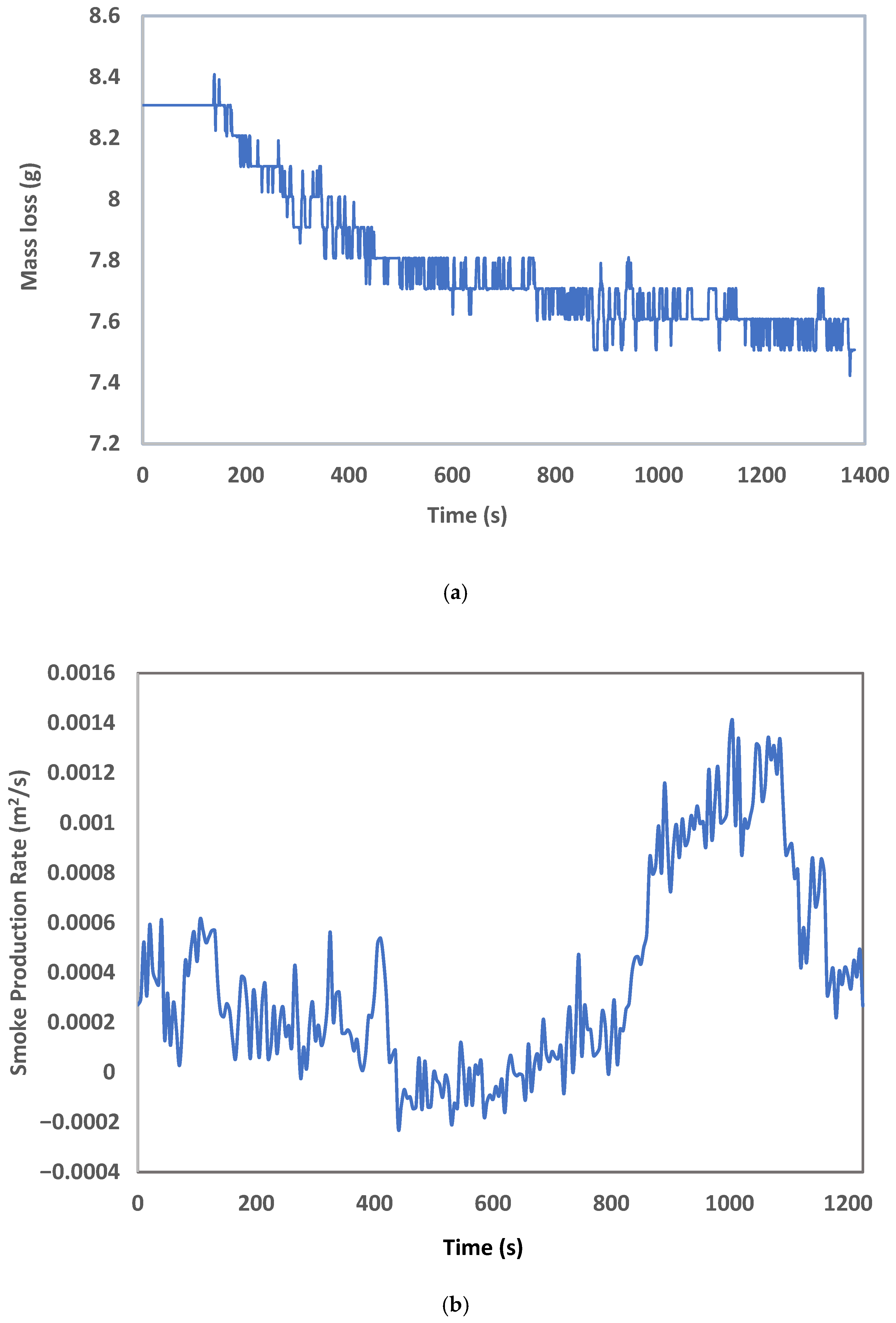
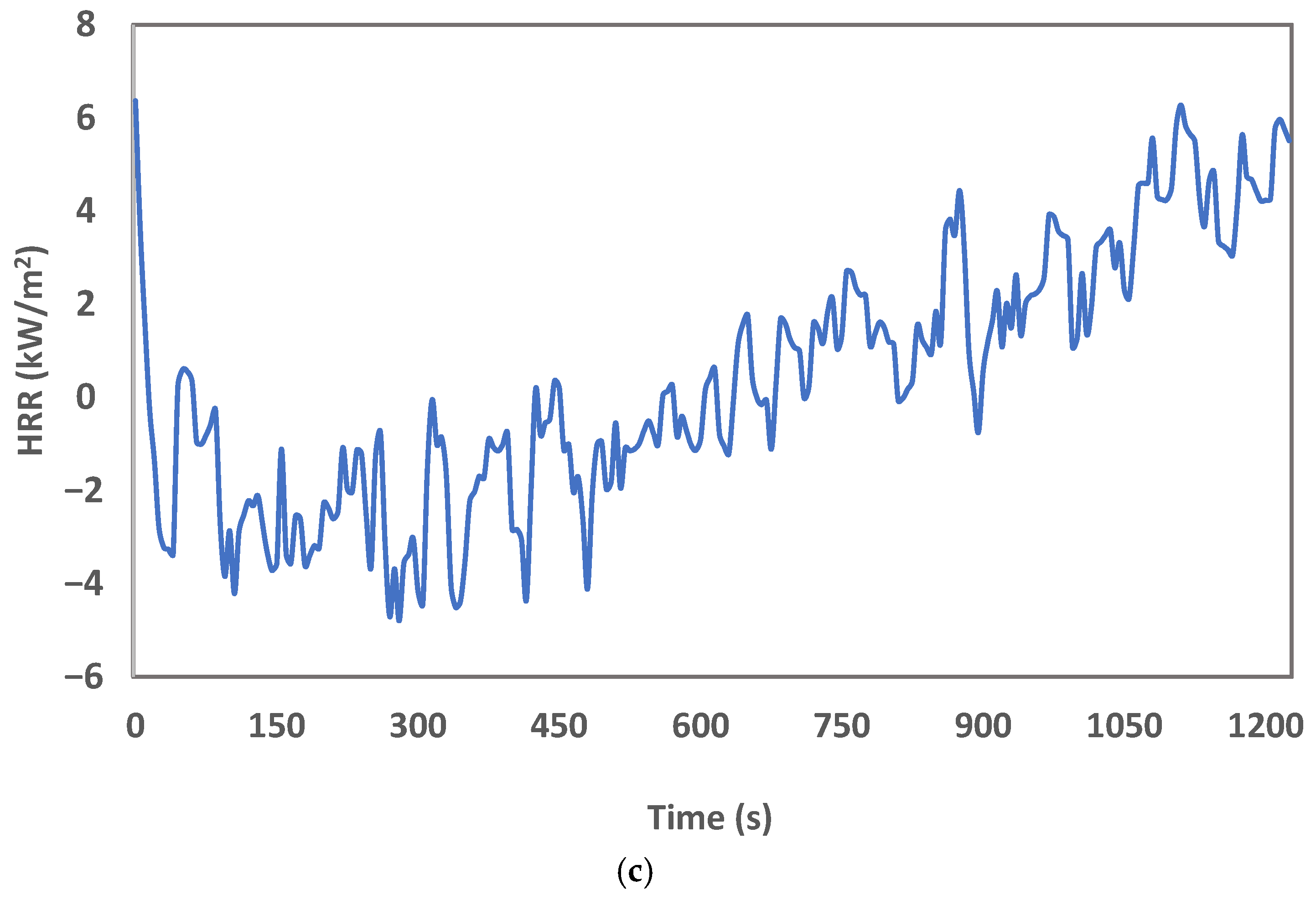
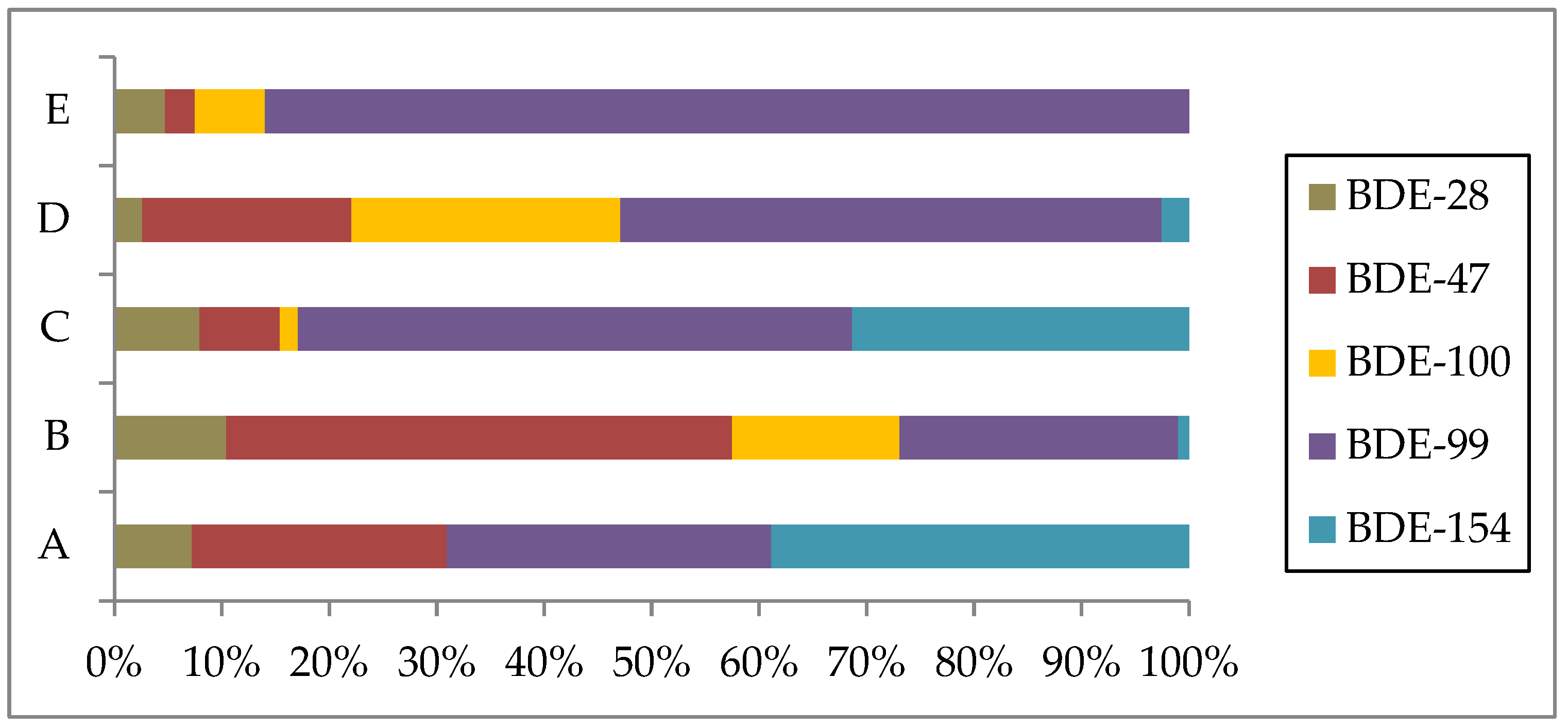
| Name | Trade Name |
|---|---|
| Melamine | Basofil |
| Polybenzimidazole | PBI |
| Para-aramid | Kevlar® |
| Meta-aramid | Normex® |
| Polybenzoxazole (PBO) | Zylon® |
| Congener Abbreviation | UIPAC Name | Number of Bromines | |
|---|---|---|---|
| 1 | BDE-28 | 2,4,4′-Tribromodiphenyl Ether | 3 |
| 2 | BDE-47 | 2,2′,4,4′-Tetrabromodiphenyl Ether | 4 |
| 3 | BDE-77 | 3,3′,4,4′-Tetrabromodiphenyl Ether | 4 |
| 4 | BDE-99 | 2,2′,4,4′,5-Pentabromodiphenyl Ether | 5 |
| 5 | BDE-100 | 2,2′,4,4′,6-Pentabromodiphenyl Ether | 5 |
| 6 | BDE-154 | 2,2′,4,4′,5,6′-Hexabromodiphenyl Ether | 6 |
| 7 | BDE-183 | 2,2′,3,4,4′,5′,6-Heptabromodiphenyl Ether | 7 |
| 8 | BDE-209 | 2,2′,3,3′,4,4′,5,5′,6,6′-Decabromodiphenyl Ether | 10 |
| Sample | Outer Shell | Thermal Liner | Moisture Barrier |
|---|---|---|---|
| A | ADVT240rsi, 39% Nomex/60% Kevlar/1% Antistatic | NVL120+2L NK70N, 7.8 oz/yd2, consisting of a 50% aramid/50% viscose FR face cloth quilted to 2 layers of needle punched 80% Aramid/20% Meta Aramid batting. | Diana 80AQ, 1 layer woven with a membrane (88% Nomex Comfort + Absorbent Monolithic Polyester) |
