A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires V: Comparison between EN 60754-1 and EN 60754-2
Abstract
:1. Introduction
1.1. The Additional Classification for Acidity and the Role of Acid Scavengers
1.2. The Background. Regulatory Context and Test Methods for Assessing the Acidity
1.3. Fire Hazards: The Role of Flame Retardants, Smoke Suppressants, and Acid Scavengers
1.4. Acid Scavengers, Their Behavior at Different Temperatures and Novel Low Smoke Acidity PVC Compounds for Cables
1.5. The Impact of Acid Scavengers when EN 60754-2 Is Run at Different Thermal Profiles
1.6. The Aim of the Research
- (1)
- EN 60754-2 has been carried out isothermally at 950 °C, and EN 60754-2 with the heating regime of EN 60754-1 (internal method 3).
- (2)
- EN 60754-2 has been conducted at isothermally 950 °C, and EN 60754-2 isothermally at 500 °C (internal method 2).
2. Materials and Methods
2.1. Materials
- -
- pH: 2.00, 4.01, 7.00, and 10.00,
- -
- conductivity: 2.0, 8.4, 14.7, 141.3 μS/mm
2.2. Test Apparatuses
| Test Apparatus | Producer | Model | Additional Info’s |
|---|---|---|---|
| Torque Rheometer | Brabender | Plastograph EC | 50 CC chamber, 30 rpm, 60 g sample mass, 160 °C per 10 min. |
| Halogen Acid Gas test apparatus | SA Associates | Standard model | Porcelain combustion boats. |
| Multimeter | Mettler Toledo | S213 standard kit | |
| Conductivity electrode | Mettler Toledo | S213 standard kit | Reference thermocouple adjusting temperature fluctuation. |
| pH electrode | Mettler Toledo | S213 standard kit | Reference thermocouple adjusting temperature fluctuation. |
| Ion Exchange Deionizer | Culligan Pharma | System 20 |
2.3. Sample Preparation
2.4. Internal Tests and International Technical Standards Used
3. Results
4. Discussion
“It is clear that the higher the temperature at which the tube furnace test is carried out, the higher the HC1 emission will be”.
