Abstract
The state of meadow vegetation in areas with different ages of experimental spring burnout (from 1 to 12 years ago) was studied in the Tomsk region for 3 years. On experimental plots of 1 m2 and 100 m2 (small- and middle-scale levels, respectively), the dynamics of species richness, total projective cover of vegetation, and aboveground phytomass reserves were characterized, and the structure of communities was analyzed. It was revealed that a single fire in the spring significantly reduces species richness for small-scale plots and increases the total projective cover for middle-scale plots. Structural differences from control plots can be traced from 1 to 4 years for different characteristics. The effects of fire are more prominent for small-scale plots. To suppress tree growth and maintain the existence of meadows, grass fires seem to be a less effective practice than mowing. At the same time, the results obtained potentially allow us to consider prescribed burning as a tool for maintaining the stability of meadow plant communities in the south of Western Siberia, preventing them from becoming overgrown with tree undergrowth, in cases with a controlled frequency of burning and the use of appropriate fire safety measures.
1. Introduction
Wildfires and anthropogenic fires are important factors that influence the dynamics of ecosystems, leading to various consequences for them. Stand-replacing forest fires lead to the replacement of forest by communities with a predominance of herbaceous plants and trigger the processes of self-restoration. At the same time, many plant communities are known to somehow depend on the fire regime [1]. In particular, it is generally accepted that treeless communities (steppes, savannas, grasslands, etc.) are largely adapted to periodic fires [2] that have played an important role in the evolution of these communities for at least the last 6–8 million years [3,4,5].
The consequences of a fire in meadows are quickly masked due to the active growth of perennial meadow grasses [2,6]. As a result, according to many environmental managers and a number of scientists, periodic fires can improve the economic condition of pastures [6,7]. Many countries use so-called “prescribed burning” as an agricultural method [8,9]. At the same time, attention is drawn to the fact that grass fires can lead to a decrease in phytodiversity [6], especially with an increase in the frequency of fires [10]. However, the consequences of a fire can vary greatly depending on many factors. For example, in a more than 50-year experiment in South Africa, in grasslands after burning that were not mowed, the number of herbaceous plant species increased by 22%. In those that were mowed twice a year, it decreased by 37% [11]. The month of a fire can also impact significantly on the subsequent dynamics of yielding capacity, community structure, and species composition [7]. The probability of damage to the underground parts of plants and the seed bank of the soil due to exposure to high temperatures during a fire largely depends on the soil moisture at the time of the fire [12,13]. At the same time, the influence of fire intensity or the temperatures reached in the flames on the subsequent state and dynamics of communities has been poorly studied [13,14]. As a result, using the example of steppe fires for almost all characteristics changing in communities after burning, one can find conflicting conclusions about the impact of fires, given in different studies [15].
Field model experiments could somewhat clarify the influence of various fire characteristics on the further dynamics of meadow ecosystems. However, most existing models and experiments were designed for either domestic fires or forest fires. The issue of ecological modeling of the role of fire in grass communities has received greater attention recently, with the emergence of evidence that fires in African savannas are “responsible” for more than half of burning-related global carbon dioxide emissions and play a critical role in the global methane cycle [16,17].
It is known that the temperature of grass fires is significantly lower than that observed during crown fires. Typically, the temperature of grass fires is estimated in the range from 450 to 650 but, in some cases, can be up to 930 °C [14,18,19]. For crown fires that destroy a tree layer, estimates are given at 900–1200 °C, and in some cases up to 1500 °C [20]. At the same time, unlike forest communities, grassland communities can be exposed to fire much more often. For example, in the protective zone of the Polistovsky Nature Reserve (Pskov region; NW Russia), meadow phytocenoses in different areas burned out from 0 to 5 times over an 18-year observation period [21]. For steppe meadows in the Bryansk region (the western part of Russia), the intervals between fires in the meadows in most cases range from 1 to 4 years [10]. In the forest-steppe zone of the Belgorod region in the “Roven’sky” national park (SW Russia), where meadows are confined mainly to unplowed gullies and river valleys, in the spring season of 2002 alone, 10% of all meadow vegetation burned out [22]. At the same time, prescribed burning of meadows is not a legally accepted agricultural practice in Russia. Most of these burnings occur spontaneously and uncontrollably, which raises the question of the impact of fires on meadow communities in different regions of Russia.
Since 2011, at the Basic Experimental Complex of the Institute of Atmospheric Optics of the Siberian Branch of the Russian Academy of Sciences (hereinafter referred to as BEC), located in the city of Tomsk (Western Siberia, Russia), experiments have been carried out to study the fire spread mechanisms associated with conducting field experiments, recording the physical parameters of the environment during prescribed fires: fire temperature, dynamics of meteorological parameters, gas and aerosol composition of the atmosphere, characteristics of turbulence in the combustion zone, etc. [18,19,23]. As part of a joint study, we decided to analyze the state of meadow communities in areas with different histories of controlled fires.
The goal of our work is to establish the degree of change and timing of restoration of the characteristics of meadow communities in the territory of the BEC after single pyrogenic events. As a null hypothesis, based on the data on the high resistance of grass vegetation to pyrogenic effects, we assumed that the characteristics of communities might be restored in the second year after the fire.
2. Materials and Methods
2.1. Study Site
The site for conducting model experiments is located at the territory of the BEC (circa 95,000 m2 in the south-eastern part of Tomsk city; 56°28′ N, 85°06′ E). The study site is located in the ancient right-bank terrace of the Ushaika River (right tributary of the Tom River, the Ob River basin), in the eastern part of the macroslope of the Tom-Yaya interfluve (152–160 m a.s.l.), and predominating slopes of 0.3–3.0°. The study region belongs to the southern part of the boreal zone, and the climate of the region is snowy and fully humid with a warm summer (Dbf; [24]). The average temperature in July and January is +17.7 and –17.0 °C, respectively. The average annual precipitation is 400 mm. This is the northernmost area of Chernozems in the Tomsk region, where these soils are sporadically found [25,26]. The loesslike loams of the Elovskaya suite serve as parent materials. This controls a low spatial variability of the soil properties and the relative simplicity of the soil cover pattern [27]. Phaeozems with a leaching water regime predominate [28]. The background vegetation at the territory of the BEC consists of polydominant meadows with a predominance in various areas of Poa pratensis, Festuca pratensis, Dactylis glomerata, Trifolium pratense, Taraxacum officinale, Galium mollugo, etc. (Table 1). The management regime in most of the BEC can be characterized as mowing 1–2 times a year with periodic cutting down of tree undergrowth.