| B | Kermel® fabric (50% Kermel® + 49% Para-aramid + 1% Antistatic Yarn. | Thermal barrier-Analite NP (Thermal: aramid (needle punched non-woven + Thermal liner: meta-aramid). | Non-woven-Aqua Tech (The Stedair® 4000 consists of a Tri-component moisture barrier constructed using a 3.2 oz/yd2 woven DuPont & trade; Nomex® containing 2% carbon fibres, laminated to a membrane comprised of an expanded PTFE matrix combined to a continuous hydrophilic and oliophoebic polymer layer). |
| C | Advanced fabric (60% Kevlar® + 40% Nomex®). | Thermal barrier-Analite NP (Thermal: aramid (needle punched non-woven + Thermal liner: meta-aramid). | Aqua Tech (The Stedair® 4000 consists of a Tri-component moisture barrier constructed using a 3.2 oz/yd2 woven DuPont & trade; Nomex® containing 2% carbon fibres, laminated to a membrane comprised of an expanded PTFE matrix combined to a continuous hydrophilic and oliophoebic polymer layer). |
| D | Advanced fabric (60% Kevlar® + 40% Nomex®). | Thermal barrier-Q8 (Thermal barrier: aramid (FR Rayon needle punched non-woven, thermal liner: 50% Meta Aramid + 50% FR Modacrylic). | Aqua Tech (The Stedair® 4000 consists of a Tri-component moisture barrier constructed using a 3.2 oz/yd2 woven DuPont & trade; Nomex® containing 2% carbon fibres, laminated to a membrane comprised of an expanded PTFE matrix combined to a continuous hydrophilic and oliophoebic polymer layer). |
