Abstract
The Australian megafires of 2019–2020 were considered catastrophic for flora and fauna, yet little is known about their impacts on reptiles. We investigated the impacts of the 2019–2020 megafires on reptiles in Morton National Park, New South Wales, in eastern Australia. To understand how fire severity affects reptile species richness and occupancy, we surveyed 28 replicate plots across unburnt areas and areas affected by high and low fire severity. We estimated reptile species richness and occupancy by performing systematic searches for reptiles during five sampling occasions in 2023, three years after the megafires. We measured vegetation structure and quantified the thermal environment in shelter sites used by reptiles. Vegetation structure varied significantly between burn severity groups. High-severity plots had the least canopy cover and the thinnest leaf litter depth but had a taller understorey with more stems. The thermal quality within reptile retreat sites did not differ between fire severity classes. Despite strong differences in post-fire vegetation structure, there was no evidence that fire severity affected reptile species richness or occupancy of the delicate skink, Lampropholis delicata. These results highlight the complexity of reptile responses to fires and contribute to increasing our understanding of the impacts of megafires on reptile communities.
1. Introduction
Fire is a natural occurrence in Australia, but in recent decades, there has been an increase in the extent and number of megafires in temperate Australia [1]. While the Australian fauna has adapted to respond to fire over evolutionary time [2], there is increasing concern that the recent increase in the frequency of high-severity fires may have detrimental impacts on reptile communities [3]. Although some reptile species appear to be able to cope with high-severity fires [4], other species may experience high levels of mortality directly from such fires [5,6] or from loss of refuge habitat burnt by fire [7].
High-severity fires can affect reptiles via direct and indirect pathways [8,9]. Evolved fire avoidance behaviours may have limited success against the rapid spread of high-severity fires, leading to direct mortality [9]. Fire can change vegetation structure, which in turn can affect the quality and availability of habitat for many species [10,11]. For example, changes to canopy openness can alter the thermal quality of habitats for rock-dwelling and forest dwelling species [12,13], while loss of leaf litter may affect habitat quality for fossorial or ground-dwelling species [14]. Loss of canopy and/or litter may also contribute to post-fire mortality from avian predation [15]. While we know something about the short-term impacts of megafires on some reptile species [16,17], little is known about the ongoing impacts of these megafires on reptile communities [18]. To better manage and conserve reptile species in fire-prone habitats, a greater understanding of population-level responses to high-severity fires is needed [19].
In this study, we investigated the effects of the 2019–2020 Australian megafires on reptile communities. The 2019–2020 megafires that occurred in eastern Australia burned across an unprecedented area of 5.8 M ha [20] and affected the habitats of many threatened species [9]. The fires were preceded by an extreme drought [21], fueled by hot winds, and characterised by their large extent of high-severity fires [22]. Our broad aim was to quantify the effects of fire severity of the 2019–2020 megafires on reptile species richness and occupancy by estimating occupancy and abundance of species in replicate monitoring plots that were unburnt and in plots that experienced either low or high-severity fire. We also assessed the vegetation structure and thermal environment available to reptiles in the three fire types in the fire severity plots. Fire severity is important in driving changes in vegetation density and structure [23], with complex habitats generally supporting a greater number of species [24]. We hypothesised that structural complexity (i.e., logs, leaf litter, and understorey vegetation) would decrease with increasing fire severity. As many reptiles have low dispersal ability and may suffer from mortality during high-severity fires or after such fires [25], we predicted that reptile species richness and occupancy would be lower at high-severity sites compared with low-severity or unburnt sites.
2. Materials and Methods
2.1. Study Area and Monitoring Plots
Morton National Park is located on the eastern escarpment of the Southern Tablelands of New South Wales, approximately 160 km south of Sydney (35° S, 150° E). Collectively, the Currowan and Morton fires burnt over 330,000 hectares of bushland, including most of Morton National Park (Figure 1).
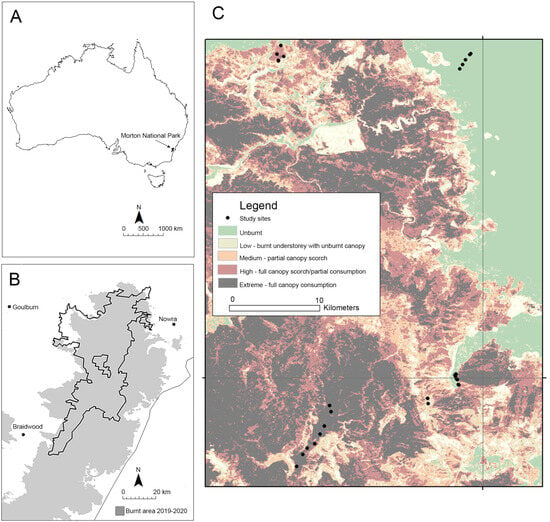
Figure 1.