Table 1.
Botanical characteristics of background communities.
2.2. Sampling and Experimental Design
On the territory of the BEC in 2011 and then from 2019 to 2022 annually, controlled burning was carried out in the experimental sites in April–May (see also Supplementary Materials), during which the temperature of the flames was measured using infrared cameras and thermocouples installed in the surface layer of the soil (Table 2; Figure 1). A detailed scheme of the experimental design can be found in [18,19]. During the pyrogenic impact, a temperature of at least 300–550 °C was recorded on the soil surface for 50–100 s. In the areas of the experimental fires, since 2019, mowing has been excluded, excepting the 2011 fire site, where the management regime was similar with the rest of the BEC territory.

Table 2.
Areas of experimental fires and characteristics of the geobotanical studies conducted.
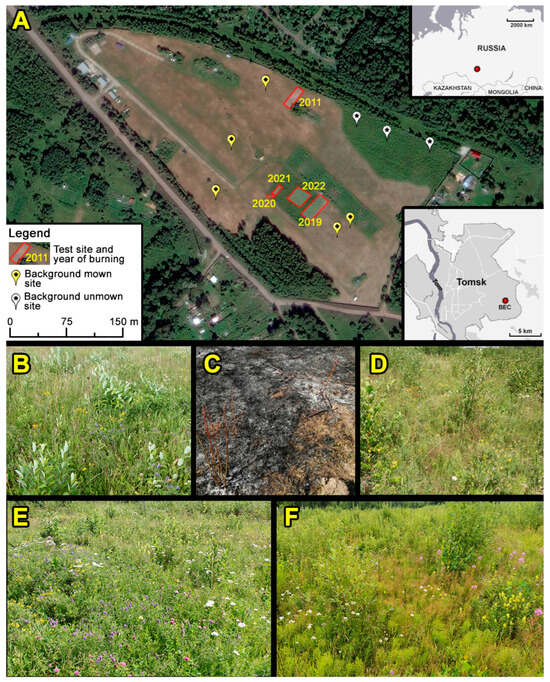
Figure 1.
Experimental sites in the territory of the BEC. (A) Scheme of the test site locations (a satellite image of maps.yandex.ru from August 2020 (accessed on 21 March 2024) after annual mowing was used as a background). (B) Fire site 2022, pre-fire condition in July 2021. (C) The same site on 30 April 2022, immediately after the fire. (D) The same site in July 2023. (E) Control plot with annual mowing, July 2022. (F) Control plot without annual mowing, July 2023.
In 2021–2023, for all experimental sites, geobotanical studies were carried out, including a description of the species composition and projective cover of plants on 1 m2 plots, complete geobotanical descriptions on 100 m2 plots (on those sites where the shape and size of the site allowed this), and the collection of above-ground phytomass on 0.25 m2 plots (Table 2). For the 2022 fire site, data for 2021 characterized the pre-fire state of vegetation. In all cases, plant coverage was assessed by eye as a percentage of the corresponding plot area. Phytomass samples were sorted into agrobotanical fractions common for agricultural exploitation of meadows (grasses, legumes, miscellaneous herbs, separately—total above-ground dead phytomass; sedges were not found) and weighed in an air-dried state (brought to constant weight at a temperature of +40 °C). Work in each field season was carried out in the middle and second half of July (13–15 July 2021; 24–27 July 2022; 17–20 July 2023) before mowing of the background areas in the territory of the BEC. In parallel with the current study, the influence of fires on the properties of the uppermost part of the humus horizon of soils was assessed at the same experimental sites [26].
2.3. Data Processing
Data collected on plots with different ages of burning were arranged into chronosequence of geobotanical descriptions with fire ages from 1 to 12. At the same time, conducting the study over three seasons made it possible to combine descriptions with the same fire age from different sites.
The collected data were analyzed according to the following criteria: species richness, total projective cover (TPC), projective cover of tree undergrowth, reserves of above-ground phytomass, and ratio of above-ground phytomass fractions (separately by agrobotanical groups). The significance of the differences was determined using the nonparametric Mann–Whitney U test with p = 0.05 selected as the threshold level. Non-parametrical multidimensional scaling (NMDS) of the array of obtained geobotanical descriptions was conducted in the R environment using a ‘vegan’ package [29] and standard commands. The application of vectors of habitat parameters (species richness, TPC, age of fire, and date of last mowing) to NMDS plot was carried out with a Bonferroni correction. All plant names in the text are given based on the World Flora Online database [30].
3. Results
3.1. Dynamics of Species Richness and TPC
When considering small-scale plots, one can identify a slight decrease in species richness 2–3 years after the fire, when there are 1–2 fewer species on average than in the control plots (Figure 2). For the chronoseries of areas that burned in different years, the differences with the control plots for 2–3 years after the fire are significant, both when compared with communities with annual mowing, and with no mowing over the last 3–4 years. Comparing these results to the pre-fire condition for the 2022 fire site, we observed a similar pattern. Species richness in the second year after the fire is lower, and the difference is close to significant (p-value = 0.09).
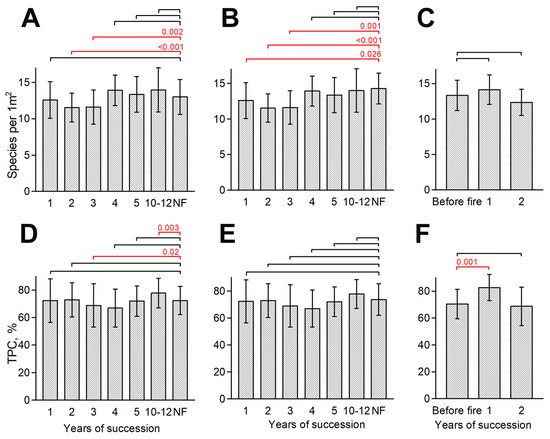
Figure 2.
Changes in species richness (A–C) and TPC (D–F) on 1 m2 plots in a chronoseries of fires of different ages compared to control (NF) plots (mowed, (A,D); unmown, (B,E)) and on the fire site in 2022 compared to the initial state (C,F). Notations: NF—did not burn. The whiskers on the histogram bars are the standard deviation. Significant p-values are shown above the histograms in red; values > 0.05 are not shown.
