Abstract
The intumescent flame retardant (IFR) technique is an alternative to halogen-based flame retardants for reducing fire hazards in polymers. However, IFR has drawbacks like unsatisfactory flame-retardant efficiency and high loading requirements. In this study, MIL-125 (Ti-based metal–organic framework) is added to ABS/IFR composites to improve flame retardancy and reduce smoke emissions. Thermogravimetric analysis (TGA) results indicate that combining ammonium polyphosphate (APP) and expandable graphite (EG) increases charred residue and slows mass loss compared with the original ABS resin. The ABS/IFR/MIL-125 system stabilizes the char layer, serving as a protective shield against combustible gases during combustion. Additionally, MIL-125 enhances performance in microscale combustion calorimetry (MCC) flammability testing. In fire tests (UL-94, limiting oxygen index (LOI), and cone calorimeter), the ABS/IFR/MIL-125 system achieves a UL-94 V0 rating and the highest LOI value of 31.5% ± 0.1%. Peak heat lease rate (PHRR) values in the cone calorimeter are reduced by 72% with 20 wt.% of additives, and smoke production decreases by 53% compared with neat ABS. These results demonstrate the efficient synergistic effects of MIL-125 and IFR additives in improving the formation and stability of the intumescent char layer, thereby protecting ABS from intense burning.
1. Introduction
Acrylonitrile butadiene styrene (ABS) is a widely used thermoplastic polymer noted for its superior mechanical properties, like impact resistance, toughness, and rigidity, compared with many other common polymers. However, a significant limitation of ABS is its inherent flammability [1]. Considerable research has been focused on improving the fire retardancy of ABS, particularly through methods such as halogen–antimony synergism [2,3]. Despite these efforts, the use of such compounds faces restrictions due to environmental regulations, and they also suffer from the disadvantage of relatively low smoke suppression [4,5].
In recent years, the quest to enhance the flame retardancy of various polymers has led to the exploration of inorganic nanoscale fillers [6]. Metal–organic frameworks (MOFs) are an innovative type of flame-retardant filler aimed at significantly improving the ignitability, thermal stability, and flame retardancy of engineering plastics [7]. Extensive literature reviews indicate that the metal compounds derived from MOFs act as catalysts, reducing smoke emissions and aiding the formation of protective char during combustion [8]. Concurrently, the organic components of MOFs—namely the ligands—enhance compatibility with the polymer matrix and introduce ignition-resistant elements or groups, such as phosphorous and nitrogen-containing groups, along with aromatic derivatives. These characteristics make MOFs a promising solution for advancing the fire safety of ABS plastics [9,10].
In the development of ABS/MOFs-based polymer nanocomposites, a limited number of researchers have reported using a solvent-casting method to integrate MOFs into the polymer matrix [11]. However, much of this research has primarily focused on the mechanical properties of the composites, with applications being restricted to areas like gas storage and 3-D printing [11,12]. Given these developments, conducting fire safety tests on ABS/MOFs nanocomposites appears crucial. Such research could yield valuable insights into effective methods for manufacturing these nanocomposites to meet the fire safety requirements of the automotive industry. Specifically, MIL-125 (Ti-based MOF) is a porous, crystalline material made up of metal ions or clusters interconnected by organic ligands (Figure 1). MIL-125 is particularly noted for its high thermal stability, a key attribute for various applications. The exceptional thermal stability of MIL-125 stems from its unique topology and the robust coordination bonds between the metal ions and the organic linkers. Due to these properties, MIL-125 is well-suited for use in a range of high-temperature environments, including catalytic processes, gas storage, and separation, as well as a supportive material for other catalysts [13,14]. In fire safety applications, the combination of metal ions and organic ligands in MIL-125 contributes to synergistic effects in flame retardancy. The metal ions can catalyze the formation of a stable char layer, while the organic ligands may contribute to forming a protective barrier that inhibits heat and mass transfer [10].
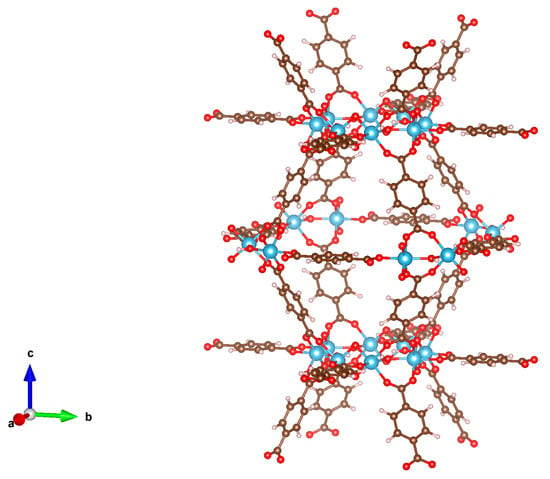
Figure 1.
Ball-and-stick structural model of MIL-125 from VESTA 3 [15]. (Brown ball for carbon atom, pink ball for hydrogen atom, red ball for oxygen atom, and blue ball for titanium atom).
One of the most effective enhancement strategies in flame retardancy involves the use of intumescent flame retardants (IFR), which are particularly prevalent in thermoplastic composites [16]. IFRs function by forming a protective carbon foam during a fire. This foam expands upon exposure to heat (intumescence) and serves as a thermal insulation layer, effectively slowing down the degradation of the polymer through pyrolysis. This type of flame retardant primarily works in the condensed phase, where it either generates its own carbon char or leverages the polymer as a carbon source [17]. Intumescent systems generally consist of three key components that synergistically produce carbon char. The first component, an acid catalyst, initiates the cross-linking of the second component, the carbon source, which then forms a thermally stable char layer. The third component, a spumific or gas-forming agent, transforms the carbon source into carbon foam [18]. The combination of ammonium polyphosphate (APP), pentaerythritol (PER), and melamine is the most commonly used system in IFR formulations [19]. In recent reports, surface modification with coupling agents and microencapsulation are two main methods to overcome the disadvantage of APP due to its inferior water resistance and poor compatibility with polymer matrix [20,21].
In addition, expandable graphite (EG) serves as an alternative to IFR, with the advantage of not requiring an acid catalyst since it acts as its own carbon source. When exposed to fire, EG expands as gas trapped between its layers is released, forming a fragmented, thermally insulating structure with a large surface area. This expansion provides significant thermal protection by blocking heat transfer [22]. According to research by Camino et al., this expansion is driven by a redox reaction between sulfuric acid and graphite [23]. Further studies by Ge et al. and Zhang et al. have noted a synergistic effect on flame retardancy when EG is combined with APP in ABS composites. Phosphorus-containing APP compounds are involved in altering the pathway of the thermal degradation of the substrate by promoting carbonization with some other additives [24,25]. However, it is important to note that high concentrations of these flame retardants can impair the mechanical or thermal properties of the material.
