Abstract
Worsening climate change and increasing temperatures generate more sever and extended wildfires, raising concerns about ecosystem services. Prescribed burns (PB) are used to reduce forest fuel loads. Improving knowledge regarding the vegetation response after PB is essential for generating common points for monitoring ecological burning effects and generating a protocol or practice guide. We compared the PB seasonality of low-intensity (spring, summer, and autumn) and unburned areas in a total of 12 plots in Pinus nigra Arnold ssp. salzmannii Mediterranean forest. Our vegetation analysis was short term (one year after each PB). We analyzed vegetation coverage, α-diversity (Pielou, Simpson, and Shannon’s index), life forms, and fire-adapted traits using the Canfield transect method, followed by statistical analyses such as non-metric multidimensional scaling (NMDS) and two-way ANOVA. α-diversity was significantly decreased (>55% of dissimilarity) in the burned plots during each season, with the lowest values after summer PB (69% of dissimilarity) when comparing the burned and unburned plots. There was a significant increase in hemicryptophytes (15−20%) and geophyte coverage (from 6% to 14%, or from 4% to 8% in certain cases) in the burned plots after PB seasonality; however, the phanerophytes were reduced (from 13% to 5%). Resprouters were more dominant after PB (an increase of 15–20%), which indicates that resprouters have a faster recovery and generate a fuel load quickly for highly flammable species such as Bromus after low-intensity burning. This suggests that low-intensity prescribed burning may not be the best methodology for these resprouting species. This study helps to understand how burning in the early season can affect inflammable vegetation and the change in fuel that is available in semi-arid landscapes. This is key to achieving the basis for the development of a standardized system that allows for the efficient management of forest services in order to reduce wildfire risks. One objective of this line of research is to observe the effects of recurrent burning in different seasons on vegetation, as well as plant−soil interaction using the microbial and enzyme soil activity.
1. Introduction
Forests provide essential ecosystem services that significantly contribute to society. They play a critical role in carbon sequestration and serve as vital habitats, particularly in biodiversity-rich regions such as the Mediterranean Basin [1]. This area is recognized as a global biodiversity hotspot and one of Europe’s most diverse biomes [2]. However, the escalating threat of wildfires, exacerbated by climate change and LULUCF (land use, land-use change, and forestry) changes, poses a severe challenge to forest ecosystems and the services they provide [3,4].
Globally, wildfires represent major disturbances in forest ecosystems [5], and they are increasingly being influenced by climate change dynamics and alterations in LULUCF [6]. These wildfires have far-reaching impacts on vegetation, water resources, air quality, and fauna, which result in the degradation of both environmental and socio-economic values [7,8]. Climate projections indicate a potential loss of 11–25% of forested areas in the Mediterranean zone by the end of the century, which would result in a significant decline in wildlife habitat and overall biodiversity [9,10].
The Mediterranean Basin, characterized by recurrent and severe fires, harbors fragile and vulnerable forest ecosystems, particularly in non-serotinous pine forests [11]. These ecosystems experience shifts in vegetation toward fire-adapted communities and shrublands due to changes in wildfire regimes [12]. Pine-dominated ecosystems constitute a main landscape feature in the Mediterranean Basin, where they cover 25% of the forested surface; the Mediterranean basin has experienced a substantial increase in wildfires, with an average annual growth rate of approximately 5% [13]. The data reveal that wildfires in the Mediterranean region have surged by over 50% in the last decade alone, posing significant challenges for ecosystems, human settlements, and biodiversity conservation [14]. The southeastern region of Europe, encompassing the Mediterranean basin, has been marked by a significant footprint from forest fires. Statistically, the area has experienced a staggering number of fire incidents over the years. For instance, in the past decade alone, the Mediterranean basin has witnessed an average of thousands of wildfires annually [15]. To mitigate the detrimental impacts of wildfires and to effectively control large-scale fire incidents, adaptive forest management practices that encompass fuel load management are crucial.
Prescribed burns (PB) have emerged as a valuable tool in adaptive forest management. PBs involve the deliberate removal of fuel loads and the modification of vegetation structure to reduce fire severity [16]. PBs can effectively reduce the available fuel in the forest ecosystem, a critical factor in fire ignition and severity [17]. In the Mediterranean Basin, PBs are typically conducted in autumn or spring by taking advantage of optimal conditions, such as the relative moisture (>40%) and ambient temperature (23–30 °C) for low-intensity burning [18]. PB has become a critical tool in fire management, particularly in the Mediterranean region of southeastern Europe, where it has shown promising results for reducing wildfire severity and protecting ecosystems. However, the seasonality of PBs can yield varying effects on vegetation recovery and post-fire dynamics [19]. Previous studies have demonstrated that the timing of PBs is critical, with spring and early summer burns promoting rapid vegetation recovery without significant impacts on plant communities [20,21,22]. One notable effect of PBs is the response of plant communities, particularly in terms of resprouting capacity and species diversity. Resprouting species, which possess the ability to regenerate from the surviving buds after fire, often exhibit increased abundance following PB due to their inherent regenerative capabilities [23]. However, the short-term, low-intensity burning associated with PB may result in a reduction in overall species diversity, as some more sensitive species may not experience the thermal shock required for their germination and establishment [24]. Striking a balance between promoting resprouter dominance and preserving species diversity is crucial when designing effective PB strategies that simultaneously address ecosystem conservation and fire-risk management.
In the Iberian mountains, Pinus nigra Arnold subsp. salzmannii (Dunal) Franco stands have been managed using diverse systems, with the shelterwood system being the predominant approach. This method involves rotation periods of 100 to 120 years and regeneration periods of 20 to 30 years [25]. Forest management practices, including using PBs, are employed to minimize external interferences and effectively reduce fuel loads. Prescribed burns for fuel reduction have been extensively practiced in various regions of southeastern Europe within the Mediterranean basin, including countries such as Spain, Italy, Greece, and Portugal, where these regions have witnessed a significant increase in the implementation of prescribed burns, with an estimated annual average of over 1000 prescribed burns conducted across the Mediterranean basin in recent years [26]. Forest management practices, including the use of PB, are employed to minimize external interferences and to effectively reduce fuel loads. However, challenges in obtaining permits and restrictions in certain areas can hinder these strategies being implemented. Nevertheless, PBs play a crucial role in mitigating disturbances such as grazing and ensure the resilience of Pinus nigra, known for its wind and drought resistance, erosion control capabilities, and extensive use in reforestation programs [27,28].
Despite the significance of PBs in fire-prone ecosystems, a critical knowledge gap exists about the short-term ecological effects of low-intensity burnings on vegetation regeneration and diversity in the Mediterranean Basin. Understanding the fire-adapted traits of existing vegetation, such as resprouters or germination of the seed bank due to thermal shock, and post-fire recovery dynamics is essential for effective ecosystem management [29]. In wildfire-prone areas with high recurrence rates, regenerative life forms characterized by root sinks, such as geophytes or hemicryptophytes, often dominate [30,31]. While several studies have examined the relevance of these factors in post-fire plant regeneration [32,33,34], only a few have integrated multiple aspects, including PB seasonality, life forms, and fire-adapted traits. These differences can be influenced by various factors, including plant phenology, fuel moisture content, and microclimatic conditions during the burn season. Studies have highlighted the role of plant functional traits, such as resprouting abilities, serotine, leaf flammability, and bark thickness, in shaping post-fire regeneration patterns. For instance, some studies have observed that species with specific fire-adaptive traits may exhibit different responses to burns depending on the timing and severity of the fire, such as the substantial increase in resprouting species, such as hemicryptophytes and geophytes, following low-intensity prescribed burns or a decrease in overall plant diversity due to the short-term impact of low-intensity burning [35,36,37]. Understanding these mechanisms is essential for effective fire management and the conservation of ecosystems [38]. By investigating the specific characteristics of vegetation communities and plant traits that contribute to these differences, we can gain insights into the dynamics of post-fire vegetation and inform management strategies. Enhancing knowledge of the PB effects on the Mediterranean Basin is crucial for preventing fires in highly flammable vegetation zones with extreme conditions and dry soils, where the aim is to minimize the potential impacts on fragile ecosystems [39]. Therefore, the present research aims to assess the short-term response of understory vegetation following low-intensity PB conducted during different seasons in a Mediterranean black pine forest in Beteta, Castilla-La Mancha, Spain.