“The lower efficiency during isothermal runs (after 3 weight loss stages) than during gradual heating runs…., coupled with the fact that there is a significantly larger weight loss in the first stage of the isothermal runs, indicates that there is a much greater likelihood of HCI being emitted before it has had the opportunity of reacting with the filler”.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PVC | Poly(vinyl chloride) |
| HCl | Hydrogen chloride |
| EU | European Union |
| CPD | Construction Product Directive |
| CPR | Construction Product Regulation |
| UPCC | Precipitated Calcium Carbonate |
| GCC | Ground Calcium Carbonate |
| Phr | Part per Hundred Resin |
| DINP | Di Iso Nonyl Phthalate |
| ESBO | Epoxidized Soy Bean Oil |
| COS | Calcium Organic Stabilizer |
| DDW | Double Deionized Water |
| M | Mean |
| SD | Standard Deviation |
| CV | Coefficient of variation |
Appendix A. A Schematic Diagram of the Sample Preparation and Testing Process
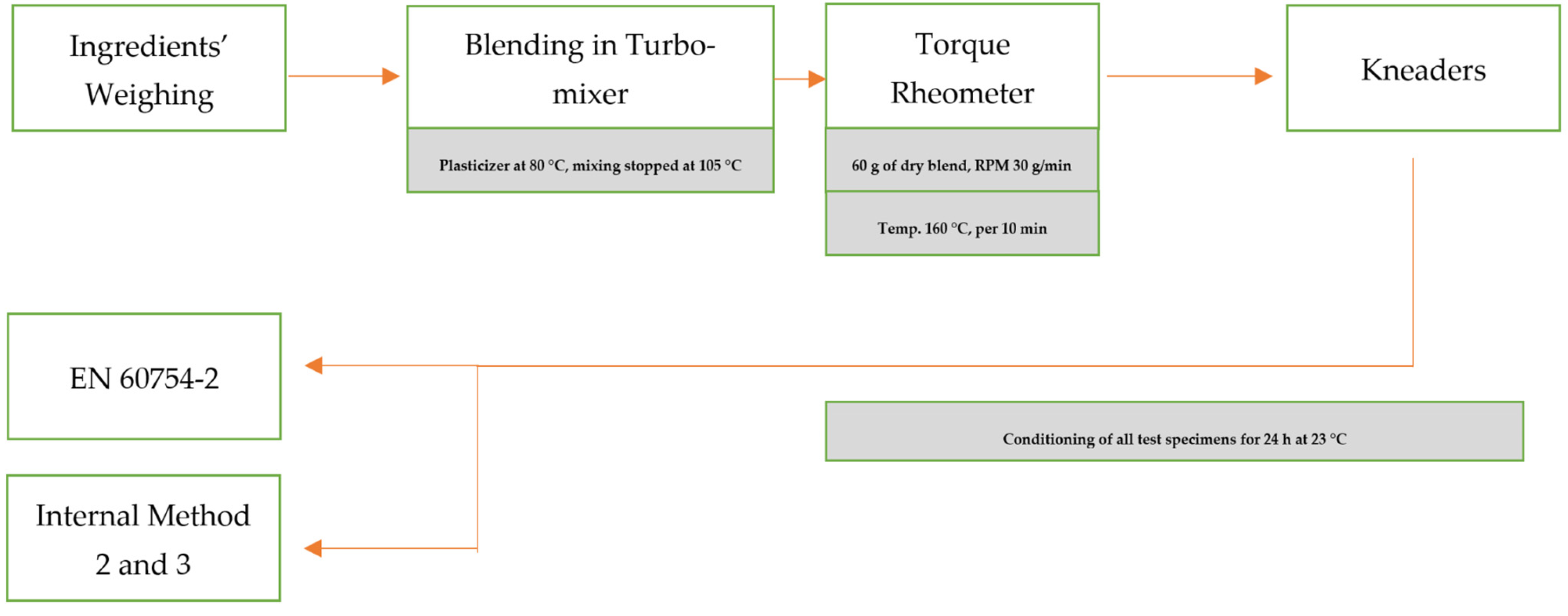
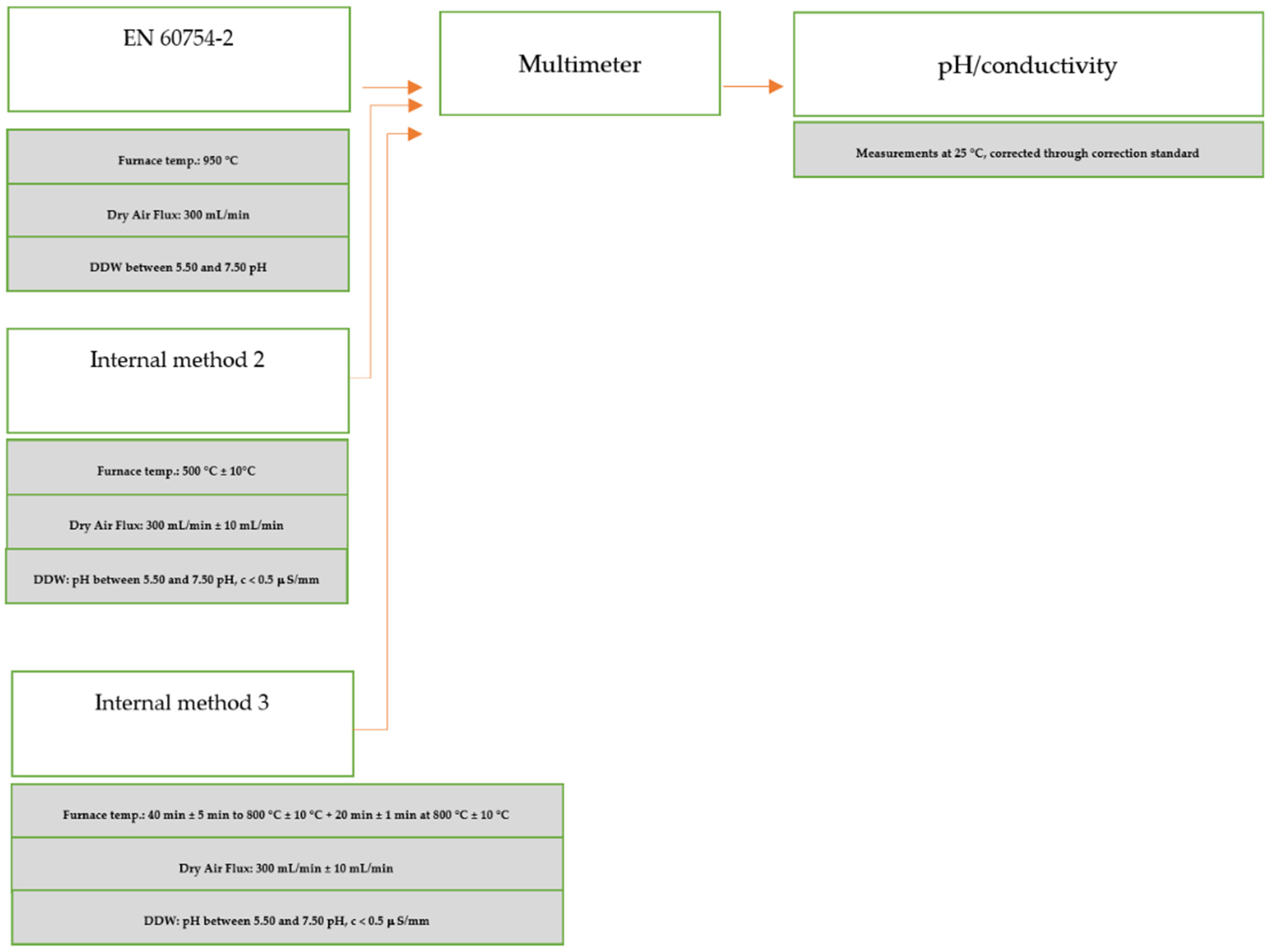
References
- Regulation (EU) No 305/2011 of the European Parliament and of the Council of 9 March 2011 Laying Down Harmonised Conditions for the Marketing of Construction Products and Repealing Council Directive 89/106/EEC. Consolidate Version. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02011R0305-20210716 (accessed on 10 June 2023).
- EN13501-1:2018; Fire Classification of Construction Products and Building Elements—Part 1: Classification Using Data from Reaction to Fire Tests. CEN: Brussels, Belgium, 2019. Available online: https://store.uni.com/ (accessed on 10 June 2023).
- EN 13501-6:2018; Fire Classification of Construction Products and Building Elements. Classification Using Data from Reaction to Fire Tests on Power, Control and Communication Cables. CEN: Brussels, Belgium, 2018. Available online: https://store.uni.com/ (accessed on 10 June 2023).