In this study, MIL-125 is proposed as a novel type of flame-retardant filler to further improve the ignitability, thermal stability, and flame retardancy of ABS engineering plastics. Through the synergistic effect of EG in the IFR system and MIL-125, the resulting ABS nanocomposite is tested with different scales of fire testing methods for flame retardancy and smoke suppression. This study will also provide new insights for developing more efficient IFR systems.
2. Materials and Methods
2.1. Materials
ABS pellets and MIL-125 powder were provided by LG Chem., Korea. Ammonium polyphosphate (APP, (NH4PO3)n, n > 1000, AFLAMMIT® PCI 202) was supplied by Thor Group. Acetone (ACS reagent, ≥99.5%) and expandable graphite (EG, flakes) with a practical size of 300 μm and expansion volume of 300 were purchased from Sigma-Aldrich Inc., St. Louis, MO, USA.
2.2. Preparation of ABS/MIL-125 Nanocomposites
Small-scale casting was used to ensure that MIL-125 was dispersed in the nanocomposite and to screen for flame retardancy. Hartings et al. reported a protocol to suspend and solvent-cast ABS and MIL-125 [11]. The process involved mixing 0.1 g of MIL-125 powder with 10 mL of acetone in a flask and then mixing with 0.9 g of ABS pellets. By adjusting the amount of MIL-125 and ABS to obtain the desired MIL-125 content, the mixture was subjected to sonication with a VWR Symphony sonicator until the ABS was dissolved at room temperature and the mixture became viscous and homogeneous. Then, the mixed solution was poured into a Teflon-coated dish and sat overnight under the hood to allow the removal of solvent [26].
2.3. Preparation of ABS/IFR/MIL-125 Composites
The bench-scale extrusion was used for ABS/IFR/MIL-125 scale-up production (Figure 2) [27]. A twin-screw extruder is a highly versatile machine widely used in the plastics and polymer industries for compounding, mixing, and processing a variety of materials, including polymers, additives, and fillers. It consists of two intermeshing, co-rotating screws housed within a barrel. These screws rotate in the same direction, and their design allows for efficient mixing, shearing, and conveying of the materials along the length of the extruder [28]. The Process 11 twin-screw extruder (Thermo Scientific, Waltham, MA, USA) is used in this study, and the extrusion condition (operation temperature ≈ 220 °C) is optimized based on the differential scanning calorimetry (DSC) result (Figure S1).
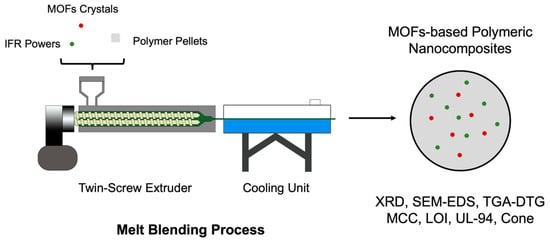
Figure 2.
Schema of melt blending process for ABS/IFR/MIL-125 using twin-screw extruder.
The IFR system used in this case is APP:EG = 1:1 [24], and the detailed recipe is listed in Table 1. The ABS pellets, APP powder, EG flakes, and MIL-125 powder were pre-mixed in a glass beaker. The mixing time is 10 min with a stirring speed of 150 rpm at room temperature. The mixed powder was then fed into the extruder through an auto-feeder. Figure 1 illustrates the process of ABS/IFR/MIL-125 extrusion.

Table 1.
Formulas of ABS/IFR/MIL-125 composites.
2.4. Characterizations
After the production, the X-ray diffraction (XRD) patterns of ABS and its composites were obtained in the scanning range of 5 to 60° using a Miniflex II (Rigaku, Tokyo, Japan) with Cu-Kα radiation (λ = 1.5406 Å). A Q500 thermoanalyzer (TA Instruments, New Castle, DE, USA) was used for the thermogravimetric analysis (TGA). The samples were heated from 30 to 700 °C at a continuous heating rate of 20 °C/min and in a nitrogen atmosphere that was maintained at a constant flow rate of 40.0 mL/min. JSM-7500F (JEOL, Tokyo, Japan) was used to obtain scanning electron microscopy (SEM) images. Using a vacuum sputter coater, the samples were initially coated with platinum and then examined at an acceleration voltage of 7 keV. Electron distribution spectroscopy (EDS) results were obtained using the Oxford EDS system at 20 kV acceleration voltage to observe the distribution of P and Ti elements from APP and MIL-125 in the ABS composites.
2.5. Fire Testing
Pyrolysis-combustion flow calorimetric (PCFC) measurements were performed by a microscale combustion calorimeter (MCC, Fire Testing Technology Ltd., Gosport, UK) to investigate the small-scale preliminary flammability and combustibility of ABS and its composites with IFR or MIL-125 [29]. For each run, the pellet-shaped sample was accurately weighed (ca. 4.00 mg) and then heated to 900 °C with a heating rate of 1 °C/s. The volatile thermal degradation products were then mixed with a stream of nitrogen (80 cm3/min) and oxygen (20 cm3/min) before entering a combustion chamber. The MCC Curve Fit 17 software (Fire Testing Technology, UK) was used to process the obtained data.
The cone calorimeter is a bench-scale flammability testing instrument that is used to evaluate the reaction-to-fire properties of materials under a well-ventilated and forced-combustion condition [30]. Following the standard operating procedures specified in ASTM E1354 [31], cone calorimeter tests were performed using an iCone classic calorimeter (Fire Testing Technology, UK). The dimensions of all samples are 100 mm × 100 mm with a thickness of 3.2 mm. During the test, a spark ignitor was utilized for the ignition of sustained combustion. The irradiance heat flux from the cone heater was 50 kW/m2, representing a severe fire exposure consistent with actual fire tests.
UL-94 (Underwriters Laboratories test standard UL-94) vertical burning test is one of the most commonly used flammability tests for plastics. It measures the ability of a plastic component to extinguish a flame after ignition and its dripping behavior in response to a small open flame or radiant heat source under laboratory-controlled conditions [32]. The UL-94 vertical combustion tests were performed using a Horizontal/Vertical Flame Chamber (Fire Testing Technology, UK) to determine the UL-94 rating following ASTM D3801 [33]. The specimen size is 130 mm × 13.0 mm with a thickness of 3.2 mm.