We hypothesize that the summer burn season may have a greater impact on vegetation due to factors such as higher temperatures, increased moisture stress, and changes in plant phenology. These hypotheses are well-grounded in the existing literature, highlighting the significant influence of burn seasonality on plant phenology, growth, and survival [40,41,42]. By exploring these specific aspects, we aim to unravel the underlying mechanisms driving the observed differences in vegetation response to burn seasons. In this study, our hypothesis is related to the following: (i) PBs’ affect in the similarity of plant communities due to short-term loss of biodiversity, as well as change in floristic composition; (ii) PBs in summer are those that most affect plant community changes and its similarity; (iii) PBs lead to an increase in resprouters due to their high regenerated capacity, while short-term low-intensity burning results in reduced diversity compared with unburned areas, likely due to the absence of thermal-shock during PB due to low intensity; and (iv) the diversity of vegetation and life form changes are higher in summer PB. By examining these variables, this study seeks to contribute to the understanding of the ecological effects of burning seasonality on vegetation and to provide insights for effective forest management strategies in fire-prone ecosystems.
2. Materials and Methods
2.1. Study Area
The research area has altitude ranges from 1250 to 1300 m.a.s.l and a 4% (±1.3) slope. The climate is Csb “Mediterranean with dry summers” according to the Köppen−Geiger climate classification [43]. The mean annual temperature is 10.2 °C (mean lowest temperatures of 1.7 °C; mean highest temperatures of 20.1 °C). The approximate rainfall is 601 mm per year [44] for the 1990–2020 period. The lowest rainfall appears in July−August with an average of 10 mm, and the most rainfall drops in October with 60 mm.
The study area soils are basic-neutral (pH 7.3) from the Beti-Iberian limb and are linked with gaps of calcareous formations without advanced horizons due to a (semi)-arid climate [45]. Soils are cambisols (CMe), which show non-significant pedogenesis derived from some rocks of aeolian, colluvial, and alluvial origins. The area is characterized by clay and iron oxide formations [46].
PBs were carried out in spring 2016, autumn 2017, and summer 2019 (Table 1), not covering areas of more than 0.1 ha as forestry management practices by the Regional Forest Service staff according to a regional burning plan. The study plots are located in the central Iberian Peninsula, close to Beteta (Spain), and represent the range of typical black pine stands with a tree density of about 1280 no./ha (±256) and a mean tree height of 7.25 m (±3.27) [47]. Understory vegetation is formed by mixed masses of Genista scorpius L., Bromus erectus Huds., Ononis spinosa L., and Pilosella castellana Boiss. & Reut., among others. The centroid in GPS coordinates (ETRS89/UTM zone 30N) of the study area is (40°33′05.1″ N 2°06′32.9″ W) (Figure 1).

Table 1.
Maximum temperatures (°T) during the prescribed fires. Mean values and standard deviations.
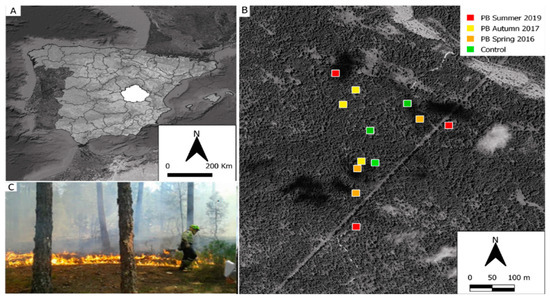
Figure 1.
(A) Province of Cuenca, Spain. (B) Plots where PBs were applied in summer (red), plots where PBs were applied in autumn (yellow), plots where PBs were applied in spring (orange), and the control plots (unburned = green). (C) Burning an experimental plot.
2.2. Experimental Design
Plots
Twelve square plots (30 × 30 m each plot) with similar conditions (ANNEX; Table S1) were set up. Three were installed where the spring PB was to be carried out (PB SPRING), three where the autumn PB was to be performed (PB AUTUMN), and three where the summer PB was to be done (PB SUMMER). The other three plots remained unburned and were used as the control (C) plots. Each plot was separated by at least 10 m to avoid pseudo-replication [48]. The following growing season after each PB (spring PB measurements in 2017; autumn PB measurements in 2018; and summer PB measurements in 2020), the vegetation strata in C and the burned plots were measured in the months (spring−summer) of the growing plant phase [49]. For the preliminary soil characterization, the soil physico-chemical properties in each plot were recorded 1 week before each PB. Checks were made to see if the variables did not differ between plots (ANNEX; Table S2). A comparison was made between the historical climate and the climate data collected during the study (ANNEX; Figure S1). This period was calculated using the methodology of Alizoti et al., 2010, to observe if the precipitation period during the study (2016–2020) deviated from the historical climate of the area, with details showing that it did not differ, but a reduction in precipitation was observed compared with the historical ones [50].
2.3. Prescribed Burnings
PBs were performed with fire lines separated by 1 m and lying perpendicularly to one another. They were carried out opposite the wind direction (a tail burn) to minimize fire severity. This methodology to initiate burning favors the front advancing and offers a shorter fire residence time on the ground, thus avoiding overheating, convection flows to the tree layer, and very high temperatures [51]. In addition, PB were conducted in different years due to the limited and specific weather windows required for carrying out the burns safely. These prescribed burns were carried out by the regional government as part of their management practices.
The fuel model was 7 (height over 1.5 m flammable shrub), according to Rothermel [52] and Albini [53]. The main understory layers were Genista scorpius L., Bromus erectus Huds., Ononis spinosa L., and Pilosella castellana Boiss. & Reut. To characterize burn intensity, six thermocouples were installed (HOBO UX120 4-Channel Analog Logger dataloggers) per plot. Eighteen dataloggers were set at four height levels: 2 cm deep below ground, at 0 cm in mineral soil, above litterfall, and 30 cm above ground.
Fire spreads fundamentally through scrubland according to the technical notebooks of the combustible model taken from the digital terrain model and the phytoclimatic atlas of Spain by MAPAMA [45].
By implementing these measures, we aimed to ensure that the prescribed burns maintained a low intensity throughout their duration. This approach helped us control the severity of the fires and minimize potential ecological impacts beyond our intended objectives. Therefore, considering the classicization of the prescribed burns, the shorter fire residence on the ground, the low fire temperature, and the minimal effect on the tree strata, we maintained a consistent focus on assessing the ecological effects of low-intensity prescribed burns across different seasons.
2.4. Vegetation Indicators
2.4.1. Plant Coverage and α-Diversity
Coverage of the vegetation strata was recorded using the linear transects methodology [54], with three transects per plot (30 × 30 m) at 5 m, 15 m, and 25 m of the corner. This method is fast, cheap, and very indicative [55].
From the recorded database, we calculated the α-diversity using three indices: (i) abundant species (Pielou Index; J), (ii) species dominance (Simpson Index; D), (iii) species abundance (Shannon Index; H°). J was measured as the observed diversity in relation to the maximum diversity (J = H°/H° max) [56]. D was calculated through the probability that two individuals were of the same species [57]. H° was calculated by the relative species abundance present (in cm), including the minimum intercept value of 1 cm [58].
2.4.2. Life Form and Fire-Adapted Traits
Based on the species measured in the line transects, the present vegetation was classified according to its strategies of adaption to fires, namely seeders or resprouters [59,60,61], and as life forms according to the Raunkiaer classification and more database studies [31,62,63], in which life forms are determined as chamaephytes, phanerophytes (subclasses: macrophanerophytes, more than 50 cm above the ground level; nanophanerophytes ≤ 50 cm), geophytes, hemicryptophytes, and therophytes. Life forms and fire strategies were calculated as the average percentages in the different short-term burning years by comparing the seasons and treatment (unburned or burned).
