Fire as a Factor in the Dynamics of Meadow Vegetation: A Model Experiment in Western Siberia
Abstract
1. Introduction
2. Materials and Methods
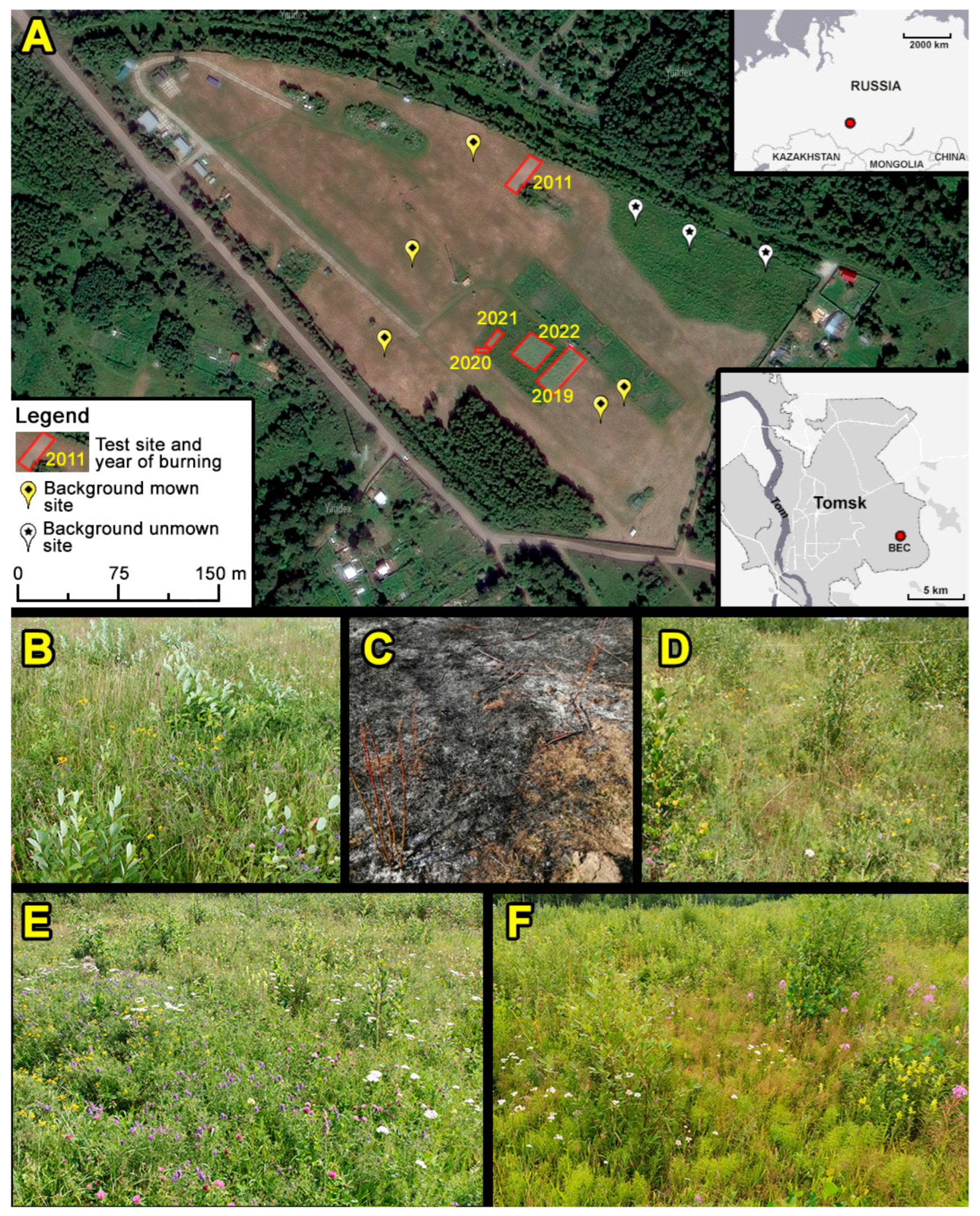
2.1. Study Site
2.2. Sampling and Experimental Design
2.3. Data Processing
3. Results
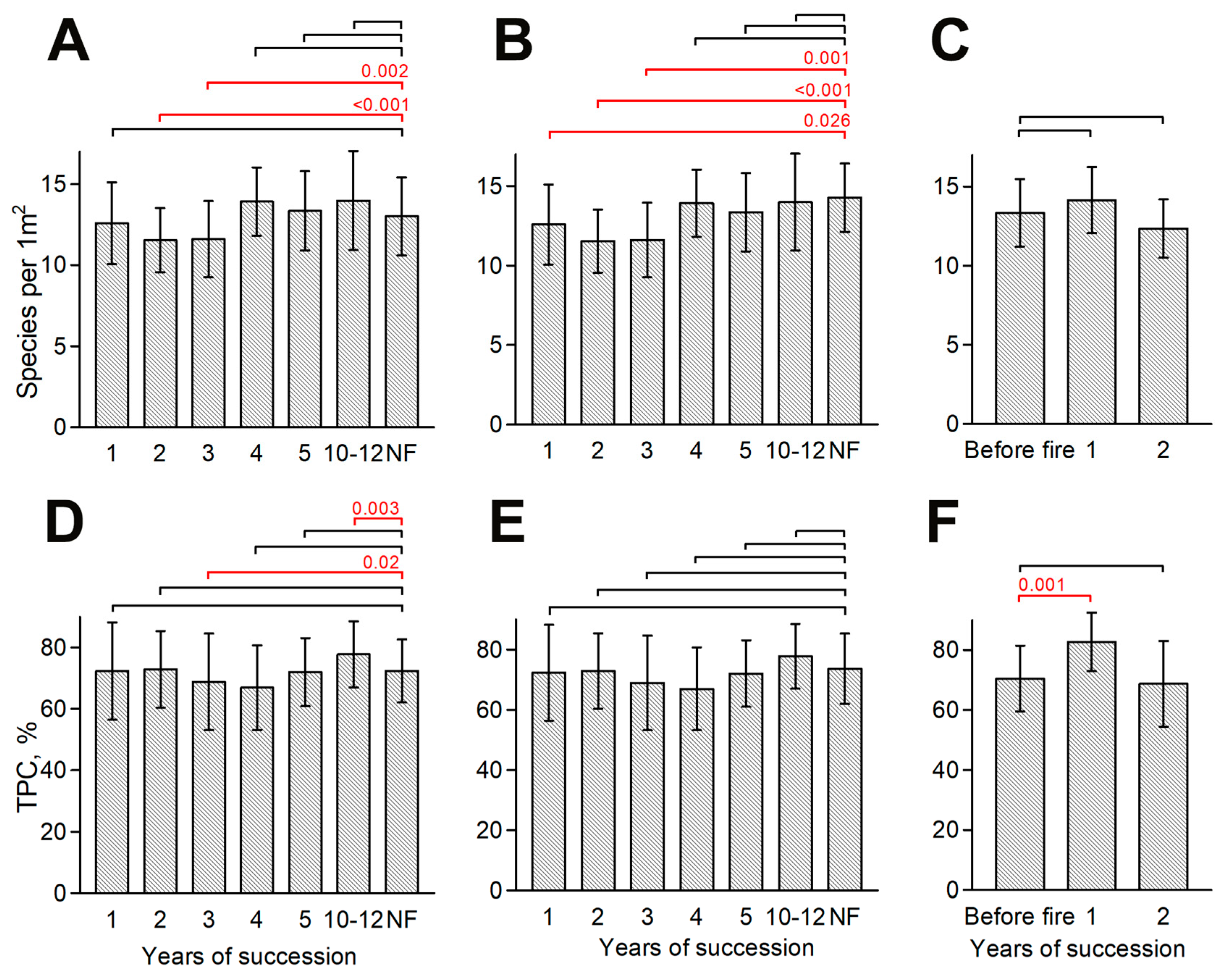
3.1. Dynamics of Species Richness and TPC
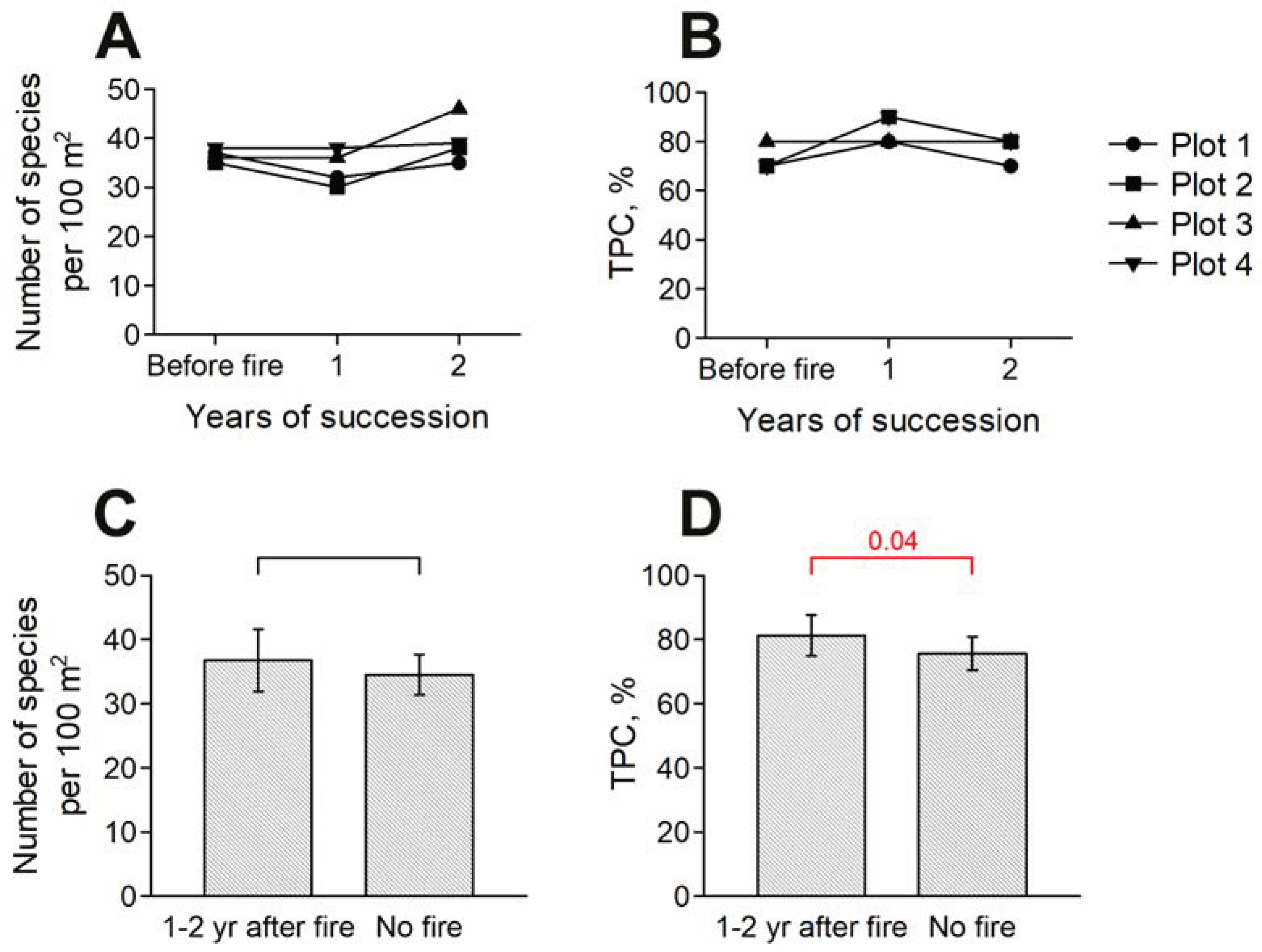
3.2. Impact of Fires on Reforestation
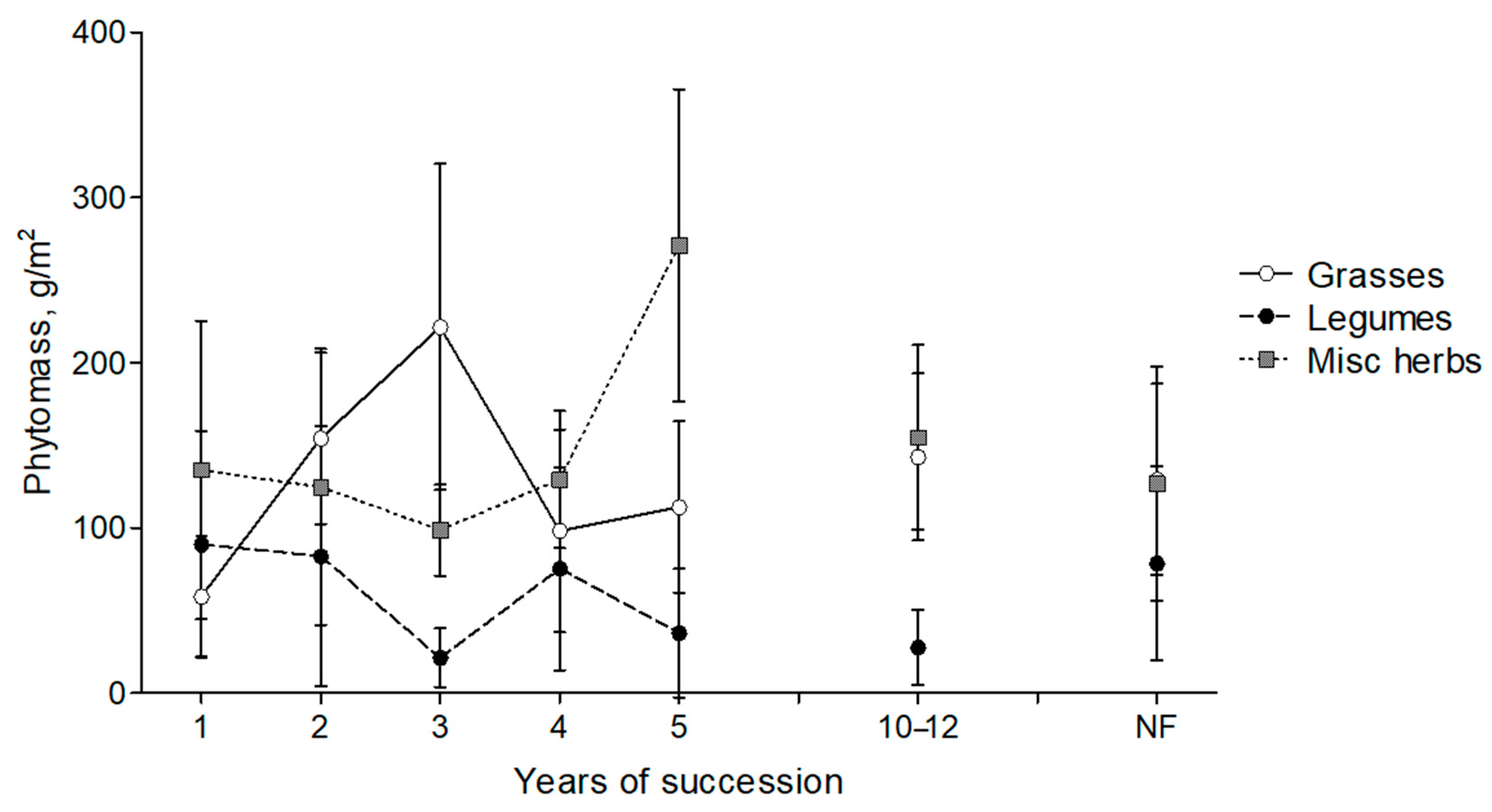
3.3. Dynamics of Aboveground Phytomass
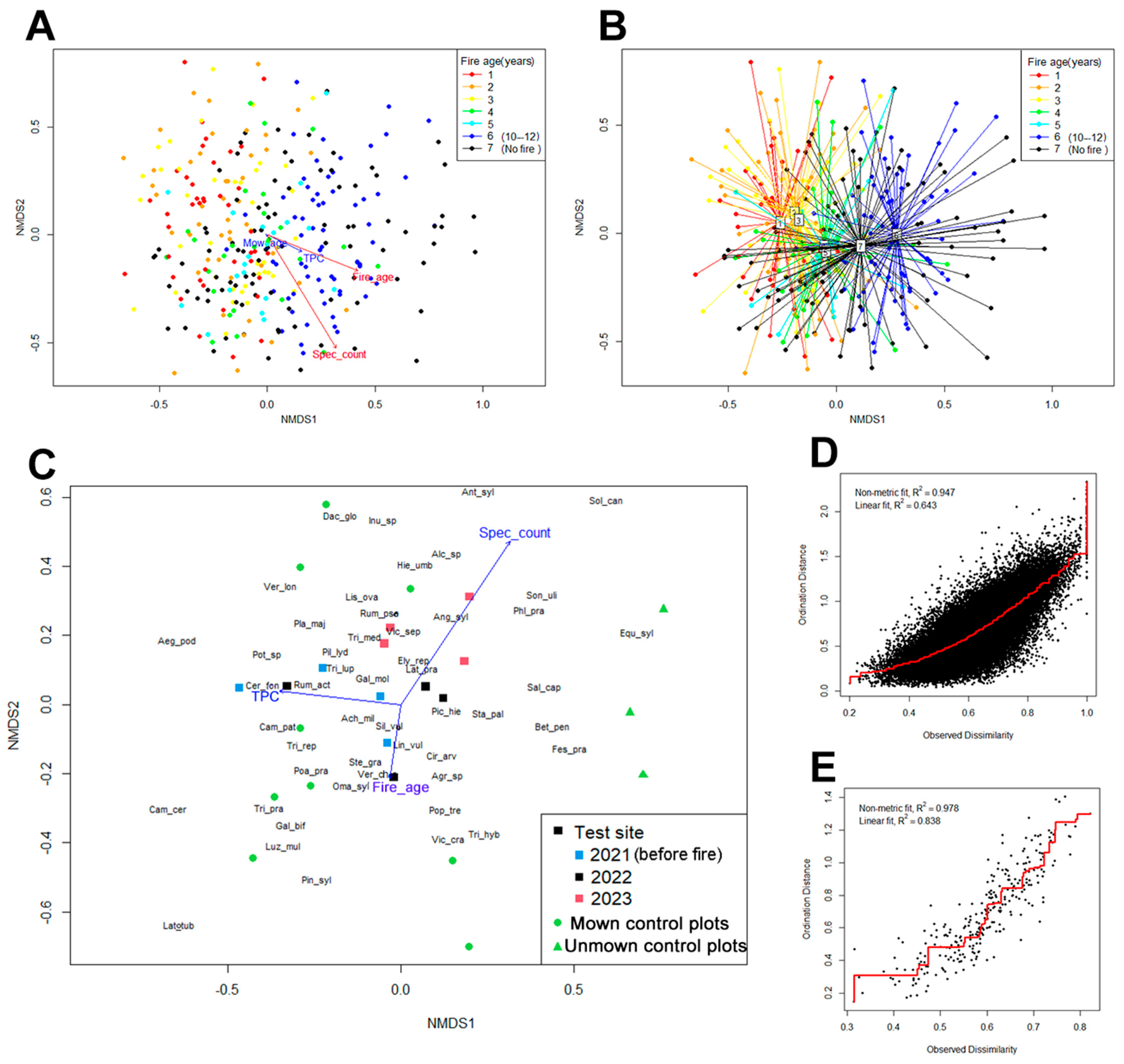
3.4. Non-Metrical Multidimensional Scaling (NMDS)
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rundel, P.W. Fire as an ecological factor. In Physiological Plant Ecology 1; Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 501–538. [Google Scholar] [CrossRef]
- Stavi, I. Wildfires in grasslands and shrublands: A review of impacts on vegetation, soil, hydrology, and geomorphology. Water 2019, 11, 1042. [Google Scholar] [CrossRef]
- Tishkov, A.A. The fires in steppes and savannas. Steppe Sci. 2003, 4, 9–22. (In Russian) [Google Scholar]
- Bond, W.J.; Woodward, F.I.; Midgley, G.F. The global distribution of ecosystems in a world without fire. New Phytol. 2005, 165, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Scheiter, S.; Higgins, S.I.; Osborne, C.P.; Bradshaw, C.; Lunt, D.; Ripley, B.S.; Taylor, L.L.; Beerling, D.J. Fire and fire-adapted vegetation promoted C4 expansion in the late Miocene. New Phytol. 