Species-Abundance Models for the Early Postfire Succession of Subalpine Shrub Grassland
Abstract
1. Introduction
2. Materials and Methods
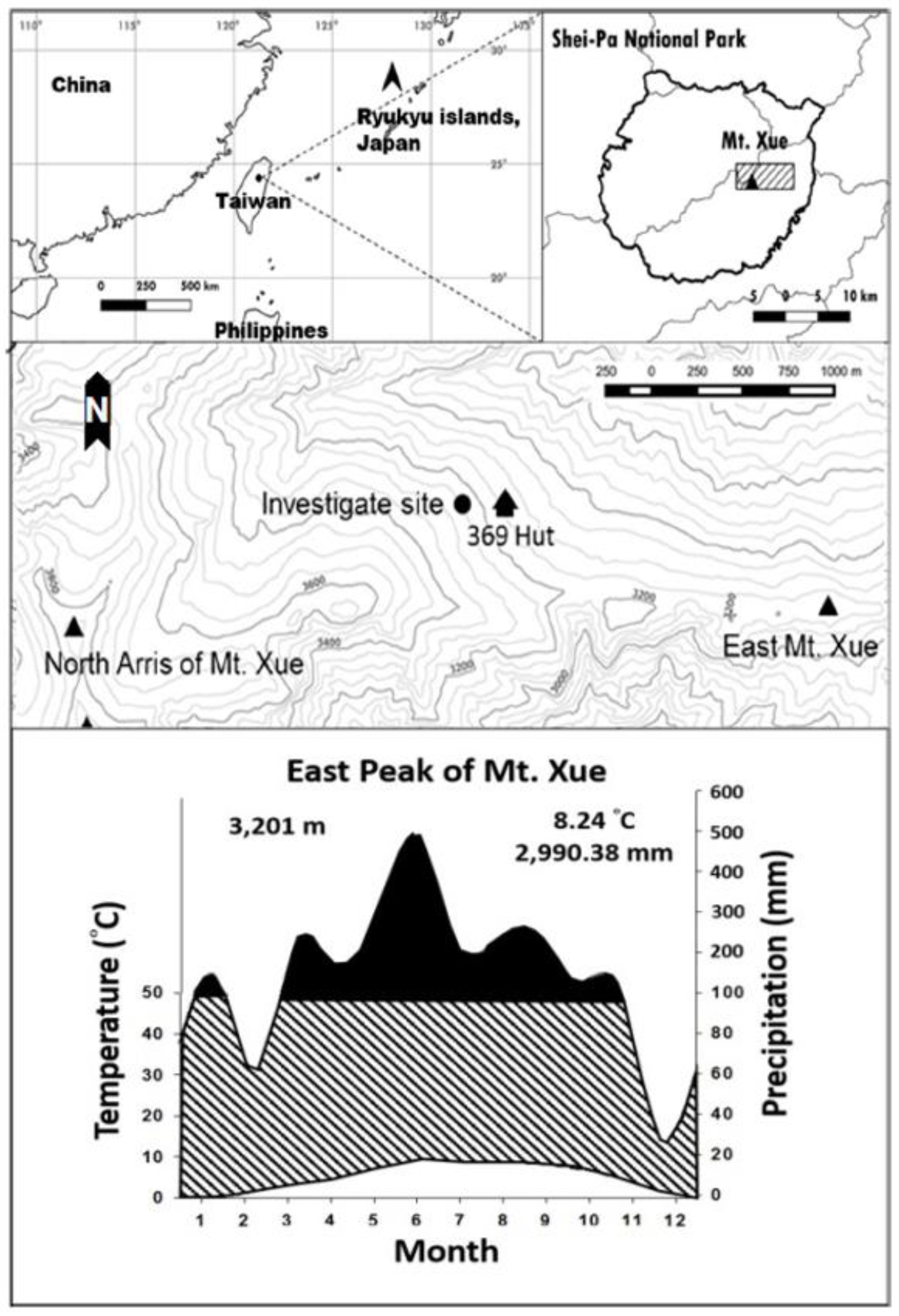
2.1. Study Area
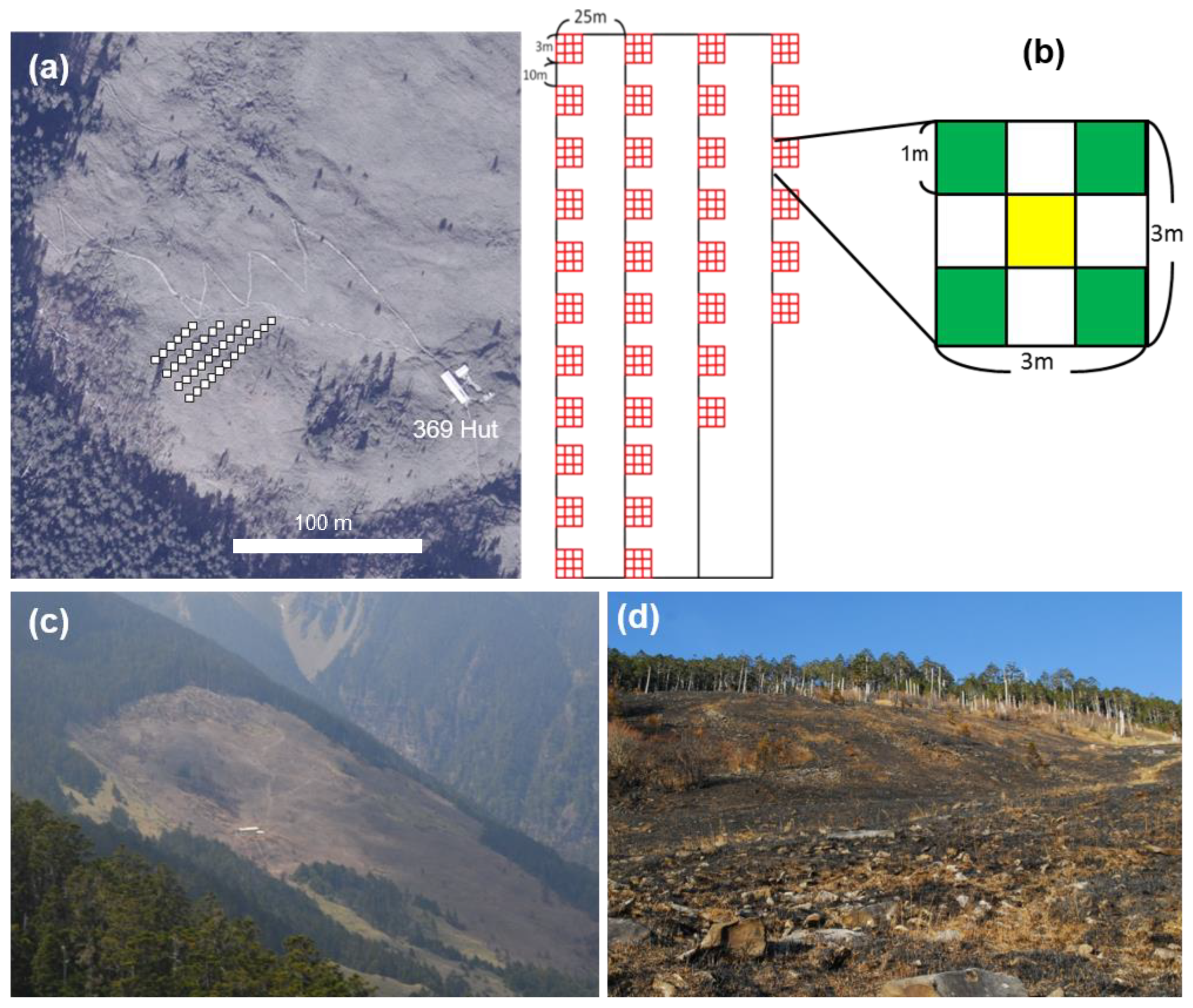
2.2. Sampling and Monitoring
2.3. Data Analysis
2.3.1. Species Diversity during Postfire Succession
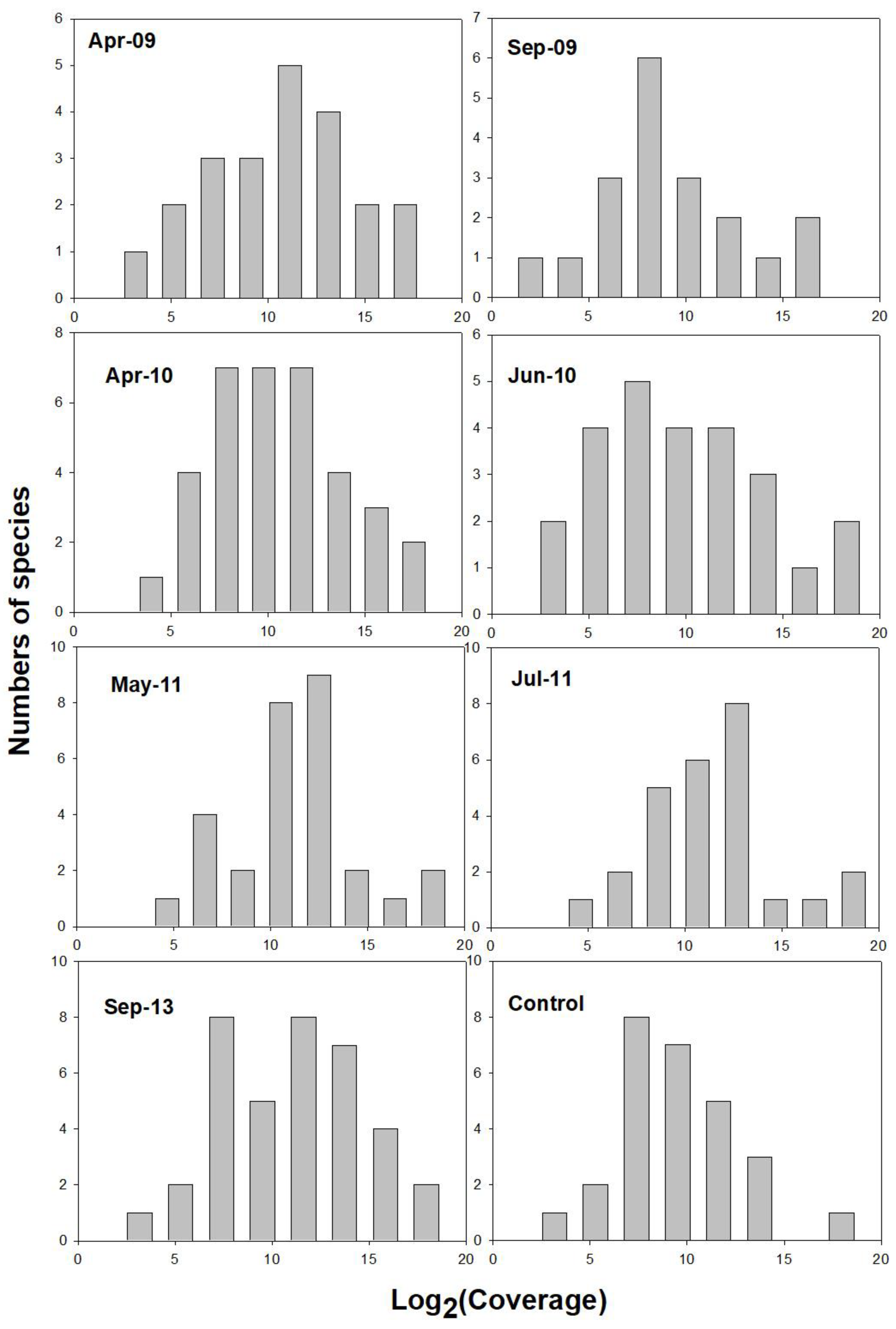
2.3.2. Log-Normal Distribution of Species-Coverage Relationships
2.3.3. Species-Abundance Model Simulation for Postfire Subalpine Shrub Grasslands
- (1)
- Preemption Model
- (2)
- Broken-stick model
- (3)
- Log-normal model
- (4)
- Zipf model
- (5)
- Zipf–Mandelbrot Model
3. Results
3.1. Short-Term Change of Postfire Species Diversity
3.2. Species–Abundance Analysis of Postfire Plant Coverage
3.3. Species-Abundance Model Simulation for Postfire Subalpine Shrub Grasslands
4. Discussion
4.1. Short-Term Change of Postfire Species Diversity
4.2. Species-Abundance Series Analysis of Postfire Plant Coverage
4.3. Species-Abundance Model Simulation for Postfire Subalpine Shrub Grasslands
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Fire Periods | Reference | Cause |
|---|---|---|
| 1903~1957 | [51] | unknown |
| 1957~1958 | ||
| 2008 | http://news.ltn.com.tw/news/life/paper/267022 (accessed on 16 December 2023) | artificial |
| 2014 | https://news.ltn.com.tw/news/society/paper/748572 (accessed on 16 December 2023) | artificial |
| 2019 | https://news.ltn.com.tw/news/life/breakingnews/2692421 (accessed on 16 December 2023) | artificial |
References
- Coop, J.D.; Massatti, R.T.; Schoettle, A.W. Subalpine vegetation pattern three decades after stand-replacing fire: Effects of landscape context and topography on plant community composition, tree regeneration, and diversity. J. Veg. Sci. 2010, 21, 472–487. [Google Scholar] [CrossRef]