The limiting oxygen index (LOI) is the minimum percentage of oxygen required in an oxygen/nitrogen mixture to sustain the combustion process [34]. The LOI values were determined using an FTT oxygen index apparatus (Fire Testing Technology, UK) following ASTM D2863 [35]. The specimen size is 125 mm × 6.5 mm with a thickness of 3.2 mm.
3. Results and Discussion
3.1. Preliminary Thermal Stability and Flammability Studies of ABS/MIL-125
3.1.1. Characterizations of ABS/MIL-125
XRD studied the crystalline phase of neat ABS, pure MIL-125 MOFs, and the ABS/MIL-125 nanocomposite. As shown in Figure 3, (101), (200), (211), (222), and (312) characteristic peaks were observed, and the most intense peak was located at 6.79° of 2θ for pure MIL-125 powders (black line). In addition, the amorphous LG ABS sample is shown as the blue line. After the casting of the ABS/MIL-125 nanocomposite, a pronounced peak (101) is observed at around 6.79°, which indicates the ABS and MIL-125 are successfully mixed together (green line).
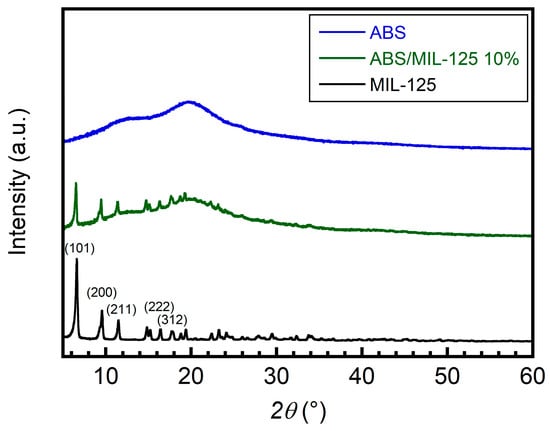
Figure 3.
XRD patterns of ABS, MIL-125, and ABS/MIL-125 nanocomposite.
SEM uses an electron beam to display samples at much higher magnifications than is possible using only light. This instrument can be used to examine the dispersions of MIL-125 and the elemental compositions of composites. It can also be used to identify the morphologies and components of combustion residues, which can assist in determining the flame-retardant mechanisms of MOFs in ABS and provide insight for further optimizations of ABS/MIL-125 nanocomposites. Figure 4 shows the surfaces of neat ABS and ABS/MIL-125 nanocomposite. The SEM micrograph of the neat ABS surface (Figure 4a) shows a smooth surface. In contrast, the SEM micrograph of the nanocomposite surface (Figure 4b) shows a slightly rougher morphology but no pronounced agglomeration of the MIL-125 crystal structure, indicating they are uniformly distributed in the ABS matrix.
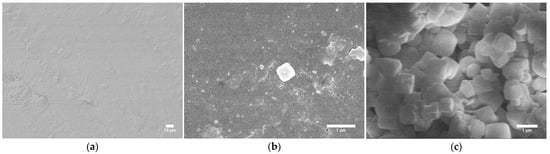
Figure 4.
SEM images of (a) neat ABS surface, (b) ABS/MIL-125 nanocomposites, (c) MIL-125 powder.
3.1.2. Thermal Stability and Flammability Studies of ABS/MIL-125
Then, the thermal stability of neat LG ABS and the ABS/MIL-125 nanocomposite was studied by TGA, and the results (including the first derivative of the TGA (DTG) curves) are shown in Table 2 and Figure 5. The onset decomposition temperature is defined as the temperature at 10 wt% weight loss (T10%). ABS in our study reached its T10% at 418 °C and the maximum thermal degradation (Tmax) at 444 °C. The thermal degradation rate and trend were similar for ABS/MIL-125 nanocomposites, with a slight delay on T10% and Tmax.

Table 2.
TGA results of ABS and different MIL-125 nanocomposites.
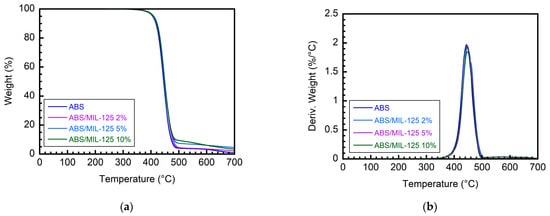
Figure 5.
(a) TGA, (b) DTG curves of ABS/MIL-125 nanocomposites with different weight ratios.
MIL-125 itself has high thermal stability (Figure S2), which helps stabilize the ABS matrix during thermal degradation. This could be the explanation for this insignificant change. When incorporated into ABS, MIL-125 increases the onset temperature of degradation, leading to a delay in the breakdown of the polymer. This higher decomposition temperature is crucial in extending the time before the material begins to degrade significantly under heat exposure.
Finally, MCC was used to investigate the flammability and fire safety properties of the ABS/MIL-125 nanocomposites. And the total heat release (THR), peak heat release (pHRR), heat release capacity (HRC), and time to reach pHRR (TpHRR) are summarized in Table 3 and Figure 6. The pHRR of the neat ABS is 657 W/g, whereas the ABS/MIL-125 nanocomposite with different weight ratios lowers the pHRR value by 20%, 22%, and 24%, respectively. The results indicate that the ABS/MIL-125 nanocomposite has light flame retardancy. The possible mechanism could be protective layer formation. MIL-125 can catalyze the formation of a char layer during the combustion process. This char acts as a physical barrier, protecting the underlying material from further thermal degradation and reducing the release of flammable volatiles. The metal ions in MIL-125, particularly titanium, can enhance cross-linking reactions that lead to the formation of a more stable and cohesive char structure.

Table 3.
MCC results of ABS and different MIL-125 nanocomposites.
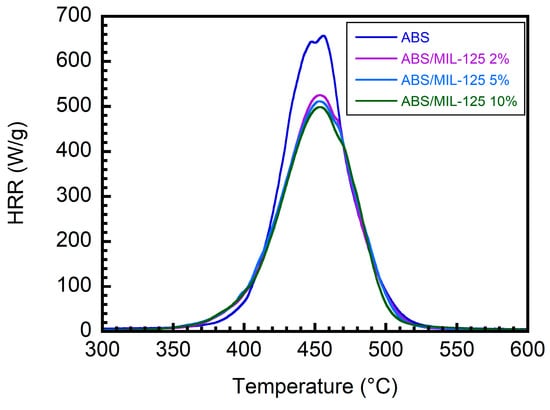
Figure 6.
MCC HRR curves of ABS/MIL-125 nanocomposites with different weight ratios.