There is no similar strong pattern identified for TPC. The coverage decreases in the 3rd–4th year of a pyrogenic succession. However, the differences in comparison with unmown control plots—which is more correct for this stage—are insignificant. Significantly higher TPC values compared to the mown control plots were noted on mown sites that burned 10–12 years ago. However, we assume that this is largely a consequence of the special microconditions of the habitat (only one site was analyzed in all three years). For the fire site 2022, the TPC in the first season after the fire was significantly higher than the initial state in the last year, which, however, is not confirmed for the compiled chronoseries from different sites. It is likely that some increase in TPC occurs in all cases, but the significance of the increase is leveled out when sites with diverse initial TPC are compared. In addition, the methodological imperfection of the visual assessment of TPC should be taken into account (value increments of 10%).
The patterns observed for small-scale plots do not correspond to those observed at a middle scale (100 m2; Figure 3). The species richness of four mid-scale plots for the first year after the experimental fire in 2022 did not change in two cases and decreased in the two others (–5 species); on average, this is –3 ± 3. However, in 2023, species richness increased at all plots from +1 to +10 species per 100 m2 compared to the first year after the fire (Figure 3, A; average increase in comparison with year 2022 and initial state is +6 ± 4 and +3 ± 5, respectively). At the same time, the increase in species richness is largely expressed as compensation for the decrease in the indicator in the first year after the fire: only one plot out of four showed a considerable increase in species richness compared to the pre-fire state, i.e., 36 species before the fire and 46 in the second year after it. On the contrary, in the first year after the fire, TPC increased by 10–20% (in 3 out of 4 cases). On average, this is +13 ± 10 (Figure 3B). In the second year, it returned to its original state. Compared to the control plots, in the first two years after the fire, TPC and species richness also increased (Figure 3C,D). Thus, on mid-scale plots, species richness and TPC tend to return to their background state in the second year after a fire.
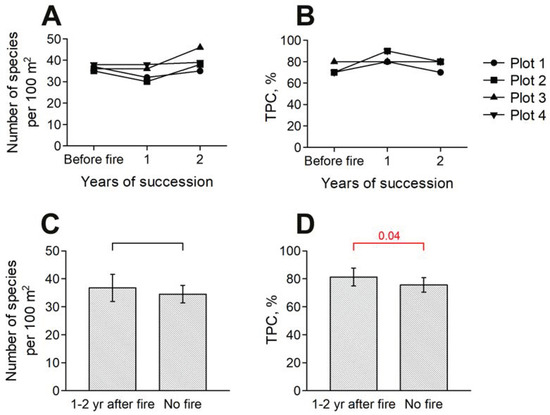
Figure 3.
Changes in species richness and TPC on mid-scale plots of the site of 2022 fire: (A)—changes in species richness compared to the pre-fire state; (B)—changes in TPC compared to the pre-fire state; (C)—change in species richness compared to the control plots; (D)—changes in TPC compared to the control plots. Notations as in Figure 2.
3.2. Impact of Fires on Reforestation
In boreal ecosystems, meadow communities developed as a result of deforestation have a tendency to self-reforest immediately after, excluding anthropogenic influence (plowing, grazing, mowing, etc.). In experimental areas where mowing was excluded after the fire, the coverage of woody undergrowth (represented by Betula pendula, Populus tremula and Salix caprea) increased during the study period (Figure 4). In the first two years after the fire, undergrowth coverage was significantly lower in unmown burned sites than in the mown control plots. In the third or fourth year, the difference disappeared (Figure 4A). In the fifth year, active regeneration of undergrowth on experimental sites lead to a significant increase of its coverage in compare with the annually mown control plots. In this case, the coverage values on experimental plots correspond to those noted for the BEC meadow communities unmown over the past 3–4 years (Figure 4B). Thus, at the level of small-scale plots, the suppressive effect on tree growth is more pronounced for recent (1–2 years) fires than for mowing.
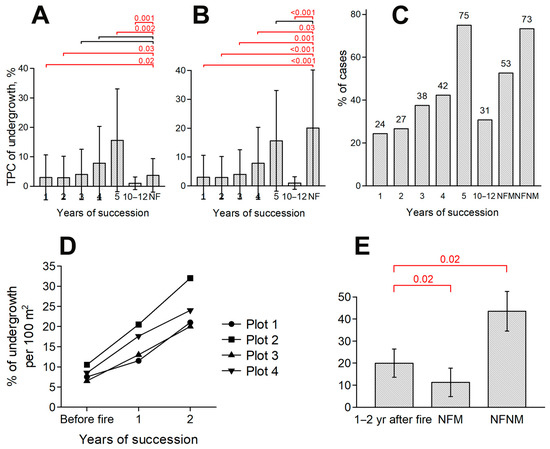
Figure 4.
Dynamics of tree undergrowth projective coverage on small-scale plots for fires of different ages (A–C) and on mid-scale plots for a fire in 2022 (D,E): A—changes of undergrowth TPC in comparison with mown control plots; (B)—changes of undergrowth TPC in comparison with unmown control plots; (C)—occurrence of undergrowth (% of total cases); (D)—dynamics of undergrowth TPC in comparison with the initial state; (E)—dynamics of undergrowth TPC in comparison with control plots. Notations are as in Figure 2; NFM –control plots with annual mowing, NFNM—unmown control plots.
For mid-scale plots, however, the opposite pattern is observed. The 2022 fire did not result in a decrease in undergrowth coverage compared to the condition of the same plots before the fire. On the contrary, over the course of two years there has been an increase in the coverage of undergrowth at all plots at an approximate rate of 10% of the plot area per year (Figure 4D). When compared with control plots, undergrowth coverage on this experimental site was significantly higher than in mowed meadow and significantly lower than in unmown meadow (Figure 4E). Thus, at the mid-scale plot level, the suppressive effect on tree growth is less pronounced for recent (within 1–2 years) fires than for mowing.
3.3. Dynamics of Aboveground Phytomass
Spring fires consume most of last year’s dead phytomass (Figure 5; p < 0.001). The decrease in green phytomass in the first year after the fire is within the range of variations in phytomass (p = 0.48). At the same time, the restoration of both live phytomass and dead phytomass to the control plots was noted in the second growing season. Further fluctuations in above-ground phytomass can be explained by the local conditions of the experimental sites and succession processes on unmown areas. At the same time, in unmown areas the ratio of live phytomass and dead phytomass continues to change, and on mown areas (control plots and an experimental site of 2011 fire) the ratio of these indicators remains approximately the same.
