Experimental Study on the Thermal Behavior Characteristics of the Oxidative Spontaneous Combustion Process of Fischer–Tropsch Wax Residue
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sample
2.2. DSC Apparatus
2.3. Thermokinetic Analysis Method
3. Experimental Results and Discussion
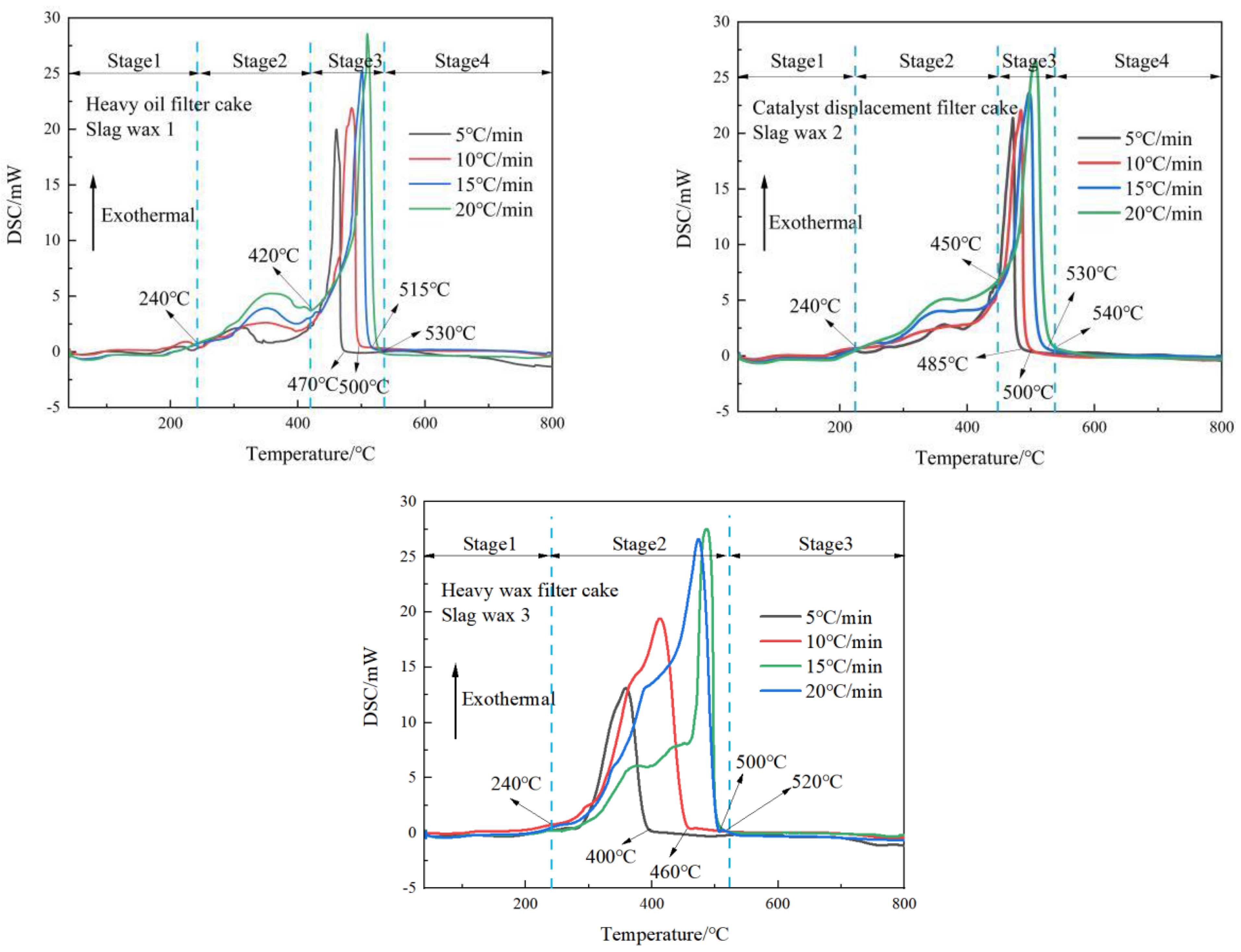
3.1. Analysis of Thermal Behavior Characteristics
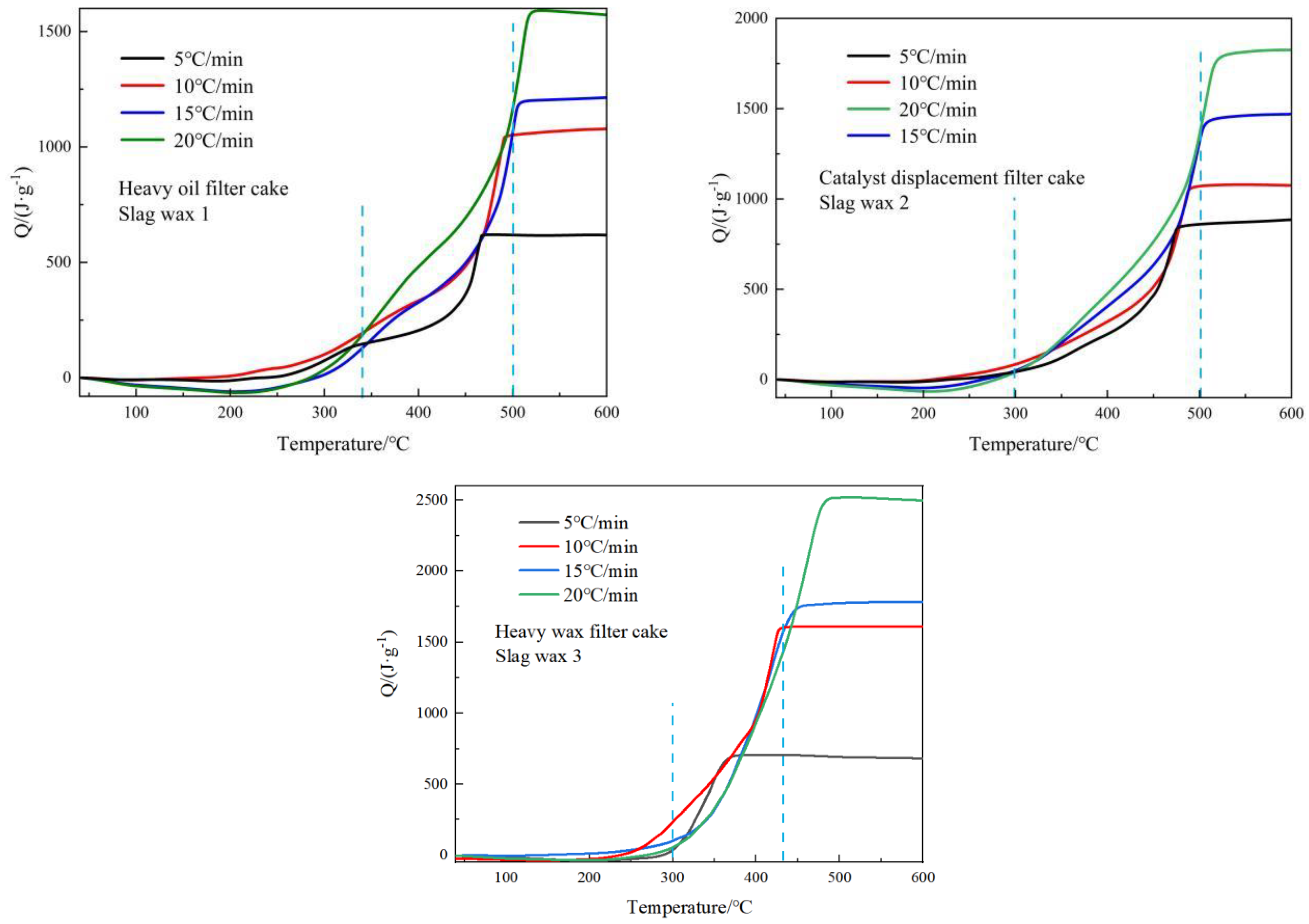
3.2. Exothermic Characteristics Analysis
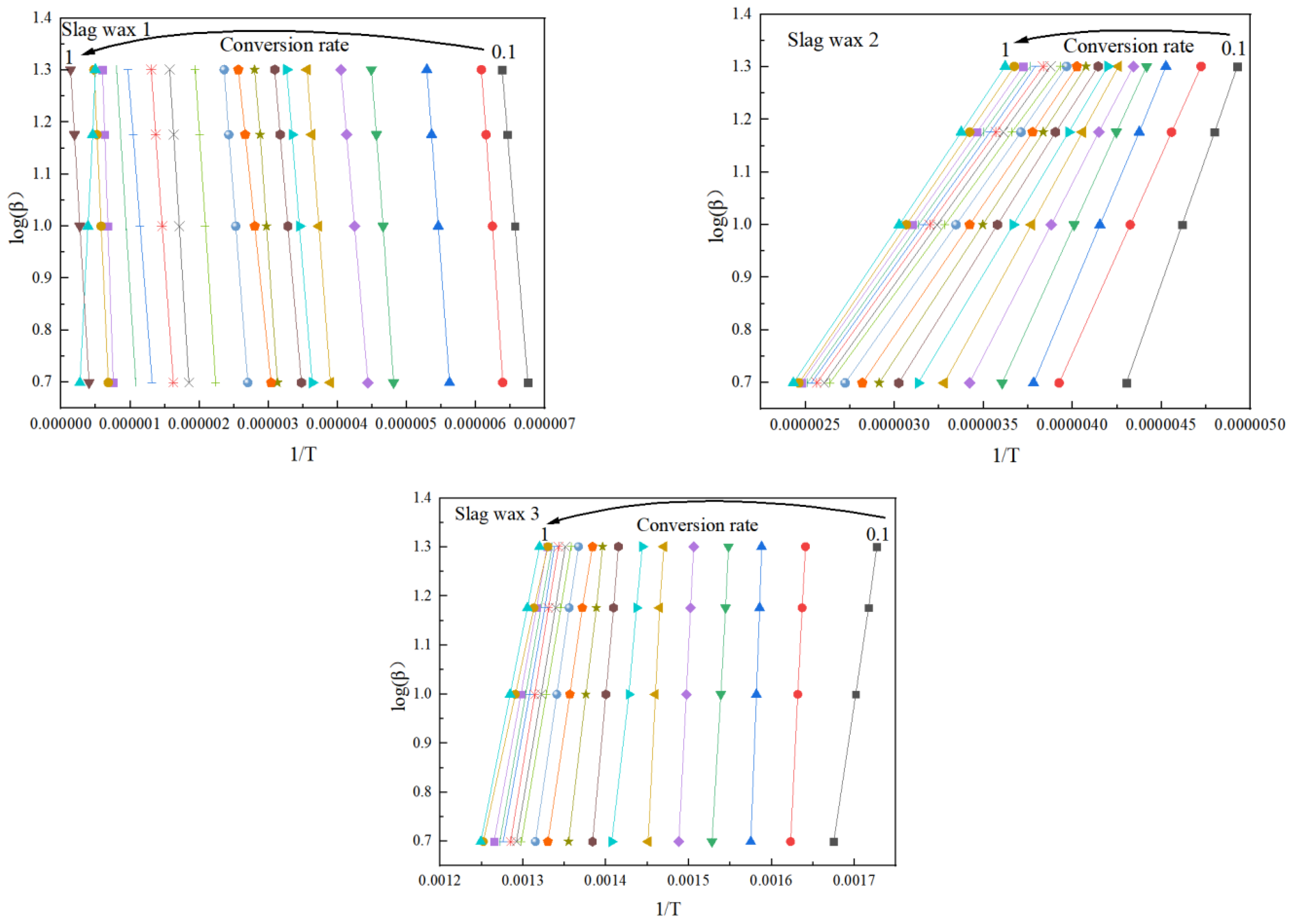
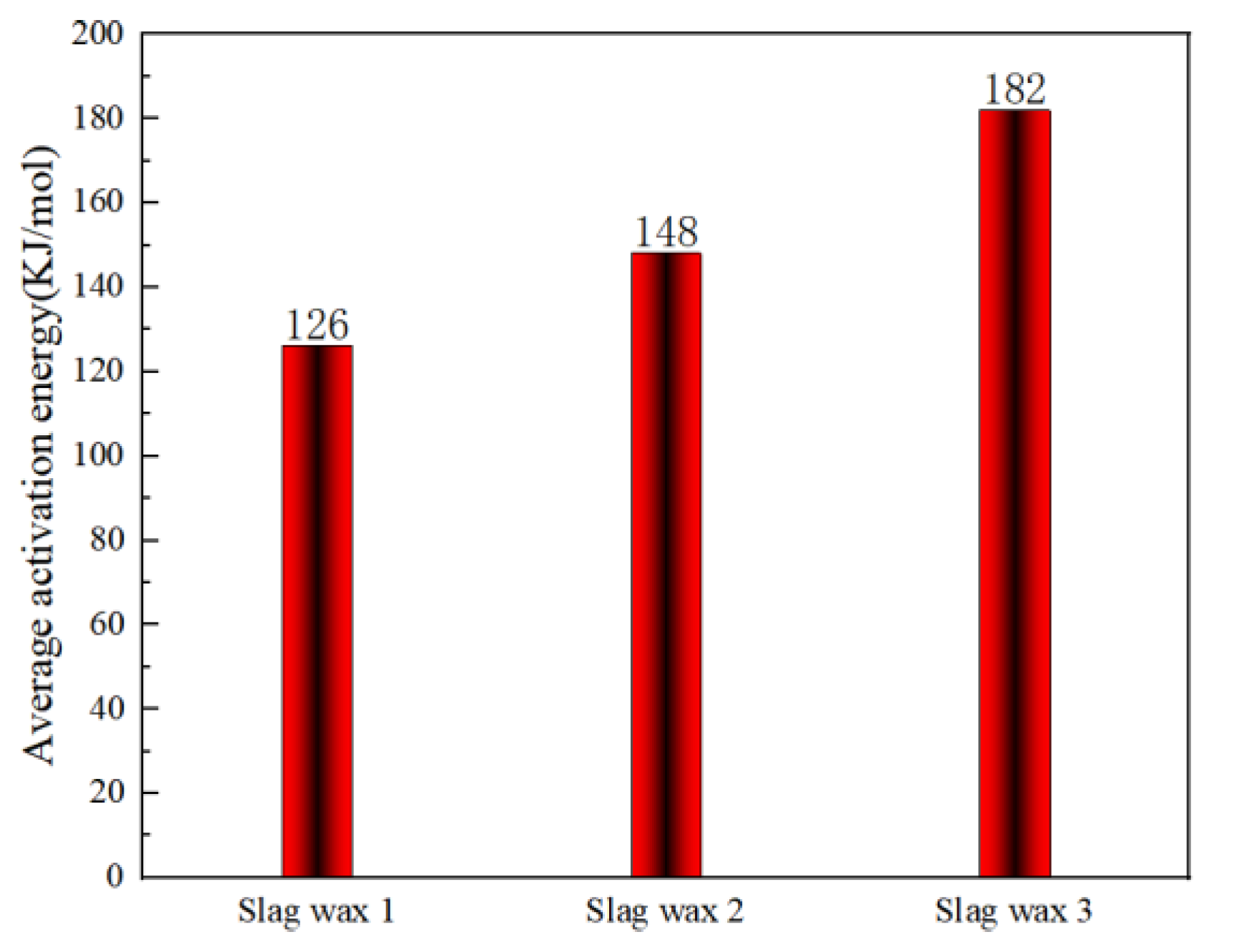
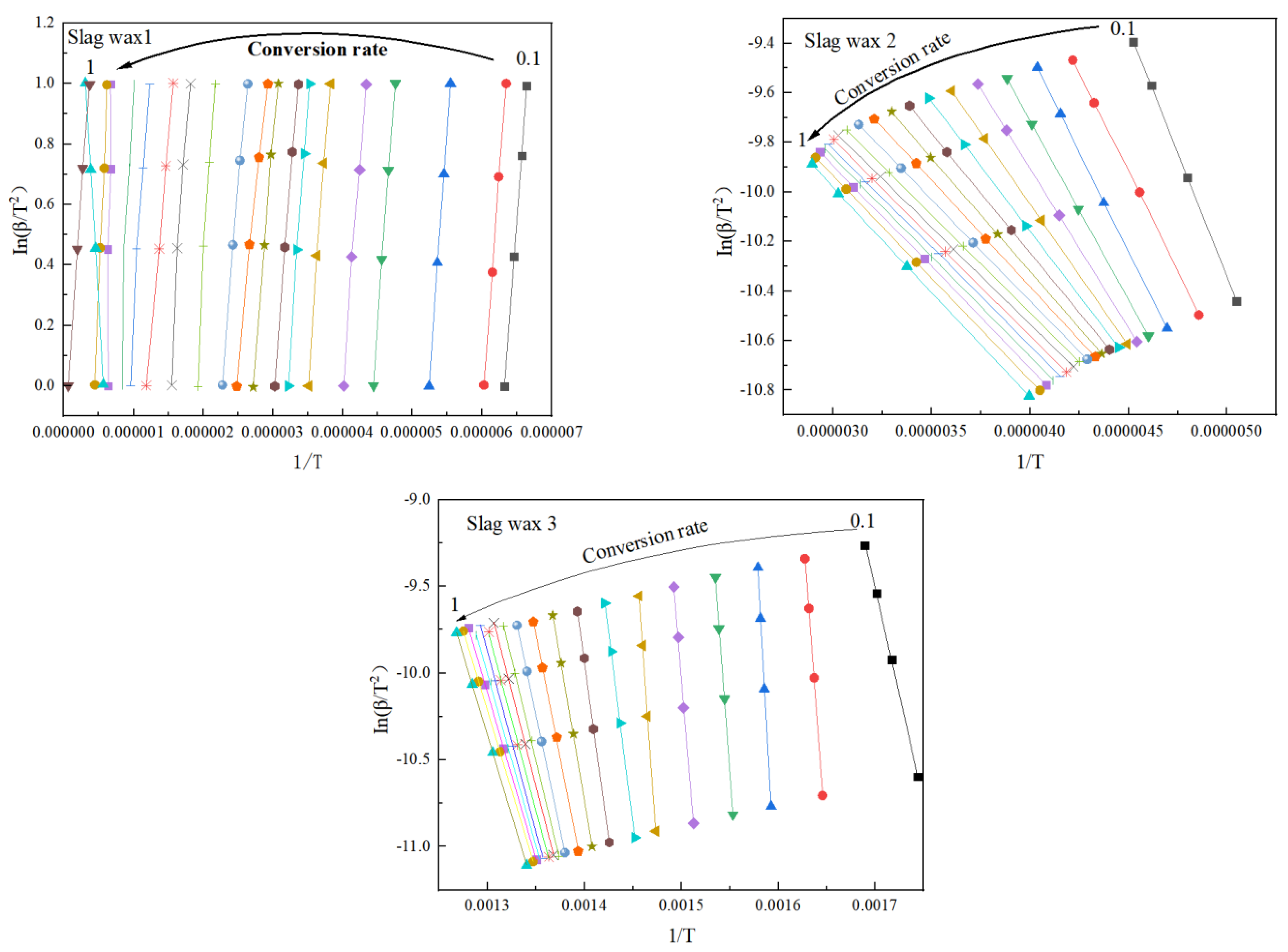
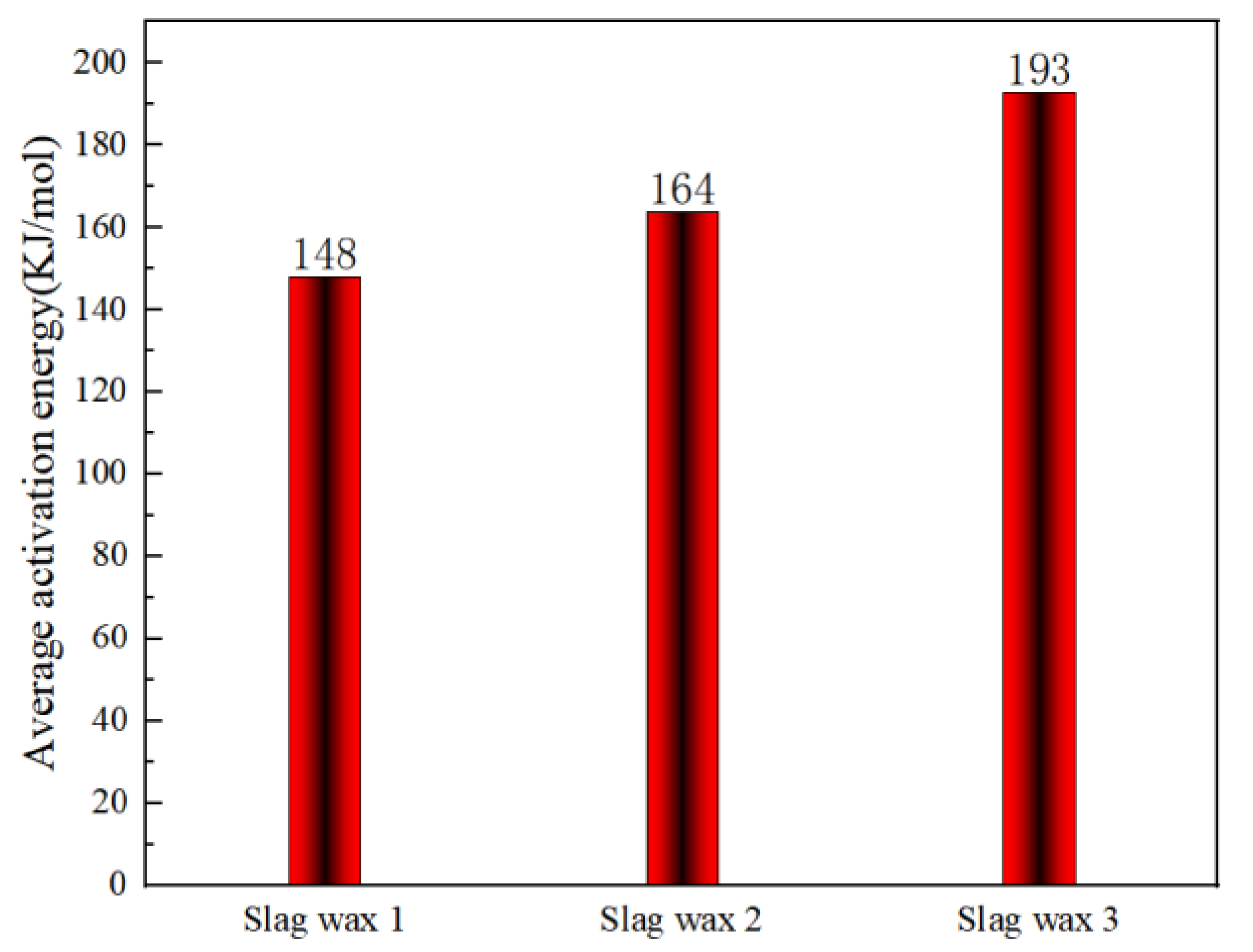
3.3. Kinetic Analysis
3.3.1. FWO
3.3.2. KAS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novikau, A. Energy security in security studies: A systematic review of twenty years of literature. Cent. Eur. J. Int. Secur. Stud. 2023, 17, 36–64. [Google Scholar] [CrossRef]
- Lin, T.; An, Y.; Yu, F.; Gong, K.; Yu, H.; Wang, C.; Sun, Y.; Zhong, L. Advances in selectivity control for Fischer–Tropsch synthesis to fuels and chemicals with high carbon efficiency. ACS Catal. 2022, 12, 12092–12112. [Google Scholar] [CrossRef]
- Okoye-Chine, C.; Mubenesha, S. The Use of Iron Ore as a Catalyst in Fischer–Tropsch Synthesis—A Review. Crystals 2022, 12, 1349. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, S.; Zhang, C.; Li, X.; Guo, Y. Adsorption decolorization and composition analysis of high melting point Fischer–Tropsch waxes. Asia-Pac. J. Chem. Eng. 2023, 18, e2857. [Google Scholar] [CrossRef]