2.5. Statistical Analyses
Shapiro−Wilk tests were performed to observe the normal distribution tendency. Bartlett statistical tests were run to determine the data homoscedasticity for variance verification in each variable. The calculation of the precipitation averages in the study periods, as well as the historical climate, were carried out to analyze the climatological change during the study following the methodology described by Alizoti et al., 2010. We ran an analysis of similarities (ANOSIM) to compare the within- and between-group (burned or control) similarities during the different burn seasons (SPRING, AUTUMN, or SUMMER); 999 replicates were used for permutation analysis to assess the significance values at a p-value threshold of 0.05. In a robust classification, the similarities between plots within the same group (season) should be substantially greater than those from different seasons. The key obtained output was the R statistic coefficient, which ranges from −1 to 1: the higher the value, the closer the similarity between the plant compositions found. R values close to 0 mean that the similarity of the between- and within-groups was irrelevant. In addition, non-metric multidimensional scaling (NMDS) was performed to graphically observe the similarity groups between plant communities; we used the Bray−Curtis dissimilarity index to calculate the distance between the data points and performed hierarchical clustering to determine the grouping of the samples. The NMDS values were square root-transformed to adjust the normal distribution. A SIMPER (similarity percentage) one-way analysis was performed to identify the similarity percentages between vegetation compositions (one-way analysis). Once again, using SIMPER pairwise tests, the most different species among seasons/treatments were individualized, and the relative contribution of each one to such differences was calculated (pairwise analysis). Marked similarity between plots of the same season or between seasons was shown by low mean square distance (R) values within the 0–100 range. We highlighted the top three species contributing the most to dissimilarity in the SIMPER results, as they demonstrate significant influence. Other species, each representing less than 3–5% contribution, were also considered, but were not explicitly listed due to their lower representativeness in the overall dissimilarity. Thus, the high mean square distance values corresponded to significant differences. Finally, we conducted one-way ANOVA to compare the significant differences of each index related to α-diversity, life forms, and fire-adapted traits classification between treatments for each season (ANOVA significance level p-value < 0.05). Combining both the ANOSIM and ANOVA analyses, we were able to gain a comprehensive understanding of how burn seasons influence vegetation responses. ANOSIM provided insights into the overall differences between the burned and control plots, while the ANOVA analysis offered more specific information on how these differences vary across different seasons. The significance level for all statistical tests was set at a 95% confidence level. The software package employed for the statistics was PRIMER-e v6.
3. Results
3.1. Analysis of Coverage Vegetation and α-Diversity
The comparison between the burned (PB) and control (C) plots in different sessions revealed significant differences based on the ANOSIM results (global R = 0.54; p < 0.01) (Table 2). Additionally, the climatic graph (ANNEX; S3) demonstrated that the climate during the study years aligned with the typical seasonal patterns of the area, indicating no significant variations between the study periods. However, it is worth noting that rainfall levels were generally lower compared with the historical climate data.

Table 2.
Analysis of similarities (ANOSIM) among treatments (C and PB plots) during each season (PB SPRING, PB AUTUMN, and PB SUMMER).
NMDS showed similarities in three different groups, where vegetation populations were similar, with all C in one, and two others between AUTUMN and SUMMER and SPRING and SUMMER (Figure 2). These findings indicate similarity between similar plant communities and before treatment, with a more distant group between groups, which was SUMMER.
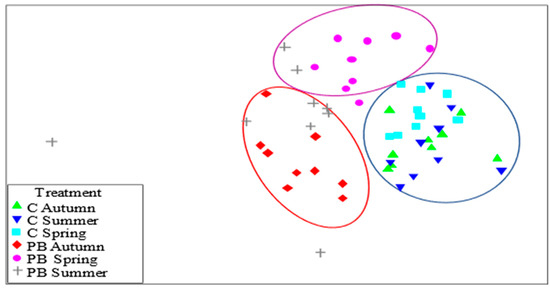
Figure 2.
NMDS graph. Three groups. CONTROL (C) plots (blue). PB AUTUMN and SUMMER burned plots (red) and PB SPRING and SUMMER burned plots (purple).
The SIMPER analysis indicated marked similarity (50%) among the C plots during each season, with two main understory species: Bromus erectus (with 20–24% contribution) and Genista scorpius (with 12–13% contribution). In the PB plots, similarity was lower (PB AUTUMN = 43.43%; PB SPRING = 44.91%; and PB SUMMER = 37.37%) (Table 3).

Table 3.
SIMPER one-way analysis with average square distances per plot and the most characteristic parameters that contribute to their similarity. The PB plots and control (C) plots during each season (AUTUMN, SPRING, and SUMMER).
However, after each burning season, marked dissimilarity (65%) was observed for the treatments (unburned and PB plots). In PB AUTUMN, a decrease from 13% to 2.7% was noted in Gesnita scorpius, while there was no Ranunculus bulbosus before burning, which increased by up to 8% after the PB, and hardly any differences were observed for Bromus erectus (20% to 17%). In PB SPRING, a reduction in Bromus erectus (26% to 15%), Pinus nigra (from 10% to 2%), and Rosa canina (from 9 to 4%) took place, with the least dissimilarity among treatments (59%). In PB SUMMER, the greatest dissimilarity was obtained (69%), with reductions in Pinus nigra (8% to 2%) and Genista scorpius (12% to 3%), and a slight reduction in Bromus erectus (21% to 18%) (Table 4).

Table 4.
SIMPER pairwise analysis, with average square distances between plots/season and species showing the most significant differences. Prescribed burning plots (PB) and control plots (C) during each season (AUTUMN, SPRING, and SUMMER).
For α-diversity, one-way ANOVAs were performed during each season by comparing treatments. J was significant (p-value = 0.03; F-statistic = 2.72), but only in PB SUMMER (Figure 3), which was the lowest. We calculated the significance of H0 during each season among treatments. Significant differences (p-value < 0.01) were obtained at each time, with higher H° values in C than in the PB plots (Figure 4). We also calculated the D index for treatments during each season by ANOVA tests. Significant differences were obtained (p-value < 0.01) and the values in the control plots were higher than in the PB ones in PB AUTUMN and PB SUMMER (Figure 5).
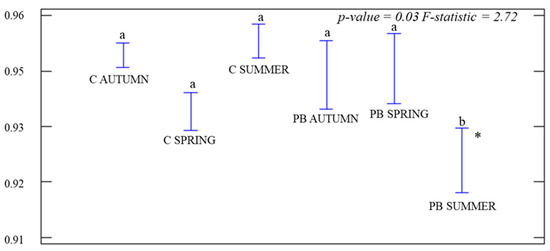
Figure 3.
ANOVA graph for the Pielou Index (J) during each season and treatment. Control plots (C AUTUMN, C SPRING, and C SUMMER). Treatment plots during each season (PB AUTUMN, PB SPRING, and PB SUMMER). Standard deviations (hanging bars) and mean values. Significance level p-value < 0.05. * The highest different value between the control and burned plots. Lowercase (a/b) indicates significant differences among treatments.
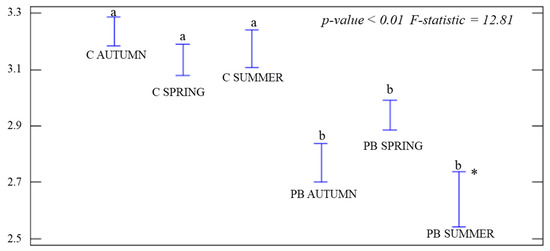
Figure 4.
ANOVA graph for the Shannon Index (H°) for each season and treatment. Control plots (C AUTUMN, C SPRING, and C SUMMER). Treatment plots during each season (PB AUTUMN, PB SPRING, and PB SUMMER). Standard deviations (hanging bars) and mean values. Significance level p-value < 0.05. * The highest different value between the control and burned plots. Lowercase (a/b) indicates significant differences among treatments.
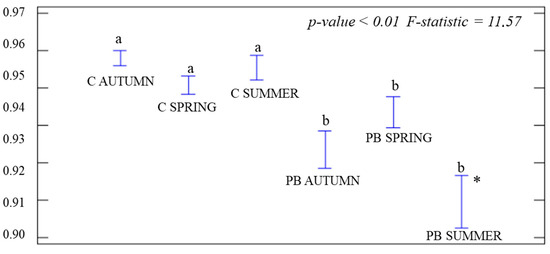
Figure 5.
ANOVA graph for the Simpson Index (D) during each season and treatment. Control plots (C AUTUMN, C SPRING, and C SUMMER). Treatment plots during each season (red circle group of Burners = PB AUTUMN, PB SPRING, and PB SUMMER). Standard deviations (hanging bars) and mean values. Significance level p-value < 0.05. * The highest different value between the control and burned plots. Lowercase (a/b) indicates significant differences among treatments.