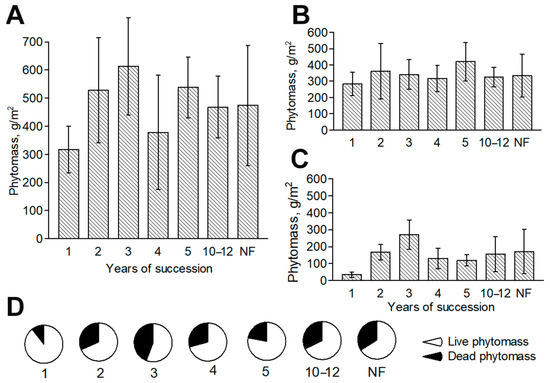
Figure 5.
Dynamics of yielding capacity of studied communities: (A)—the total above-ground phytomass (sum of live and dead phytomass) for studied pyrogenic chronosequence; (B)—live phytomass; (C)—dead phytomass; (D)—ratio of live and dead phytomass during succession. NF—mown control plots.
Spring fire affects primarily the phytomass of grasses, which significantly decreases in the first year after the fire (Figure 6; p = 0.003) while maintaining the average phytomass stocks of other fractions at the background level. At the same time, in the second or third year, the reserve of the grasses restores to control values and may even exceed them. Starting from 4 years after the fire, the proportion of miscellaneous herbs (forbs) increases. This dynamic also demonstrates the restoration of the ratio of fractions to a level corresponding to the control plots in the second year after the fire.
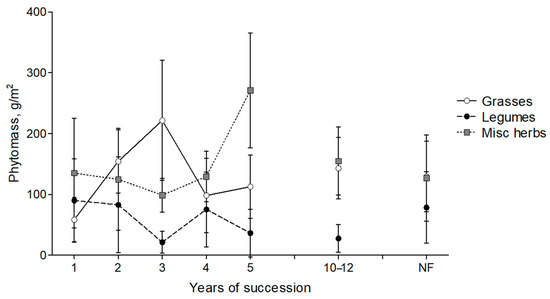
Figure 6.
Dynamics of aboveground phytomass of grasses, legumes and miscellaneous herbs in the experimental sites and in mown control plots (NF) of the BEC.
3.4. Non-Metrical Multidimensional Scaling (NMDS)
The results of NMDS of the data from small-scale plots (1 m2) and mid-scale plots (100 m2) are shown on Figure 7. Points on the ordination plane correspond to geobotanical descriptions, placed according to the similarity of its species composition; half-changes of plant communities are measured along the axes. The vectors correspond to the direction of growth of habitat parameters in the array of geobotanical descriptions.
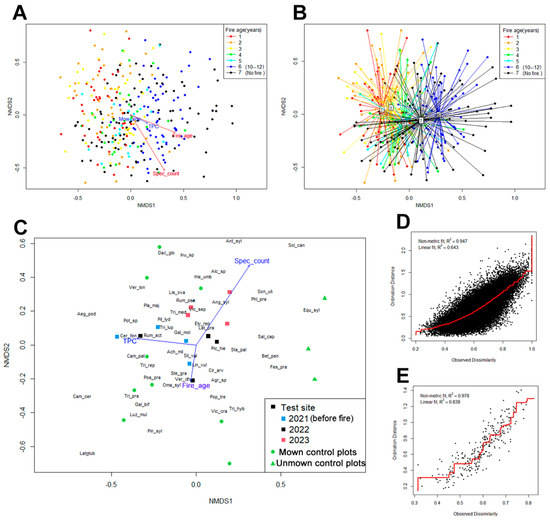
Figure 7.
NMDS of geobotanical data (plant species composition): (A)—for 1 m2 plots grouped by time since fire with overlay of vectors of changes in description parameters (stress level is about 0.23); (B)—the same graph with the centroids of the groups, centroids are marked with numbers according to their order in legend; (C)—for 100 m2 plots at the 2022 fire site and control plots with the imposition of abbreviations of plant species names (the age of the fire on the site for control plots is conditionally set as 20 years; stress level is 0.14–0.16); (D)—stress plot for figures (A,B); (E)—stress plot for figure (C).
Descriptions of the meadow community on the BEC do not cluster in NMDS, being more or less evenly distributed along the ordination plane. Grouping descriptions by age of the fire, it is possible to identify some isolation of descriptions up to and including 3 years after the fire, with group centroids located close to each other. Descriptions of sites 4–12 years after the fire (groups 4–6 in Figure 7B) are more consistent with the pool of descriptions of unburnt control plots (group 7 in Figure 7B). The age of the fire (vector Fire_age) is largely aligned with the growth vector of TPC and the growth vector of species richness per 1 m2 (Spec_count). It indicates an increase in the quantitative values of these characteristics on the scale of 1 m2 plots during pyrogenic succession.
At the same time, the NMDS of data from 100 m2 plots for 2022 fire site (Figure 7C), in which we can compare the current pre- and post-fire state of the same sites, demonstrates in general a less significant impact of the fire on the composition of phytocenoses. In the first growing season, the location of the group of descriptions is similar to pre-fire. In the next season, it shifts towards an increase in species richness and the appearance of large meadow weeds (Hieracium umbellatum, Solidago canadensis, Sonchus arvensis subsp. uliginosus, etc.) and meadow-edge species (Angelica sylvestris, Anthriscus sylvestris, etc.), which can be interpreted as a result of the lack of mowing and the beginning of successional processes of replacing the meadow community with forest. Further analysis of NMDS diagrams is placed in the following Section 4.
4. Discussion
Our study showed that the negative impact of a single spring fire on species richness was more pronounced for small-scale plots for 3 years after burning. This involves, first of all, a decrease of species richness (in average 1 species less per 1 m2), and its restoration to background level in the 4th–5th years can be explained not only with the restoration of meadows after fires, but also with successful reforestation in the absence of mowing that leads to the appearance and increasing role of forest-edge species. Similar results were obtained for meadow communities on mountain slopes in southwestern Germany [31]. After prescribed burning there, a gradual increase in the number of species (2 or 3 species more on average) per 1 m2 during the following 3 years was reported. More significant fluctuations in species composition can be caused not only by differences in observed communities, but also in the research methodology: prescribed burning was carried out in the middle of winter [31], which is impossible in the snowy humid climate of Western Siberia. As numerous authors have pointed out [7,32,33], fire seasons play an important role in consequences for plant cover.