From the preliminary results, we can conclude that MIL-125 Ti-MOFs has the potential to be an effective flame retardant additive. However, the application of MIL-125 as a single additive has limited flame retardancy. As we discussed previously, MOFs can form a variety of multicomponent composite systems with other conventional flame retardants and achieve better performance. In this study, the ABS/IFR/MIL-125 system is finally selected. The IFR component is APP:EG = 1:1. All other preliminary tests for a proper IFR system are recorded in Supplementary Materials.
3.2. Flame Retardancy Studies of ABS/IFR/MIL-125
3.2.1. Characterizations of ABS/IFR/MIL-125
After the extrusion, we want to confirm that both the APP, EG, and MIL-125 MOF structures are successfully dispersed in the ABS matrix. The crystalline phases of pure APP, pure EG, pure MIL-125 MOFs, and the ABS/IFR/MIL-125 composite were studied by XRD. As shown in Figure 7, pronounced peaks for APP (around 14.7° and 15.5°) and EG (around 26.5° and 54.7°) were found in both pure APP/EG and ABS composite. This indicates that APP and EG were successfully mixed with ABS. However, the pronounced peaks for MIL-125 were not obvious in the ABS composite. Considering the amount of MIL-125 applied in the ABS matrix is small (2 wt.%), further confirmation is needed for MIL-125.
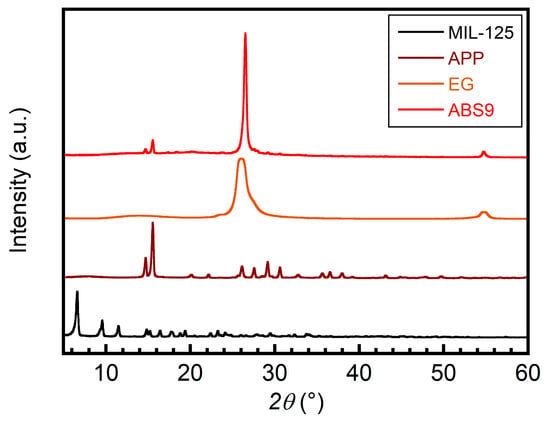
Figure 7.
XRD patterns of APP, EG, MIL-125, and ABS/IFR/MIL-125 composite.
Then SEM was used to identify the morphologies of the composites, which can assist in the determination of the flame-retardant mechanisms of MOFs in ABS and provide insight for further optimizations of ABS/IFR/MIL-125 composites. Figure 8 shows the surfaces of neat ABS and ABS/IFR/MIL-125 composites. The SEM micrograph of the neat ABS surface (Figure 8a) shows a smooth surface, whereas the SEM micrograph of the composite surface (Figure 8b) shows a rougher morphology with a layered structure, indicating EG is successfully mixed in the ABS matrix. However, the APP and MIL-125 structures were not observed for visual verification. Higher-resolution SEM and EDS are needed to verify the existence of APP and MIL-125.
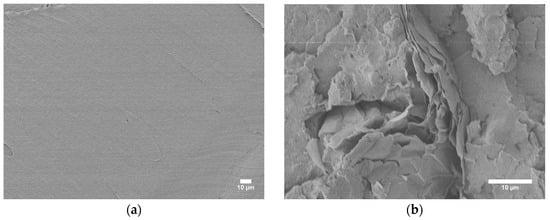
Figure 8.
SEM images of (a) neat ABS surface, (b) ABS/IFR/MIL-125 composites.
EDS is an analytical technique used for the elemental analysis or chemical characterization of a sample. It relies on the interaction of some source of X-ray excitation and a sample. Its characterization capabilities are due in large part to the fundamental principle that each element has a unique atomic structure, allowing a unique set of peaks on its electromagnetic emission spectrum. In our case, the P element from APP and the Ti element from MIL-125 were the unique elements for each component. Figure 9b,c suggests a uniform distribution of P and Ti in the ABS. As a result, we conclude that APP, EG, and MIL-125 were successfully mixed with ABS.
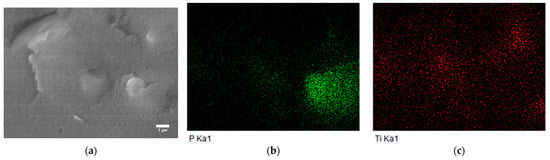
Figure 9.
(a) SEM image of ABS composites and EDS mapping of (b) P (c) Ti elements.
3.2.2. Thermal Stability and Flammability Studies of ABS/IFR/MIL-125
Then, the thermal stability of ABS samples is related to flame-retardant additives. TGA is used to study the thermal degradation properties of pure ABS and its composites, as shown in Table 4. The pure ABS resin decomposes for 5% at around 392 °C and shows only one-step decomposition to 700 °C, leaving negligible char (2.0 wt.%) above 700 °C. The TGA and DTG curves of ABS samples are separately shown in Figure 10, and some important data are tabulated in Table 4. The thermogram of samples containing APP/EG exhibits two main pyrolysis peaks at about 275 and 423 °C, respectively. The first step is an intumescent process of APP/EG appearing in the temperature range of 250 to 320 °C. The second one occurs in the range of 380–480 °C and can be attributed to the thermal degradation of ABS. The char residues of the ABS/IFR/MIL-125 system at 700 °C are about 31 wt.%, which is more than that of pure ABS. The maximum decomposition rates (Deriv. Weight, %/°C) of different samples are listed in Table 4. Compared with the Deriv. Weight of pure ABS, one can conclude that the samples containing APP/EG make Deriv. Weight decrease, thus indicating a slower decomposition rate when flame retardants are added. It should be noted that T5% of ABS samples containing flame-retardant additives are below that of pure ABS. The earlier decomposition of the flame retardants (APP/EG) may explain this point.

Table 4.
TGA results of ABS and different ABS/IFR/MIL-125 composites.
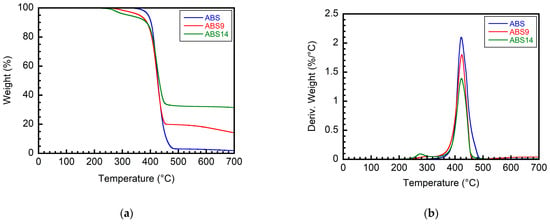
Figure 10.
(a) TGA, (b) DTG curves of ABS/IFR/MIL-125 composites with different weight ratios.