- EN 50575:2014+A1:2016; Power, Control and Communication Cables—Cables for General Applications in Construction Works Subject to Reaction to Fire Requirements. CENELEC: Brussels, Belgium, 2016. Available online: https://my.ceinorme.it/home.html (accessed on 10 June 2023).
- EN 60754-2:2014/A1:2020; Test on Gases Evolved during Combustion of Materials from Cables—Part 2: Determination of Acidity (by pH Measurement) and Conductivity. CENELEC: Brussels, Belgium, 2020. Available online: https://my.ceinorme.it/home.html (accessed on 10 June 2023).
- Sarti, G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires. I: An Overview of the Theory, Test Methods, and the European Union Regulatory Status. Fire 2022, 5, 127. [Google Scholar] [CrossRef]
- Sarti, G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires. II: Some Examples of Acid Scavengers at High Temperatures in the Condensed Phase. Fire 2022, 5, 142. [Google Scholar] [CrossRef]
- EN 60754-1; Test on Gases Evolved during Combustion of Materials from Cables—Part 1: Determination of the Halogen Acid Gas Content. CENELEC: Brussels, Belgium, 2014. Available online: https://my.ceinorme.it/home.html (accessed on 1 August 2022).
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. FAM 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Babrauskas, V. Specimen Heat Fluxes for Bench-scale Heat Release Rate Testing. Fire Mater. 1995, 19, 243–252. [Google Scholar] [CrossRef]
- Schartel, B.; Kebelmann, K. Fire Testing for the Development of Flame Retardant Polymeric Materials from: Flame Retardant Polymeric Materials, a Handbook; CRC Press: Boca Raton, FL, USA, 2019; Available online: https://www.routledgehandbooks.com/doi/10.1201/b22345-3 (accessed on 10 June 2023).
- Hirschler, M. Poly(vinyl chloride) and its fire properties. FAM 2017, 41, 993–1006. [Google Scholar] [CrossRef]
- Babrauskas, V.; Peacock, R.D. Heat release rate: The single most important variable in fire hazard. Fire Saf. J. 1992, 18, 255–272. [Google Scholar] [CrossRef]
- Hirschler, M. Fire safety, smoke toxicity, and acidity. In Proceedings of the Flame Retardants—Interscience Communications, London, UK, 14–15 February 2006. [Google Scholar]
- Bassi, I.; Delchiaro, F.; Bandinelli, C.; Mazzocchetti, L.; Salatelli, E.; Sarti, G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires, IV: The Impact of Acid Scavengers at High Temperatures on Flame Retardance and Smoke Emission. Fire 2023, 6, 259. [Google Scholar] [CrossRef]
- Sarti, G.; Piana, M. PVC in cables for building and construction. Can the “European approach” be considered a good example for other countries? Acad. Lett. 2022, 5453. [Google Scholar] [CrossRef]
- Sarti, G.; Piana, M. PVC cables and smoke acidity: A review comparing performances of old and new compounds. In Proceedings of the AMI International Wire and Cable Conference, Duesseldorf, Germany, 3–5 March 2020. [Google Scholar]
- Sarti, G.; Piana, M. Smoke acidity and a new generation of PVC formulations for cables. In Proceedings of the AMI PVC Formulation Conference, Cologne, Germany, 16–18 September 2021. [Google Scholar]
- Sarti, G. Developing and improving fire performance and safety in PVC. In Proceedings of the Future of PVC Compounding, Production & Recycling, AVLANTE, Online Conference, 24–25 February 2022. [Google Scholar]
- Commercial GCC Purchased by Umbriafiller. Available online: https://elastomeri-polimeri.hu/pdf/umbria/riochim.pdf (accessed on 1 June 2023).
- Kipouros, G.J.; Sadoway, D.R. A thermochemical analysis of the production of anhydrous MgCl2. J. Light Met. 2001, 1, 111–117. [Google Scholar] [CrossRef]
- Galwey, A.K.; Laverty, G.M. The thermal decomposition of magnesium chloride dihydrate. Thermochim. Acta 1989, 138, 115–127. [Google Scholar] [CrossRef]
- Commercial PCC Purchased by Imerys. Available online: https://www.imerys-performance-minerals.com/system/files/2021-02/DATPCC_Winnofil_S_LSK_EN_2019-07.pdf (accessed on 1 June 2023).
- Chandler, L.A.; Hirschler Smith, G.F. A heated tube furnace test for the emission of acid gas from PVC wire coating materials: Effects of experimental procedures and mechanistic considerations. Eur. Polym. J. 1987, 23, 51–61. [Google Scholar] [CrossRef]
- Bassi, I. Characterization of PVC Compounds and Evaluation of Their Fire Performance, Focusing on the Comparison between EN 60754-1 and EN 60754-2 in the Assessment of the Smoke Acidity. Master’s Thesis, University of Bologna, Bologna, Italy, October 2021. Available online: https://www.pvc4cables.org/images/assessment_of_the_smoke_acidity.pdf (accessed on 1 June 2023).


