1. Introduction
Until the 19th century, wood was the most used fuel by mankind. However, this energy source was overtaken, first by mineral coal and later by oil and natural gas [
1]. The fossil fuels have, until then, sustained the world’s energy needs. However, the decrease in energy reserves, as well as the emissions of pollutant gases from their use, have caused concern worldwide [
2]. These challenges have forced most economies to mitigate the use of fossil fuels and to replace them, in part, with alternative energy sources of renewable origin, like biomass.
Today, biomass can be converted into useful energy through various conversion technologies, which allows the obtaining of fuels in different physical states presenting high applicability [
3,
4]; however, this renewable energy source, considered traditional, is often characterized as inefficient [
5].
Biomass is a very heterogeneous fuel and, as such, presents significant differences in its properties, namely in moisture content and chemical composition [
6]. In general, biomass presents some undesirable characteristics when used as fuel. This is the case due to its high moisture content, its irregular shape and size and its low volumetric specific energy; these are the main reasons why its transport, handling and storage are problematic [
7]. The transformation of this bulky substance into a densified material improves handling and reduces transportation and storage costs [
8].
Pelletizing performs an increase in bulk density applying mechanical pressure at appropriate temperature conditions. Pellets will have low moisture content, regular size and shape and higher bulk density [
8].
The global wood pellets market began to develop over a decade ago, and since then the production has been progressively increasing. According to the latest statistics published by Bioenergy Europe [
9], world pellets production in 2021 was 6.8% higher than the previous year, at approximately 46 million tons. The commercial and residential sector is responsible for the largest consumption of pellets: in 2021 it will account for 51 percent of the total value [
9].
The biomass combustion process occurs along a sequence of several distinct phases: drying, pyrolysis, ignition and combustion of the solid carbonaceous residue [
3]. The knowledge of the ignition behavior of biofuels during their handling, transport and storage is of high importance to avoid serious incidents [
10].
In the last years, some works have been developed in the characterization of the ignition and flammability of biomass fuels, mainly due to fire and dust explosibility hazards during handling, transport and storage.
Tognotti et al. [
11] experimentally determined the ignition temperature of a South African coal using two distinct cylindrical beds. The data obtained were compared with thermal ignition models. Additionally, Grokjaer et al. [
12] experimentally determined the ignition temperature of biomasses, specifically wheat straw, at 21% (
v/
v) oxygen. Ferrero et al. [
13] developed a mathematical model that predicts the heating of wood chip piles in open air to prevent the auto ignition of these materials during storage. These authors found that microbial processes are responsible for the highest heat production in the early stages of storage. Jones et al. [
14] evaluated the ignition risk of seven biomasses and classified them according to ignition risk using kinetic parameters. These authors found that slow combustion occurred, ignition only took place in the carbonaceous residue and combustion of the released volatiles was not observed.
Shan et al. [
15] studied the ignition and combustion characteristics of single pellets obtained from
Pinus bungeana and rice husk. The combustion tests were performed at 873, 973 and 1073 K, using air and different O
2/CO
2 atmospheres. The study revealed that the atmospheres with higher O
2 content present shorter and more stable flames. The air replacement by O
2/CO
2, with the same oxygen concentration, led to an increase in ignition delay, internal ignition temperature and the volatile combustion time. In a similar experimental setup, and for the same combustion conditions, Wang et al. [
16] analyzed the ignition and combustion characteristics of pellets blends made by two different coals, anthracite and bituminous, with rice husk in different ratios. In the ignition study, the rice husk content had a more pronounced effect on the anthracite blends. Moreover, the behavior of the oxidant atmosphere was the same reported in the previous work.
Restuccia et al. [
17] evaluated the self-heating ignition behavior of wheat biomass with different particle sizes. The experimental study was conducted in an isothermal oven, and the minimum ignition temperatures were determined as a function of sample volume. The results indicated that the critical temperature for self-heating ignition was similar for particles between 300 μm and pellet size.
The flame propagation characteristics and the lean flammability limit of four commercial pellets in three size ranges, <63 μm, 63–500 μm and <500 μm, were determined in a modified Hartmann dust explosion tube by Saeed et al. [
18]. The authors concluded that the pellets’ explosibility characteristics depended on their composition, namely particle size, ash and moisture content, which is quite variable. They also proved that mixtures of fine particles had greater reactivity and promoted ignition.
Kuznetsov et al. [
19] developed an experimental study on the characteristics of pellet ignition of fuel mixtures based on coal dust and grounded wood. The tests were carried out at 600, 700 and 800 °C. The heating process, the ignition and the combustion of these fuels were analyzed using a high-speed video camera. The results indicated that the ignition delay times strongly decreased with the proportion of wood in the pellets.
Rezaei et al. [
20] evaluated the emission of combustible gases of western red cedar and spruce/pine/Douglas fir sawdust mixtures at 140 °C and found that the western red cedar released a lower amount of carbon monoxide, methane and hydrogen. However, the concentration of the released gas mixture from the fuels tested did not reach a flammable level.