- Chernyak, S.; Burtsev, A.; Maksimov, S.; Kupreenko, S.; Maslakov, K.; Savilov, S. Structural evolution, stability, deactivation and regeneration of Fischer-Tropsch cobalt-based catalysts supported on carbon nanotubes. Appl. Catal. A-Gen. 2020, 603, 117741. [Google Scholar] [CrossRef]
- Liang, C.; Yin, Z.; Sun, Y.; Xu, Y.; Yao, k.; Liu, Z.; Zhu, M. Pyrolysis of waste Fischer-Tropsch wax: An experimental study. J. Clean. Prod. 2022, 350, 131529. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, S.; Xie, Z.; Zhu, K.; Xu, X.; Ding, X.; Glowacz, A. Investigation of the pyrophoric tendency of the powder of corrosion products in an oil tank. Powder Technol. 2018, 339, 296–305. [Google Scholar] [CrossRef]
- Deng, J.; Liu, T.; Yao, M.; Yi, X.; Bai, G.; Huang, Q.; Li, Z. Comparative study of the combustion and kinetic characteristics of fresh and naturally aged pine wood. Fuel 2023, 343, 127962. [Google Scholar] [CrossRef]
- Kubicka, D.; Černý, R. Upgrading of Fischer-Tropsch waxes by fluid catalytic cracking. Ind. Eng. Chem. Res. 2012, 51, 8849–8857. [Google Scholar] [CrossRef]
- Zhao, J.; Li, R.; Song, J.; Lu, S.; Shu, C. Effect of oxygen concentration on the heat release behaviour of bituminous coal over the complete spontaneous combustion process. J. Therm. Anal. Calorim. 2024, 149, 10227–10240. [Google Scholar] [CrossRef]