3.2. Analysis of Life Forms and Fire-Adapted Traits Classification
Regarding life forms (Raunkiaer classification), an ANOVA test was run in spring. Significant differences were observed in phanerophytes (also subclasses) and geophytes (p-value < 0.01), which showed a reduction in all of the life forms and seasons, except for geophytes and hemicryptophytes. In PB SPRING, the only life form with a significant increase was geophytes, which implied a mean increase from 4% to 16%. The reduction in the percentage of macrophanerophytes went from 13% to 5% and was significant. In PB AUTUMN, there were significant differences in chamaephytes, hemicryptophytes, and phanerophytes (both subclasses) (p-value < 0.01), with a lower percentage value for chamaephytes and phanerophytes between the C and PB plots, but a marked increase in hemicryptophytes from 46% to 74%. In PB SUMMER, significant differences were observed in chamaephytes, hemicryptophytes, and phanerophytes (both subclasses) (p-value < 0.01), and lower values were for chamaephytes. However, hemicryptophytes increased from 44% to 64%. There was a slight increase in geophytes (6% to 9%) and therophytes (4% to 8%), but with no significant differences (Figure 6).
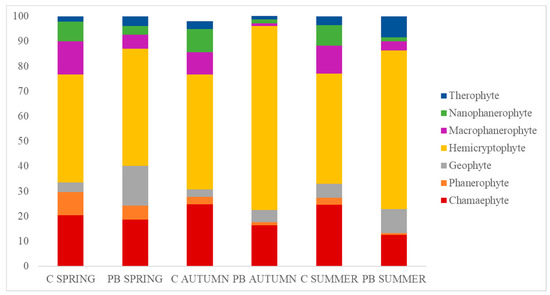
Figure 6.
Accumulative graph of the life forms coverage (% = y axis) in the unburned (C) and burned (PB) plots during different seasons (x axis).
Regarding the evaluated results of the fire-adapted traits, significant differences (p-value < 0.01) were found in both resprouters and seeders during each PB season when comparing the PB plots to the C ones. In PB SPRING, the percentage of seeders was lowered from 29% to 15%, while the resprouters increased from 71% to 85% when comparing the measurement taken in the PB plots to that of the C plots. In PB AUTUMN, once again seeders reduced from 40% to 20% in the PB plots versus the C plots, and the percentage of resprouters increased from 60% to 80%. PB SUMMER was similar to PB AUTUMN, with a significant reduction in seeders in the PB plots compared with the C plots, which decreased from 42% to 21%, and resprouters rose from 58% to 79% (Figure 7).
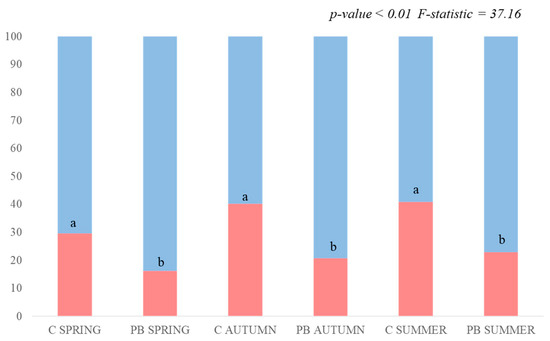
Figure 7.
Accumulative graph of the coverage percentage (y-axis) in the unburned (C) and burned (PB) plots during different seasons (x-axis) for fire-adapted traits. Legend: red bars = seeder strategy coverage percentage; blue bars = resprouter strategy coverage percentage. Lowercase (a/b) indicates significant differences among treatments.
4. Discussion
In this research, the choice of PB seasons was determined based on the measured variables by following a specific burning methodology whose aim is to achieve low-intensity burns with minimal severity [47]. It was concluded that the observed changes in the measured variables were primarily attributed to the PB treatments applied during each respective season, which may have influenced, or not, the vegetation dynamics. By establishing a comparable initial situation and conducting samplings at consistent time points following PB treatments, this research aimed to discern the specific effects of PB seasonality on vegetation recovery. These findings will contribute to a better understanding of the ecological impacts of PBs in forest ecosystems and inform about effective management strategies.
A consistent initial situation was established among plots when considering various aspects, such as forest ecosystem characteristics, management practices, geology, floristic composition, and soil data (ANNEX, Table S1). This careful selection ensured that there were no significant differences between the C plots when the experiment design was implemented and provides a uniform starting point to effectively address the research objectives. Both the C and PB plots were sampled year after applying PB during each season by specifically focusing on the short-term recovery of vegetation [49]. This standardized timeframe allowed for a comparative analysis to be conducted across all the plots to ensure consistency in the assessment of vegetation dynamics.
4.1. Coverage Vegetation and α-Diversity
The analysis using NMDS revealed a high level of homogeneity among the studied areas, which indicates a consistent composition of plant communities within the C plots prior to applying PBs. However, after implementing PBs, a dissimilarity between the C and PB plots became evident, which supports the findings from previous studies [64,65,66]. This observed dissimilarity persisted across all three PB seasons, which suggests a significant impact on vegetation composition. Following PB treatments, a notable reduction in vegetal coverage occurred for the species with high flammability and a dominant presence in the study area, such as Bromus erectus and Genista scorpius. This reduction was particularly marked in summer when combined with water stress in semi-arid environments, and significantly influenced vegetation coverage [67]. The study area, situated in the Mediterranean Basin, is characterized by environmental constraints, including high temperatures, low rainfall, and nutrient-poor soils. These factors contribute to a short growing season for vegetation [68], which likely plays a role in the observed uniformity of fire-adapted plant communities following disturbances [69].
In α-diversity terms, the J index showed significant differences only for the summer burn season. This index, which measures the proportion of observed diversity relative to the maximum expected diversity, is directly associated with community evenness [70]. The summer burn plots obtained the lowest α-diversity values, which can be attributed to species migration to unoccupied areas facilitated by fire or the limited impact on the seed bank due to low-intensity PBs. Similar shifts in species composition shortly after disturbances, particularly fires, have been documented in previous studies [71,72,73]. This dominance can be influenced by factors such as fire seasonality, intensity, and post-fire recovery dynamics of the vegetation community [74,75]. Fire-adapted traits of species should also be considered, as fire-adapted species tend to thrive in post-fire environments [76]. This finding aligns with the hypothesis that ecological succession drives changes in plant communities following disturbances [77]. Shannon and Pielou emphasize equitability, while the Simpson index highlights dominance [78,79]. These differences reflect varying disturbance impacts and community responses to prescribed burns.
To gain a comprehensive understanding, multiple factors influencing diversity patterns, such as fire intensity, microclimate variations, soil nutrient availability, and interspecific interactions, should be considered [80]. Analyzing these aspects will lead to a more holistic interpretation of the observed results and their ecological implications. Fire, by eliminating both fire-sensitive and non-fire-sensitive species, has a profound influence on floristic composition, which leads to a certain level of homogeneity at small scales (burned area), but heterogeneity at larger scales [81]. Furthermore, other studies have indicated that low-severity fires can enhance species richness [82,83].
4.2. Raunkiaer and Fire-Adapted Traits Classification
Our study reveals a significant increase in perennial vegetation, specifically hemicryptophytes and geophytes, following low-intensity PBs. These plant forms exhibit rapid growth rates and possess extensive root systems that efficiently absorb nutrients from the soil [39]. Notably, the regeneration process of these life forms predominantly occurs underground, where temperatures remain relatively moderate [84,85].
It is particularly interesting to note that the most substantial increments in hemicryptophytes took place after the autumn burning, where percentages increased from 46% to 74%, and also during the summer season, with an increase from 44% to 64%. These variations can be attributed to various factors, including local microclimate conditions and biotic processes, such as soil−plant interactions triggered by ash deposition and nutrient input [86]. Consequently, these factors contribute to altering the response of functional vegetation traits [87], which reinforces the observed changes in hemicryptophyte abundance. The differential response of hemicryptophytes to prescribed burns in different seasons may be influenced by specific local microclimate conditions and biotic processes, such as soil−plant interactions triggered by ash deposition and nutrient input [88,89]. The absence of a similar response in the spring burning season could be attributed to factors such as the timing of the burn not coinciding with optimal growth conditions for hemicryptophytes or different ecological conditions resulting from spring burns [90]. Further research is needed to explore these factors and their implications for hemicryptophyte dynamics and response to fire events across different burn seasons.