- Turner, M.G.; Braziunas, K.H.; Hansen, W.D.; Harvey, B.J. Short-interval severe fire erodes the resilience of subalpine lodgepole pine forests. Proc. Natl. Acad. Sci. USA 2019, 116, 11319–11328. [Google Scholar] [CrossRef] [PubMed]
- Bashirzadeh, M.; Abedi, M.; Shefferson, R.P.; Farzam, M. Post-Fire Recovery of Plant Biodiversity Changes Depending on Time Intervals since Last Fire in Semiarid Shrublands. Fire 2023, 6, 103. [Google Scholar] [CrossRef]
- Tilman, D. Resource Competition and Community Structure. In Monographs in Population Biology; Princeton University Press: Princeton, NJ, USA, 1982; Volume 17, pp. 1–296. [Google Scholar]
- Lu, K.C. Effects of Wildfires on the Main Forest Ecosystems in Taiwan. Ph.D. Thesis, National Chung-Hsing University, Taichung, Taiwan, 1989. [Google Scholar]
- Lai, G.S. Dynamic Structures of Ecotones between Subalpine Coniferous forests and Grasslands in Taiwan. Ph.D. Thesis, National Chung-Hsing University, Taichung, Taiwan, 1992. [Google Scholar]
- Lai, G.S. The fire ecology of subalpine in Taiwan. In Seminar of Fire Ecology and Management; Forestry Bureau: Taipei, Taiwan, 2003; pp. 49–52. [Google Scholar]
- Overbeck, G.E.; Müller, S.C.; Pillar, V.D.; Pfadenhauer, J. Small-scale dynamics after fire in South Brazilian humid subtropical grassland. J. Veg. Sci. 2005, 16, 655–664. [Google Scholar] [CrossRef]
- Wang, W.; Tsai, S.T.; Chiu, C.A.; Hsu, C.K.; Tzeng, H.Y.; Lu, K.C. Species and life-form diversity along the altitudinal gradient on the Mt. Shei Eastern Trail. Q. J. For. Res. 2013, 5, 139–152. [Google Scholar]