2012, 195, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Ilyina, V.N. Pyrogenic impact on vegetation cover. Samar. Luka Probl. Reg. Glob. Ecol. 2011, 20, 4–30. (In Russian) [Google Scholar]
- Molchanov, V.I. Impact of Pyrogenic Factor on Peculiarities of Structure and Yielding of Meadow Communities in SW Transbaikal. Ph.D. Thesis, Irkutsk State Transport University, Ulan-Ude, Russia, 2012. (In Russian). [Google Scholar]
- Arkle, R.S.; Pilliod, D.S. Prescribed fires as ecological surrogates for wildfires: A stream and riparian perspective. For. Ecol. Manag. 2010, 259, 893–903. [Google Scholar] [CrossRef]
- Harper, A.R.; Doerr, S.H.; Santin, C.; Froyd, C.A.; Sinnadurai, P. Prescribed fire and its impacts on ecosystem services in the UK. Sci. Total Environ. 2018, 624, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Ruchinskaya, E.V. Structural and Species Diversity of Vegetation of Steppe Meadows in Nemoral Forests Zone (on the Example of the Natural Monument “Melovitsky Slopes”, Bryansk Region). Ph.D. Thesis, Moscow Pedagogical State University, Moscow, Russia, 2019. (In Russian). [Google Scholar]
- Fynn, R.W.S.; Morris, C.D.; Edwards, T.J. Effect of burning and mowing on grass and forb diversity in a long- term grassland experiment. Appl. Veg. Sci. 2004, 7, 1–10. [Google Scholar] [CrossRef]