| Raw Materials | Trade Name | FR50.0 [phr] | FR50.1 [phr] | FR50.2 [phr] | FR50.3 [phr] | FR50.4 [phr] | FR50.5 [phr] |
|---|---|---|---|---|---|---|---|
| PVC | Inovyn 271 PC | 100 | 100 | 100 | 100 | 100 | 100 |
| DINP | Diplast N | 50 | 50 | 50 | 50 | 50 | 50 |
| ESBO | Reaflex EP/6 | 2 | 2 | 2 | 2 | 2 | 2 |
| Antioxidant | Arenox A10 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| COS | RPK B-CV/3037 | 3 | 3 | 3 | 3 | 3 | 3 |
| CaCO3 | Riochim | 90 | 0 | 0 | 0 | 0 | 0 |
| Al(OH)3 | Apyral 40 CD | 0 | 90 | 0 | 0 | 0 | 0 |
| Mg(OH)2 | Ecopyren 3.5 | 0 | 0 | 90 | 0 | 0 | 0 |
| UPCC | Winnofil S | 0 | 0 | 0 | 90 | 0 | 0 |
| HTAS 1 | AS-1B | 0 | 0 | 0 | 0 | 90 | 0 |
| HTAS 2 | AS-6B | 0 | 0 | 0 | 0 | 0 | 90 |
| Raw Materials | Trade Name | FR50.6 [phr] | FR50.7 [phr] | FR50.8 [phr] | FR950.9 [phr] |
|---|---|---|---|---|---|
| PVC | Inovyn 271 PC | 100.0 | 100.0 | 100.0 | 100.0 |
| DINP | Diplast N | 50.0 | 50.0 | 50.0 | 50.0 |
| ESBO | Reaflex EP/6 | 2.0 | 2.0 | 2.0 | 2.0 |
| Mg(OH)2 | Kisuma 5A | 30.0 | 30.0 | 30.0 | 30.0 |
| Antioxidant | Arenox A10 | 0.1 | 0.1 | 0.1 | 0.1 |
| COS | RPK B-CV/3037 | 3.0 | 3.0 | 3.0 | 3.0 |
| HTAS 1 | AS-1B | 123.0 | 123.0 | 0.0 | 0.0 |
| HTAS 2 | AS-6B | 0.0 | 0.0 | 123.0 | 123.0 |
| Anti Pinking | RPK B-NT/8014 | 0.0 | 6.0 | 0.0 | 6.0 |
| Raw Materials | Trade Name | FR50.10 [phr] | FR50.11 [phr] | FR50.12 [phr] | FR950.13 [phr] |
|---|---|---|---|---|---|
| PVC | Inovyn 271 PC | 100.0 | 100.0 | 100.0 | 100.0 |