- Deng, J.; Qu, G.; Ren, S.; Wang, C.; Su, H.; Yuan, Y.; Duan, X.; Yang, N.; Wang, J. Effect of water soaking and air drying on the thermal effect and heat transfer characteristics of coal oxidation at the low-temperature oxidation stage. Energy 2024, 288, 129705. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Z.; Zhai, X.; Wen, H. Thermodynamic behaviors of coal spontaneous combustion under different CO2 concentration. Int. J. Coal Prep. Util. 2023, 43, 1583–1596. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, L.; Liu, H. Experimental research on the activation energy analysis of coal spontaneous combustion based on DSC. Disaster Adv. 2013, 6, 278–283. [Google Scholar]
- Yang, F.; Lai, Y.; Song, Y. Determination of the influence of pyrite on coal spontaneous combustion by thermodynamics analysis. Process Saf. Env. 2019, 129, 163–167. [Google Scholar] [CrossRef]
- Li, X.; Jin, Z.; Bai, G. Experimental study on the effect of acidity on coal spontaneous combustion at different oxygen concentrations. Energy Source Part A 2020, 1–10. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, T.; Jia, H.; Hu, D.; Wang, L. Study of the mutual coupling characteristics of the oxidation thermal effect and microstructural evolution of gas-containing coal. Sci. Total Env. 2024, 924, 171574. [Google Scholar] [CrossRef]
- Qin, B.; Dou, G.; Wang, D. Thermal analysis of vitamin C affecting low-temperature oxidation of coal. J. Wuhan Univ. Technol. 2016, 31, 519–522. [Google Scholar] [CrossRef]