Regarding fire-adapted traits, our research reveals a noteworthy rise in the vegetation cover percentage of resprouters in the PB plots compared with the C ones during each season, which was consistently observed across all of the scenarios. This increase in resprouters is attributed to the low intensity and severity of PBs, which prevent the application of underground heat needed for the germination of the existing seed bank [36]. Resprouters effectively capitalize on the nutrient-rich environment created by post-burning ashes; hence, their recovery and prevalence are enhanced [91]. These findings reinforce the observations made regarding life form dynamics and the observed increases that primarily result from ground-level sprouting mechanisms.
The results obtained in our study highlight the importance of the microclimate in shaping vegetation responses following prescribed burns. Microclimatic conditions, such as temperature, moisture, and light availability, play a crucial role in determining the success of post-fire regeneration and the establishment of different plant forms. The observed increases in hemicryptophytes and geophytes can be attributed to favorable microclimatic conditions that promote their underground regeneration and nutrient uptake [92,93]. Additionally, the prevalence of resprouters in the prescribed burn plots suggests that the microclimatic conditions created by low-intensity burns facilitate their recovery and persistence [94]. Understanding the intricate relationship between microclimate and vegetation dynamics is essential for effective management strategies and the conservation of biodiversity in fire-prone ecosystems.
4.3. Future Evaluations on the Effects of Preventive Treatments in Forest Management
Despite the growing understanding of the short-term effects, there is still a lack of knowledge regarding the long-term implications of prescribed burns on vegetation communities, highlighting the need for further research in this area, and we will ensure that we address the potential limitations of our study and the importance of long-term monitoring in assessing community changes beyond the first year of succession. To fully understand the long-term implications of PBs on vegetation communities, including changes in life form coverage, diversity, and fire adaptation, further research is needed. This should consider the dynamic nature of post-fire succession, the potential influence of competition and light availability, and the persistence of the observed differences over time. By addressing these aspects, we can develop more informed management strategies for fire-prone ecosystems and advance our understanding of vegetation dynamics in response to changing environmental conditions.
In our study focusing on burning seasons, we recognize the importance of considering plant physiological differences throughout the growing season as potential explanations for our results. Plant physiology, including photosynthetic activity, water-use efficiency, and nutrient uptake, influences plant responses to environmental factors such as prescribed burning. Seasonal variations in physiological traits have been documented to affect plant growth and survival [95]. Moreover, fire-adapted species thriving in post-fire environments have been well studied [96,97]. Understanding the seasonal dynamics of plant physiology could provide further insights into the mechanisms driving observed patterns, contributing to more effective ecosystem management strategies.
Based on our results, we recommend the following management guidelines: (1) careful consideration of burning season based on specific objectives; (2) incorporation of spatial heterogeneity in prescribed burning; (3) regular monitoring and adaptive management; and (4) engaging stakeholders to support conservation efforts. These guidelines can optimize vegetation responses and enhance the resilience of fire-prone ecosystems.
5. Conclusions
PB aims to reduce fuel availability and promote the establishment of fire-adapted vegetation. In our study, we investigated the effects of PB on plant communities one year after treatment. The results revealed significant differences between PB and control plots, indicating changes in community composition following low-intensity fire application. While dominant species persisted, their relative abundance decreased. Notably, we observed an increase in hemicryptophytes and geophytes, which are indicative of fire-adapted traits. This shift towards fire-adapted vegetation enhances the ecosystem resilience to future fires.
Our findings have important implications for improving fire prevention strategies in fire-prone ecosystems. However, to better understand the impact of these changes on biodiversity patterns, including life form classifications and fire-adapted traits, further research is needed, especially considering the influence of changing climatic conditions. Our study contributes to a better understanding of the ecological consequences of PB, the seasonality of their application, and their impact on vegetation diversity in fire-prone ecosystems.
To facilitate the practical application of our research, we recommend developing a common framework for a practical guide or monitoring protocol to assess the ecological effects of PB. Future research should focus on deepening our understanding of PB effects across different seasons, examining medium- to long-term changes, exploring plant−soil interactions, and evaluating the impacts of PB on soil physical-chemical and microbiological properties. Additionally, assessing the regeneration of chemical compounds and the photosynthetic rate in vegetation following PB would provide valuable insights.
Continuing this research line will contribute to a comprehensive understanding of the ecological implications of PB, enabling informed decision making and the development of effective strategies for managing fire-prone ecosystems. Moreover, it will support the establishment of monitoring protocols to assess the ecological effects of PB and improve the management of fire-prone ecosystems. It is important to acknowledge the study’s limitations and consider the long-term implications. Further research is needed to explore the dynamics of post-fire succession, including changes in life form coverage, diversity, and fire adaptation, and to determine the persistence of observed differences over time. By addressing these knowledge gaps, we can develop more precise and informed management strategies for fire-prone ecosystems, considering the complex interplay of ecological factors in shaping post-fire vegetation dynamics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fire6080283/s1, Figure S1: Graph of precipitation (mm) during the study period (2016–2020) compared to the historical average (1980–2010) of the study area, Beteta (Spain). Dashed lines depict the historical average. The continuous line refers to the precipitation for the study period (2016–2020). The gray area denotes the deviation between the historical average and that of the study. 2016 Spring Prescribed Burn = PB SPRING; 2017 Prescribed Burn = PB AUTUMN; 2019 Summer Prescribed Burn = PB SUMMER; Table S1: Preliminary characterization of terrain and vegetation in the research area. Mean values and standard deviations; Table S2: Preliminary characterization (Pre-burned) of physico-chemical soil parameters in the research area. Mean values and standard deviations.
Author Contributions
Á.F.-C.: conceptualization, methodology, formal analysis, writing—original draft preparation. J.d.l.H.-I.: funding acquisition, supervision, and project administration. M.E.L.-B.: conceptualization and writing—review and editing. P.A.P.-Á.: validation and methodology. E.P.-M.: validation and methodology. J.G.-R.: validation and methodology. D.M.: validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by regional funds by the “Junta de Comunidades Castilla-La Mancha (PRESFIRE: SBPLY/19/180501/000130/1)”, and by the Spanish Agricultural Research and Technology Institute (“INIA”) with a link to a Research National Project “VIS4FIRE (RTA2017-0042-C05-03 MCIN/AEI/10.13039/501100011033 “FEDER a way of making Europe”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support this study will be shared upon reasonable request to the corresponding author.