| DINP | Diplast N | 50.0 | 50.0 | 50.0 | 50.0 |
| ESBO | Reaflex EP/6 | 2.0 | 2.0 | 2.0 | 2.0 |
| Antioxidant | Arenox A10 | 0.1 | 0.1 | 0.1 | 0.1 |
| COS | RPK B-CV/3037 | 3.0 | 3.0 | 3.0 | 3.0 |
| Mg(OH)2 | Kisuma 5A | 30.0 | 30.0 | 30.0 | 30.0 |
| UPCC | Winnofil S | 90.0 | 90.0 | 0.0 | 0.0 |
| Fumed Silica | Cabosil H5 | 0.0 | 15.0 | 0.0 | 15.0 |
| HTAS 3 | AS-0B | 0.0 | 0.0 | 123.0 | 123.0 |
| Raw Materials | Trade Name | FR50.14 [phr] | FR50.15 [phr] | FR50.16 [phr] | FR50.17 [phr] |
|---|---|---|---|---|---|
| PVC | Inovyn 271 PC | 100.0 | 100.0 | 100.0 | 100.0 |
| DINP | Diplast N | 50.0 | 50.0 | 50.0 | 50.0 |
| ESBO | Reaflex EP/6 | 2.0 | 2.0 | 2.0 | 2.0 |
| Mg(OH)2 | Ecopyren 3.5 | 30.0 | 30.0 | 30.0 | 30.0 |
| Antioxidant | Arenox A10 | 0.1 | 0.1 | 0.1 | 0.1 |
| COS | RPK B-CV/3037 | 3.0 | 3.0 | 3.0 | 3.0 |
| ATO | RI004 | 10.0 | 10.0 | 10.0 | 10.0 |
| CaCO3 | Riochim | 0.0 | 0.0 | 0.0 | 65.0 |
| HTAS 1 | AS-1B | 123.0 | 0.0 | 0.0 | 0.0 |
| HTAS 2 | AS-6B | 0.0 | 123.0 | 0.0 | 0.0 |
| HTAS 3 | AS-0B | 0.0 | 0.0 | 123.0 | 0.0 |
| Technical Standard | Measurement | Temperature | Note |
|---|---|---|---|
| EN 60754-2 | pH and conductivity | Isothermal at 950 °C | The general method, according to the 2014 version. |
| Internal method 2 | pH and conductivity | Isothermal at 500 °C | The general method, according to the 2014 version. |
| Internal method 3 | pH and conductivity | 23–800 °C in 40 min 800 °C per 20 min | EN 60754-2 carried out with the thermal profile of EN 60754-1 |