Location of Morton National Park, NSW in south-eastern Australia (A), showing the extent of the 2019–2020 fires (B), and locations of fire monitoring plots with respect to fire severity (C). The grey shading indicates burnt area, while the dots indicate locations of monitoring plots.
In October and November 2020, we established 28 replicate reptile monitoring plots within high-severity burnt areas (10 plots), low-severity burnt areas (5 plots), and in unburnt areas (13 plots) adjacent to the burnt area (Figure 1). Reptile monitoring plots were 10 × 10 m in size, were located 30 to 70 m from vehicle tracks, and were spaced between 200 m and 2 km apart. As smaller lizards have small home ranges and limited dispersal ability [22], plots were far enough apart to reduce the chances that the same individuals would be detected from multiple plots. We selected sites with similar vegetation types and elevation that were located on sandstone plateau tops at similar distances (100–200 m) from the nearest rock outcrops. Vegetation at the sites was open woodland, with dominant species comprising grey gums Eucalyptus punctata, scribbly gums E. tereticornis, Sydney peppermints E. piperita, blue-leaf stringybarks E. agglomerata, and red-bloodwoods Corymbia gummifera [26].
Fire severity was determined from satellite imagery obtained from the fire extent and severity mapping (FESM) data [27], which uses the amount of biomass lost as a proxy for burn intensity. The FESM data are obtained from high-resolution Sentinel 2 satellite imagery, using a semi-automated approach to map fire extent and severity through a machine learning framework. The fire-severity classes are defined as unburnt, low (burnt understorey/unburnt canopy), moderate (partial canopy scorch), high (full canopy scorch), and extreme (full canopy consumption). The mean accuracy of this approach is very high: >95% for the unburnt and extreme severity, >80% for low severity, and >85% for the high severity class [28]. Sites were visited from May to November 2020 to ground truth the severity classes obtained from the FESM data. To ground truth, we visually assessed the degree of canopy scorch and burnt understorey at each site. Because there was little variation in fire severity in accessible areas, we were unable to intersperse plots with different fire severity classes in the same areas (Figure 1).
2.2. Reptile Surveys
We used artificial refuges of tin and roof tiles to detect reptiles. When plots were established in 2020, we placed two pieces of roofing iron (86 × 120 cm) and four terracotta or concrete roof tiles (42 × 35 cm) in each plot. To increase the likelihood of detecting arboreal skinks and geckos, a sheet of artificial bark (Consolidated Alloys foam expansion joint, 150 mm wide × 10 mm thick) was wrapped around the base of one tree per plot [29].
Standardised timed surveys were conducted between May and September 2023. To allow us to estimate occupancy, we surveyed each plot on five occasions, separated by a minimum of two-week intervals. To eliminate time of day bias on detections, the order in which sites were surveyed was rotated on each trip [30]. Searches consisted of lifting tiles and tin, peeling back artificial bark, and raking leaf litter around logs and trees for five minutes, and an opportunistic search that involved lifting any natural cover (bark, logs, and rocks) and scanning for active reptiles for five minutes. Any reptiles encountered were identified to species level using a published key [31].
2.3. Vegetation Surveys
Vegetation structure was quantified by randomly placing a 1 × 1 m quadrat (constructed from 15 mm PVC-U pressure pipe) on the ground inside each plot. Five quadrat surveys were conducted per plot. For each quadrat, we recorded the percentage of canopy cover (ground level and above understorey by visual estimate), litter depth (millimetres, using a ruler), percentage of litter cover (visual estimate), number of understorey plant stems, height of understorey (metres, using tape measure), and number of logs and rocks [13,32].
2.4. Quantifying the Thermal Environment
Vegetation structure is influenced by fire and can also influence the thermal environment available to reptiles [13]. To determine if retreat sites had different thermal regimes across plots, we placed two temperature loggers (Tempmate -S1 Pro Single-Use Temp Data Logger, ±0.3 °C accuracy, 86 × 40 × 8.7 mm), one under tile and one under tin, at each fire plot. Loggers recorded temperatures at 10-min intervals from 10 August to 10 September 2023.
2.5. Statistical Analyses
Vegetation Structure—Data were tested for normality using the Shapiro–Wilk test and visual inspection of boxplots, histograms, and Q-Q plots. All variables (except understorey height) displayed a non-normal distribution. Arcsine transformations were applied to all percentage variables, and log10, square, and inverse transformations were investigated on all other variables. However, these were unsuccessful, and therefore non-parametric analyses were used [33]. The variables number of rocks and logs were excluded from analysis due to sparse data points. Correlations between habitat variables were examined using Spearman’s correlation [34]. For two correlated variables (with a strong negative or positive correlation coefficient ≥ 0.7), the variable that was most relevant for reptile habitat [35] was included and the other excluded. Kruskal–Wallis H tests were conducted to establish if habitat attributes (dependent variables) differed significantly between burn severity groups (independent variable). Post hoc pairwise comparisons were performed using a Bonferroni correction for multiple comparisons [36] to determine where differences between groups occurred.