| E | KANOX® HM02RP, >50% Para-aramid | KANOX® GORNOX quilt with MAZIC® ST02 KANOX | PTFE/Aramid spunlace |
| Target Compounds | Sample A | Sample B | Sample C | Sample D | Sample E |
|---|---|---|---|---|---|
| BDE 28 | 0.0439 | 0.0750 | 0.0989 | 0.0153 | 0.0476 |
| BDE 47 | 0.144 | 0.338 | 0.0931 | 0.115 | 0.0279 |
| BDE 99 | 0.183 | 0.186 | 0.642 | 0.296 | 0.866 |
| BDE 100 | <LOD | 0.112 | 0.0212 | 0.147 | 0.0657 |
| BDE 154 | 0.236 | 0.00739 | 0.390 | 0.0150 | <LOD |
| BDE 209 | <LOD | <LOD | <LOD | <LOD | <LOD |
| Sample Type | Number of Samples | PBDE Congener | ∑PBDE (ng/g) | Reference |
|---|---|---|---|---|
| Bunker gear (volatiles) | 5 | 28; 47; 99; 100; 154; 209 | 0.59–1.25 | Present study |
| Bunker gear (off-gassing) | 4 | 28; 47; 99; 100; 153; 154; 183 | 550 | [1] |
| Curtains and car interior foam | 8 | 28; 66; 100; 119; 153; 197; 206; 209 | 2436.5–13,876 | [29] |
| Carpets and curtains | 61 | 28; 47; 66; 100; 99; 85; 154; 153; 183; 209 | 0.3–10 | [30] |
| Carpet, PUF and upholstery textiles | 13 | 28; 47; 99; 100; 153; 154; 183; 209 | 0.8–1.5 | [19] |
| Curtains | 10 | N/A | 11–120 × 106 | [31] |