- Ruprecht, E.; Lukács, K.; Domokos, P.; Kuhn, T.; Fenesi, A. Hydration status influences seed fire tolerance in temperate European herbaceous species. Plant Biol. 2016, 18, 295–300. [Google Scholar] [CrossRef]
- Neary, G.D.; Leonard, M.J. Effects of fire on grassland soils and water: A review. In Grasses and Grassland Aspects; Kindomihou, V.M., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Fidelis, A.; Cartay, M.; Blanco, C.; Müller, S.; Pillar, V.; Pfadenhauer, J. Fire intensity and severity in Brazilian Campos grasslands. Interciencia 2010, 35, 739–745. [Google Scholar]
- Dusaeva, G.K.; Kalmykova, O.G. Influence of fires on vegetable cover of steppes: Literature review. Bull. Mosc. Soc. Naturalists. Biol. Ser. 2021, 126, 26–38. (In Russian) [Google Scholar]
- Lipsett-Moore, G.J.; Wolff, N.H.; Game, E.T. Emissions mitigation opportunities for savanna countries from early dry season fire management. Nat. Commun. 2018, 9, 2247. [Google Scholar] [CrossRef] [PubMed]
- Laris, P.; Koné, M.; Dembélé, F.; Yang, L.; Jacobs, R.; Laris, Q. Methane gas emissions from savanna fires: What analysis of local burning regimes in a working West African landscape tell us. Biogeosciences 2021, 18, 6229–6244. [Google Scholar] [CrossRef]