- Dong, X.; Wang, F.; Guo, L.; Han, T. Study on the Influence of Coal Structure and Oxidation Performance by Endogenous Bacterium. Fire 2023, 6, 339. [Google Scholar] [CrossRef]
- Bekhouche, S.; Trache, D.; Akbi, H.; Abdelaziz, A.; Tarchoun, A.F.; Boudouh, H. Thermal decomposition behavior and ki-netic study of nitrocellulose in presence of ternary nanothermites with different oxi-dizers. FirePhysChem 2023, 3, 208–216. [Google Scholar] [CrossRef]
- Pal, Y.; Mahottamananda, S.N.; Subha, S.; Palateerdham, S.K.; Ingenito, A. Thermal decomposition kinetics and combustion performance of paraffin-based fuel in the presence of CeO2 catalyst. FirePhysChem 2023, 3, 217–226. [Google Scholar] [CrossRef]
- Chalghoum, F.; Jouini, M.; Abdelaziz, A.; Tarchoun, A.F.; Boukeciat, H.; Bekhouche, S.; Benziane, M.; Trache, D. Effect of the accelerated aging process on the thermal decomposition of LiAlH4-based composite solid propellants. FirePhysChem, 2024; in press. [Google Scholar] [CrossRef]
- Mohalik, N.; Mandal, S.; Ray, S.; Khan, A.; Mishra, D.; Pandey, J. TGA/DSC study to characterise and classify coal seams conforming to susceptibility towards spontaneous combustion. Int. J. Min. Sci. Technol. 2022, 32, 75–88. [Google Scholar] [CrossRef]
- Daoudi, M.; Triki, A.; Redjaimia, A. DSC study of the kinetic parameters of the metastable phases formation during non-isothermal annealing of an Al–Si–Mg alloy. J. Therm. Anal. Calorim. 2011, 104, 627–633. [Google Scholar] [CrossRef]