The above results show that the combination of APP and EG can not only increase the charred residue at high temperatures but also slow down the mass loss rate compared with the original ABS resin and stabilize the char layer, which could act as a protective shield against combustible gases during combustion. Thus, the flame-retardant property has been highly enhanced. Generally, a close connection exists between the weight percent of char residue and the LOI value. For example, an increase in the weight percent of char residue always accompanies an improvement in the LOI value. This will be thoroughly discussed in the next section.
Next, MCC was used to investigate the flammability of the ABS/IFR/MIL-125 composites, and the results are summarized in Table 5 and Figure 11. The pHRR of the neat ABS is 657 W/g, whereas the ABS/IFR/MIL-125 composite with different weight ratios lowers the pHRR values. In detail, ABS15 lowers the pHRR to 454 W/g. If fewer IFRs were added to the system (e.g., ABS10), the pHRR deduction would only be 506 W/g. However, the presence of MIL-125 (in ABS14 and ABS9) will improve the performance of flammability testing, no matter how many IFRs are added to the system.

Table 5.
MCC results of ABS and different ABS/IFR/MIL-125 composites.
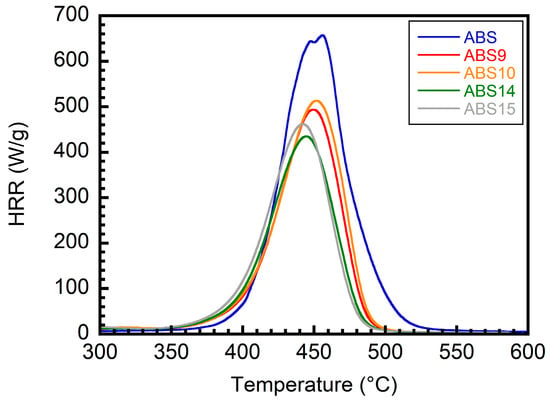
Figure 11.
MCC HRR curves of ABS/IFR/MIL-125 composites with different weight ratios.
3.2.3. Flame Retardancy Studies
To evaluate the flame retardancy of ABS resins, LOI and UL-94 tests were conducted. The data obtained are given in Table 6, which indicates that pure ABS is a flammable polymeric material with an LOI value of only 17.9% ± 0.1%. When mixed APP/EG at a 30 wt.% (1:1) level, the LOI of systems is remarkably enhanced above 31.3% ± 0.1%. When lowering the concentration of IFRs to 20 wt.%, the LOI value is still much higher than the atmosphere (20.5%). And with the presence of MIL-125, the LOI value can be improved from 25.9% ± 0.1% to 27.0% ± 0.1%.

Table 6.
Flame retardancy (LOI, UL-94) of ABS and different ABS/IFR/MIL-125 composites.
In addition, as shown in the UL-94 test in Table 6, pure ABS burns too fast to classify the level. However, all the samples with the EG and APP mixture can pass the UL-94 test, which indicates we can lower the IFR concentration to reach better mechanical properties. It needs to be pointed out that the UL-94 rating is only V1, with 20 wt.% IFRs (APP:EG = 1:1). But if you replace 2 wt.% IFRs with MIL-125, the UL-94 rating can go back to V0 (the best rating). With the synergistic effect of MIL-125, we successfully lowered the total amount of additives added to ABS composites.
Finally, a cone calorimeter was used to investigate the flame retardancy and smoke suppression of the ABS/IFR/MIL-125 composites. Based on the flame retardancy test results (UL-94, MCC, and LOI), ABS14 and ABS9 composites obtained better flame retardancy than their own contrast sample. Furthermore, the fire safety of the ABS14 composite is the highest. However, as mentioned in the introduction part, one of the IFR drawbacks is the high loading requirement. In total, adding 30 wt.% of IFR (APP/EG) is already reported to be effective in ABS [25]. To balance the fire safety performance and high loading requirement of IFR, we selected a good flame retardancy and lower loading volume sample, ABS9, to perform the following fire behaviors under the cone calorimeter.
The cone calorimeter results are summarized in Table 7 and Figure 12. A cone calorimeter directly corresponds to the burning intensity of a fire, and the heat release rate (HRR) data are widely accepted to be the most critical factor in determining the fire size and controlling fire development. As shown in Figure 12a, the peak heat release rate (PHRR) of the neat ABS sample is high at about 1020.41 kW/m2 compared with those of the flame-retardant composites. After this peak value, its HRR decays sharply. When virtually no flaming burning is observed, the total heat release for the cone (THRc) reaches a plateau value of approximately 101.40 MJ/m2. For ABS9, the HRR curve of the sample with a typical insulating intumescent coating is observed. After ignition, the HRR initially rises sharply, but an intumescent protective layer soon forms on the burning ABS’s outer surface. This layer acts as a shield, protecting the substrate from external heat exposure and preventing the transport of flammable volatiles to the flame. The char layer directly safeguards the ABS, thereby slowing down its combustion. Consequently, the sample reaches a PHRR of 288.18 kW/m2. Compared with the neat ABS sample, this intumescent system significantly prolongs the burning time of ABS and effectively reduces the PHRR by 72%.

Table 7.
Cone calorimeter results of ABS and ABS/IFR/MIL-125 composite.
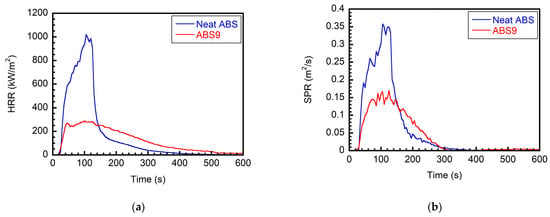
Figure 12.
(a) HRR (b) SPR curves of ABS and ABS/IFR/MIL-125 composites in cone calorimeters.
In addition to the heat release, the accompanying smoke during the burning process may pose significant hazards to exposed individuals via asphyxiation or toxicity. Three different types of parameters related to smoke production can be obtained from a cone calorimeter, including (1) smoke release rate (SPR, in m2/s), which is related to the extinction coefficient and flow rate through the duct; (2) total smoke release (TSR, in m2), which is the integration of SPR with respect to time; and (3) smoke extinction area (SEA, in m2/g), which is defined as the ratio of smoke production to mass loss during the test period [30]. The smoke production results have a similar trend (Figure 12b). The peak smoke release rate (pSPR) values of the ABS/IFR/MIL-125 composites reach the maximum reduction (53%) at 20 wt.% filler weight ratios.