- Grishin, A.M.; Filkov, A.I.; Loboda, E.L.; Reyno, V.V.; Kozlov, A.V.; Kuznetsov, V.T.; Kasymov, D.P.; Andreyuk, S.M.; Ivanov, A.I.; Stolyarchuk, N.D. A field experiment on grass fire effects on wooden constructions and peat layer ignition. Int. J. Wildl. Fire 2014, 23, 445–449. [Google Scholar] [CrossRef]
- Loboda, E.; Kasymov, D.; Agafontsev, M.; Reyno, V.; Gordeev, Y.; Tarakanova, V.; Martynov, P.; Loboda, Y.; Orlov, K.; Sav-in, K.; et al. Effect of Small-Scale Wildfires on the Air Parameters near the Burning Centers. Atmosphere 2021, 12, 75. [Google Scholar] [CrossRef]
- Andreev, Y.A.; Bryukhanov, A.V. Prevention, Monitoring and Control of Wildfires (on the Example of the Altai-Sayan Ecoregion); ‘Gorod’: Krasnoyarsk, Russia, 2011; 271p. (In Russian) [Google Scholar]
- Borodulina, V.P.; Komarova, A.F.; Cherednichenko, O.V. Calamagrostis epigeios meadows in the buffer zone of the Polistovsky Nature Reserve (Pskov region). Divers. Plant World 2019, 1, 44–61. (In Russian) [Google Scholar] [CrossRef]
- Ukrainskiy, P.A. Dynamics of the spectral properties of overgrown burned grass areas. Curr. Probl. Remote Sens. Earth Space 2013, 10, 229–238. (In Russian) [Google Scholar]