TPC seems to be more stable characteristic, even for the first season after the fire. Restoration of a Lithuanian meadow lasted 46 days after a spring fire [34]. At the same time, the rate of recovery is directly affected by the amount of precipitation [34,35]. These data are consistent with ours. In 2021 and 2022, we noted the restoration of the TPC of vegetation progressed to background level in 2.5 months after the fire, which was facilitated by the humid climate of the region, with a sufficiently large amount of precipitation in May–June. The slight increase of TPC in the 4th–5th years also can be explained with reforestation, as in the case of species richness. On the mid-scale 100 m2 plots (more often used in geobotanical studies at least in in countries of the former Soviet Union), the negative impact of fires is much less pronounced, and the differences from the control plots are insignificant. However, we should note that the size of a fire can also impact the characteristics of plant communities and time of restoration after the fire. Thus, our results may be more applicable to relatively small-scaled fires.
The observed rapid restoration of vegetation cover after fires in treeless communities may be possibly related to the climate of the area, especially with the increasing continentality. We have previously noted research that claims fairly rapid restoration of meadow vegetation after a fire in the boreal forest zone of the northern hemisphere [34], which is consistent with our data. Semi-natural grasslands in the Mediterranean climate of Europe also restore quickly after fire [36,37]. At the same time, in the steppes of the Republic of Tuva (southern Siberia, 700 km to SE from the BEC) [32], the restoration period for vegetation was 4–6 years, and the structure of vegetation after a fire changed less in the meadow steppes (the most northern type of steppes, typically having conditions of higher moisture). In the even more arid communities of the desert steppes in Kalmykia [38] and northern deserts in western Kazakhstan [39], fire leads to changes of subshrub communities to communities with the predominance of turf-grasses, and the period of pyrogenic succession of vegetation at least exceeds 10 years.
Fires are often considered as one of the key factors in maintaining the species composition and structure of treeless communities, including meadows, by preventing the regeneration of woody vegetation [1,40]. However, in southwestern Germany, J.G. Goldammer and H. Page [31] found the insufficient effectiveness of prescribed burning to maintain meadows and inhibit reforestation in the long perspective, although a 2–3-year period of burning was capable of delaying the reforestation at an early stage. Additionally, it was reported that the best options for maintaining temperate meadows in Scandinavia are periodic mowing and moderate grazing, while annual burning significantly reduced species richness over several years and caused successional changes that are untypical for mown and grazed areas [41,42]. In another three-year experiment carried out in temperate grasslands in Scandinavia, mowing had a beneficial effect on plant species diversity, while in areas exposed to spring fires, the number of species did not differ significantly from that observed in fallow areas [43]. Similarly, burning did not increase species richness in the experiment conducted in North America [44]. The effect of fire on plant biodiversity can obviously vary for communities in different edaphoclimatic conditions. For example, an obvious increase in species richness after the fire was noted in semi-natural grasslands in the Mediterranean [37] and Japan [45]. Although we identified a significant decrease in species richness in the first years after the fire for small-scale plots, this decrease of an average of 1–2 species can hardly be called considerable. At the same time, our data did not reveal a significant effect of mowing on the species richness of the BEC meadows. However, due to the limited scope of our study, we cannot exclude the influence of other factors on the composition of the studied communities such as, for example, vicinism with the introduction of diaspores from neighboring areas [46].
The pyrogenic dynamics of meadow communities in Siberia currently remains poorly studied, despite the prevalence of forest fires in the region and their large scale. However, individual publications make it possible to evaluate the quantitative values of the yielding capacity of the communities under consideration in comparison with other meadows in the region. For meadows of the Priob forest-steppe in the Novosibirsk region (bordering the Tomsk region where the BEC is located), the reserves of above-ground phytomass with temporary conservation and light agricultural use are 420–530 g/m2 [47]. This corresponds to the yielding capacity of both the background communities of the BEC territory and the experimental sites in the second year after a fire. For floodplain meadows on the Ob River in the area of the Tomsk carbon polygon “Kaibasovo”, on average, slightly lower values of above-ground phytomass are given (300–400 g/m2) [48]. Meadow communities of the Nazarovskaya Depression (300 km to East) in the Krasnoyarsk Territory bordering the Tomsk region are also characterized by lower yielding capacity, averaging 250–350 g/m2, but in some areas reaching 500 g/m2 [49]. Thus, the yielding capacity of the studied communities is restored relatively quickly (in the second growing season after the fire) to background values. This is indirectly consistent with the opinion of Heinl et al. [50] that a positive effect of a single fire on biomass growth is more typical for poor habitats, while in nutrient-rich habitats (such as the chernozem in our study), this is practically not observed.
NMDS analyses show some difference of results for the 1 m2 and 100 m2 plots. It is worth noting that for 1 m2 plots, the vectors of fire age, species richness, and TPC are almost co-directed, while for 10 × 10 m plots, the vectors of fire age and species richness are almost oppositely directed, and the TPC vector is orthogonal to the fire age vector. Partly, the differences between small- and middle-scale plots in the direction of the species richness and TPC vectors relatively to the fire age vector might be explained by the imprecision of fire age labeling in control plots (20 years has been established as the approximate age of the availability of detailed photographs on the territory of the BEC). At the same time, as described above, the decrease in species richness after the fire and the further dynamics of its growth are more clearly expressed on small-scale plots than on mid-scale plots.
Some inconsistency in the results obtained for small-scale plots and mid-scale plots (for example, regarding the timing of restoration of species richness or suppression of tree growth) may, on the one hand, be due to the difference in the significance of factors influencing the species composition of communities when using plots of various sizes. In view of this, for example, for geobotanical studies of European meadows Chytrý and Otýpková [51] recommended plots of 16 m2 in size, intermediate in relation to ours. At the same time, the observed differences in the effect of fire on different scales can be explained by natural causes. Due to the relatively low intensity and transience of grass fires, small unburnt patches remain in the burnt area (see Figure 1C). Therefore, at the mid-scale, we observe an increase in the heterogeneity of site conditions and, as a consequence, an increase in species richness in the second year. At the same time, a small-scale plot is more likely to describe a section of the territory where the vegetation has completely burned out, so the decrease in species diversity caused by fire is more noticeable. The lack of influence of fire on tree undergrowth in mid-scale plots for 2021–2023 can be explained in the same way.