Glushkov et al. [
21] analyzed the temperature during the ignition and combustion of coal pellets through a high-speed two-color pyrometry technology. Below 1073 K, the volatiles were released slowly, and the heat and mass transfer was dominant during the ignition process. At 1173 K, the volatiles and the carbonaceous residue were ignited simultaneously, leading to a stable combustion with complete burnout. However, for temperatures above 1173 K, the fuel ignition was affected.
The co-combustion characteristics and the kinetic and ash fusion temperatures of clean coal, biomass pellets and their blends were studied by Yuan et al. [
22]. Compared with pure fuels, the blends showed a better combustion performance, and the flammability of these fuels improved. This effect was obtained due to the addition of biomass pellets into clean coal.
Castells et al. [
23] evaluated olive-derived biomasses’ flammability and explosibility properties, namely olive stone and exhausted olive cake. The authors verified that these biomasses present a high risk of forming explosive atmospheres. The thermal behavior of both materials was studied with TGA tests, which revealed that olive stone demonstrated a greater propensity for oxidation and a medium risk of self-inflammation.
Kuznetsov et al. [
24] conducted experimental and theoretical studies of the ignition processes of dry particles of five different woody biomasses, specifically birch, larch, pine, aspen and cedar. It was verified that the temperature of the oxidizing agent affects the nature of the wood ignition process. For ambient temperatures below 873 K, the ignition occurs in the gas region under the particle; nevertheless, for ambient temperatures above 1073 K, the ignition process occurs in the gaseous phase, around the wood particle. The authors also show that the orientation of wood fibers, in space relative to the direction of the heat flow, does not influence the characteristics of the ignition process.
During the scientific literature review, relevant works in biomass ignition and flammability were identified as mentioned above; however, there is a lack of experimental data regarding the ignition of volatiles being released from ligneous pellets during their path along a traveling grate. In the present work, six types of biomass pellets were used to investigate their ignition characteristics in a traveling grate furnace, using five average furnace temperatures, namely 360, 385, 410, 435 and 460 °C. The ignition behavior was studied measuring the ignition time, i.e., the time elapsed between placing the pellets on the traveling grate and the ignition moment of the volatiles released from the pellet batch. Therefore, the pellet ignition, in this particular context, is the ignition of volatiles released from the pellets in the initial phase of their path along the traveling grate.
2. Materials and Methods
2.1. Pellets Preparation
The pellets were produced from six different species: Pinus pinaster, Acacia dealbata, Cytisus scoparius, Cistus ladanifer, Paulownia cotevisa and Eucalyptus globulus. With the exception of Pinus pinaster, the other species were used as raw material for the production of pellets used in the tests, at the laboratories of the Polytechnic Institute of Viseu, Portugal.
The Pinus pinaster pellets were acquired through a Portuguese company, while the species Acacia dealbata, Cytisus scoparius, Cistus ladanifer, Paulownia cotevisa and Eucalyptus globulus were collected in a forest surrounding the city of Viseu, Portugal, and dried in a solar kiln, with the moisture content monitored throughout the process. A knife mill, Retsch SM 100, from Retsch GmbH, Germany, was used to ground the raw material using a sieve with an average diameter of 7 mm, in order to obtain the necessary raw material for the pellets’ production. After characterization, regarding particle size and moisture content, 6 mm diameter pellets were produced using a Tojaltec pelletizer press machine form Tojaltec, Portugal.
Subsequently, the pellets were manually cut into smaller and uniform-sized particles with average lengths between 14–15 and 7–8 mm, respectively. The pellets exact dimensions, length and diameter were determined using a digital caliper with a resolution of 0.1 mm. The mass of each pellet, which allowed the calculation of the average pellet density, was determined using a Sartorius BP 310 P digital balance from Sartorius, Göttingen, Germany.
Figure 1 illustrates the pellets used, with lengths between 14 and 15 mm.
In addition to pellet dimensions, the average pellet density, moisture content and volatile content of the pellets were also determined. The pellet density was determined by the method of dimensions [
25]. This method is based on the relationship between the mass of a pellet and the corresponding volume, considering it as a cylinder.
Table 1 shows the average dimensions (length and diameter), the mass and the density of the pellets used in the ignition tests.
The results show minor differences between the experimental pellets’ dimensions, mass and density.
Regarding proximate analysis, the moisture content of the pellets was obtained according to FprEN 14774-2:2009; the pellet samples were dried in a Venticell 50 L oven, from MMM Medcenter Einrichtungen GmbH, Germany, at a temperature of 105 ± 2 °C, until a constant mass was achieved. The moisture content, dry basis, was determined according to Equation (1):
where
represents the initial wet mass and
the dry mass.
The volatile content was determined according to EN 15148:2009 by the Department of Environmental Engineering of the School of Technology and Management of Viseu. The process consists in placing each sample in a silica crucible, previously weighed and then heated in a muffle, at a temperature of 900 ± 10 °C, for approximately 7 min.
Table 2 shows the moisture content and the volatile content of the tested pellets. Regarding the moisture content, the different species show some similarities; Eucalyptus pellets had a higher moisture content at 8.81%, and Pinus pinaster pellets had lower, at 7.24%. However, in the volatile content, some differences were found. Paulownia was the species that presented a higher volatile content at 83.87%, and Cistus presented a lower value, at 78.84%.