- Su, H.J. Studies on the climate and vegetation types of the natural forests in Taiwan. (II). Altitudinal vegetation zones in relation to temperature gradient. Q. J. Chin. For. 1984, 17, 57–73. [Google Scholar]
- Wang, W.; Chiu, C.A.; Tsai, S.T.; Hsu, C.K.; Tzeng, H.Y.; Lu, K.C. Vegetation research along the East Trail of Mt. Shei. Q. J. For. Res. 2011, 32, 15–34. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Lu, F.Y.; Ou, C.H.; Lai, G.S. Plant succession and competition mechanism of Yushania niitakayamensis at alpine in Taiwan. Q. J. Chin. For. 1984, 17, 1–32. [Google Scholar]
- McGill, B.J.; Etienne, R.S.; Gray, J.S.; Alonso, D.; Anderson, M.J.; Benecha, H.K.; Dornelas, M.; Enquist, B.J.; Green, J.L.; He, F.; et al. Species abundance distributions: Moving beyond single prediction theories to integration within an ecological framework. Eco. Lett. 2007, 10, 995–1015. [Google Scholar] [CrossRef]
- Fisher, R.A.; Corbet, A.S.; Williams, C.B. The relation between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 1943, 12, 42–58. [Google Scholar] [CrossRef]
- MacArthur, R.H. On the relative abundance of bird species. Proc. Natl. Acad. Sci. USA 1957, 43, 293–295. [Google Scholar] [CrossRef]
- Zhang, J.T. Quantitative Ecology; Science Press: Beijing, China, 2004; pp. 1–370. [Google Scholar]
- Yin, Z.Y.; Ren, H.; Peng, S.L.; Guo, Q.F.; Zeng, L.H.; He, X. Dynamics and modeling of species abundance distribution during natural restoration of degraded hilly grassland in south China. Ecol. Environ. Sci. 2009, 1, 222–228. [Google Scholar] [CrossRef]