Occupancy—To estimate the probability of detection (p) and occupancy (Psi), we ran single-season occupancy models in the programme MARK [37,38]. We included a group variable in the input file so that we could assess whether occupancy or detection varied between fire treatments. Multiple models were run where detection (p) and occupancy (Psi) were time dependent, constant, or varied with fire treatment. To identify the most parsimonious model from the candidate set, the Akaike information criterion (AIC) was used [39]. Due to low count data, we could only run occupancy analysis for Lampropholis delicata.
Species richness—A one-way ANOVA was conducted to test if species richness (total number of species observed per plot) varied between fire severity levels. Data were checked for the assumptions of normality using the Shapiro–Wilk test and visual inspection of Q-Q plots, histograms, and boxplots. Homogeneity of variances was tested using Levene’s test of equality of variances.
Temperature—For each data logger, we calculated the daily minimum, maximum, and average temperature. We also estimated the daily number of minutes during which temperatures were within the preferred temperature range (Tpref, 25–35 °C) for the skink Lampropholis delicata, i.e., the range where skinks achieve >80% of their maximum running speed [40]. Data were checked for normality and homogeneity of variance as discussed previously, and repeated measures ANOVAs were used to determine whether temperature varied across days or across fire severity treatments. Because the assumption of sphericity was violated (with epsilon < 0.3), we used the Greenhouse–Geisser correction for determining the significance of F-tests [41]. Unless stated otherwise, statistical analyses were carried out using SPSS (version 29.0.1.0).
3. Results
3.1. Vegetation Structure
Kruskal–Wallis tests indicated that ranks of all habitat variables (except trees per plot) differed between fire severity levels. Litter depth was similar in unburnt and low-severity plots but was significantly lower in high-severity plots (χ2 = 58.94, p < 0.001, Figure 2A). Vegetation cover was higher in high-severity plots than in unburnt and low-severity plot groups (χ2 = 15.24, p < 0.001, Figure 2B). The number of stems was highest in high-severity plots and was similar between unburnt and low plots (χ2 = 18.10, p < Figure 2C). Understorey height was greater in high-severity plots than in unburnt or low-severity plots (χ2 = 58.94, p < 0.001, Figure 2D). Above-understorey canopy cover was lower in high-severity plots than in low-severity or unburnt plots (χ2 = 54.24, p < 0.001, Figure 2E). The number of trees varied across plots (χ2 = 23.44, p < 0.001) but did not differ across burn severity categories (Figure 2F).
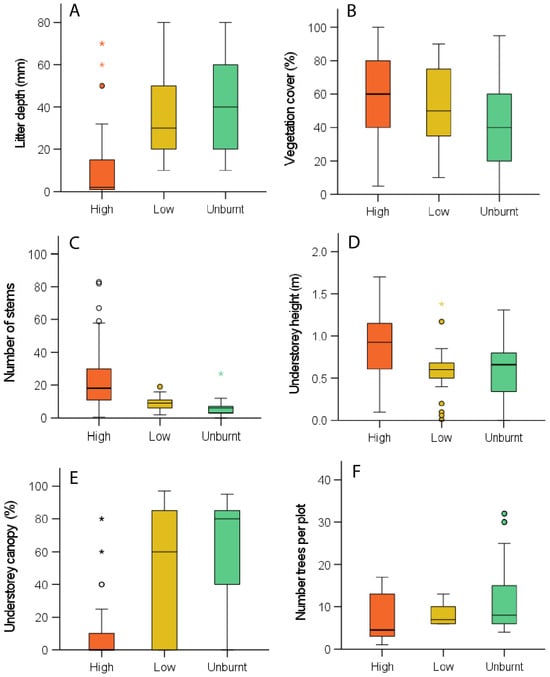
Figure 2.
Vegetation structure at fire monitoring plots at high and low-severity burnt sites, and unburnt sites. Figure shows leaf litter depth (A), percent vegetation cover (B), number of stems (C), understorey height (D), canopy cover above understorey (E), and number of trees per plot (F). Box and whisker plots display the lower and upper interquartile values and the median, while whiskers show the minimum and maximum values (excluding outliers). Circles and stars indicate outliers.
3.2. Reptile Species Richness
We detected 44 individuals from six lizard species and one snake species. The number of plots in each fire severity class in which each species was detected is shown in Table 1. Lampropholis delicata was the most common species with 18 captures, whilst Amalosia lesueurii was the least common lizard with only two captures. Drysdalia rhodogaster was the only snake species encountered, with two captures. There was no difference in species richness across the fire severity classes (F2,25 = 1.48, p = 0.25). Three different species were found at unburnt plots, four species were found at low-severity plots, and five species were found at high-severity plots.