2.2. Experimental Setup
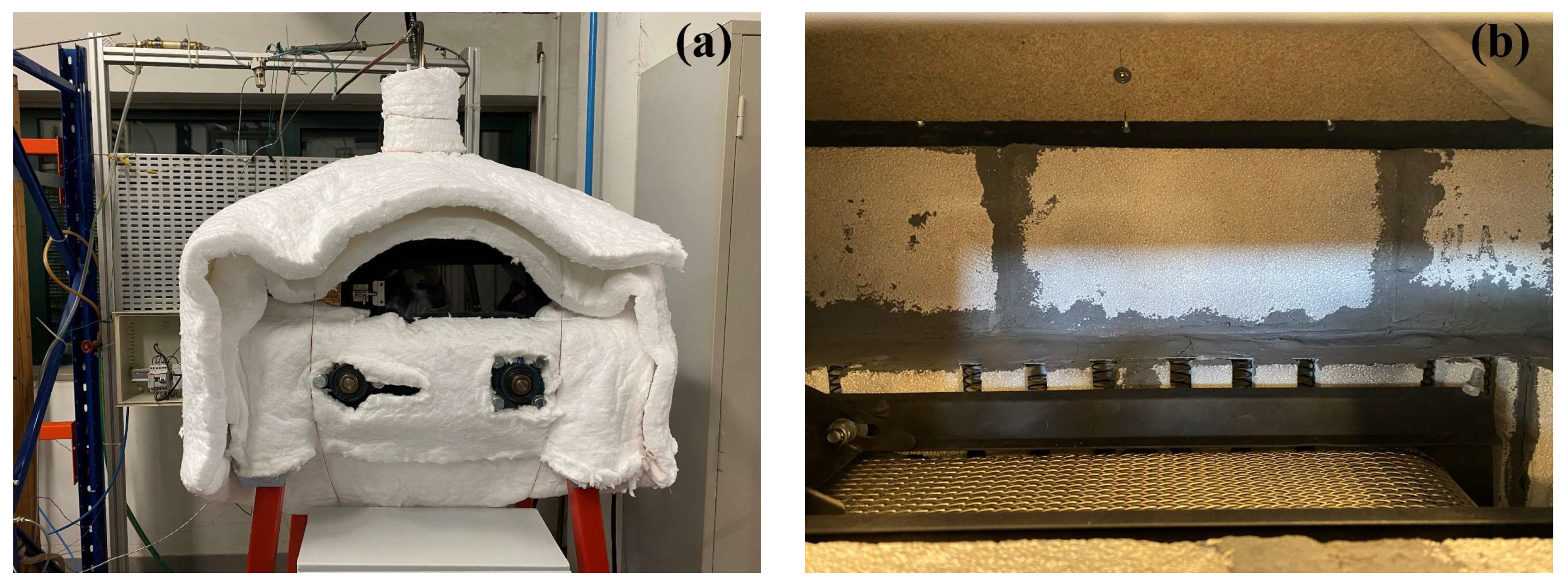
The experimental setup used in the pellet ignition tests consisted of a traveling grate furnace, as seen in
Figure 2a, and a set of instruments that allowed the measurement and control of the furnace temperature, as well as the air mass flow rate used in the tests. The furnace was designed to carry out experiments of burning small batches of char pellets in a traveling bed. However, in the initial installation procedures of this new laboratory reactor and its start-up tests, which were carried out not only with carbonized pellets but also with pellets just as they had been produced and therefore not yet carbonized, ignition events of the wood pellets on their way through the traveling grate were detected.
The traveling grate furnace was constructed of ST37 steel with 610 mm length and 320 mm width internal dimensions. The front part had a high temperature glass door that was 23 mm long and 9 mm wide. The fuel supply was fulfilled from the left side, through a gutter, and the ashes exit was on the right side. The traveling grate was made from a refractory stainless steel that was 30 cm long and 9 cm wide, as seen in
Figure 2b.