Acknowledgments
The authors thank GEACAM (“Empresa Pública de Gestión Ambiental de Castilla-La Mancha”) for its support and for conducting the PB and the Castilla-La Mancha Regional Forestry Service. They are also grateful to Helen Warburton for revising the English language. Álvaro Fajardo has a predoctoral-contract from the University of Castilla-La Mancha with the European Social Fund (2023-PRED-21287). This research was funded by regional funds by the “Junta de Comunidades Castilla-La Mancha (PRESFIRE: SBPLY/19/180501/000130/1)”, and by Spanish Agricultural Research and Technology Institute (“INIA”) with a link to a Research National Project “VIS4FIRE (RTA2017-0042-C05-03 MCIN/AEI/10.13039/501100011033 “FEDER a way of making Europe”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Tittensor, D.P.; Walpole, M.; Hill, S.L.L.; Boyce, D.G.; Britten, G.L.; Burgess, N.D.; Butchart, S.H.M.; Leadley, P.W.; Regan, E.C.; Alkemade, R.; et al. A mid-term analysis of progress toward international biodiversity targets. Science 2014, 346, 241–244. [Google Scholar] [CrossRef]
- Poorter, L.; van der Sande, M.T.; Thompson, J.; Arets, E.J.M.M.; Alarcón, A.; Álvarez-Sánchez, J.; Ascarrunz, N.; Balvanera, P.; Barajas-Guzmán, G.; Boit, A.; et al. Diversity enhances carbon storage in tropical forests. Glob. Ecol. Biogeogr. 2015, 24, 1314–1328. [Google Scholar] [CrossRef]
- Cassell, B.A.; Scheller, R.M.; Lucash, M.S.; Hurteau, M.D.; Loudermilk, E.L. Widespread severe wildfires under climate change lead to increased forest homogeneity in dry mixed-conifer forests. Ecosphere 2019, 10, e02934. [Google Scholar] [CrossRef]
- Roces-Díaz, J.V.; Santín, C.; Martínez-Vilalta, J.; Doerr, S.H. A global synthesis of fire effects on ecosystem services of forests and woodlands. Front. Ecol. Environ. 2022, 20, 170–178. [Google Scholar] [CrossRef]
- Anav, A.; Mariotti, A. Sensitivity of natural vegetation to climate change in the Euro-Mediterranean area. Clim. Res. 2011, 46, 277–292. [Google Scholar] [CrossRef]
- Cantón, Y.; Solé-Benet, A.; de Vente, J.; Boix-Fayos, C.; Calvo-Cases, A.; Asensio, C.; Puigdefábregas, J. A review of runoff generation and soil erosion across scales in semiarid south-eastern Spain. J. Arid. Environ. 2011, 75, 1254–1261. [Google Scholar] [CrossRef]
- Barredo, J.I.; Caudullo, G.; Dosio, A. Mediterranean habitat loss under future climate conditions: Assessing impacts on the Natura 2000 protected area network. Appl. Geogr. 2016, 75, 83–92. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J. Global Change and Forest Disturbances in the Mediterranean Basin: Breakthroughs, Knowledge Gaps, and Recommendations. Forests 2021, 12, 603. [Google Scholar] [CrossRef]
- Halofsky, J.E.; Peterson, D.L.; Harvey, B.J. Changing wildfire, changing forests: The effects of climate change on fire regimes and vegetation in the Pacific Northwest, USA. Fire Ecol. 2020, 16, 4. [Google Scholar] [CrossRef]
- Chick, M.P.; Nitschke, C.R.; Cohn, J.S.; Penman, T.D.; York, A. Factors influencing above-ground and soil seed bank vegetation diversity at different scales in a quasi-Mediterranean ecosystem. J. Veg. Sci. 2018, 29, 684–694. [Google Scholar] [CrossRef]
- Barbéro, M.; Loisel, R.; Quézel, P.; Richardson, D.; Romane, F. Pines of the Mediterranean basin. In Ecology and Biogeography of Pinus; Cambridge University Press: Cambridge, UK, 1998; p. 8. [Google Scholar]
- Change, M.E.C.; Cramer, W.; Guiot, J.; Marini, K.; Azzopardi, B.; Balzan, M.V.; Cherif, S.; Doblas-Miranda, E.; dos Santos, M.; Dobrinski, P. Climate and Environmental Change in the Mediterranean Basin—Current Situation and Risks for the Future; First Mediterranean Assessment Report; MedECC: Lisbon, Portugal, 2020. [Google Scholar]
- EFFIS. Forest Fires in Europe, Middle East and North Africa 2021. 2023. Available online: https://effis.jrc.ec.europa.eu/ (accessed on 12 July 2023).
- Vadilonga, T.; Úbeda, X.; Germann, P.F.; Lorca, M. Effects of prescribed burnings on soil hydrological parameters. Hydrol. Process. 2008, 22, 4249–4256. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Wildfires and global change. Front. Ecol. Environ. 2021, 19, 387–395. [Google Scholar] [CrossRef]
- Clark, D.A.; Brown, S.; Kicklighter, D.W.; Chambers, J.Q.; Thomlinson, J.R.; Ni, J. Measuring Net Primary Production in Forests: Concepts and Field Methods. Ecol. Appl. 2001, 11, 356–370. [Google Scholar] [CrossRef]
- Vázquez-Veloso, A.; Dejene, T.; Oria-de-Rueda, J.A.; Guijarro, M.; Hernando, C.; Espinosa, J.; Madrigal, J.; Martín-Pinto, P. Prescribed burning in spring or autumn did not affect the soil fungal community in Mediterranean Pinus nigra natural forests. For. Ecol. Manag. 2022, 512, 120161. [Google Scholar] [CrossRef]
- Reinking, D.L. Fire regimes and avian responses in the central tallgrass prairie. Stud. Avian Biol. 2005, 30, 116. [Google Scholar]
- García-Llamas, P.; Suárez-Seoane, S.; Fernández-Manso, A.; Quintano, C.; Calvo, L. Evaluation of fire severity in fire prone-ecosystems of Spain under two different environmental conditions. J. Environ. Manag. 2020, 271, 110706. [Google Scholar] [CrossRef]
- Bowd, E.J.; Egidi, E.; Lindenmayer, D.B.; Wardle, D.A.; Kardol, P.; Cary, G.J.; Foster, C. Direct and indirect effects of fire on microbial communities in a pyrodiverse dry-sclerophyll forest. J. Ecol. 2022, 110, 1687–1703. [Google Scholar] [CrossRef]
- Quevedo, L.; Rodrigo, A.; Espelta, J.M. Post-fire resprouting ability of 15 non-dominant shrub and tree species in Mediterranean areas of NE Spain. Ann. For. Sci. 2007, 64, 883–890. [Google Scholar] [CrossRef]
- Moreira, F.; Ascoli, D.; Safford, H.; Adams, M.A.; Moreno, J.M.; Pereira, J.M.C.; Catry, F.X.; Armesto, J.; Bond, W.; González, M.E.; et al. Wildfire management in Mediterranean-type regions: Paradigm change needed. Environ. Res. Lett. 2020, 15, 011001. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Heydari, M.; Miralles, I.; Zema, D.A.; Manso, R. Effects of Skidding Operations after Tree Harvesting and Soil Scarification by Felled Trees on Initial Seedling Emergence of Spanish Black Pine (Pinus nigra Arn. ssp. salzmannii). Forests 2020, 11, 767. [Google Scholar] [CrossRef]
- Valor, T.; Battipaglia, G.; Piqué, M.; Altieri, S.; González-Olabarria, J.R.; Casals, P. The effect of prescribed burning on the drought resilience of Pinus nigra ssp. salzmannii Dunal (Franco) and P. sylvestris L. Ann. For. Sci. 2020, 77, 13. [Google Scholar] [CrossRef]
- del Cerro Barja, A.; Borja, M.L.; García, E.M.; Serrano, F.L.; Abellán, M.A.; Morote, F.G.; López, R.N. Influence of stand density and soil treatment on the Spanish Black Pine (Pinus nigra Arn. ssp. Salzmannii) regeneration in Spain. For. Syst. 2009, 18, 167–180. [Google Scholar]
- Martín-Alcón, S.; Coll, L. Unraveling the relative importance of factors driving post-fire regeneration trajectories in non-serotinous Pinus nigra forests. For. Ecol. Manag. 2016, 361, 13–22. [Google Scholar] [CrossRef]
- Keane, R.E.; Agee, J.K.; Fulé, P.; Keeley, J.E.; Key, C.; Kitchen, S.G.; Miller, R.; Schulte, L.A.; Keane, R.E.; Agee, J.K.; et al. Ecological effects of large fires on US landscapes: Benefit or catastrophe? Int. J. Wildland Fire 2008, 17, 696–712. [Google Scholar] [CrossRef]