Table 1.
Reptile species which were detected during five surveys carried out from May to September 2023. The table shows the number of unburnt plots, low- and high-severity fire plots in which the species was detected.
3.3. Occupancy Estimates
For L. delicata, the best-supported model was one in which occupancy was constant and detection was time-dependent (Table 2). From this model, occupancy was estimated at 0.82 ± 0.35, while detection rates increased through time (sample 1 and 3, p = 0.09, SE = 0.07, sample 2, p = 0, sample 4, p = 0.17, SE = 0.34, sample 5, p = 0.30, SE = 0.16). There was some support from the data for one other model (delta AIC < 2.0, Table 1), in which occupancy and detection were constant. Based on AIC weights, there was no support that occupancy of L. delicata varied with fire severity.

Table 2.
Results showing models for occupancy (Psi) and detection rates (p) of Lampropholis delicata across the three fire severity classes using single-species occupancy analyses in programme MARK. The table shows AICc values and weights, model likelihood, number of parameters (N) and model deviance. The best-supported model is shown with grey shading.
3.4. Thermal Profiles of Reptile Retreat-Sites
Temperatures in retreat sites showed strong daily fluctuations due to changes in the weather (for all independent variables, repeated measures ANOVA showed a significant effect of days). The average number of minutes per day during which retreat-site temperatures fell within the preferred temperature range (Tpref) for skinks (25–35 °C) was similar across burn severity groups (F2,47 = 0.28, p = 0.76). On average, the minutes per day within Tpref were 66.1 min (SE = 18.6) in low-severity plots, 67.4 min (SE = 10.5) in unburnt plots, and 78.9 min (SE = 12.8) in high-severity plots. Mean daily minimum temperatures were lower in retreat sites in high-severity fire plots (4.48 °C ± 0.02) than in unburnt (6.76 °C ± 0.19) or low-severity plots (6.35 °C ± 0.31, F2,48 = 31.71, p < 0.0001). There was a significant day x fire severity interaction (F7, 170 = 5.09, p < 0.001); inspection of plots of daily mean temperatures showed that on all but four days, minimum temperatures were lower in high-severity plots than in other plots. Mean daily maximum temperatures were similar in low-severity plots (mean = 24.6 °C ± 2.3), unburnt plots (26.9 °C ± 1.4), and high-severity plots (28.6 °C ± 1.7, F2, 48 = 0.97, p = 0.39). Average daily temperatures were also similar in high (11.20 °C ± 1.38) and low-severity plots (11.70 °C ± 1.57) and in unburnt plots (12.35 ± 1.51, F2, 48 = 2.39, p = 0.10).
4. Discussion
We found that fire severity influenced vegetation structure in open woodland, but this did not translate to differences in reptile species richness. Our prediction that habitat variables would decrease with increasing fire severity was both supported and contradicted. Canopy openness was lowest in high fire severity plots, supporting our prediction and the findings of previous studies [13,42]. Few trees escape canopy scorch in high-severity fires [43], and three years after the 2019–2020 megafires, plots in high-severity burnt sites still had less canopy cover than those in unburnt sites. In agreement with previous studies, leaf litter depth was lower in burnt plots than in unburnt plots [30].
Contradictory to our predictions, there were more understorey stems and a higher understorey in high-severity fire plots than in low-severity fire or unburnt plots. This result supports a recent study in Eucalypt forests in the Sydney region that found that high-severity fire plots had a denser and higher understorey than low-severity fire plots [42]. Another study in Victoria found that two years after high-severity fires, burnt plots had a higher density of Eucalyptus saplings than unburnt plots [44]. A study in drier sclerophyll forests in the Warrumbungle National Park also recorded higher densities of Acacia shrubs two years post-fire compared to before fire. In our study, high-severity plots had no vegetation cover when they were established in late 2020, but there was above-average rainfall in 2021 and 2022. Hence, the removal of canopy and vegetation in high-severity plots would have allowed light to reach the forest floor, allowing epicormic buds or lignotubers of shrubs and trees to resprout. Furthermore, many Australian plants retain a seedbank in the soil or on plants, and high-severity fire is known to promote mass germination of fire-dependent species [45].
Although vegetation structure varied with fire severity, we found no difference in reptile species richness between plots with different fire severity, a result that supports previous studies [14,46]. However, other studies have found higher species richness in unburnt sites than in burnt sites [47,48]. Our findings in this respect may reflect sparse data, as we only captured 44 reptiles (Table 1). While we detected few animals, species richness was similar to that recorded by other studies in south-eastern Australia, which had much greater sampling effort [49]. We note that other studies on reptiles, using similar detection methods, have also reported very low detections; for example, a long-term study carried out in Booderee National Park, roughly 30 km from our southernmost monitoring plots, reported very low detections for almost all species [49]. For example, from 2011–2022, 24 individual Ctenotus taeniolatus were detected in 19 of 2429 surveys (0.78%) [49], which is similar to our results (2 detected from 140 surveys = 1.42%).