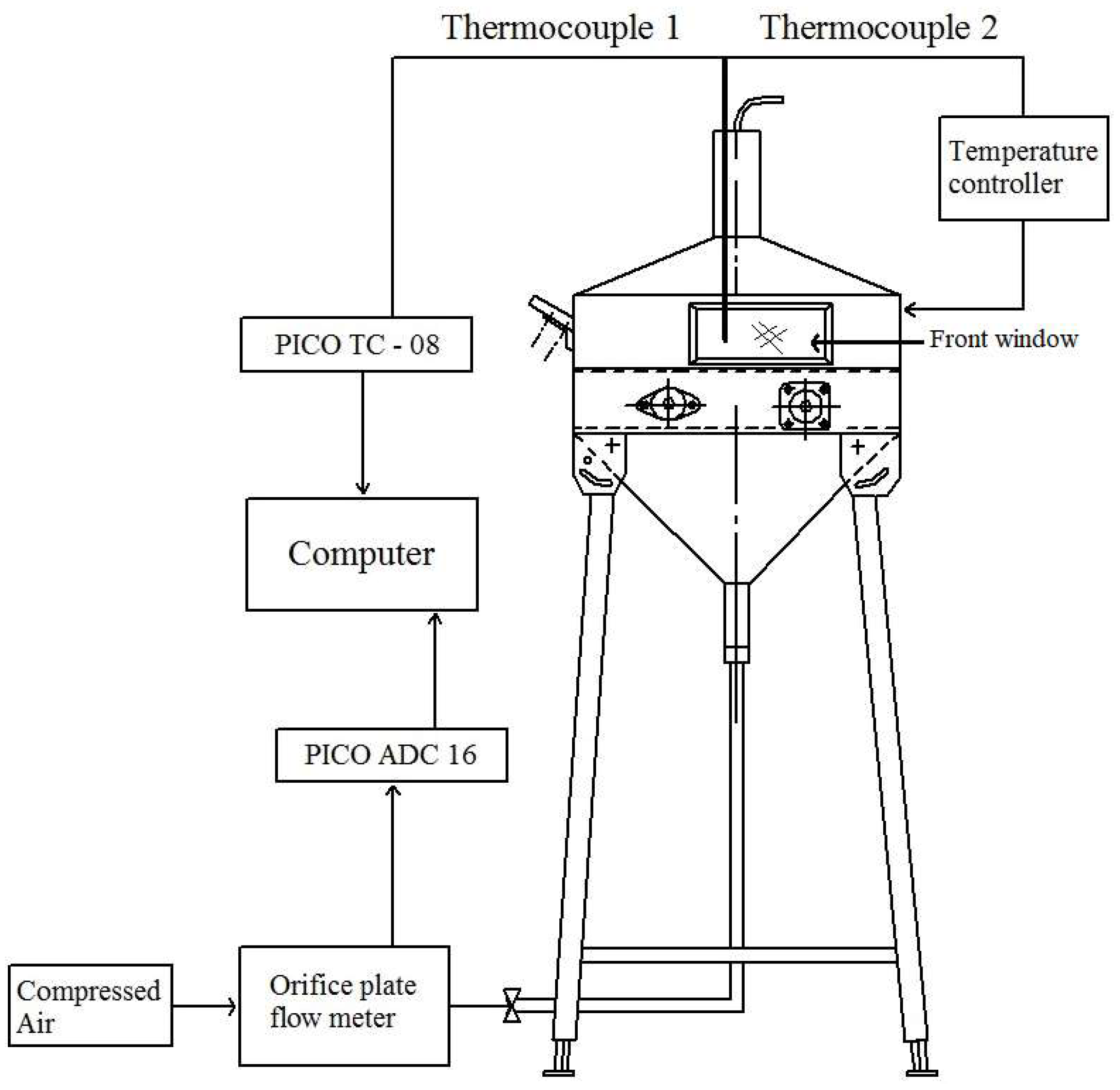
The furnace heating system consisted of a 4.5 kW electric resistance made from a 1.14 mm diameter Kanthal wire, inserted in refractory bricks and placed around the traveling grate. The furnace was also thermally insulated with a Kaowool ceramic blanket. The experimental setup is schematically represented in
Figure 3.
The furnace temperature was measured by 2 K-type thermocouples, coated with 3 mm diameter and 1500 mm long stainless steel sheaths positioned in the middle of the traveling grate at a height of 10 mm above the bed. One of the thermocouples was connected to an Omron ESCSV temperature controller, and the other one was connected to a Pico TC-08 data logger. The air mass flow rate, coming from the compressed air network, properly filtered and dehumidified, was measured with an orifice plate flow meter, using both a U-tube water pressure manometer and an Omega PX143-2,5BD5V differential pressure transducer, connected to the PicoLog recorder through an ADC-16 data logger. The orifice plate flow meter was previously calibrated by the tracer gas dilution method.
The acquisition systems were connected to a computer via RS232 ports, and the PicoLog software from Pico Technology Ltd, Pico Technology, United Kingdom, was used to visualize and store the acquired data. The used sampling rate was 1.0 Hz.
The average uncertainty values for the experimental measurements were determined and are shown in
Table 3. These values refer to the total uncertainty obtained by quantifying the random and systematic errors associated with the measurements, data acquisition and conversion processes.
2.3. Experimental Procedure
Initially, for the 6 types of pellets, all with an average length between 14 and 15 mm, ignition tests were performed with batches of 6 g to study the influence of the initial mass and the average length on the ignition time. In a second set of tests, experiments were performed only with the Cistus ladanifer and Paulownia cotevisa species: 6 g loads were used, consisting of pellets with average lengths ranging from 7 to 8 mm and 8 g loads, with average lengths from 14 to 15 mm.
The start-up of the experimental installation began with the connection of the furnace heating system and the temperature controller, adjusted according to the desired test temperatures. The water level in the U-tube water pressure manometer was adjusted, and the pressure transducer was connected to measure the air mass flow rate through the corresponding orifice plate, as well as two data acquisition systems.