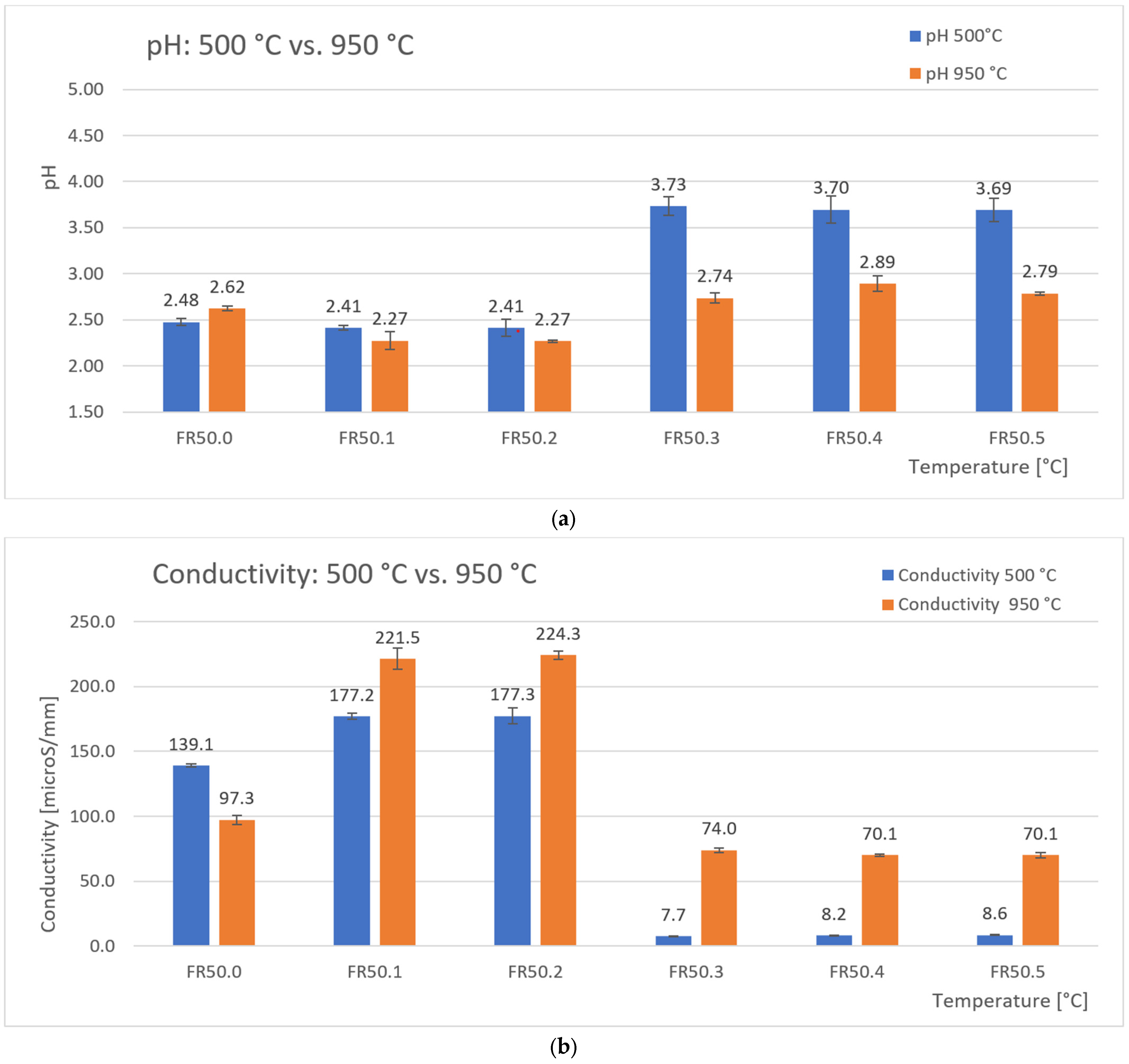
| Formulation | FR50.0 | FR50.1 | FR50.2 | FR50.3 | FR50.4 | FR50.5 |
|---|---|---|---|---|---|---|
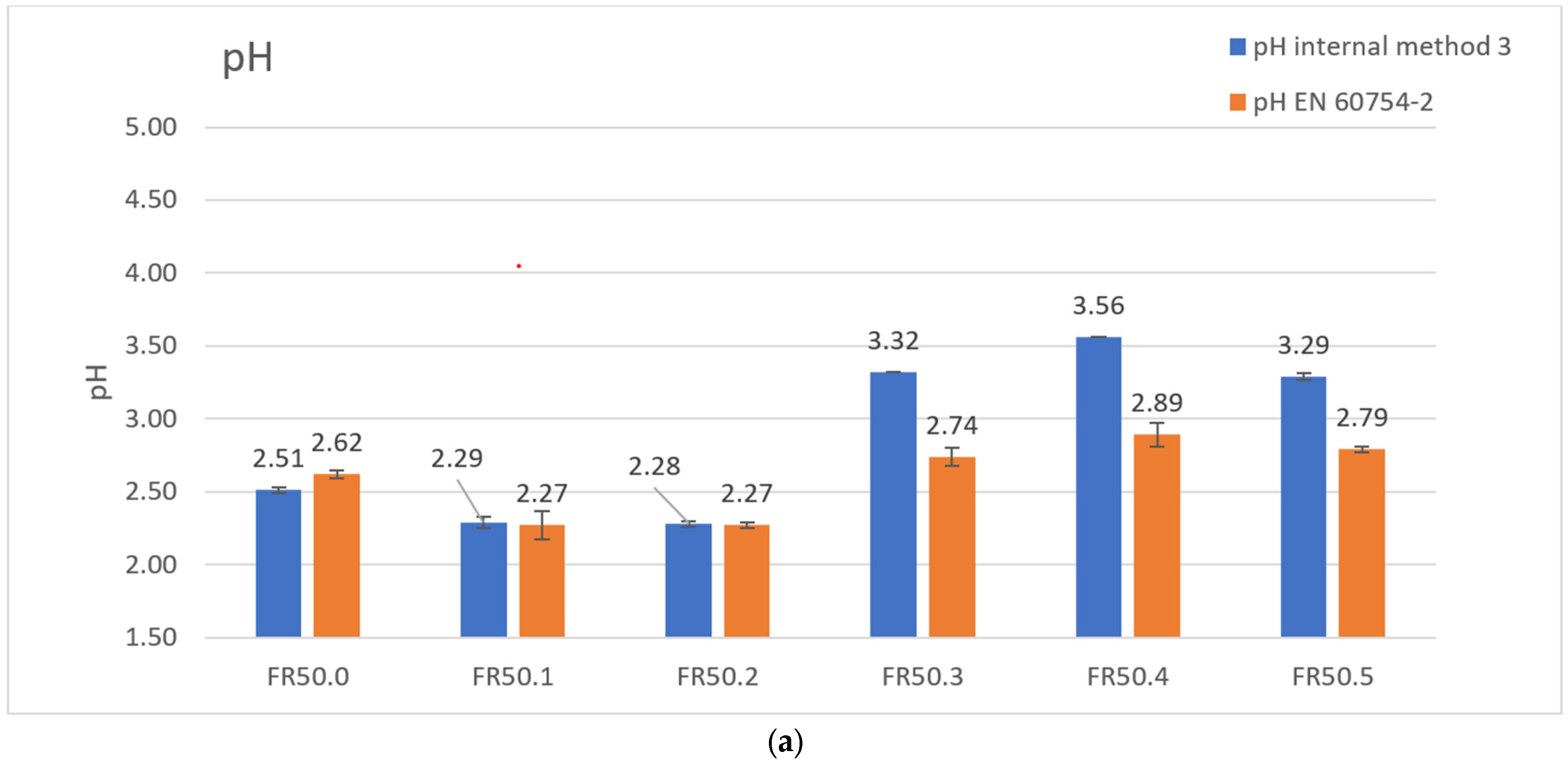
| pH | 2.62 | 2.27 | 2.27 | 2.74 | 2.89 | 2.79 |
| SDpH | 0.03 | 0.10 | 0.02 | 0.06 | 0.08 | 0.02 |
| CVpH [%] | 1.15 | 4.41 | 0.88 | 2.19 | 2.77 | 0.72 |
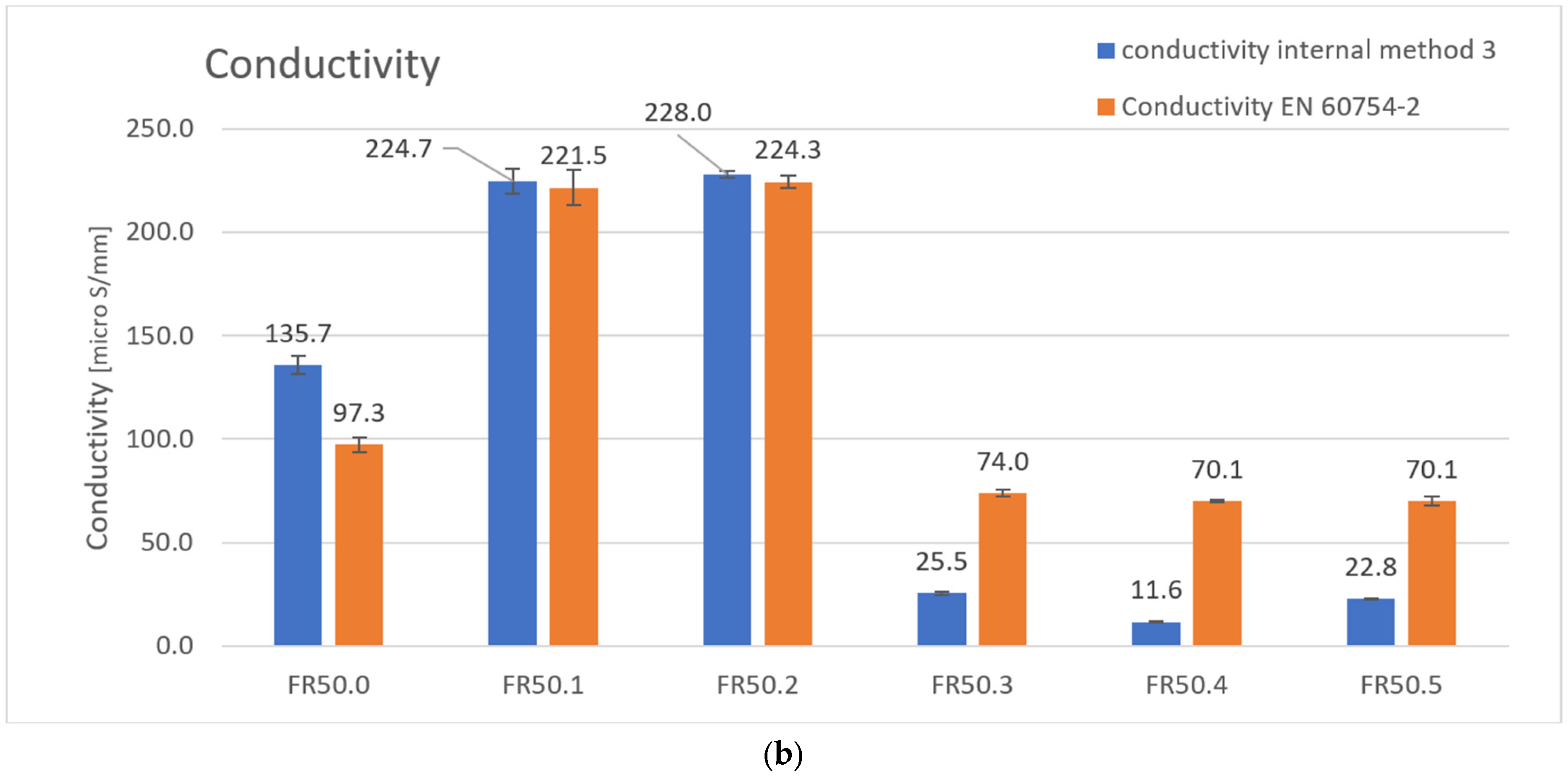
| Conductivity [μS/mm] | 97.3 | 221.5 | 224.3 | 74.0 | 70.1 | 70.1 |
| SDc | 3.7 | 8.4 | 3.1 | 1.6 | 0.7 | 2.0 |
| CVc [%] | 3.8 | 3.8 | 1.4 | 2.2 | 1.0 | 2.9 |
| Formulation | FR50.0 | FR50.1 | FR50.2 | FR50.3 | FR50.4 | FR50.5 |
|---|---|---|---|---|---|---|
| pH | 2.51 | 2.29 | 2.28 | 3.32 | 3.56 | 3.29 |
| SDpH | 0.02 | 0.04 | 0.02 | 0.00 | 0.00 | 0.02 |
| CVpH [%] | 0.80 | 1.75 | 0.88 | 0.00 | 0.00 | 0.61 |
| Conductivity [μS/mm] | 135.7 | 224.7 | 228.0 | 25.5 | 11.6 | 22.8 |
| SDc | 4.4 | 6.1 | 1.5 | 0.7 | 0.2 | 0.1 |
| CVc [%] | 3.2 | 2.7 | 0.7 | 2.7 | 1.7 | 0.4 |
| Formulation | FR50.0 | FR50.1 | FR50.2 | FR50.3 | FR50.4 | FR50.5 |
|---|---|---|---|---|---|---|
| pH at 500 °C | 2.48 | 2.41 | 2.41 | 3.73 | 3.70 | 3.69 |
| SDpH | 0.04 | 0.03 | 0.09 | 0.10 | 0.15 | 0.13 |
| CVpH [%] | 1.61 | 1.24 | 3.73 | 2.68 | 4.05 | 3.52 |
| Conductivity at 500 °C [μS/mm] | 139.1 | 177.2 | 177.3 | 7.7 | 8.2 | 8.6 |
| SDc | 1.2 | 2.5 | 6.2 | 0.3 | 0.4 | 0.3 |
| CVc [%] | 0.9 | 1.4 | 3.5 | 3.9 | 4.9 | 3.5 |