- Li, Q.; Tu, J.; Xiong, Z.P.; Lu, Z.X.; Liu, C.J. Survey of research on species abundance pattern. J. Yunnan Agric. Univ. 2011, 26, 117–123. [Google Scholar]
- Tsai, S.T.; Lu, K.C.; Tzeng, H.Y. Analyzing vegetation dynamics of the broad-leaved secondary forest by species abundance models at Mt. Showchu in the Hue-Sun Forest Station. Q. J. For. Res. 2011, 33, 9–22. [Google Scholar] [CrossRef]
- Huang, L.; Yang, H.; An, X.; Yu, Y.; Yu, L.; Huang, G.; Liu, X.; Chen, M.; Xue, Y. Species Abundance Distributions Patterns between Tiankeng Forests and Nearby Non-Tiankeng Forests in Southwest China. Diversity 2022, 14, 64. [Google Scholar] [CrossRef]
- Zang, Z.; Zeng, Y.; Wang, D.; Shi, F.; Dong, Y.; Liu, N.; Liang, Y. Species-Abundance Distribution Patterns of Plant Communities in the Gurbantünggüt Desert, China. Sustainability 2022, 14, 12957. [Google Scholar] [CrossRef]
- Antão, L.H.; Magurran, A.E.; Dornelas, M. The Shape of Species Abundance Distributions Across Spatial Scales. Front. Ecol. Evol. 2021, 9, 626730. [Google Scholar] [CrossRef]
- Cheng, J.J.; Mi, X.C.; Ma, K.P.; Zhang, J.T. Responses of species-abundance distribution to varying sampling scales in a subtropical broad-leaved forest. Biodivers. Sci. 2011, 19, 168–177. [Google Scholar] [CrossRef]
- Wilson, J.B.; Wells, T.C.E.; Trueman, C.; Jones, G.; Atkinson, M.D.; Crawley, M.J.; Dodd, M.E.; Silvertown, J. Are there assembly rules for plant species abundance: An investigation in relation to soil resources and successional trends. J. Eco. 1996, 84, 527–538. [Google Scholar] [CrossRef]
- Central Weather Bureau. 2022. Available online: https://www.cwb.gov.tw/V8/C/ (accessed on 31 December 2022).
- Ho, C.S. Geological Introduction of Taiwan; Central Geological Survey (MOEA): Taipei, Taiwan, 2003; pp. 1–163. [Google Scholar]