Lastly, two important parameters that provide a comprehensive evaluation of a material’s fire safety are the fire propagation index (FPI) and the fire growth index (FGI). The FPI, which is the ratio of the time to ignition (TTI) to PHRR, indicates the material’s tendency to flashover in a high-temperature environment. On the other hand, the FGI, which is the ratio of the PHRR to the time to PHRR, represents the rate at which the fire spreads. Additionally, the maximum average rate of heat emission (MAHRE) measures the peak value of cumulative heat emission per unit time, serving as an effective indicator of the material’s fire spread tendency [16]. As shown in Table 7, with the IFR and MIL-125 additives, ABS9 has lower FGI, FPI, and MARHE values than the neat ABS. This indicates that it has a lesser tendency for fire spread and flashover.
3.2.4. Carbonaceous Residue Analysis
In the condensed phase, the quality of the char plays a crucial role in determining flame-retarding efficiency. SEM analysis was conducted to examine the morphologies of the char residues and evaluate the impact of the char layer during combustion. Figure 13a,b shows flaws and cracks on the surface of the char from ABS10 without MIL-125, observed at various magnifications. This is believed to be a significant factor in the relatively low flame retardancy of ABS10 among all samples. In addition, the typical worm-like expansive carbon layer is formed by EG. In contrast, the char residue of ABS9, which contained 2 wt.% of MIL-125, displayed a noticeable change, with a more compact, uniform, and smooth structure (Figure 13c,d). The surface also featured some folds that could serve as a support structure and enhance the strength of the surface layer.
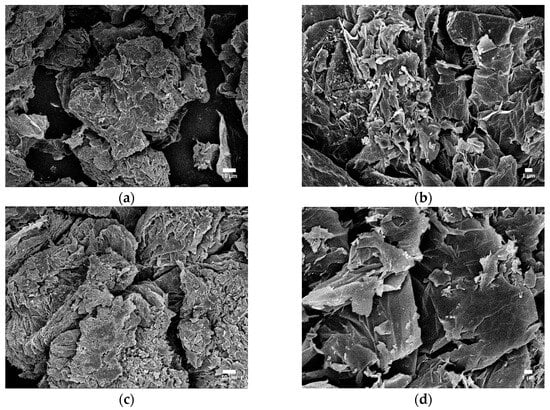
Figure 13.
SEM images of the char residue of ABS10 (a,b) and ABS9 (c,d) after cone calorimeter tests.
A similar phenomenon was observed in the SEM image for ABS15 and ABS14 (Figure 14). Compared with the previous results (low concentration of IFRs), the SEM image with lower magnifications (Figure 14a,c) was similar, with a compact, uniform, and smooth surface. This compact carbon char also explains why ABS15 and ABS14 have better overall flame retardancy than ABS10 and ABS9. Under higher magnifications (Figure 14b,d), we can observe that the carbon char surface of ABS14 with MIL-125 is slightly smoother than ABS15.
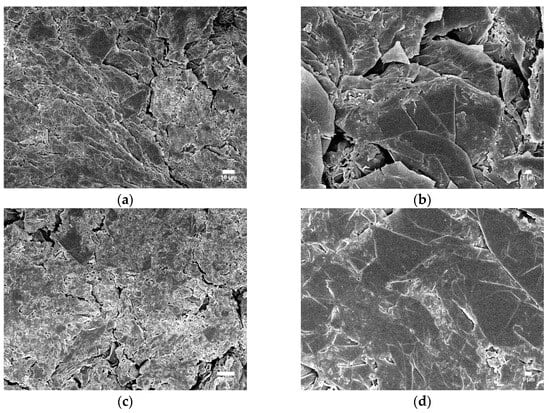
Figure 14.
SEM images of the char residue of ABS15 (a,b) and ABS14 (c,d) after cone calorimeter tests.
3.2.5. Proposed Mechanism
Based on the experimental results mentioned in the previous sections, the possible mechanism of flame retardancy for ABS/APP/EG/MIL-125 can be explained through a synergistic process involving physical and chemical interactions between the different components.
The first step is initial thermal degradation and char formation. Upon exposure to heat, APP begins to decompose, releasing phosphoric acid. This acid acts as a catalyst, promoting the dehydration of the polymer and leading to the formation of a carbonaceous char layer. The phosphoric acid also reacts with the carbon source, EG, to further enhance char formation [20,21]. Simultaneously, EG undergoes thermal expansion due to the release of trapped gases between its layers. This expansion contributes to forming a thick, thermally insulating intumescent layer on the surface of the burning ABS, which significantly reduces heat transfer to the underlying material.
MIL-125, with its titanium-based framework, plays a crucial role in stabilizing the char layer. The metal ions (Ti4+) in MIL-125 can catalyze further cross-linking within the char structure, making it more cohesive and robust. This stable char layer acts as a physical barrier, protecting the underlying ABS from further thermal degradation and reducing the release of flammable volatiles. Furthermore, the presence of MIL-125 enhances the formation of the intumescent layer by interacting with the decomposition products of APP and EG. This interaction leads to a more compact and uniform char layer, which is more effective in insulating the polymer and slowing down the burning process [16]. In addition, MIL-125 has a porous structure that can adsorb volatile organic compounds (VOCs) released during the thermal degradation of ABS. This adsorption reduces the amount of combustible gas available for ignition, thereby lowering smoke production and improving overall fire safety.
Overall, the combined char layer formed by APP, EG, and MIL-125 acts as an excellent thermal insulator. This layer prevents the underlying ABS from reaching its decomposition temperature quickly, thereby reducing the HRR. In addition, the protective char layer, along with the catalytic action of MIL-125, delays the ignition and prolongs the time before PHRR is reached, contributing to the overall flame retardancy of the composite.
4. Conclusions and Future Work
In this study, MIL-125, a common type of MOF, was incorporated into ABS/IFR composites as a booster to further improve their flame retardancy and reduce smoke emissions. The TGA/DTG results show that a combination of APP and EG can not only increase the charred residue at high temperatures but also slow down the mass loss rate compared with the original ABS resin. The ABS/IFR/MIL-125 system also stabilizes the char layer, which could act as a protective shield against combustible gases during combustion. Thus, the flame retardancy of the ABS composites has been highly enhanced. In addition, the presence of MIL-125 will improve the performance of MCC flammability testing. In the fire testing of UL-94, LOI, and cone calorimeter, the ABS/IFR/MIL-125 system shows a great UL-94 test result with a V0 rating, and the highest LOI value is 31.5% ± 0.1%. Lastly, the PHRR values in the cone calorimeter of the ABS/IFR/MIL-125 composites are largely reduced (72%) when applied to 20 wt.% of flame-retardant additives. The smoke production results have a similar trend, with a reduction of 53%. Therefore, these behaviors demonstrate the efficient synergistic effects between MIL-125 and the IFR additives on improving the formation and stability of the intumescent char layer and, hence, protecting ABS from intensive burning.