- Khedri, S.; Elyasi, S. Kinetic analysis for thermal cracking of HDPE: A new isoconversional approach. Polym. Degrad. Stabil. 2016, 129, 306–318. [Google Scholar] [CrossRef]
- Sopaci, S.; Nazir, H.; Emir, E.; Atakol, O.; Oz, S. Thermal kinetic analysis, theoretical thermodynamic calculations and antimicrobial activity of three new energetic materials. J. Therm. Anal. Calorim. 2018, 131, 3105–3120. [Google Scholar] [CrossRef]
- Wang, K.; Hu, L.; Deng, J.; Zhang, Y. Multiscale thermal behavioral characterization of spontaneous combustion of pre-oxidized coal with different air exposure time. Energy 2023, 262, 125397. [Google Scholar] [CrossRef]
- Hernowo, P.; Steven, S.; Restiawaty, E.; Bindar, Y. Nature of mathematical model in lignocellulosic biomass pyrolysis process kinetic using volatile state approach. J. Taiwan Inst. Chem. E 2022, 139, 104520. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Wang, G.; Xu, T.; Chai, Y.; Zheng, C.; Xu, R. Kinetics of petroleum coke/biomass blends during co-gasification. Int. J. Min. Met. Mater. 2016, 23, 1001–1010. [Google Scholar] [CrossRef]
- Aghili, A.; Shabani, A. A modification to the Friedman and Ortega isoconversional methods for evaluation of the activation energy as a function of conversion and temperature. Thermochim. Acta 2024, 736, 179748. [Google Scholar] [CrossRef]

| Author | Research Contents | Research Conclusions |
|---|---|---|