The ignition experiments began when the furnace temperature was stabilized. Each pellets batch, previously weighed and characterized, was placed in the feeder and introduced, through the feed zone, into the furnace. In so doing, the ceramic blanket cover, which sealed the furnace entrance, had to be removed, and the feeder was quickly introduced into the furnace to deposit the pellet batch at the beginning of the traveling grate, as shown in
Figure 4. As soon as the pellets fell a chronometer was activated, the feeder was removed from inside the furnace and the burner feed was covered again. The instant the pellet batch ignited, visually detected through the furnace front window, as seen in
Figure 3, the chronometer was stopped, and the time was recorded. The ignition time is the time elapsed between placing the batch of pellets on the traveling grate and the volatiles’ ignition moment.
The pellet batches were burned in the traveling grate furnace at set point temperatures of 360, 385, 410, 435 and 460 °C. For all the experiments, the mass flow rate of the supplied air was approximately 43 mg/s, with the interstitial air velocity being approximately equal to 0.016 m/s. The average traveling grate velocity in all tests was 1.1 mm/s.
For each ignition experiment, the evolution of the furnace temperature and the air mass flow rate were continuously measured and registered. At furnace temperatures above 460 °C, the ignition delay is so short that it cannot be correctly measured with this experimental setup.
2.4. Furnace Temperature Measurement
Since the thermocouples positioned inside the burner were in a gaseous environment, and the temperature measured was the radiation equilibrium temperature and not the real temperature of this environment, a temperature correction according to Holman (2012) was necessary. A Testo 835-T2 high-temperature infrared thermometer was used to measure the furnace walls temperature in the range measured during the ignition tests. This procedure was carried out by three persons simultaneously, so that it was done as quickly and accurately as possible. Thus, when the furnace door was opened, the temperature measured by the thermocouple was recorded and, with the high-temperature infrared thermometer, the temperature of the furnace walls was also measured, through the front furnace window.
Table 4 shows the corrected temperatures determined for the temperature ranges used in the experiments.
Although the traveling bed furnace set point temperatures chosen for the experiment were, as referred to before, 360, 385, 410, 435 and 460 °C, taking into account the corrected temperatures for each case, the actual ignition tests values were, respectively, 359, 381, 403, 424 and 443 °C.
3. Results
As previously mentioned, the ignition tests consisted in introducing batches of pellets, of 6 and 8 g, inside the traveling grate furnace that was heated to the desired temperature, to determine the time between the introduction instant and the moment of the ignition of the volatiles released from that batch of pellets. The moment of ignition of a batch of
Pinus pinaster pellets is illustrated in
Figure 5.
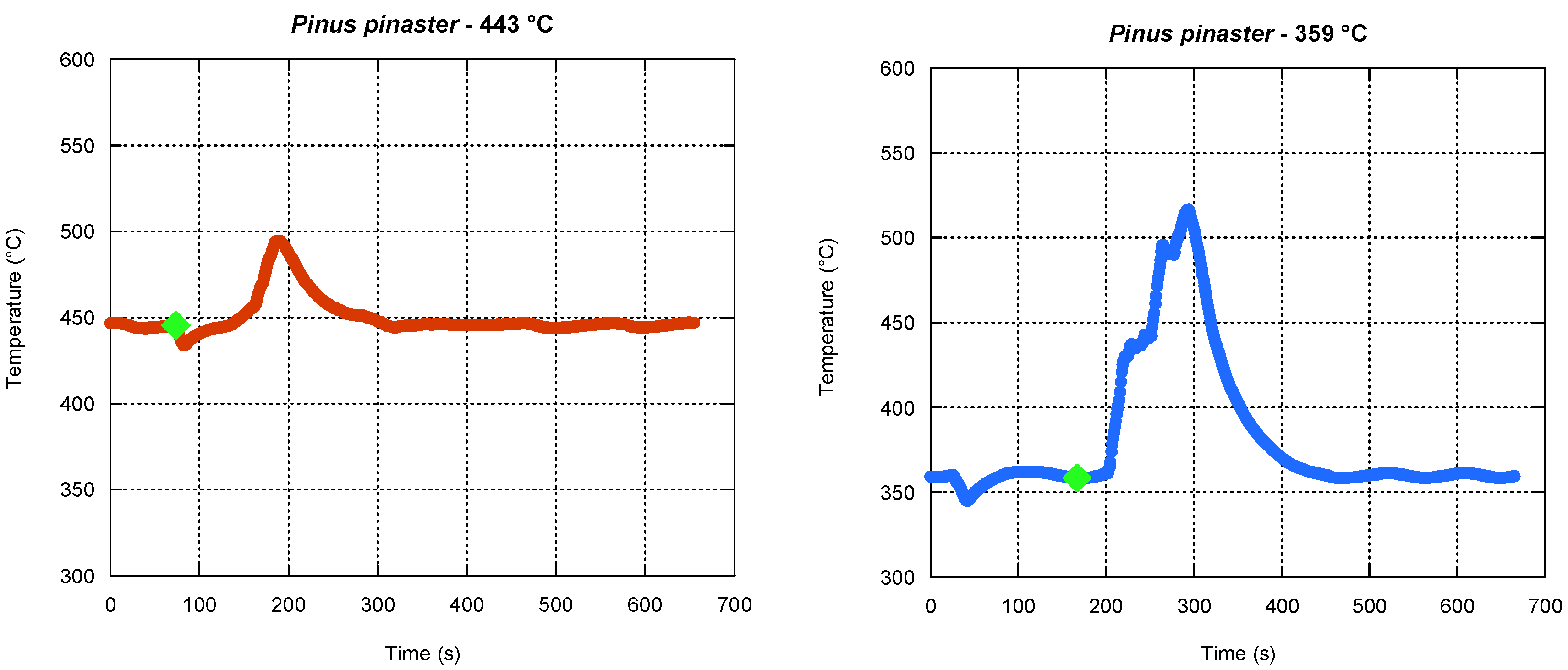
The furnace temperature evolution during the ignition tests was continuously recorded. As an example,
Figure 6 shows temperature evolution curves during two ignition tests performed with
Pinus pinaster, with the furnace at 359 and 443 °C, respectively. The ignition moment was represented in both graphs by a green square.