In the future, mechanical properties (e.g., tensile strength and elongation at break) tests will be another important part of the ABS composite [36,37]. We will investigate methods to improve the mechanical properties of the ABS/IFR/MIL-125 composites, such as adding reinforcing agents or using surface treatments on MIL-125 particles to improve their dispersion within the polymer matrix. In addition, we also want to improve the incompatibility issues between ABS and IFRs to balance mechanical properties, flame retardancy, and smoke suppression. This could involve experimenting with surface modifications of APP and assessing their impact on overall composite performance [21].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fire7080284/s1, [38,39]. Figure S1: DSC results of ABS pellets and Tg at around 220 °C; Figure S2: TGA result of MIL-125 powder; and Figure S3: UL-94 test result for the IFR (APP + LIG)/MIL-125 system. (a) put the burner under the specimen; (b) maintain the position for 10 s and withdraw the burner; (c) burn for about 40 s; Figure S4: The residue of the (a) ABS/IFR (APP + LIG) and (b) ABS/IFR (APP + LIG)/MIL-125; Figure S5: The residue of the ABS/IFR (APP + EG)/MIL-125; Figure S6: UL-94 test result for the IFR (APP + EG)/MIL-125 system. (a) put the burner under the specimen; (b) maintain the position for 10 s; (c) withdraw the burner.
Author Contributions
Conceptualization, Q.W.; Methodology, Z.Z.; Validation, Z.Z., Y.Q. and R.S.; Formal analysis, Z.Z., Y.Q., R.S. and K.-Y.W.; Writing—original draft preparation, Z.Z.; Writing—review and editing, Z.Z. and Q.W.; Supervision, H.-C.Z. and Q.W.; Project administration, Q.W.; Funding acquisition, Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the LG Chem Global Innovation Contest, GIC 2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Material, further inquiries can be directed to the corresponding author.
Acknowledgments
LG Chem: Korea provided the neat polymer ABS and nanofiller MIL-125. Parts of the characteristic experiments were conducted with help from Rong Ma from the Department of Chemical Engineering and Mingzhen Zhao from the Department of Materials Science & Engineering at Texas A&M University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Owen, S.R.; Harper, J.F. Mechanical, Microscopical and Fire Retardant Studies of ABS Polymers. Polym. Degrad. Stab. 1999, 64, 449–455. [Google Scholar] [CrossRef]
- Wilkie, C.A.; Morgan, A.B. Fire Retardancy of Polymeric Materials; CRC Press: Boca Raton, FL, USA, 2009; ISBN 1420084003. [Google Scholar]
- Rybiński, P.; Janowska, G. Influence Synergetic Effect of Halloysite Nanotubes and Halogen-Free Flame-Retardants on Properties Nitrile Rubber Composites. Thermochim. Acta 2013, 557, 24–30. [Google Scholar] [CrossRef]
- Vest, N.A.; Afonso, A.O.; Rodriguez-Melendez, D.; Ponis, J.; Smith, D.L.; Iverson, E.T.; Zhang, Z.; Marquez, J.A.D.; Banerjee, S.; Wang, Q.; et al. Polyelectrolyte Complex for Flame Retardant Silk. Polym. Degrad. Stab. 2023, 216, 110491. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Q.; Nie, Y.; Zhang, J.; Yang, L. A Review of Combustion and Flame Spread over Thermoplastic Materials: Research Advances and Prospects. Fire 2023, 6, 125. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, H.; Zhu, Z.; Zhu, X.; Quan, Y.; Zhang, Z.; Wu, H.-M.; Wu, J.-L.; Kang, W.-H.; Wang, Q.; et al. Multifunctional Polyethylene Nanocomposites Based on Polyethylene-Grafted α-Zirconium Phosphate Nanoplatelets. Polymer 2022, 261, 125422. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Metal-Organic Frameworks for Flame Retardant Polymers Application: A Critical Review. Compos. Part. A Appl. Sci. Manuf. 2020, 139, 106113. [Google Scholar] [CrossRef]
- Quan, Y.; Shen, R.; Schweizer, C.; Parajuli, P.; Zhang, Z.; Kulatilaka, W.; Wang, Q. Synergistic Effects of Zeolitic Imidazolate Frameworks (ZIFs) with Different Transition Metals on Intumescent Flame-Retarded Polypropylene Composites: A Comparative Study. J. Mater. Sci. Technol. 2023, 155, 102–110. [Google Scholar] [CrossRef]
- Pan, Y.-T.; Zhang, Z.; Yang, R. The Rise of MOFs and Their Derivatives for Flame Retardant Polymeric Materials: A Critical Review. Compos. B Eng. 2020, 199, 108265. [Google Scholar] [CrossRef]
- Hou, Y.; Xu, Z.; Chu, F.; Gui, Z.; Song, L.; Hu, Y.; Hu, W. A Review on Metal-Organic Hybrids as Flame Retardants for Enhancing Fire Safety of Polymer Composites. Compos. B Eng. 2021, 221, 109014. [Google Scholar] [CrossRef]
- Kreider, M.C.; Sefa, M.; Fedchak, J.A.; Scherschligt, J.; Bible, M.; Natarajan, B.; Klimov, N.N.; Miller, A.E.; Ahmed, Z.; Hartings, M.R. Toward 3D Printed Hydrogen Storage Materials Made with ABS-MOF Composites. Polym. Adv. Technol. 2018, 29, 867–873. [Google Scholar] [CrossRef]
- Bible, M.; Sefa, M.; Fedchak, J.A.; Scherschligt, J.; Natarajan, B.; Ahmed, Z.; Hartings, M.R. 3D-Printed Acrylonitrile Butadiene Styrene-Metal Organic Framework Composite Materials and Their Gas Storage Properties. 3D Print. Addit. Manuf. 2018, 5, 63–72. [Google Scholar] [CrossRef]
- Kim, S.-N.; Kim, J.; Kim, H.-Y.; Cho, H.-Y.; Ahn, W.-S. Adsorption/Catalytic Properties of MIL-125 and NH2-MIL-125. Catal. Today 2013, 204, 85–93. [Google Scholar] [CrossRef]