Our results allow us to discuss the advisability of periodic—but not annual—prescribed grass burning in the spring as a method of grassland management in the region under study. According to the results of the analysis of mid-scale plots, spring burning of meadows seems to be a less effective method of reducing the cover of tree undergrowth compared to annual mowing. This is consistent with the findings of studies of grasslands in Scandinavia [42,43]. According to the results of the experiment by Halpern et al. [45], to maintain the long-term existence of meadows, periodic cutting down of trees without burning the territory is sufficient. This result is also confirmed by the existing practice of periodic cutting of undergrowth at the BEC. However, due to labor costs, this practice is unlikely to be applicable for large areas. Our results for small-scale plots, indicating a significant lower coverage of undergrowth in burned areas in the first 2 years after a fire, suggest that the prescribed fire method is not meaningless for controlling tree undergrowth if mowing is impossible or inappropriate. However, in this case, it is necessary to establish intervals between burnings that allow the characteristics of meadow vegetation to be restored.
Our study reveals a high tolerance of Siberian meadows to fire impact that was poorly discovered before, and our data are comparable with other pyrogenic studies in meadow communities in boreal forests. The “pyrogenic fingerprint,” in most parameters of communities in our study, such as species richness, TPC, and above-ground phytomass of the community, persists for no more than 1 year for mid-scale plots and no more than 3–4 years for small-scale plots; based on the ratio of phytomass fractions, one can also assume a 3–4-year recovery period for the community. Thus, the null hypothesis is only partially confirmed, since the restoration of vegetation parameters in the second season is typical only for the mid-scale plots. However, in total, based on the results of our experiment, the impact of single fires on the meadow vegetation of Siberia at the cenotic and subcenotic levels can generally be considered insignificant. This corresponds with the opinion of J.M. Greele and J.H. Langenheim [52] that in fire-dependent communities a single fire should not be regarded as a factor of disturbance, although with an increase in the frequency of fires it may become so. Heinl et al. [50] also reported that single fires have little effect on species composition and projective cover. However, we should mention that both studies involved communities of biomes that significantly differ from ours.
The recovery period for the main characteristics of meadow communities, according to data for mid-scale plots in the second year after a fire, correlates with the traditional two-year period of prescribed fires [53]. At the same time, our data indicate that to restore some characteristics of vegetation in burnt areas (in particular, species richness, the ratio of phytomass fractions, and the participation of undergrowth in the vegetation cover), a period of 3–4 years is required. This is consistent with known data for other treeless communities, such as Mediterranean heathlands [54] or wetland communities [55,56] and mountain grasslands in North America [45]. Gordjin and O’Connor [53] support these findings by emphasizing that to achieve the greatest biodiversity with the traditional two-year burn period, it is necessary to maintain sites with longer intervals between burns within the treated area. This regime of prescribed fires seems to be the gentlest for biodiversity and, therefore, the most appropriate based on the results of our study.
5. Conclusions
It was established that a single controlled spring fire on the territory of the BEC did not significantly change meadow vegetation. The period for the restoration of vegetation coverage indicators ranged from 1 to 3–4 years after a fire. Most of them restored in the second growing season. The differences between burned and unburned plots were visible for longer in small-scale plots than in mid-scale plots. In other words, these differences are expressed for longer at the subcenotic level than at the cenotic level.
This allows us to consider periodic spring fires as a tool for maintaining meadow communities where it is necessary; that is, in conditions where forest regeneration is not suppressed by mowing or grazing. However, a necessary condition for such management practices is compliance with the intervals between burnings.
6. Patents
The array of raw geobotanical data collected in this research was registered as patent No. 2024620154 (registered 12 January 2024) with the Russian Federal Service for Intellectual Property (Rospatent).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fire7040115/s1, Satellite images of BEC territory in 2007–2022 with comments.
Author Contributions
S.L.: Conceptualization, Methodology, Software, Formal analysis, Field work, Visualisation, Data curation, and Writing—original draft preparation, review, and editing; D.K.: Field work, Resources, and Supervision; I.S.: Conceptualization, Formal analysis, and Writing—review and editing; G.K.: Formal analysis, Writing—review and editing, and Translation; M.A.: Field work, Resources, and Supervision; T.K.: Conceptualization, Project administration, and Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number No. 22-27-00329.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request or in a patent No. 2024620154.
Acknowledgments
The authors thank the research team of the Institute of Atmospheric Optics of the Siberian Branch of the Russian Academy of Sciences for their assistance in performing experiments on the territory of the BEC.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rundel, P.W. Fire as an ecological factor. In Physiological Plant Ecology 1; Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 501–538. [Google Scholar] [CrossRef]
- Stavi, I. Wildfires in grasslands and shrublands: A review of impacts on vegetation, soil, hydrology, and geomorphology. Water 2019, 11, 1042. [Google Scholar] [CrossRef]