From the analysis of the previous figures, the temperature evolution presents a similar trend. There is a slight drop when the batch is introduced into the burner, and the maximum temperature reached is higher in the tests performed at 359 °C. This is related to the fact that at 359 °C the batch takes longer to ignite and, therefore, its position on the traveling grate at the time of ignition will be closer to the thermocouple. For furnace temperatures above 443 °C, the time ignition was so short that they could not be determined with a minimum of precision and, as such, those results were discarded.
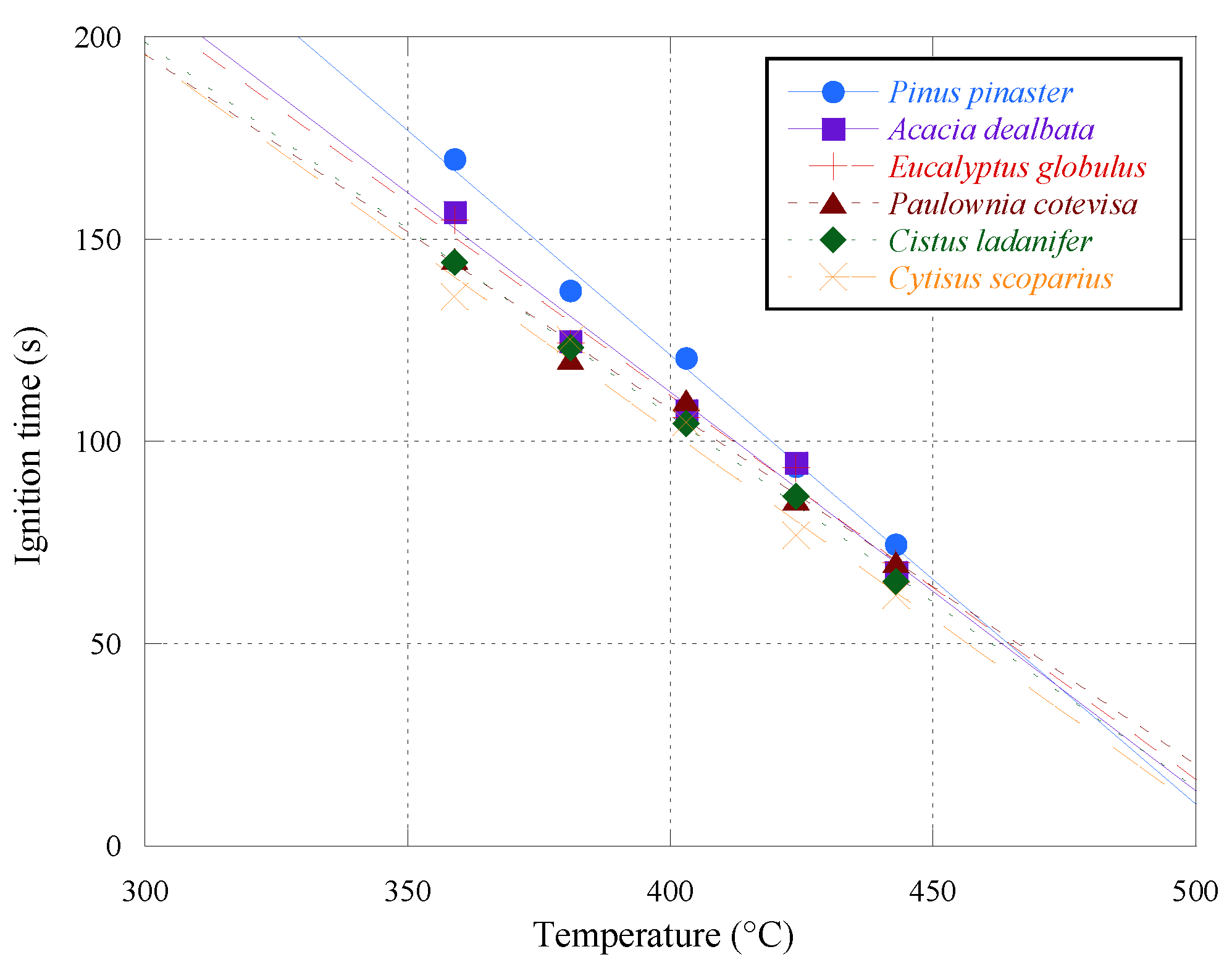
The results obtained regarding the ignition time for batches of 6 g and 14–15 mm average length are graphically represented in
Figure 7, including the six species and the five temperatures used, and they are also shown in
Table 5.