- Pausas, J.G.; Carbó, E.; Neus Caturla, R.; Gil, J.M.; Vallejo, R. Post-fire regeneration patterns in the eastern Iberian Peninsula. Acta Oecol. 1999, 20, 499–508. [Google Scholar] [CrossRef]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Being the Collected Papers of C. Raunkiaer; Clarendon Press: Oxford, UK, 1934. [Google Scholar]
- Teixeira, A.M.C.; Curran, T.J.; Jameson, P.E.; Meurk, C.D.; Norton, D.A. Post-Fire Resprouting in New Zealand Woody Vegetation: Implications for Restoration. Forests 2020, 11, 269. [Google Scholar] [CrossRef]
- Martinez, J.L.; Lucas-Borja, M.E.; Plaza-Alvarez, P.A.; Denisi, P.; Moreno, M.A.; Hernández, D.; González-Romero, J.; Zema, D.A. Comparison of Satellite and Drone-Based Images at Two Spatial Scales to Evaluate Vegetation Regeneration after Post-Fire Treatments in a Mediterranean Forest. Appl. Sci. 2021, 11, 5423. [Google Scholar] [CrossRef]
- Carrillo-García, C.; Girola-Iglesias, L.; Guijarro, M.; Hernando, C.; Madrigal, J.; Mateo, R.G. Ecological niche models applied to post-megafire vegetation restoration in the context of climate change. Sci. Total Environ. 2023, 855, 158858. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Akhalkatsi, M.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, R.F.; et al. Recent Plant Diversity Changes on Europe’s Mountain Summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Epicormic Resprouting in Fire-Prone Ecosystems. Trends Plant Sci. 2017, 22, 1008–1015. [Google Scholar] [CrossRef]
- Rabin, S.S.; Gérard, F.N.; Arneth, A. The influence of thinning and prescribed burning on future forest fires in fire-prone regions of Europe. Environ. Res. Lett. 2022, 17, 055010. [Google Scholar] [CrossRef]
- Ewing, A.L.; Engle, D.M. Effects of Late Summer Fire on Tallgrass Prairie Microclimate and Community Composition. Am. Midl. Nat. 1988, 120, 212–223. [Google Scholar] [CrossRef]
- Moghli, A.; Santana, V.M.; Baeza, M.J.; Pastor, E.; Soliveres, S. Fire Recurrence and Time Since Last Fire Interact to Determine the Supply of Multiple Ecosystem Services by Mediterranean Forests. Ecosystems 2022, 25, 1358–1370. [Google Scholar] [CrossRef]
- Keeley, J.E.; Pausas, J.G.; Keeley, J.E.; Pausas, J.G. Distinguishing disturbance from perturbations in fire-prone ecosystems. Int. J. Wildland Fire 2019, 28, 282–287. [Google Scholar] [CrossRef]
- Stevens, J.T.; Haffey, C.M.; Coop, J.D.; Fornwalt, P.J.; Yocom, L.; Allen, C.D.; Bradley, A.; Burney, O.T.; Carril, D.; Chambers, M.E.; et al. Tamm Review: Postfire landscape management in frequent-fire conifer forests of the southwestern United States. For. Ecol. Manag. 2021, 502, 119678. [Google Scholar] [CrossRef]
- Thomsen, A.M.; Ooi, M.K.J. Shifting season of fire and its interaction with fire severity: Impacts on reproductive effort in resprouting plants. Ecol. Evol. 2022, 12, e8717. [Google Scholar] [CrossRef] [PubMed]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- AEMET. Weather Data from Beteta Station (Cuenca-Spain) 2000–2022; State Meteorological Agency of Spanish Government (AEMET): Madrid, Spain, 2022; Available online: http://www.aemet.es/ (accessed on 3 May 2022).
- IGN. Mapa de Suelos de España: Escala 1:1.000.000; Instituto Geográfico Nacional: Madrid, Spain, 2006. [Google Scholar]
- Working Group WRB IUSS. Base Referencial Mundial del Recurso Suelo 2014, Actualización 2015. Informes Sobre Recursos Mundiales de Suelos 106, FAO, Roma. 2016; ISBN 978-92-5-308369-5. Available online: https://www.fao.org/home/en/ (accessed on 13 April 2022).
- Espinosa, J.; Martin-Benito, D.; Rodríguez de Rivera, Ó.; Hernando, C.; Guijarro, M.; Madrigal, J. Tree Growth Response to Low-Intensity Prescribed Burning in Pinus nigra Stands: Effects of Burn Season and Fire Severity. Appl. Sci. 2021, 11, 7462. [Google Scholar] [CrossRef]
- Blaser, S.; Prati, D.; Senn-Irlet, B.; Fischer, M. Effects of forest management on the diversity of deadwood-inhabiting fungi in Central European forests. For. Ecol. Manag. 2013, 304, 42–48. [Google Scholar] [CrossRef]
- Rossetti, I.; Cogoni, D.; Calderisi, G.; Fenu, G. Short-Term Effects and Vegetation Response after a Megafire in a Mediterranean Area. Land 2022, 11, 2328. [Google Scholar] [CrossRef]
- Alizoti, P.G.; Kilimis, K.; Gallios, P. Temporal and spatial variation of flowering among Pinus nigra Arn. clones under changing climatic conditions. For. Ecol. Manag. 2010, 259, 786–797. [Google Scholar] [CrossRef]
- Vega, J.A.; Landsberg, J.; Bará, S.; Paysen, T.; Fontúrbel, M.T.; Alonso, M. Efectos del fuego prescrito bajo arbolado de P. Pinaster en suelos forestales de Galicia y Andalucía. Cuad. Soc. Esp. Cienc. For. 2000, 9, 123–136. [Google Scholar]
- Rothermel, R.C. A Mathematical Model for Predicting Fire Spread in Wildland Fuels; Research Paper INT-116 1972; US Department of Agriculture, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1972; 40p.
- Albini, F.A. Estimating Wildfire Behavior and Effects; General Technical Reports INT-GTR-30; U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1976; Volume 30, 92p.
- Canfield, R.H. Application of the Line Interception Method in Sampling Range Vegetation. J. For. 1941, 39, 388–394. [Google Scholar]
- Pokswinski, S.; Gallagher, M.R.; Skowronski, N.S.; Loudermilk, E.L.; Hawley, C.; Wallace, D.; Everland, A.; Wallace, J.; Hiers, J.K. A simplified and affordable approach to forest monitoring using single terrestrial laser scans and transect sampling. MethodsX 2021, 8, 101484. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Springer: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Paula, S.; Arianoutsou, M.; Kazanis, D.; Tavsanoglu, Ç.; Lloret, F.; Buhk, C.; Ojeda, F.; Luna, B.; Moreno, J.M.; Rodrigo, A.; et al. Fire-related traits for plant species of the Mediterranean Basin. Ecology 2009, 90, 1420. [Google Scholar] [CrossRef]
- Fares, S.; Bajocco, S.; Salvati, L.; Camarretta, N.; Dupuy, J.-L.; Xanthopoulos, G.; Guijarro, M.; Madrigal, J.; Hernando, C.; Corona, P. Characterizing potential wildland fire fuel in live vegetation in the Mediterranean region. Ann. For. Sci. 2017, 74, 1. [Google Scholar] [CrossRef]
- Tavşanoğlu, Ç.; Pausas, J.G. A functional trait database for Mediterranean Basin plants. Sci. Data 2018, 5, 180135. [Google Scholar] [CrossRef] [PubMed]
- Gachet, S.; Véla, E.; Tatoni, T. BASECO: A floristic and ecological database of Mediterranean French flora. Biodivers. Conserv. 2005, 14, 1023–1034. [Google Scholar] [CrossRef]
- Santana, V.M.; Alday, J.G.; Baeza, M.J. Effects of fire regime shift in Mediterranean Basin ecosystems: Changes in soil seed bank composition among functional types. Plant Ecol. 2014, 215, 555–566. [Google Scholar] [CrossRef]
- Manseau, M.; Huot, J.; Crete, M. Effects of Summer Grazing by Caribou on Composition and Productivity of Vegetation: Community and Landscape Level. J. Ecol. 1996, 84, 503–513. [Google Scholar] [CrossRef]
- Elliott, K.J.; Hendrick, R.L.; Major, A.E.; Vose, J.M.; Swank, W.T. Vegetation dynamics after a prescribed fire in the southern Appalachians. For. Ecol. Manag. 1999, 114, 199–213. [Google Scholar] [CrossRef]