Weather conditions may also have affected our ability to detect some species. Our study was carried out from autumn to spring, when temperatures are cooler and when larger heliothermic reptiles (e.g., varanids, agamids) are less likely to be active [50]. Thus, it is not surprising that we failed to detect these species. Other studies have also found that weather conditions can influence the likelihood of detecting reptiles [49,50]. For example, Evans et al. found that the likelihood of detecting reptiles varied with weather and time since fire. In that study, the probability of detecting the most common species, Lampropholis delicata, declined from 50% to just 10% over the period 2011 to 2022 [49]. Hence, the low detections of reptiles in unburnt plots in our study might reflect declines caused by the drought that preceded the megafires and/or variability in detection due to weather. Additionally, species may respond less to fire severity than other variables such as rainfall or microhabitat features [51].
Previous studies have found that opportunities for reptile thermoregulation can vary with fire severity [13]. Although high-severity fire plots had less canopy cover than other sites, the thermal quality of retreat sites did not vary substantially across fire severity classes. Mean daily temperatures, maximum temperatures, and minutes within Tpref were similar across treatment plots. This counterintuitive result likely occurred because although high-severity plots had less canopy cover than other plots, they had more stems and a higher understorey than plots within unburnt or low-severity burnt areas. Thus, shading from understorey vegetation may have contributed to the similarity in thermal regimes across fire severity classes. While our focus was on retreat-site temperatures, we note that there was heterogeneity in vegetation cover in plots, which active reptiles could have exploited to thermoregulate. This might explain the similarity in species richness across the fire treatments.
The occupancy of the most abundant species, Lampropholis delicata, was unaffected by fire severity. Because it is a generalist, this species can cope with a wide range of vegetation and habitat types. Thermal conditions in retreat sites used L. delicata were similar across the plots, which would have favoured this species. Similarly, another generalist, Lampropholis guichenoti, was detected at both unburnt and high-severity plots. Interestingly, a predator of these skinks, the mustard-bellied snake Drysdalia rhodogaster, was detected at burnt and unburnt sites. Broader-scale surveys after the black-summer fires also found that the occupancy of D. rhodogaster was also unaffected by fire severity [52].
Sparse data meant that we could make few inferences about the effects of the 2019–2020 fires (and fire severity) on other reptile species. The red-throated skink Acritoscincus platynotus was detected at burnt sites but was not detected at unburnt plots. The diurnally active skink Ctenotus taeniolatus was only detected at high-severity plots, a finding consistent with previous studies, which found that this species prefers open habitats [53]. By contrast, Saproscincus mustelina, a litter-dependent species that prefers moist shaded sites [31], was only detected at two low-severity plots, which had dense leaf litter. As litter-dependent species might be at greater risk of mortality during fires or post-fire predation, longer-term monitoring is needed to evaluate the impacts of the 2019–2020 fires on such species [18].
5. Conclusions
We found no significant effects of fire severity on reptile species richness, suggesting that the reptile assemblage was resilient to high-severity fire. While high-severity fire influenced vegetation structure, this did not translate to significant differences in the thermal quality of the habitat. Further studies quantifying the responses of reptiles to fire severity in other fire-prone regions in Australia affected by megafires are necessary to determine if our findings are only locally or more generally applicable.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fire7100349/s1, Table S1: Excel file with data used in all statistical analyses.
Author Contributions
Conceptualization and methodology, M.L.A., M.L., B.R.M. and J.K.W.; statistical analysis, M.L.A. and J.K.W.; field work, M.L.A.; writing—original draft preparation, M.L.A.; writing—review and editing, M.L.A., M.L, B.R.M. and J.K.W.; supervision, J.K.W. and B.R.M.; project administration, J.K.W.; funding acquisition, J.K.W. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a federally funded grant entitled “Wildfire impact on threatened reptiles” (GA-2000634) under Tranche 2 of the Wildlife and Habitat Bushfire Recovery Program 2019-20.
Institutional Review Board Statement
The study on animals was approved by the University of Technology Sydney Animal Care and Ethics Committee (protocol: #ETH21-6233), and the research was approved by the NSW National Parks and Wildlife Service (SL 102573).
Data Availability Statement
Data can be downloaded in the Supplementary Information Section.