- Tishkov, A.A. The fires in steppes and savannas. Steppe Sci. 2003, 4, 9–22. (In Russian) [Google Scholar]
- Bond, W.J.; Woodward, F.I.; Midgley, G.F. The global distribution of ecosystems in a world without fire. New Phytol. 2005, 165, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Scheiter, S.; Higgins, S.I.; Osborne, C.P.; Bradshaw, C.; Lunt, D.; Ripley, B.S.; Taylor, L.L.; Beerling, D.J. Fire and fire-adapted vegetation promoted C4 expansion in the late Miocene. New Phytol. 2012, 195, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Ilyina, V.N. Pyrogenic impact on vegetation cover. Samar. Luka Probl. Reg. Glob. Ecol. 2011, 20, 4–30. (In Russian) [Google Scholar]
- Molchanov, V.I. Impact of Pyrogenic Factor on Peculiarities of Structure and Yielding of Meadow Communities in SW Transbaikal. Ph.D. Thesis, Irkutsk State Transport University, Ulan-Ude, Russia, 2012. (In Russian). [Google Scholar]
- Arkle, R.S.; Pilliod, D.S. Prescribed fires as ecological surrogates for wildfires: A stream and riparian perspective. For. Ecol. Manag. 2010, 259, 893–903. [Google Scholar] [CrossRef]
- Harper, A.R.; Doerr, S.H.; Santin, C.; Froyd, C.A.; Sinnadurai, P. Prescribed fire and its impacts on ecosystem services in the UK. Sci. Total Environ. 2018, 624, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Ruchinskaya, E.V. Structural and Species Diversity of Vegetation of Steppe Meadows in Nemoral Forests Zone (on the Example of the Natural Monument “Melovitsky Slopes”, Bryansk Region). Ph.D. Thesis, Moscow Pedagogical State University, Moscow, Russia, 2019. (In Russian). [Google Scholar]
- Fynn, R.W.S.; Morris, C.D.; Edwards, T.J. Effect of burning and mowing on grass and forb diversity in a long- term grassland experiment. Appl. Veg. Sci. 2004, 7, 1–10. [Google Scholar] [CrossRef]
- Ruprecht, E.; Lukács, K.; Domokos, P.; Kuhn, T.; Fenesi, A. Hydration status influences seed fire tolerance in temperate European herbaceous species. Plant Biol. 2016, 18, 295–300. [Google Scholar] [CrossRef]
- Neary, G.D.; Leonard, M.J. Effects of fire on grassland soils and water: A review. In Grasses and Grassland Aspects; Kindomihou, V.M., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Fidelis, A.; Cartay, M.; Blanco, C.; Müller, S.; Pillar, V.; Pfadenhauer, J. Fire intensity and severity in Brazilian Campos grasslands. Interciencia 2010, 35, 739–745. [Google Scholar]
- Dusaeva, G.K.; Kalmykova, O.G. Influence of fires on vegetable cover of steppes: Literature review. Bull. Mosc. Soc. Naturalists. Biol. Ser. 2021, 126, 26–38. (In Russian) [Google Scholar]
- Lipsett-Moore, G.J.; Wolff, N.H.; Game, E.T. Emissions mitigation opportunities for savanna countries from early dry season fire management. Nat. Commun. 2018, 9, 2247. [Google Scholar] [CrossRef] [PubMed]
- Laris, P.; Koné, M.; Dembélé, F.; Yang, L.; Jacobs, R.; Laris, Q. Methane gas emissions from savanna fires: What analysis of local burning regimes in a working West African landscape tell us. Biogeosciences 2021, 18, 6229–6244. [Google Scholar] [CrossRef]
- Grishin, A.M.; Filkov, A.I.; Loboda, E.L.; Reyno, V.V.; Kozlov, A.V.; Kuznetsov, V.T.; Kasymov, D.P.; Andreyuk, S.M.; Ivanov, A.I.; Stolyarchuk, N.D. A field experiment on grass fire effects on wooden constructions and peat layer ignition. Int. J. Wildl. Fire 2014, 23, 445–449. [Google Scholar] [CrossRef]
- Loboda, E.; Kasymov, D.; Agafontsev, M.; Reyno, V.; Gordeev, Y.; Tarakanova, V.; Martynov, P.; Loboda, Y.; Orlov, K.; Sav-in, K.; et al. Effect of Small-Scale Wildfires on the Air Parameters near the Burning Centers. Atmosphere 2021, 12, 75. [Google Scholar] [CrossRef]
- Andreev, Y.A.; Bryukhanov, A.V. Prevention, Monitoring and Control of Wildfires (on the Example of the Altai-Sayan Ecoregion); ‘Gorod’: Krasnoyarsk, Russia, 2011; 271p. (In Russian) [Google Scholar]
- Borodulina, V.P.; Komarova, A.F.; Cherednichenko, O.V. Calamagrostis epigeios meadows in the buffer zone of the Polistovsky Nature Reserve (Pskov region). Divers. Plant World 2019, 1, 44–61. (In Russian) [Google Scholar] [CrossRef]
- Ukrainskiy, P.A. Dynamics of the spectral properties of overgrown burned grass areas. Curr. Probl. Remote Sens. Earth Space 2013, 10, 229–238. (In Russian) [Google Scholar]
- Grishin, A.M.; Filkov, A.I.; Loboda, E.L.; Reyno, V.V.; Kuznetsov, V.T. Full-scale experiments on influence of the grassland fire on wooden constructions and peat layers. Pozharnaya Bezop./Fire Saf. 2013, 3, 52–57. (In Russian) [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Kuznetsov, K.A. Soils of Tomsk region (preliminary reports). Vopr. Geogr. Sib. 1949, 2, 69–86. (In Russian) [Google Scholar]
- Semenkov, I.N.; Lednev, S.A.; Klink, G.V.; Kasymov, D.P.; Agafontsev, M.V.; Kostrova, S.N.; Koroleva, T.V. Impact of spring fires on the properties of the humus layer of chernozem (SE of Western Siberia). Eurasian Soil Sci. 2024, 57, 493–501. [Google Scholar] [CrossRef]
- Loiko, S.V.; Kritskov, I.V.; Kulikova, O.R.; Istigechev, G.I. Influence of a relief and peasant nature management on chromaticity of the humic horizons in a foothill subtaiga of the southeast of Western Siberia. In Proceedings of the Reflection Bio-Geo-Antroposferal Interactions in Soils and Soil Cover Collection of Materials V International Scientific Conference, Dedicated to the 85th Anniversary of the Opening of the First University Department of Soil Science in Siberia, Tomsk State University, Tomsk, Russia, 7–11 September 2015. (In Russian). [Google Scholar]
- Fridland, V.M. Soil Map of the RSFSR. Scale 1:2,500,000; Glavnoe Upravlenie Geodezii i Kartografii: Moscow, Russian, 1988. (In Russian)
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.3-5. Available online: http://CRAN.R-project.org/package=vegan (accessed on 29 January 2024).
- World Flora Online Database. Available online: https://www.worldfloraonline.org (accessed on 29 January 2024).
- Page, H.; Goldammer, J.G. Fire History of Central Europe: Implications for Prescribed Burning in Landscape Management and Nature Conservation. Paper presented at the Baltic Exercise for Fire Information and Resources Exchange (BALTEX FIRE 2000), Kuopio, Finland, June 2000. Available online: https://gfmc.online/programmes/natcon/BAL-PAP3-2.PDF (accessed on 28 February 2024).