- Grishin, A.M.; Filkov, A.I.; Loboda, E.L.; Reyno, V.V.; Kuznetsov, V.T. Full-scale experiments on influence of the grassland fire on wooden constructions and peat layers. Pozharnaya Bezop./Fire Saf. 2013, 3, 52–57. (In Russian) [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Kuznetsov, K.A. Soils of Tomsk region (preliminary reports). Vopr. Geogr. Sib. 1949, 2, 69–86. (In Russian) [Google Scholar]
- Semenkov, I.N.; Lednev, S.A.; Klink, G.V.; Kasymov, D.P.; Agafontsev, M.V.; Kostrova, S.N.; Koroleva, T.V. Impact of spring fires on the properties of the humus layer of chernozem (SE of Western Siberia). Eurasian Soil Sci. 2024, 57, 493–501. [Google Scholar] [CrossRef]
- Loiko, S.V.; Kritskov, I.V.; Kulikova, O.R.; Istigechev, G.I. Influence of a relief and peasant nature management on chromaticity of the humic horizons in a foothill subtaiga of the southeast of Western Siberia. In Proceedings of the Reflection Bio-Geo-Antroposferal Interactions in Soils and Soil Cover Collection of Materials V International Scientific Conference, Dedicated to the 85th Anniversary of the Opening of the First University Department of Soil Science in Siberia, Tomsk State University, Tomsk, Russia, 7–11 September 2015. (In Russian). [Google Scholar]
- Fridland, V.M. Soil Map of the RSFSR. Scale 1:2,500,000; Glavnoe Upravlenie Geodezii i Kartografii: Moscow, Russian, 1988. (In Russian)
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.3-5. Available online: http://CRAN.R-project.org/package=vegan (accessed on 29 January 2024).