- Yue, K.; Zhang, X.; Jiang, S.; Chen, J.; Yang, Y.; Bi, F.; Wang, Y. Recent Advances in Strategies to Modify MIL-125 (Ti) and Its Environmental Applications. J. Mol. Liq. 2021, 335, 116108. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Shen, R.; Quan, Y.; Zhang, Z.; Ma, R.; Wang, Q. Metal–Organic Framework as an Efficient Synergist for Intumescent Flame Retardants against Highly Flammable Polypropylene. Ind. Eng. Chem. Res. 2022, 61, 7292–7302. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. An Overview of Flame Retardancy of Polymeric Materials: Application, Technology, and Future Directions. Fire Mater. 2013, 37, 259–279. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, S.; Wang, X.; Han, Y.; Li, J.; Jian, X. The Analysis of Synergistic Effects of Zeolites Applied in Intumescent Halogen-Free Flame-Retardant ABS Composites. Polym. Plast. Technol. Eng. 2008, 47, 613–618. [Google Scholar] [CrossRef]
- Lim, K.-S.; Bee, S.-T.; Sin, L.T.; Tee, T.-T.; Ratnam, C.T.; Hui, D.; Rahmat, A.R. A Review of Application of Ammonium Polyphosphate as Intumescent Flame Retardant in Thermoplastic Composites. Compos. B Eng. 2016, 84, 155–174. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, B.; Liu, Y.; Cheng, B.; Wang, J.; Yan, J.; Huang, F. Novel Bio-Based Nanosheets: Improving the Fire Safety, Electromagnetic Shielding and Mechanical Properties of Polylactic Acid. Compos. Part. A Appl. Sci. Manuf. 2024, 179, 108044. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, B.; Shang, S.; Zhang, H.; Shi, Y.; Yu, B.; Qi, C.; Dong, H.; Chen, X.; Yang, X. Surface Modification of Ammonium Polyphosphate by Supramolecular Assembly for Enhancing Fire Safety Properties of Polypropylene. Compos. B Eng. 2020, 181, 107588. [Google Scholar] [CrossRef]
- Duquesne, S.; Delobel, R.; Le Bras, M.; Camino, G. A Comparative Study of the Mechanism of Action of Ammonium Polyphosphate and Expandable Graphite in Polyurethane. Polym. Degrad. Stab. 2002, 77, 333–344. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A.; Simioni, F.; Camino, G. Expandable Graphite as an Intumescent Flame Retardant in Polyisocyanurate–Polyurethane Foams. Polym. Degrad. Stab. 2002, 77, 195–202. [Google Scholar] [CrossRef]
- Ge, L.-L.; Duan, H.-J.; Zhang, X.-G.; Chen, C.; Tang, J.-H.; Li, Z.-M. Synergistic Effect of Ammonium Polyphosphate and Expandable Graphite on Flame-Retardant Properties of Acrylonitrile-Butadiene-Styrene. J. Appl. Polym. Sci. 2012, 126, 1337–1343. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Fang, Z. Synergistic Effects of Expandable Graphite and Ammonium Polyphosphate with a New Carbon Source Derived from Biomass in Flame Retardant ABS. J. Appl. Polym. Sci. 2013, 128, 2424–2432. [Google Scholar] [CrossRef]
- Sun, R.; Elabd, Y.A. Synthesis and High Alkaline Chemical Stability of Polyionic Liquids with Methylpyrrolidinium, Methylpiperidinium, Methylazepanium, Methylazocanium, and Methylazonanium Cations. ACS Macro Lett. 2019, 8, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Shen, R.; Ma, R.; Zhang, Z.; Wang, Q. Sustainable and Efficient Manufacturing of Metal-Organic Framework-Based Polymer Nanocomposites by Reactive Extrusion. ACS Sustain. Chem. Eng. 2022, 10, 7216–7222. [Google Scholar] [CrossRef]
- Quan, Y.; Tanchak, R.N.; Zhang, Z.; Wang, Q. Efficient and Sustainable Synthesis of ZIF-67 for Synergistically Improving Reaction-to-Fire Properties of Biomass-Based Polypropylene Composites. J. Therm. Anal. Calorim. 2024, 149, 2585–2592. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, H.; Quan, Y.; Ma, R.; Pentzer, E.B.; Green, M.J.; Wang, Q. Thermal Stability and Flammability Studies of MXene–Organic Hybrid Polystyrene Nanocomposites. Polymers 2022, 14, 1213. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Zhang, Z.; Tanchak, R.N.; Wang, Q. A Review on Cone Calorimeter for Assessment of Flame-Retarded Polymer Composites. J. Therm. Anal. Calorim. 2022, 147, 10209–10234. [Google Scholar] [CrossRef]
- ASTM E1354-23; Standard Test Method for Heat and Visible Smoke Release Rates for Materials and Products Using an Oxygen Consumption Calorimeter. American Society for Testing and Materials: West Conshohocken, PA, USA, 2023.
- Rodriguez-Melendez, D.; Vest, N.A.; Kolibaba, T.J.; Quan, Y.; Zhang, Z.; Iverson, E.T.; Wang, Q.; Grunlan, J.C. Boron-Based Polyelectrolyte Complex Nanocoating for Fire Protection of Engineered Wood. Cellulose 2024, 31, 3083–3094. [Google Scholar] [CrossRef]
- ASTM D3801–20a; Standard Test Method for Measuring the Comparative Burning Characteristics of Solid Plastics in a Vertical Position. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- Shen, R.; Fan, T.; Quan, Y.; Ma, R.; Zhang, Z.; Li, Y.; Wang, Q. Thermal Stability and Flammability of Cotton Fabric with TiO2 Coatings Based on Biomineralization. Mater. Chem. Phys. 2022, 282, 125986. [Google Scholar] [CrossRef]
- ASTM D2863-19; Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-Like Combustion of Plastics (Oxygen Index). American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- Sun, Y.; Liu, Y.; Zou, Y.; Wang, J.; Bai, F.; Yan, J.; Huang, F. Poorly-/Well-Dispersed Fe3O4: Abnormal Influence on Electromagnetic Wave Absorption Behavior of High-Mechanical Performance Polyurea. Chem. Eng. J. 2024, 493, 152833. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, B.; Liu, Y.; Yan, J.; Xu, Z.; Cheng, B.; Huang, F.; Wang, J. Bio-Inspired Surface Manipulation of Halloysite Nanotubes for High-Performance Flame Retardant Polylactic Acid Nanocomposites. Nano Res. 2024, 17, 1595–1606. [Google Scholar] [CrossRef]
- Andrade, P.H.M.; Dhainaut, J.; Volkringer, C.; Loiseau, T.; Moncomble, A.; Hureau, M.; Moissette, A. Stability of Iodine Species Trapped in Titanium-Based MOFs: MIL-125 and MIL-125_NH2. Small 2024. [Google Scholar] [CrossRef]
- Réti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability Properties of Intumescent PLA Including Starch and Lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).