- Titlyanova, A.A.; Sambuu, A.D. Successions in Grasslands; Publishing House SB RAS: Novosibirsk, Russia, 2016; 191p. (In Russian) [Google Scholar]
- Pereira, P.; Francos, M.; Ubeda, X.; Brevik, E. Fire impacts in European Grassland ecosystems. In Wildfires. Perspectives, Issues and Challenges of the 21st Century; Nova Science Publishers, Inc.: New York, NY, USA, 2017. [Google Scholar]
- Pereira, P.; Cerdà, A.; Jordán, A.; Zavala, L.; Mataix-Solera, J.; Arcenegui, V.; Misiune, I.; Keesstra, S.; Novara, A. Vegetation recovery after a grassland fire in Lithuania. The effects of fire severity, slope position and aspect. Land Degrad. Dev. 2015, 27, 1523–1534. [Google Scholar] [CrossRef]
- Balázs, D.; Valkó, O.; Török, P.; Végvári, Z.; Hartel, T.; Schmotzer, A.; Kapocsi, I.; Tóthmérész, B. Grassland fires in Hungary–Experiences of nature conservationists on the effects of fire on biodiversity. Appl. Ecol. Environ. Res. 2014, 12, 267–283. [Google Scholar] [CrossRef]
- Rossetti, I.; Cogoni, D.; Calderisi, G.; Fenu, G. Short-Term Effects and Vegetation Response after a Megafire in a Mediterranean Area. Land 2022, 11, 2328. [Google Scholar] [CrossRef]
- Bonanomi, G.; Idbella, M.; Abd-ElGawad, A.M.; Motti, R.; Ippolito, F.; Santorufo, L.; Adamo, P.; Agrelli, D.; De Marco, A.; Maisto, G.; et al. Impact of prescribed burning, mowing and abandonment on a Mediterranean grassland: A 5-year multi-kingdom comparison. Sci. Total Environ. 2022, 834, 155442. [Google Scholar] [CrossRef] [PubMed]
- Klink, G.V.; Lednev, S.A.; Semenkov, I.N.; Konyushkova, M.V.; Karpachevskiy, A.M.; Chemidov, M.M.; Ulanova, S.S.; Fedorova, N.L.; Sharapova, A.V.; Bogun, S.A.; et al. Influence of Fires on Desert Plant Communities at the Chernye Zemli (SW Russia). Fire 2024, 7, 96. [Google Scholar] [CrossRef]
- Ivanov, V.V. Steppes of Western Kazakhstan in Connection with the Dynamics of Their Cover; Akademiya Nauk USSR: Moskva, Russia; Saint Petersburg, Russia, 1958. (In Russian) [Google Scholar]
- Keeley, J.E.; Rundel, P.W. Fire and the Miocene expansion of C4 grasslands. Ecol. Lett. 2005, 8, 683–690. [Google Scholar] [CrossRef]
- Hansson, M.; Fogelfors, H. Management of a semi-natural grassland; results from a 15-year-old experiment in southern Sweden. J. Veg. Sci. 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Wahlman, H.; Milberg, P. Management of semi-natural grassland vegetation: Evaluation of a long-term experiment in southern Sweden. Ann. Bot. Fenn. 2002, 39, 159–166. [Google Scholar]
- Antonsen, H.; Olsson, P.A. Relative importance of burning, mowing and species translocation in the restoration of a former boreal hayfield: Responses of plant diversity and the microbial community. J. Appl. Ecol. 2005, 42, 337–347. [Google Scholar] [CrossRef]
- Halpern, C.B.; Haugo, R.D.; Antos, J.A.; Kaas, S.S.; Kilanowski, A.L. Grassland restoration with and without fire: Evidence from a tree-removal experiment. Ecol. Appl. 2012, 22, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.K.; Ushimaru, A. Traditional burning and mowing practices support high grassland plant diversity by providing intermediate levels of vegetation height and soil pH. Appl. Veg. Sci. 2016, 19, 567–577. [Google Scholar] [CrossRef]
- Zonneveld, I. Vicinism and mass effect. J. Veg. Sci. 2009, 6, 441–444. [Google Scholar] [CrossRef]
- Zvereva, G.K.; Lomova, T.G. The evaluation of vegetation condition in the meadows of the Ob river forest-steppe in the context of its economic use. Bull. Altai State Agric. Univ. 2019, 11, 75–83. (In Russian) [Google Scholar]
- Shepeleva, L.F.; Kolesnichenko, L.G.; Pudova, M.S. Dynamics of the aboveground phytomass of the Ob floodplain mead-ows in the area of the Tomsk carbon polygon (Kaibasovo). Environ. Dyn. Glob. Clim. Chang. 2022, 13, 104–119. [Google Scholar]
- Dubynina, S.S. The productivity of the phytomass of meadow plant communities of the Nazarovskaya Basin under different conditions of use. Adv. Curr. Nat. Sci. 2018, 10, 102–107. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Heinl, M.; Sliva, J.; Tacheba, B. Vegetation changes after single fire-events in the Okavango Delta wetland, Botswana. S. Afr. J. Bot. 2004, 70, 695–704. [Google Scholar] [CrossRef]
- Chytrý, M.; Otýpková, Z. Plot sizes used for phytosociological sampling of European vegetation. J. Veg. Sci. 2003, 14, 563–570. [Google Scholar] [CrossRef]
- Greenlee, J.M.; Langenheim, J.H. Historic Fire Regimes and Their Relation to Vegetation Patterns in the Monterey Bay Area of California. Am. Midl. Nat. 1990, 124, 239–253. [Google Scholar] [CrossRef]
- Gordijn, P.J.; O’Connor, T.G. Multidecadal effects of fire in a grassland biodiversity hotspot: Does pyrodiversity enhance plant diversity? Ecol. Appl. 2021, 31, e02391. [Google Scholar] [CrossRef]
- Cespedes, B.; Luna, B.; Perez, B.; Urbieta, I.R.; Moreno, J.M. Burning season effects on the short-term post-fire vegetation dynamics of a Mediterranean heathland. Appl. Veg. Sci. 2014, 17, 86–96. [Google Scholar] [CrossRef]
- Norton, D.A.; De Lange, P.J. Fire and Vegetation in a Temperate Peat Bog: Implications for the Management of Threatened Species. Conserv. Biol. 2003, 17, 138–148. [Google Scholar] [CrossRef]
- Jules, E.S.; Ellison, A.M.; Gotelli, N.J.; Lillie, S.; Meindl, G.A.; Sanders, N.J.; Young, A.N. The Influence of fire on a rare serpentine plant assemblage: A 5-year study of Darlingtonia fens. Am. J. Bot. 2011, 98, 801–811. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).