- World Flora Online Database. Available online: https://www.worldfloraonline.org (accessed on 29 January 2024).
- Page, H.; Goldammer, J.G. Fire History of Central Europe: Implications for Prescribed Burning in Landscape Management and Nature Conservation. Paper presented at the Baltic Exercise for Fire Information and Resources Exchange (BALTEX FIRE 2000), Kuopio, Finland, June 2000. Available online: https://gfmc.online/programmes/natcon/BAL-PAP3-2.PDF (accessed on 28 February 2024).
- Titlyanova, A.A.; Sambuu, A.D. Successions in Grasslands; Publishing House SB RAS: Novosibirsk, Russia, 2016; 191p. (In Russian) [Google Scholar]
- Pereira, P.; Francos, M.; Ubeda, X.; Brevik, E. Fire impacts in European Grassland ecosystems. In Wildfires. Perspectives, Issues and Challenges of the 21st Century; Nova Science Publishers, Inc.: New York, NY, USA, 2017. [Google Scholar]
- Pereira, P.; Cerdà, A.; Jordán, A.; Zavala, L.; Mataix-Solera, J.; Arcenegui, V.; Misiune, I.; Keesstra, S.; Novara, A. Vegetation recovery after a grassland fire in Lithuania. The effects of fire severity, slope position and aspect. Land Degrad. Dev. 2015, 27, 1523–1534. [Google Scholar] [CrossRef]
- Balázs, D.; Valkó, O.; Török, P.; Végvári, Z.; Hartel, T.; Schmotzer, A.; Kapocsi, I.; Tóthmérész, B. Grassland fires in Hungary–Experiences of nature conservationists on the effects of fire on biodiversity. Appl. Ecol. Environ. Res. 2014, 12, 267–283. [Google Scholar] [CrossRef]
- Rossetti, I.; Cogoni, D.; Calderisi, G.; Fenu, G. Short-Term Effects and Vegetation Response after a Megafire in a Mediterranean Area. Land 2022, 11, 2328. [Google Scholar] [CrossRef]
- Bonanomi, G.; Idbella, M.; Abd-ElGawad, A.M.; Motti, R.; Ippolito, F.; Santorufo, L.; Adamo, P.; Agrelli, D.; De Marco, A.; Maisto, G.; et al. Impact of prescribed burning, mowing and abandonment on a Mediterranean grassland: A 5-year multi-kingdom comparison. Sci. Total Environ. 2022, 834, 155442. [Google Scholar] [CrossRef] [PubMed]
- Klink, G.V.; Lednev, S.A.; Semenkov, I.N.; Konyushkova, M.V.; Karpachevskiy, A.M.; Chemidov, M.M.; Ulanova, S.S.; Fedorova, N.L.; Sharapova, A.V.; Bogun, S.A.; et al. Influence of Fires on Desert Plant Communities at the Chernye Zemli (SW Russia). Fire 2024, 7, 96. [Google Scholar] [CrossRef]
- Ivanov, V.V. Steppes of Western Kazakhstan in Connection with the Dynamics of Their Cover; Akademiya Nauk USSR: Moskva, Russia; Saint Petersburg, Russia, 1958. (In Russian) [Google Scholar]
- Keeley, J.E.; Rundel, P.W. Fire and the Miocene expansion of C4 grasslands. Ecol. Lett. 2005, 8, 683–690. [Google Scholar] [CrossRef]
- Hansson, M.; Fogelfors, H. Management of a semi-natural grassland; results from a 15-year-old experiment in southern Sweden. J. Veg. Sci. 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Wahlman, H.; Milberg, P. Management of semi-natural grassland vegetation: Evaluation of a long-term experiment in southern Sweden. Ann. Bot. Fenn. 2002, 39, 159–166. [Google Scholar]
- Antonsen, H.; Olsson, P.A. Relative importance of burning, mowing and species translocation in the restoration of a former boreal hayfield: Responses of plant diversity and the microbial community. J. Appl. Ecol. 2005, 42, 337–347. [Google Scholar] [CrossRef]
- Halpern, C.B.; Haugo, R.D.; Antos, J.A.; Kaas, S.S.; Kilanowski, A.L. Grassland restoration with and without fire: Evidence from a tree-removal experiment. Ecol. Appl. 2012, 22, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.K.; Ushimaru, A. Traditional burning and mowing practices support high grassland plant diversity by providing intermediate levels of vegetation height and soil pH. Appl. Veg. Sci. 2016, 19, 567–577. [Google Scholar] [CrossRef]
- Zonneveld, I. Vicinism and mass effect. J. Veg. Sci. 2009, 6, 441–444. [Google Scholar] [CrossRef]