Acknowledgments
We thank Bruce Gray from Nowra NPWS for granting fire trail access, and Maddison Gatenby, Lauren Crofts, Dylan Archer, Joshua Ward, and Tess Paterson for their assistance with the field work. We thank the three anonymous reviewers for their helpful comments and suggestions, which helped us to improve the earlier version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tran, B.N.; Tanase, M.A.; Bennett, L.T.; Aponte, C. High-severity wildfires in temperate Australian forests have increased in extent and aggregation in recent decades. PLoS ONE 2020, 15, e0242484. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Parr, C.L. Towards an understanding of the evolutionary role of fire in animals. Evol. Ecol. 2018, 32, 113–125. [Google Scholar] [CrossRef]
- Legge, S.; Woinarski, J.C.Z.; Scheele, B.C.; Garnett, S.T.; Lintermans, M.; Nimmo, D.G.; Whiterod, N.S.; Southwell, D.M.; Ehmke, G.; Buchan, A.; et al. Rapid assessment of the biodiversity impacts of the 2019–2020 Australian megafires to guide urgent management intervention and recovery and lessons for other regions. Divers. Distrib. 2021, 28, 571–591. [Google Scholar] [CrossRef]
- Santos, X.; Belliure, J.; Gonçalves, J.F.; Pausas, J.G. Resilience of reptiles to megafires. Ecol. Appl. 2022, 32, e2518. [Google Scholar] [CrossRef] [PubMed]
- Tomas, W.M.; Berlinck, C.N.; Chiaravalloti, R.M.; Faggioni, G.P.; Strüssmann, C.; Libonati, R.; Abrahão, C.R.; do Valle Alvarenga, G.; de Faria Bacellar, A.E.; de Queiroz Batista, F.R.; et al. Distance sampling surveys reveal 17 million vertebrates directly killed by the 2020’s wildfires in the Pantanal, Brazil. Sci. Rep. 2021, 11, 23547. [Google Scholar] [CrossRef]
- Griffiths, A.D.; Christian, K.A. The effects of fire on the frillneck lizard (Chlamydosaurus kingii) in northern Australia. Aust. J. Ecol. 1996, 21, 386–398. [Google Scholar] [CrossRef]
- Ward, M.; Tulloch, A.I.T.; Radford, J.Q.; Williams, B.A.; Reside, A.E.; Macdonald, S.L.; Mayfield, H.J.; Maron, M.; Possingham, H.P.; Vine, S.J.; et al. Impact of 2019-2020 mega-fires on Australian fauna habitat. Nat. Ecol. Evol. 2020, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, D.G.; Carthey, A.J.R.; Jolly, C.J.; Blumstein, D.T. Welcome to the Pyrocene: Animal survival in the age of megafire. Glob. Chang. Biol. 2021, 27, 5684–5693. [Google Scholar] [CrossRef]
- Jolly, C.J.; Dickman, C.R.; Doherty, T.S.; van Eeden, L.M.; Geary, W.L.; Legge, S.M.; Woinarski, J.C.Z.; Nimmo, D.G. Animal mortality during fire. Glob. Chang. Biol. 2022, 28, 2053–2065. [Google Scholar] [CrossRef]
- Nimmo, D.G.; Kelly, L.T.; Farnsworth, L.M.; Watson, S.J.; Bennett, A.F. Why do some species have geographically varying responses to fire history? Ecography 2014, 37, 805–813. [Google Scholar] [CrossRef]
- Driscoll, D.A.; Smith, A.L.; Blight, S.; Maindonald, J. Reptile responses to fire and the risk of post-disturbance sampling bias. Biodivers. Conserv. 2012, 21, 1607–1625. [Google Scholar] [CrossRef]
- Pringle, R.M.; Webb, J.K.; Shine, R. Canopy structure, microclimate, and habitat selection by a nocturnal snake. Hoplocephalus bungaroides. Ecology 2003, 84, 2668–2679. [Google Scholar] [CrossRef]
- Elzer, A.L.; Pike, D.A.; Webb, J.K.; Hammill, K.; Bradstock, R.A.; Shine, R. Forest-fire regimes affect thermoregulatory opportunities for terrestrial ectotherms. Austral Ecol. 2013, 38, 190–198. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Wood, J.T.; MacGregor, C.; Michael, D.R.; Cunningham, R.B.; Crane, M.; Montague-Drake, R.; Brown, D.; Muntz, R.; Driscoll, D.A. How predictable are reptile responses to wildfire? Oikos 2008, 117, 1086–1097. [Google Scholar] [CrossRef]