- Yen, C.H. Soil properties of alpine in Xue Mountain. In Alpine Ecosystem Integrative Survey in Xue Mountain; Lu, K.C., Ou, C.H., Eds.; Shei-Pa National Park Headquarters: Miaoli, Taiwan, 2009; Chapter 3; pp. 1–30. [Google Scholar]
- Cheng, T.W.; Tzeng, H.Y.; Chiu, C.A.; Liu, S.Q.; Wang, C.M.; Tseng, Y.H. Studies on the life form of vascular plants along the East Trail of Xue Mountain. J. Nat. Park 2012, 22, 41–51. [Google Scholar]
- Wu, C.Y.; Tzeng, H.Y.; Qiu, Q.A.; Wang, C.M.; Liu, S.C.; Tseng, Y.H. Flowering phenology of East Xue Trail of Xue Mountain. Q. J. For. Res. 2013, 35, 223–240. [Google Scholar]
- Wilson, J.B. Methods for fitting dominance/diversity curves. J. Veg. Sci. 1991, 2, 35–46. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Fang, J.Y. A review on the elevational patterns of plant species diversity. Sheng Wu Duo Yang Xing 2004, 12, 20–28. [Google Scholar] [CrossRef]
- Liu, T.S.; Su, H.J. Forest Ecology; The Commercial Press: Taipei, Taiwan, 1983; pp. 1–462. [Google Scholar]
- Midgley, J.J. Why the world’s vegetation is not totally dominated by resprouting plants; because resprouters are shorter than reseeders. Ecography 1996, 19, 92–95. Available online: https://www.jstor.org/stable/3683094 (accessed on 10 October 2023). [CrossRef]
- Hughes, R.G. Theories and models of species abundance. Am. Nat. 1986, 128, 879–899. Available online: https://www.jstor.org/stable/2461769 (accessed on 10 October 2023). [CrossRef]
- Motomura, I. A statistical treatment of associations. Jpn. J. Zool. 1932, 44, 379–383. Available online: https://cir.nii.ac.jp/crid/1570009750645470336 (accessed on 10 October 2023).