- Zvereva, G.K.; Lomova, T.G. The evaluation of vegetation condition in the meadows of the Ob river forest-steppe in the context of its economic use. Bull. Altai State Agric. Univ. 2019, 11, 75–83. (In Russian) [Google Scholar]
- Shepeleva, L.F.; Kolesnichenko, L.G.; Pudova, M.S. Dynamics of the aboveground phytomass of the Ob floodplain mead-ows in the area of the Tomsk carbon polygon (Kaibasovo). Environ. Dyn. Glob. Clim. Chang. 2022, 13, 104–119. [Google Scholar]
- Dubynina, S.S. The productivity of the phytomass of meadow plant communities of the Nazarovskaya Basin under different conditions of use. Adv. Curr. Nat. Sci. 2018, 10, 102–107. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Heinl, M.; Sliva, J.; Tacheba, B. Vegetation changes after single fire-events in the Okavango Delta wetland, Botswana. S. Afr. J. Bot. 2004, 70, 695–704. [Google Scholar] [CrossRef]
- Chytrý, M.; Otýpková, Z. Plot sizes used for phytosociological sampling of European vegetation. J. Veg. Sci. 2003, 14, 563–570. [Google Scholar] [CrossRef]
- Greenlee, J.M.; Langenheim, J.H. Historic Fire Regimes and Their Relation to Vegetation Patterns in the Monterey Bay Area of California. Am. Midl. Nat. 1990, 124, 239–253. [Google Scholar] [CrossRef]
- Gordijn, P.J.; O’Connor, T.G. Multidecadal effects of fire in a grassland biodiversity hotspot: Does pyrodiversity enhance plant diversity? Ecol. Appl. 2021, 31, e02391. [Google Scholar] [CrossRef]
- Cespedes, B.; Luna, B.; Perez, B.; Urbieta, I.R.; Moreno, J.M. Burning season effects on the short-term post-fire vegetation dynamics of a Mediterranean heathland. Appl. Veg. Sci. 2014, 17, 86–96. [Google Scholar] [CrossRef]
- Norton, D.A.; De Lange, P.J. Fire and Vegetation in a Temperate Peat Bog: Implications for the Management of Threatened Species. Conserv. Biol. 2003, 17, 138–148. [Google Scholar] [CrossRef]
- Jules, E.S.; Ellison, A.M.; Gotelli, N.J.; Lillie, S.; Meindl, G.A.; Sanders, N.J.; Young, A.N. The Influence of fire on a rare serpentine plant assemblage: A 5-year study of Darlingtonia fens. Am. J. Bot. 2011, 98, 801–811. [Google Scholar] [CrossRef] [PubMed]


| Botanical Characteristics and Vascular Plant Species | Type of Community | |||||
|---|---|---|---|---|---|---|
| Mowed, n = 9 | Non-Mowed, n = 3 | |||||
| Med | Min | Max | Med | Min | Max | |
| Main characteristics | ||||||
| Number of species per 100 m2 | 33 | 29 | 37 | 37 | 33 | 39 |
| Total projective cover, % | 80 | 70 | 80 | 80 | 70 | 80 |
| Species projective cover, % | ||||||
| Achillea millefolium L. | 1 | r | 5 | + | + | 1 |
| Agrostis sp. | 5 | 1 | 10 | 10 | 10 | 20 |
| Alchemilla filicaulis Buser | + | + | + | - | ||
| Angelica sylvestris L. | + | r | 1 | + | r | + |
| Anthriscus sylvestris (L.) Hoffm. | + | + | + | r | r | r |
| Artemisia vulgaris L. | - | r | r | r | ||
| Betula pendula Roth | + | + | 1 | 5 | 1 | 10 |
| Bromus inermis Leyss. | 1 | + | 3 | - | ||
| Campanula cervicaria L. | + | r | + | - | ||
| Campanula patula L. | + | r | + | - | ||
| Cirsium arvense (L.) Scop. | + | + | 1 | 1 | + | 1 |
| Dactylis glomerata L. | 5 | + | 40 | 1 | 1 | 5 |
| Deschampsia cespitosa (L.) P. Beauv. | - | + | + | + | ||
| Dianthus deltoides L. | + | r | + | - | ||
| Elymus repens (L.) Gould | + | + | 1 | + | + | + |
| Epilobium angustifolium L. | - | 3 | 1 | 10 | ||
| Equisetum sylvaticum L. | 1 | + | 5 | 10 | 5 | 20 |
| Euphrasia sp. | - | r | r | r | ||
| Lolium pratense (Huds.) Darbysh. | + | + | 1 | 1 | + | 10 |
| Fragaria vesca L. | - | r | r | r | ||
| Galega orientalis Lam. | r | r | r | - | ||
| Galeopsis bifida Boenn. | r | r | r | - | ||
| Galium mollugo L. | 5 | 1 | 10 | 1 | + | 3 |
| Geum sp. | + | + | + | - | ||
| Hieracium umbellatum L. | + | + | + | + | + | + |
| Hypericum perforatum L. | + | + | 10 | + | + | + |
| Lathyrus tuberosus L. | + | + | + | - | ||