| Formulation | FR50.6 | FR50.7 | FR50.8 | FR50.9 |
|---|---|---|---|---|
| pH | 4.17 | 4.18 | 4.31 | 4.14 |
| SDpH | 0.08 | 0.11 | 0.07 | 0.17 |
| CVpH [%] | 1.92 | 2.63 | 1.62 | 4.11 |
| Conductivity [μS/mm] | 3.2 | 2.8 | 2.5 | 3.9 |
| SDc | 0.1 | 0.4 | 0.1 | 1.0 |
| CVc [%] | 3.1 | 14.3 | 4.0 | 25.6 |
| Formulation | FR50.6 | FR50.7 | FR50.8 | FR50.9 |
|---|---|---|---|---|
| pH | 4.29 | 4.46 | 4.73 | 4.44 |
| SDpH | 0.01 | 0.09 | 0.35 | 0.28 |
| CVpH [%] | 0.23 | 2.02 | 7.40 | 6.31 |
| Conductivity [μS/mm] | 1.8 | 1.8 | 1.3 | 2.3 |
| SDc | 0.0 | 0.4 | 0.3 | 0.7 |
| CVc [%] | 0.0 | 22.2 | 23.1 | 30.4 |
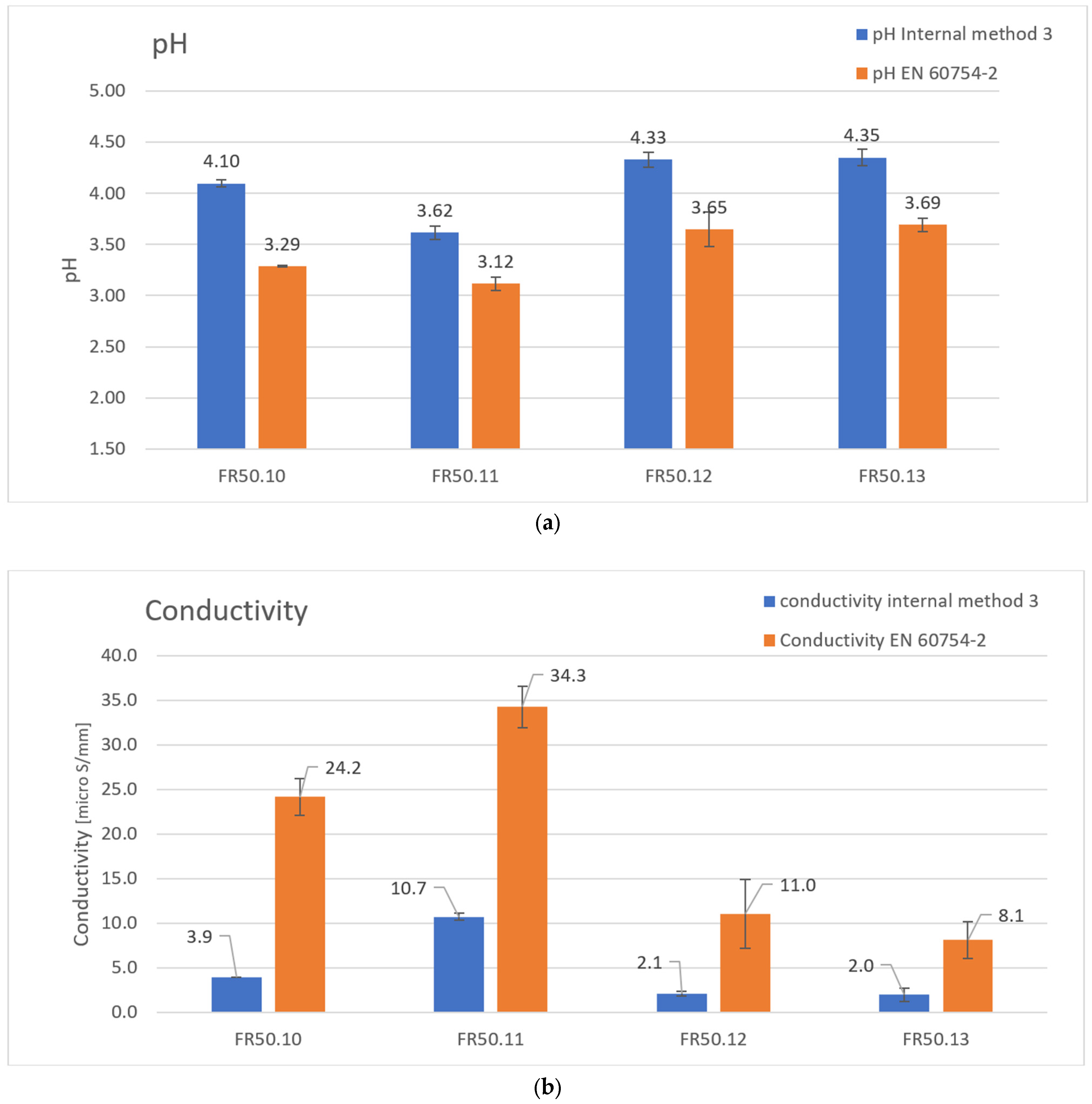
| Formulation | FR50.10 | FR50.11 | FR50.12 | FR50.13 |
|---|---|---|---|---|
| pH | 3.29 | 3.12 | 3.65 | 3.69 |
| SDpH | 0.01 | 0.06 | 0.17 | 0.07 |
| CVpH [%] | 0.30 | 1.92 | 4.66 | 1.90 |
| Conductivity [μS/mm] | 24.2 | 34.3 | 11.0 | 8.1 |
| SDc | 2.1 | 2.3 | 3.9 | 2.1 |
| CVc [%] | 8.7 | 6.8 | 35.2 | 25.9 |
| Formulation | FR50.10 | FR50.11 | FR50.12 | FR50.13 |
|---|---|---|---|---|
| pH | 4.10 | 3.62 | 4.33 | 4.35 |
| SDpH | 0.04 | 0.06 | 0.07 | 0.08 |
| CVpH [%] | 0.98 | 1.66 | 1.62 | 1.84 |
| Conductivity [μS/mm] | 3.9 | 10.7 | 2.1 | 2.0 |
| SDc | 0.3 | 1.6 | 0.7 | 0.2 |
| CVc [%] | 7.7 | 15.0 | 33.3 | 10.0 |
| Formulation | FR50.14 | FR50.15 | FR50.16 | FR50.17 |
|---|---|---|---|---|
| pH | 4.18 | 4.20 | 4.03 | 2.63 |
| SDpH | 0.08 | 0.09 | 0.08 | 0.10 |
| CVpH [%] | 1.91 | 2.14 | 1.99 | 3.80 |