- Webb, J.K.; Shine, R. Differential effects of an intense wildfire on survival of sympatric snakes. J. Wildl. Manag. 2008, 72, 1394–1398. [Google Scholar] [CrossRef]
- Webb, J.K.; Jolly, C.J.; Hinds, M.; Adams, C.; Cuartas-Villa, S.; Lapwong, Y.; Letnic, M. Effects of the Australian 2019–2020 megafires on a population of endangered broad-headed snakes Hoplocephalus bungaroides. Austral Ecol. 2023, 48, 24–30. [Google Scholar] [CrossRef]
- Letnic, M.; Roberts, B.; Hodgson, M.; Ross, A.K.; Cuartas, S.; Lapwong, Y.; Price, O.; Sentinella, N.; Webb, J.K. Fire severity influences the post-fire habitat structure and abundance of a cool climate lizard. Austral Ecol. 2023, 48, 1440–1453. [Google Scholar] [CrossRef]
- Price, O.F.; Mikac, K.; Wilson, N.; Roberts, B.; Critescu, R.H.; Gallagher, R.; Mallee, J.; Donatiou, P.; Webb, J.; Keith, D.A.; et al. Short-term impacts of the 2019–20 fire season on biodiversity in eastern Australia. Austral Ecol. 2023, 48, 3–11. [Google Scholar] [CrossRef]
- Driscoll, D.A.; Lindenmayer, D.B.; Bennett, A.F.; Bode, M.; Bradstock, R.A.; Cary, G.J.; Clarke, M.F.; Dexter, N.; Fensham, R.; Friend, G.; et al. Fire management for biodiversity conservation: Key research questions and our capacity to answer them. Biol. Conserv. 2010, 143, 1928–1939. [Google Scholar] [CrossRef]
- Boer, M.M.; Resco de Dios, V.; Bradstock, R.A. Unprecedented burn area of Australian mega forest fires. Nat. Clim. Chang. 2020, 10, 171–172. [Google Scholar] [CrossRef]
- King, A.D.; Pitman, A.J.; Henley, B.J.; Ukkola, A.M.; Brown, J.R. The role of climate variability in Australian drought. Nat. Clim. Chang. 2020, 10, 177–179. [Google Scholar] [CrossRef]
- Collins, L.; Bradstock, R.A.; Clarke, H.; Clarke, M.F.; Nolan, R.H.; Penman, T.D. The 2019/2020 mega-fires exposed Australian ecosystems to an unprecedented extent of high-severity fire. Environ. Res. Lett. 2021, 16, 044029. [Google Scholar] [CrossRef]
- Keeley, J.E.; Fotheringham, C.J.; Baer-Keeley, M. Determinants of postfire recovery and succession in mediterranean-climate shrublands of California. Ecol. Appl. 2005, 15, 1515–1534. [Google Scholar] [CrossRef]
- Santos, X.; Badiane, A.; Matos, C. Contrasts in short- and long-term responses of Mediterranean reptile species to fire and habitat structure. Oecologia 2016, 180, 205–216. [Google Scholar] [CrossRef]
- Doherty, T.S.; Geary, W.L.; Jolly, C.J.; Macdonald, K.J.; Miritis, V.; Watchorn, D.J.; Cherry, M.J.; Conner, L.M.; González, T.M.; Legge, S.M.; et al. Fire as a driver and mediator of predator–prey interactions. Biol. Rev. Camb. Philos. Soc. 2022, 97, 1539–1558. [Google Scholar] [CrossRef]
- Black, D.E. The Vegetation of the Ettrema and Northern Budawangs Wilderness Areas, Morton National Park, New South Wales. Master’s Thesis, Department of Geology, University of Wollongong, Wollongong, Australia, 1995. [Google Scholar]
- State Government of NSW and NSW Department of Climate Change, Energy, the Environment and Water. Fire Extent and Severity Mapping (FESM). In The Sharing and Enabling Environmental Data Portal; 2020. Available online: https://datasets.seed.nsw.gov.au/dataset/33c2ee86-d2f7-4aaf-8c40-76b6d393a35c (accessed on 27 August 2024).