| Zhao et al. [10] | Using DSC experiments, the effect of oxygen concentration on the spontaneous combustion and heat release behavior of bituminous coal was studied. | Oxygen increases the apparent activation energy during the spontaneous combustion of bituminous coal and promotes heat release during the spontaneous combustion process. |
| Deng et al. [11] | Using DSC experiments, the effect of moisture content in coal-on-coal oxidation and spontaneous combustion was studied. | Moisture in coal can promote the occurrence of spontaneous combustion. |
| Wang et al. [12] | Using DSC experiments, the effect of CO2 on the thermal behavior of coal spontaneous combustion was studied. | CO2 primarily inhibits the heat release stage during the spontaneous combustion of coal. |
| Zhu et al. [13] | Using DSC experiments, the effects of particle size, heating rate, and oxygen concentration on the spontaneous combustion behavior of coal were studied. | The smaller the coal particle size, the lower the heating rate, and the higher the oxygen concentration, the greater the tendency of the coal to undergo spontaneous combustion. |
| Yang et al. [14] | Using DSC experiments, the effect of pyrite on the spontaneous combustion of coal was studied. | Pyrite increases the probability of spontaneous combustion in coal. |
| Li et al. [15] | Using DSC experiments, the effect of acidity on the spontaneous combustion of coal was studied. | Under the same oxygen concentration, the heat release of neutral coal is greater than that of acidic coal. |