| Conductivity [μS/mm] | 3.0 | 3.2 | 4.0 | 92.8 |
| SDc | 0.5 | 0.2 | 0.5 | 3.2 |
| CVc [%] | 16.7 | 6.3 | 12.5 | 3.4 |
| Formulation | FR50.14 | FR50.15 | FR50.16 | FR50.17 |
|---|---|---|---|---|
| pH | 4.31 | 4.59 | 4.62 | 2.66 |
| SDpH | 0.01 | 0.01 | 0.02 | 0.07 |
| CVpH [%] | 0.23 | 0.22 | 0.43 | 2.63 |
| Conductivity [μS/mm] | 1.7 | 1.1 | 1.1 | 91.6 |
| SDc | 0.0 | 0.1 | 0.1 | 0.9 |
| CVc [%] | 0.0 | 9.1 | 9.1 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassi, I.; Bandinelli, C.; Delchiaro, F.; Piana, M.; Sarti, G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires V: Comparison between EN 60754-1 and EN 60754-2. Fire 2023, 6, 326. https://doi.org/10.3390/fire6080326
Bassi I, Bandinelli C, Delchiaro F, Piana M, Sarti G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires V: Comparison between EN 60754-1 and EN 60754-2. Fire. 2023; 6(8):326. https://doi.org/10.3390/fire6080326
Chicago/Turabian StyleBassi, Iacopo, Claudia Bandinelli, Francesca Delchiaro, Marco Piana, and Gianluca Sarti. 2023. "A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires V: Comparison between EN 60754-1 and EN 60754-2" Fire 6, no. 8: 326. https://doi.org/10.3390/fire6080326
APA StyleBassi, I., Bandinelli, C., Delchiaro, F., Piana, M., & Sarti, G. (2023). A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires V: Comparison between EN 60754-1 and EN 60754-2. Fire, 6(8), 326. https://doi.org/10.3390/fire6080326