| Camping tents | 11 | 47; 99; 100; 154; 153; 209 | <0.01–7103 | [32] |
| Curtains | 2 | 15; 33/28/16; 47; 99; 100; 153; 154; 175/183; 209 | 7.4–9.1 | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokoana, V.; Asante, J.K.O.; Okonkwo, J. Emission of Brominated Flame-Retarding Compounds from Polymeric Textile Materials Used in Firefighter Protective Garment During Thermal Exposure. Fire 2025, 8, 418. https://doi.org/10.3390/fire8110418
Mokoana V, Asante JKO, Okonkwo J. Emission of Brominated Flame-Retarding Compounds from Polymeric Textile Materials Used in Firefighter Protective Garment During Thermal Exposure. Fire. 2025; 8(11):418. https://doi.org/10.3390/fire8110418
Chicago/Turabian StyleMokoana, Vincent, Joseph K. O. Asante, and Jonathan Okonkwo. 2025. "Emission of Brominated Flame-Retarding Compounds from Polymeric Textile Materials Used in Firefighter Protective Garment During Thermal Exposure" Fire 8, no. 11: 418. https://doi.org/10.3390/fire8110418
APA StyleMokoana, V., Asante, J. K. O., & Okonkwo, J. (2025). Emission of Brominated Flame-Retarding Compounds from Polymeric Textile Materials Used in Firefighter Protective Garment During Thermal Exposure. Fire, 8(11), 418. https://doi.org/10.3390/fire8110418