| Pan et al. [16] | Using DSC experiments, the effect of gas on the spontaneous combustion of coal was studied. | As the coal temperature continues to rise, the pore connectivity of high-pressure gas-containing coal is enhanced, increasing the risk of spontaneous combustion. |
| Qin et al. [17] | Using DSC experiments, the effect of vitamin C on the spontaneous combustion of coal was studied. | Vitamin C is more effective than water at inhibiting the spontaneous combustion of coal, but it decomposes at around 200 °C. |
| Dong et al. [18] | Using DSC experiments, the effect of endogenous bacteria on coal combustion was studied. | Endogenous bacteria reduce total heat release during the coal combustion process. |
| Slimane et al. [19] | Using DSC experiments, characterization of the prepared energetic composite material NC/MgAl MXOY was performed. | The effect of oxidants on the decomposition behavior of NC/MgAl MXOY energetic composite materials was discovered. |
| Yash et al. [20] | Thermal decomposition kinetics and combustion performance of paraffin-based fuel was performed in the presence of the CeO2 catalyst and Al additive. | The fuel sample with added additives and catalysts degrades faster and decomposes at a higher rate than the original paraffin sample. |
| Fateh et al. [21] | Using DSC experiments, the thermal properties of the studied propellant samples were measured. | The effect of the aging process on the thermal degradation of aged samples is significantly greater than that of non-aged samples. |