From
Figure 7, as expected, it is shown that as the furnace temperature increases there is a decrease in the pellet ignition time. In general, the evolution of the ignition time presents a linear trend.
Pinus pinaster was the species that presented a higher ignition time, and this difference was more evident with the tests performed at 359 °C, where the average ignition time of
Pinus pinaster was approximately 170 s.
Cytisus scoparius, in the same conditions, was the species that took the least time to ignite. With the tests performed at 359 °C, the average ignition time for this species was 136 s.
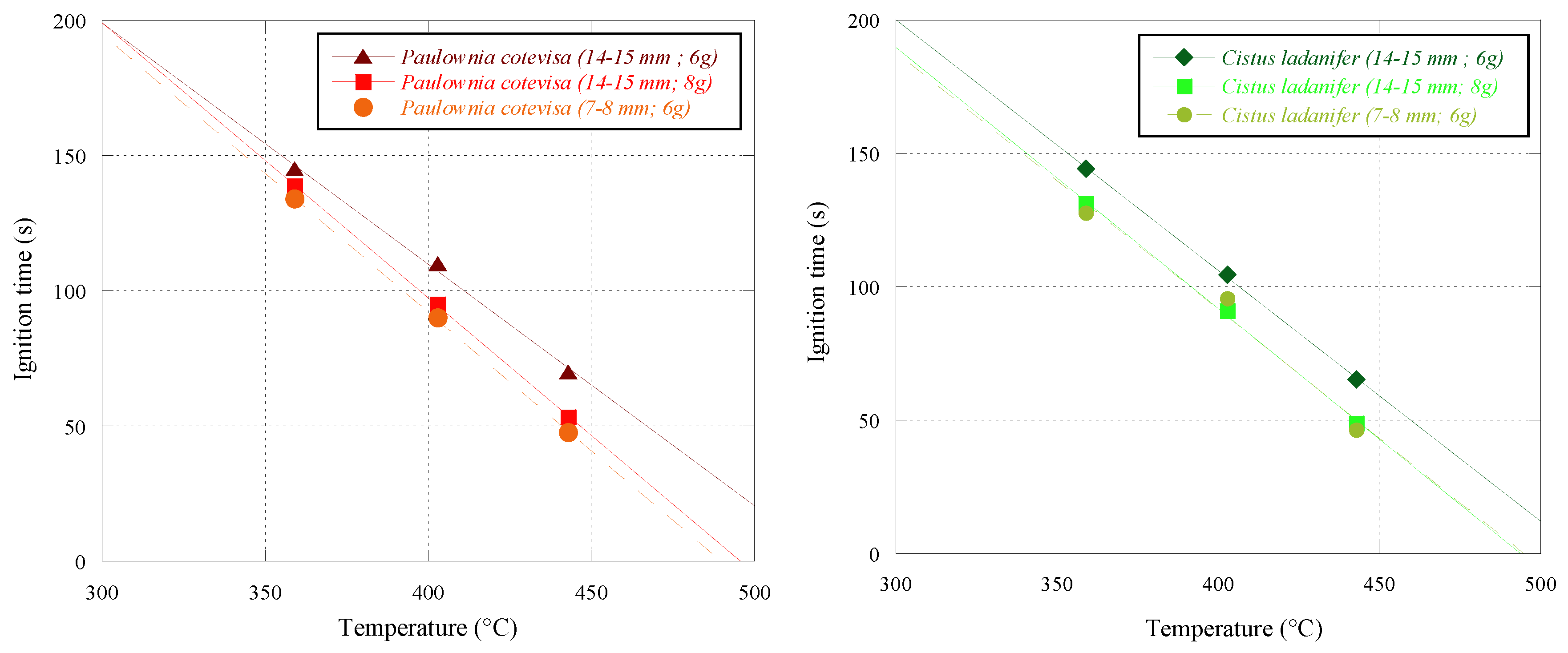
Regarding the experiments performed with
Paulownia cotevisa and
Cistus ladanifer, using batches of 8 g with 14–15 mm particles and batches of 6 g with 7–8 mm particles,
Figure 8 represents the ignition times obtained at temperatures of 359, 403 and 443 °C. The ignition time, in these figures, is also shown, for both species, with pellet batches of 6 g with an average length of 14–15 mm, as previously presented in
Figure 7.
For a better interpretation, the ignition times of both pellets are also presented in
Table 6. The results showed that for the same pellets, batch mass and a smaller particle size led to a shorter ignition time. Likewise, for both species, in the batches with the same pellets sizes of 14–15 mm, an increase in its mass, from 6 to 8 g, led to a lower ignition time.