- Preston, F.W. The commonness and rarity of species. Ecology 1948, 29, 254–283. [Google Scholar] [CrossRef]
- May, R.M. Patterns of species abundance and diversity. In Ecology and Evolution of Communities; Lody, M.L., Diamond, J.M., Eds.; Harvard University Press: Cambridge, MA, USA, 1975; pp. 81–120. [Google Scholar]
- Barange, M.; Campos, B. Models of species abundance: A critique of and an alternative to the dynamics model. Mar. Ecol. Prog. Ser. 1991, 69, 293–298. Available online: https://digital.csic.es/bitstream/10261/281973/1/Barange_et_al_1991.pdf (accessed on 10 October 2023). [CrossRef]
- Frontier, S. Diversity and structure in aquatic ecosystems. Oceanogr. Mar. Biol. 1985, 23, 253–312. [Google Scholar]
- Moradizadeh, H.; Heydari, M.; Omidipour, R.; Mezbani, A.; Prévosto, B. Ecological effects of fire severity and time since fire on the diversity partitioning, composition and niche apportionment models of post-fire understory vegetation in semi-arid oak forests of Western Iran. Ecol. Eng. 2020, 143, 105694. [Google Scholar] [CrossRef]
- Bell, D.T. Ecological response syndromes in the flora of southwestern Western Australia: Fire resprouters versus reseeders. Bot. Rev. 2001, 67, 417–440. [Google Scholar] [CrossRef]
- Sritharan, M.S.; Scheele, B.C.; Blanchard, W.; Foster, C.N.; Werner, P.A.; Lindenmayer, D.B. Plant rarity in fire-prone dry sclerophyll communities. Sci. Rep. 2022, 12, 12055. [Google Scholar] [CrossRef] [PubMed]
- Vogl, R.J. Effects of fire on grasslands. In Fire and Ecosystems; Kozlowski, T.T., Ahlgren, C.E., Eds.; Academic: New York, NY, USA, 1974; pp. 139–194. [Google Scholar]
- Connell, J.H. Diversity in tropical rain forest and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Huston, M.A. A general hypothesis of species diversity. Am. Nat. 1979, 113, 81–101. Available online: https://www.jstor.org/stable/2459944 (accessed on 10 October 2023). [CrossRef]
- Denslow, J.S. Gap partitioning among tropical rainforest trees. Biotropica 1980, 12, 47–55. [Google Scholar] [CrossRef]
- Lin, C.C.; Chiou, C.R.; Chou, C.Y. Identifying and evaluating fire severity: A case study of the Wulin fire. Taiwan J. For. Sci. 2014, 20, 203–213. [Google Scholar] [CrossRef]
- Pan, C.C.; Tseng, Y.H.; Chiu, C.A.; Tzeng, H.Y. Phenology of Rhododendron pseudochrysanthum in Mt. Xue. Q. J. For. Res. 2013, 35, 71–86. [Google Scholar]
- Deng, N.; Song, Q.; Ma, F.; Tian, Y. Patterns and Driving Factors of Diversity in the Shrub Community in Central and Southern China. Forests 2022, 13, 1090. [Google Scholar] [CrossRef]
- Chiarucci, A.; Wilson, J.B.; Anderson, B.J.; De Dominicis, V. Cover versus biomass as an estimate of species abundance: Does it make a difference to the conclusions? J. Veg. Sci. 1999, 10, 35–42. [Google Scholar] [CrossRef]
- Chen, M.Y.; Shih, Y.Y. Studies on the effects of wildfires on the vegetation at Fansan and Shesan areas. Nat. Park J. 1999, 8, 155–165. [Google Scholar]