| Sample | Industrial Analysis (wt.%) | Elemental Analysis (wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | C | H | O | N | |
| Slag Wax 1 | 0.36 | 10.78 | 82.60 | 6.26 | 81.92 | 13.99 | 4.04 | 0.05 |
| Slag Wax 2 | 0.63 | 13.67 | 85.57 | 0.13 | 83.23 | 16.45 | 0.29 | 0.03 |
| Slag Wax 3 | 0.77 | 8.65 | 90.32 | 0.26 | 83.95 | 15.90 | 0.13 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Deng, J.; Yao, M.; Yong, X.; Zhao, T.; Yi, X.; He, Y. Experimental Study on the Thermal Behavior Characteristics of the Oxidative Spontaneous Combustion Process of Fischer–Tropsch Wax Residue. Fire 2024, 7, 348. https://doi.org/10.3390/fire7100348
Liu T, Deng J, Yao M, Yong X, Zhao T, Yi X, He Y. Experimental Study on the Thermal Behavior Characteristics of the Oxidative Spontaneous Combustion Process of Fischer–Tropsch Wax Residue. Fire. 2024; 7(10):348. https://doi.org/10.3390/fire7100348
Chicago/Turabian StyleLiu, Tongshuang, Jun Deng, Min Yao, Xiaojing Yong, Tiejian Zhao, Xin Yi, and Yongjun He. 2024. "Experimental Study on the Thermal Behavior Characteristics of the Oxidative Spontaneous Combustion Process of Fischer–Tropsch Wax Residue" Fire 7, no. 10: 348. https://doi.org/10.3390/fire7100348
APA StyleLiu, T., Deng, J., Yao, M., Yong, X., Zhao, T., Yi, X., & He, Y. (2024). Experimental Study on the Thermal Behavior Characteristics of the Oxidative Spontaneous Combustion Process of Fischer–Tropsch Wax Residue. Fire, 7(10), 348. https://doi.org/10.3390/fire7100348