| Lathyrus pratensis L. | 2 | + | 3 | 5 | + | 5 |
| Leucanthemum ircutianum DC | + | + | 3 | 1 | 1 | 1 |
| Linaria vulgaris Mill. | + | r | 1 | + | + | + |
| Lupinus polyphyllus Lindl. | - | 1 | + | 1 | ||
| Luzula multiflora (Ehrh.) Lej. | + | + | + | - | ||
| Myosotis arvensis Hill | + | r | + | + | + | + |
| Omalotheca sylvatica (L.) Sch.Bip & F.W.Schultz | + | + | + | r | r | r |
| Phleum pratense L. | 1 | + | 5 | 5 | 5 | 10 |
| Picris hieracioides Sm. | 1 | r | 5 | 3 | 1 | 5 |
| Pilosella novosibirskensis Tupitz. | 1 | + | 5 | 1 | 1 | 1 |
| Pinus sylvestris L. | + | r | + | - | ||
| Plantago major L. | + | + | + | r | r | r |
| Platanthera bifolia (L.) Rich. | r | r | + | - | ||
| Poa pratensis L. | 3 | + | 10 | + | + | + |
| Populus tremula L. | 1 | + | 1 | 1 | 1 | 1 |
| Potentilla sp. | + | + | + | - | ||
| Prunella vulgaris L. | + | r | + | - | ||
| Ranunculus acris L. | + | + | 1 | + | + | + |
| Rhinanthus serotinus (Schönh.) Oborny | - | 1 | + | 1 | ||
| Rosa acicularis Lindl. | - | + | + | + | ||
| Rumex acetosa L. | + | r | + | - | ||
| Rumex acetosella L. | r | r | r | - | ||
| Rumex pseudonatronatus (Borbás) Murb. | + | r | + | - | ||
| Salix caprea L. | 3 | + | 5 | 20 | 10 | 30 |
| Silene vulgaris (Moech) Garcke | + | + | + | + | + | + |
| Solidago canadensis L. | - | + | + | + | ||
| Sonchus arvensis subsp. uliginosus (M.Bieb.) Nyman | - | + | + | + | ||
| Stachys palustris L. | + | r | + | + | + | + |
| Stellaria graminea L. | 3 | + | 5 | + | + | + |
| Taraxacum officinale F.H.Wigg. | 5 | 1 | 30 | 5 | 1 | 10 |
| Trifolium hybridum L. | 1 | + | 5 | + | + | + |
| Trifolium lupinaster L. | r | r | r | - | ||
| Trifolium medium L. | + | + | + | 1 | + | 1 |
| Trifolium pratense L. | 3 | 1 | 40 | + | + | 5 |
| Trifolium repens L. | 1 | + | 3 | + | + | + |
| Urtica dioica L. | + | r | + | + | + | + |
| Valeriana officinalis L. | + | + | + | - | ||
| Veronica chamaedrys L. | 1 | + | 5 | + | + | 1 |
| Veronica longifolia L. | + | r | + | - | ||
| Vicia cracca L. | 5 | + | 20 | 3 | 1 | 5 |
| Vicia sepium L. | + | + | + | + | + | + |
| Viola sp. | + | + | + | - | ||
| Site | Y | A | T | Time, s | Geobotanical Researches (N of Replicas) | M | ||
|---|---|---|---|---|---|---|---|---|
| 2021 | 2022 | 2023 | ||||||
| 1 | 2011 | 300 | 600–750 | No data | a (25) | a (20), c (9) | a (20), c (5) | + |
| 2 | 2019 | 500 | 300–550 | 50–100 | a (25) | a (20), c (9) | a (20), c (5) | - |
| 3 | 2020 | 30 | 550–750 | 50–100 | a (10) | - | a (6), c (3) | - |
| 4 | 2021 | 65 | 500–700 | 150–400 | a (21) | a (15), c (9) | a (15), c (5) | - |
| 5 | 2022 | 400 | 300–600 | 100–200 | a (20), b (4) | a (20), b (4), c (9) | a (20), b (4), c (5) | - |
| 6 (C) | - | - | - | - | a (21), b (3) | a (15), b (3), c (9) | a (30) *, b (6) *, c (6) | + or - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lednev, S.; Kasymov, D.; Semenkov, I.; Klink, G.; Agafontsev, M.; Koroleva, T. Fire as a Factor in the Dynamics of Meadow Vegetation: A Model Experiment in Western Siberia. Fire 2024, 7, 115. https://doi.org/10.3390/fire7040115
Lednev S, Kasymov D, Semenkov I, Klink G, Agafontsev M, Koroleva T. Fire as a Factor in the Dynamics of Meadow Vegetation: A Model Experiment in Western Siberia. Fire. 2024; 7(4):115. https://doi.org/10.3390/fire7040115
Chicago/Turabian StyleLednev, Sergey, Denis Kasymov, Ivan Semenkov, Galya Klink, Mikhail Agafontsev, and Tatyana Koroleva. 2024. "Fire as a Factor in the Dynamics of Meadow Vegetation: A Model Experiment in Western Siberia" Fire 7, no. 4: 115. https://doi.org/10.3390/fire7040115
APA StyleLednev, S., Kasymov, D., Semenkov, I., Klink, G., Agafontsev, M., & Koroleva, T. (2024). Fire as a Factor in the Dynamics of Meadow Vegetation: A Model Experiment in Western Siberia. Fire, 7(4), 115. https://doi.org/10.3390/fire7040115