| Parameters | Survey Period | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Feb-09 | Apr-09 | Sep-09 | Apr-10 | Jun-10 | May-11 | Jul-11 | Sep-13 | Control | |
| Season 1 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 2 |
| Number of species | 5 | 13 | 24 | 24 | 36 | 27 | 33 | 29 | 27 |
| Coverage (%) | 0.21 | 0.93 | 25.91 | 13.14 | 43.95 | 42.47 | 48.45 | 64.80 | 100.00 |
| Phanerophytes (%) | 0.00 | 0.00 | 0.00 | 5.26 | 3.23 | 4.17 | 6.90 | 8.33 | 14.29 |
| Chamaephytes (%) | 0.00 | 18.18 | 22.73 | 26.32 | 16.13 | 20.83 | 17.24 | 8.33 | 9.52 |
| Hemicryptophytes (%) | 75.00 | 54.55 | 54.55 | 52.63 | 61.29 | 54.17 | 58.62 | 66.67 | 57.14 |
| Geophytes (%) | 25.00 | 27.27 | 22.73 | 15.79 | 19.35 | 20.83 | 17.24 | 16.67 | 19.05 |
| Survival strategy 2 | 100/0 | 92/8 | 79/21 | 81/19 | 62/38 | 70/30 | 63/37 | 61/39 | 41/59 |
| Ferns quotient | 6.25 | 4.55 | 2.27 | 2.63 | 2.42 | 3.13 | 2.59 | 4.17 | 5.71 |
| Gleason index | 1.01 | 2.62 | 4.83 | 4.83 | 7.24 | 5.43 | 6.64 | 5.84 | 7.32 |
| Shannon index | 0.44 | 0.57 | 1.10 | 0.96 | 1.11 | 0.87 | 1.08 | 1.03 | 1.08 |
| Evenness index | 0.27 | 0.22 | 0.35 | 0.30 | 0.31 | 0.26 | 0.31 | 0.31 | 0.33 |
| Survey Period | S 1 | Species-Abundance Models | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Broken Stick | Preemption | Log-Normal | Zipf | Zipf–Mandelbrot | |||||||
| Dev. | χ2 | Dev. | χ2 | Dev. | χ2 | Dev. | χ2 | Dev. | χ2 | ||
| Feb-09 | 1 | 0.29 | 0.25 | 0.82 | 2.04 | 0.01 | 0.01 | 0.00 * | 0.00 | 0.00 | 0.01 |
| Apr-09 | 1 | 1.15 | 1.27 | 2.42 | 4.48 | 0.03 * | 0.03 | 0.03 | 0.02 | 0.04 | 0.04 |
| Apr-10 | 1 | 25.33 | 26.39 | 36.14 | 48.65 2 | 6.11 | 7.58 | 7.04 | 8.68 | 4.82 * | 5.72 |
| May-11 | 1 | 111.30 | 124.90 | 135.84 | 186.24 | 12.60 | 15.03 | 13.99 | 16.50 | 8.59 * | 9.68 |
| Sep-09 | 2 | 30.67 | 30.36 | 44.70 | 54.26 | 4.22 | 4.50 | 7.12 | 6.87 | 3.84 * | 3.64 |
| Jun-10 | 2 | 66.53 | 67.15 | 64.37 | 69.46 | 11.22 | 11.64 | 17.01 | 16.94 | 9.47 * | 9.29 |
| Jul-11 | 2 | 104.47 | 25.62 | 115.11 | 24.23 | 6.70 | 0.25 | 7.98 | 0.93 | 4.49 * | 0.49 |
| Sep-13 | 2 | 157.85 | 68.51 | 184.94 | 71.86 | 25.43 | 1.94 | 27.32 | 6.40 | 18.12 * | 3.15 |
| Control | 2 | 90.03 | 143.08 | 107.12 | 217.77 | 4.07 | 15.38 | 3.29 * | 3.61 | 5.83 | 10.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liao, M.-C.; Tzeng, H.-Y. Species-Abundance Models for the Early Postfire Succession of Subalpine Shrub Grassland. Fire 2024, 7, 21. https://doi.org/10.3390/fire7010021
Wang W, Liao M-C, Tzeng H-Y. Species-Abundance Models for the Early Postfire Succession of Subalpine Shrub Grassland. Fire. 2024; 7(1):21. https://doi.org/10.3390/fire7010021
Chicago/Turabian StyleWang, Wei, Min-Chun Liao, and Hsy-Yu Tzeng. 2024. "Species-Abundance Models for the Early Postfire Succession of Subalpine Shrub Grassland" Fire 7, no. 1: 21. https://doi.org/10.3390/fire7010021
APA StyleWang, W., Liao, M.-C., & Tzeng, H.-Y. (2024). Species-Abundance Models for the Early Postfire Succession of Subalpine Shrub Grassland. Fire, 7(1), 21. https://doi.org/10.3390/fire7010021