- Grant, C.D.; Loneragan, W.A. The effects of burning on the understorey composition of rehabilitated bauxite mines in Western Australia: Community changes and vegetation succession. For. Ecol. Manag. 2001, 145, 255–279. [Google Scholar] [CrossRef]
- Chu, C.-J.; Maestre, F.T.; Xiao, S.; Weiner, J.; Wang, Y.-S.; Duan, Z.-H.; Wang, G. Balance between facilitation and resource competition determines biomass–density relationships in plant populations. Ecol. Lett. 2008, 11, 1189–1197. [Google Scholar] [CrossRef]
- Heydari, M.; Faramarzi, M.; Pothier, D. Post-fire recovery of herbaceous species composition and diversity, and soil quality indicators one year after wildfire in a semi-arid oak woodland. Ecol. Eng. 2016, 94, 688–697. [Google Scholar] [CrossRef]
- Hashemi, A.; Aghbash, F.G.; Zarafshar, M.; Bazot, S. 80-years livestock transit impact on permanent path soil in Zagros oak forest, Iran. Appl. Soil Ecol. 2019, 138, 189–194. [Google Scholar] [CrossRef]
- Valdez, C.G.; Guzmán, M.A.; Valdés, A.; Forougbakhch, R.; Alvarado, M.A.; Rocha, A.; Valdez, C.G.; Guzmán, M.A.; Valdés, A.; Forougbakhch, R.; et al. Estructura y diversidad de la vegetación en un matorral espinoso prístino de Tamaulipas, México. Rev. Biol. Trop. 2018, 66, 1674–1682. [Google Scholar] [CrossRef]
- Mattingly, W.B.; Orrock, J.L.; Collins, C.D.; Brudvig, L.A.; Damschen, E.I.; Veldman, J.W.; Walker, J.L. Historical agriculture alters the effects of fire on understory plant beta diversity. Oecologia 2015, 177, 507–518. [Google Scholar] [CrossRef]
- Mahood, A.L.; Balch, J.K. Repeated fires reduce plant diversity in low-elevation Wyoming big sagebrush ecosystems (1984–2014). Ecosphere 2019, 10, e02591. [Google Scholar] [CrossRef]
- Moya, D.; Sagra, J.; Lucas-Borja, M.E.; Plaza-Álvarez, P.A.; González-Romero, J.; De Las Heras, J.; Ferrandis, P. Post-Fire Recovery of Vegetation and Diversity Patterns in Semiarid Pinus halepensis Mill. Habitats after Salvage Logging. Forests 2020, 11, 1345. [Google Scholar] [CrossRef]
- Novak, L.; Scholl, J.P.; Kiefer, G.; Iler, A.M. Prescribed burning has limited effects on the population dynamics of rare plants. Conserv. Sci. Pract. 2022, 4, e12792. [Google Scholar] [CrossRef]
- Vidal-Cordero, J.M.; Angulo, E.; Molina, F.P.; Boulay, R.; Cerdá, X. Long-term recovery of Mediterranean ant and bee communities after fire in southern Spain. Sci. Total Environ. 2023, 887, 164132. [Google Scholar] [CrossRef] [PubMed]
- Valkó, O.; Deák, B. Increasing the potential of prescribed burning for the biodiversity conservation of European grasslands. Curr. Opin. Environ. Sci. Health 2021, 22, 100268. [Google Scholar] [CrossRef]
- Hanes, T.L. Succession after Fire in the Chaparral of Southern California. Ecol. Monogr. 1971, 41, 27–52. [Google Scholar] [CrossRef]
- DeJong, T.M. A Comparison of Three Diversity Indices Based on Their Components of Richness and Evenness. Oikos 1975, 26, 222–227. [Google Scholar] [CrossRef]
- James, V.; Frankie, L.; Kumar, R.; Karan, N.M. Species-area and species-individual relationships for tropical trees. J. Ecol. 1996, 84, 549–562. [Google Scholar]
- Fuerst-Bjeliš, B. Mediterranean Identities: Environment, Society, Culture; IntechOpen: London, UK, 2017. [Google Scholar]
- Myers, J.A.; Chase, J.M.; Crandall, R.M.; Jiménez, I. Disturbance alters beta-diversity but not the relative importance of community assembly mechanisms. J. Ecol. 2015, 103, 1291–1299. [Google Scholar] [CrossRef]
- Strand, E.K.; Satterberg, K.L.; Hudak, A.T.; Byrne, J.; Khalyani, A.H.; Smith, A.M.S. Does burn severity affect plant community diversity and composition in mixed conifer forests of the United States Intermountain West one decade post fire? Fire Ecol. 2019, 15, 25. [Google Scholar] [CrossRef]
- Moradizadeh, H.; Heydari, M.; Omidipour, R.; Mezbani, A.; Prévosto, B. Ecological effects of fire severity and time since fire on the diversity partitioning, composition and niche apportionment models of post-fire understory vegetation in semi-arid oak forests of Western Iran. Ecol. Eng. 2020, 143, 105694. [Google Scholar] [CrossRef]
- Martínez-Sánchez, J.J.; Ferrandis, P.; Trabaud, L.; Galindo, R.; Franco, J.A.; Herranz, J.M. Comparative root system structure of post-fire Pinus halepensis Mill. and Cistus monspeliensis L. saplings. Plant Ecol. 2003, 168, 309–320. [Google Scholar] [CrossRef]
- Dymov, A.A.; Startsev, V.V.; Milanovsky, E.Y.; Valdes-Korovkin, I.A.; Farkhodov, Y.R.; Yudina, A.V.; Donnerhack, O.; Guggenberger, G. Soils and soil organic matter transformations during the two years after a low-intensity surface fire (Subpolar Ural, Russia). Geoderma 2021, 404, 115278. [Google Scholar] [CrossRef]
- Condit, R.; Ashton, P.S.; Baker, P.; Bunyavejchewin, S.; Gunatilleke, S.; Gunatilleke, N.; Hubbell, S.P.; Foster, R.B.; Itoh, A.; LaFrankie, J.V.; et al. Spatial Patterns in the Distribution of Tropical Tree Species. Science 2000, 288, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Verdú, M. Fire Reduces Morphospace Occupation in Plant Communities. Ecology 2008, 89, 2181–2186. [Google Scholar] [CrossRef]
- Bond, W.J.; Keeley, J.E. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef]
- Tangney, R.; Paroissien, R.; Le Breton, T.D.; Thomsen, A.; Doyle, C.A.T.; Ondik, M.; Miller, R.G.; Miller, B.P.; Ooi, M.K.J. Success of post-fire plant recovery strategies varies with shifting fire seasonality. Commun. Earth Environ. 2022, 3, 126. [Google Scholar] [CrossRef]
- Pausas, J.G.; Bond, W.J. Alternative Biome States in Terrestrial Ecosystems. Trends Plant Sci. 2020, 25, 250–263. [Google Scholar] [CrossRef]
- Ruprecht, E.; Fenesi, A.; Fodor, E.I.; Kuhn, T. Prescribed burning as an alternative management in grasslands of temperate Europe: The impact on seeds. Basic Appl. Ecol. 2013, 14, 642–650. [Google Scholar] [CrossRef]
- Pausas, J.G.; Ribeiro, E. Fire and plant diversity at the global scale. Glob. Ecol. Biogeogr. 2017, 26, 889–897. [Google Scholar] [CrossRef]
- Huerta, S.; Fernández-García, V.; Calvo, L.; Marcos, E. Soil Resistance to Burn Severity in Different Forest Ecosystems in the Framework of a Wildfire. Forests 2020, 11, 773. [Google Scholar] [CrossRef]
- Stewart, J.A.E.; van Mantgem, P.J.; Young, D.J.N.; Shive, K.L.; Preisler, H.K.; Das, A.J.; Stephenson, N.L.; Keeley, J.E.; Safford, H.D.; Wright, M.C.; et al. Effects of postfire climate and seed availability on postfire conifer regeneration. Ecol. Appl. 2021, 31, e02280. [Google Scholar] [CrossRef] [PubMed]
- Węgrzyn, M.H.; Fałowska, P.; Alzayany, K.; Waszkiewicz, K.; Dziurowicz, P.; Wietrzyk-Pełka, P. Seasonal Changes in the Photosynthetic Activity of Terrestrial Lichens and Mosses in the Lichen Scots Pine Forest Habitat. Diversity 2021, 13, 642. [Google Scholar] [CrossRef]
- Pereira-Silva, E.F.L.; Casals, P.; Sodek, L.; Delitti, W.B.C.; Vallejo, V.R. Post-fire nitrogen uptake and allocation by two resprouting herbaceous species with contrasting belowground traits. Environ. Exp. Bot. 2019, 159, 157–167. [Google Scholar] [CrossRef]
- Paroissien, R.; Ooi, M.K.J. Effects of fire season on the reproductive success of the post-fire flowerer Doryanthes excelsa. Environ. Exp. Bot. 2021, 192, 104634. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).