- Gibson, R.; Danaher, T.; Hehir, W.; Collins, L. A remote sensing approach to mapping fire severity in south-eastern Australia using sentinel 2 and random forest. Remote Sens. Environ. 2020, 240, 111702. [Google Scholar] [CrossRef]
- Michael, D.R.; Florance, D.; Crane, M.; Blanchard, W.; Lindenmayer, D.B. Barking up the right tree: Comparative use of arboreal and terrestrial artificial refuges to survey reptiles in temperate eucalypt woodlands. Wildl. Res. 2018, 45, 185–192. [Google Scholar] [CrossRef]
- Foster, C.N.; Barton, P.S.; Sato, C.F.; Wood, J.T.; MacGregor, C.I.; Lindenmayer, D.B. Herbivory and fire interact to affect forest understory habitat, but not its use by small vertebrates. Anim. Conserv. 2016, 19, 15–25. [Google Scholar] [CrossRef]
- Wilson, S.; Swan, G. A Complete Guide to Reptiles of Australia; Reed New Holland Publishers: Sydney, Australia, 2021. [Google Scholar]
- Doherty, T.S.; Davis, R.A.; van Etten, E.J.B.; Collier, N.; Krawiec, J. Response of a shrubland mammal and reptile community to a history of landscape-scale wildfire. Int. J. Wildland Fire 2015, 24, 534–543. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Spearman, C. The proof and measurement of association between two things. Am. J. Psychol. 1904, 15, 72–101. [Google Scholar] [CrossRef]
- Bakus, J.G. Types of data, standardizations and transformations, introduction to biometrics, experimental design. In Quantitative Analysis of Marine Biological Communities; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 62–122. [Google Scholar]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.; Hines, J.E. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence; Elsevier Science & Technology: San Diego, CA, USA, 2017. [Google Scholar]
- MacKenzie, D.I.; Nichols, J.D.; Lachman, G.B.; Droege, S.; Royle, J.A.; Langtimm, C.A. Estimating site occupancy rates when detection probabilities are less than one. Ecology 2002, 83, 2248–2255. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Goulet, C.T.; Thompson, M.B.; Chapple, D.G. Repeatability and correlation of physiological traits: Do ectotherms have a “thermal type”? Ecol. Evol. 2017, 7, 710–719. [Google Scholar] [CrossRef]
- Blanca, M.J.; Arnau, J.; García-Castro, F.J.; Alarcón, R.; Bono, R. Repeated measures ANOVA and adjusted F-tests when sphericity is violated: Which procedure is best? Front. Psychol. 2023, 14, 1192453. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.W.; Price, O.F.; Jenkins, M.E. High severity fire promotes a more flammable eucalypt forest structure. Austral Ecol. 2022, 47, 519–529. [Google Scholar] [CrossRef]
- Denham, A.J.; Vincent, B.E.; Clarke, P.J.; Auld, T.D. Responses of tree species to a severe fire indicate major structural change to Eucalyptus–Callitris forests. Plant Ecol. 2016, 217, 617–629. [Google Scholar] [CrossRef]
- Bassett, M.; Leonard, S.W.J.; Chia, E.K.; Clarke, M.F.; Bennett, A.F. Interacting effects of fire severity, time since fire and topography on vegetation structure after wildfire. For. Ecol. Manag. 2017, 396, 26–34. [Google Scholar] [CrossRef]
- Gordon, C.E.; Price, O.F.; Tasker, E.M.; Denham, A.J. Acacia shrubs respond positively to high severity wildfire: Implications for conservation and fuel hazard management. Sci. Total Environ. 2017, 575, 858–868. [Google Scholar] [CrossRef]
- Lindsay, M.N.; Lewis, D.B.; Halstead, N.; Gainsbury, A.M. Fire severity effects on the herpetofaunal diversity of the Florida scrub, a biodiversity hotspot. Biodivers. Conserv. 2023, 32, 1857–1878. [Google Scholar] [CrossRef]
- Legge, S.; Murphy, S.; Heathcote, J.; Flaxman, E.; Augusteyn, J.; Crossman, M. The short-term effects of an extensive and high-intensity fire on vertebrates in the tropical savannas of the central Kimberley, northern Australia. Wildl. Res. 2008, 35, 33–43. [Google Scholar] [CrossRef]
- Taylor, J.E.; Ellis, M.V.; Williams, N.; Kloecker, U. Responses of birds and reptiles in Warrumbungle National Park after the extensive 2013 wildfire. Proc. Linn. Soc. N. S. W. 2020, 142, S155–S208. [Google Scholar]
- Evans, M.J.; MacGregor, C.; Lindenmayer, D. A misleading tail: A long-term study of reptile responses to multiple disturbances undermined by a change in surveying techniques. PLoS ONE 2024, 19, e0305518. [Google Scholar] [CrossRef] [PubMed]
- Spence-Bailey, L.M.; Nimmo, D.G.; Kelly, L.T.; Bennett, A.F.; Clarke, M.F. Maximising trapping efficiency in reptile surveys: The role of seasonality, weather conditions and moon phase on capture success. Wildl. Res. 2010, 37, 104–115. [Google Scholar] [CrossRef]
- Letnic, M.; Dickman, C.R.; Tischler, M.K.; Tamayo, B.; Beh, C.L. The responses of small mammals and lizards to post-fire succession and rainfall in arid Australia. J. Arid Environ. 2004, 59, 85–114. [Google Scholar] [CrossRef]
- Hodgson, M.; Ross, A.; Lapwong, Y.; Cuartas, S.; Roberts, B.; Price, O.; Webb, J.K.; Sentinella, N.; Lee, J.; Laffan, S.; et al. A cryptic elapid snake persists in the wake of catastrophic wildfires. Oryx 2024, in press.
- Taylor, J.E.; Fox, B.J. Disturbance effects from fire and mining produce different lizard communities in eastern Australian forests. Austral Ecol. 2001, 26, 193–204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).