4. Discussion
In general, 6 g pellet batches with 7–8 mm length are the fastest to ignite, compared to the two conditions previously reported. It can be seen that for the same mass of a pellets batch, the size of the constituent particles of the pellet has a significant influence on the ignition time. The smaller the particle size, the faster the ignition time. In the case of the tests performed with the 8 g batches and 14–15 mm particles, for both species, the ignition time was shorter than the one initially obtained with the 6 g batches.
The fact that a batch of pellets with greater mass is used implies a greater amount of volatile matter and, consequently, a higher fuel equivalence ratio, since the air flow rate has been kept constant.
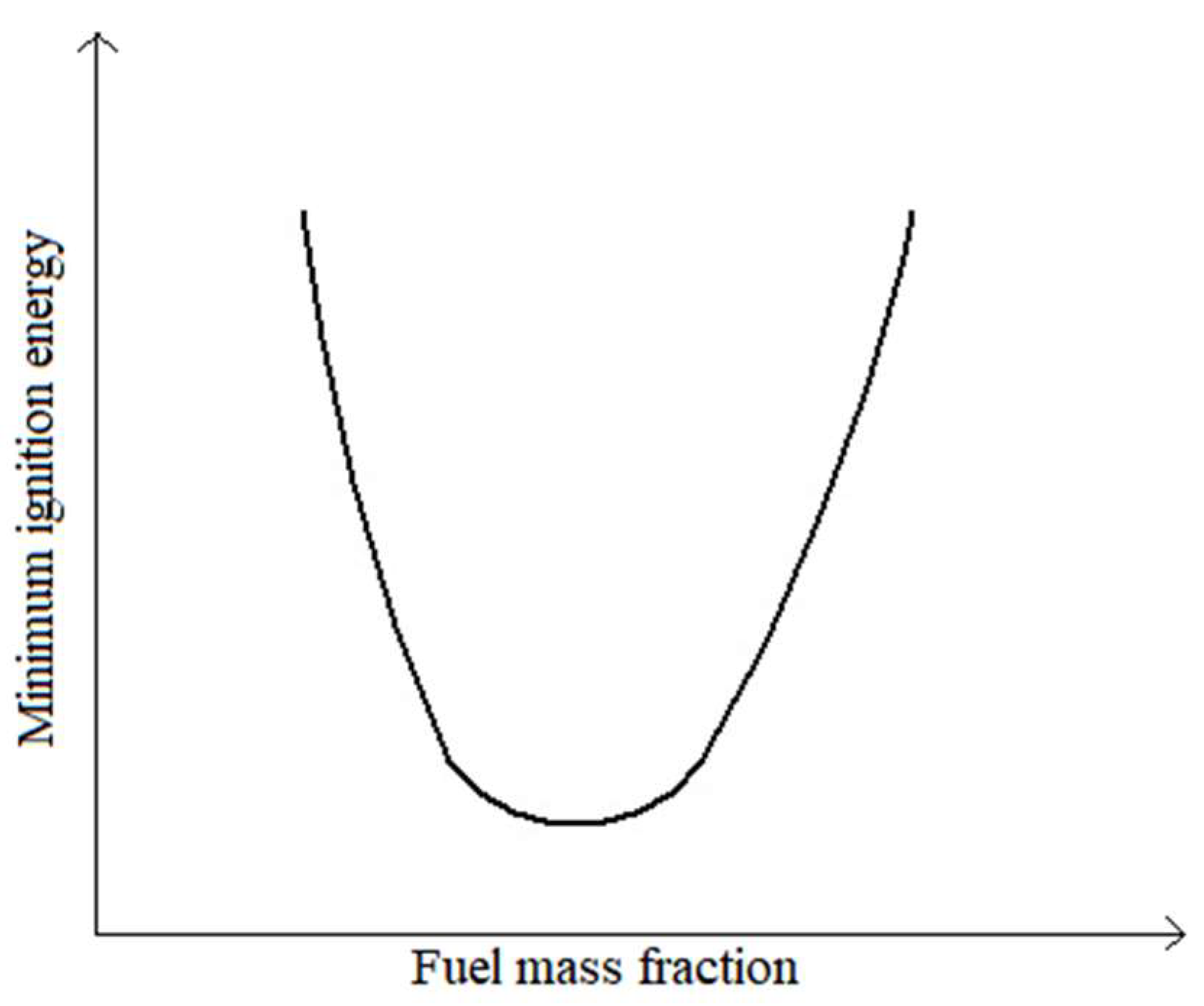
According to the literature [
26,
27,
28], characteristic fuel flammability curves, a variation of minimum ignition energy, MIE, as a function of fuel mass fraction, exhibit a U-shaped configuration, as sketched in
Figure 9, where a generic configuration for the relationship between minimum ignition energy and fuel volume fraction in the reactant mixture is presented. These curves can have at least two ordinate axis parameterizations: the minimum ignition energy or the ignition delay. The bottom point of this “U” shaped curve will represent the minimum, and for this minimum the critical fuel fraction in the mixture is defined, which will lead to this limit situation.
Thus, increasing the mass of the pellet charges to the size of 14–15 mm made it possible to increase the fuel mass fraction in the reagent mixture, which may have required a lower minimum ignition energy and thus a shorter ignition time. This is true as long as the composition of the reactant mixture is in a zone of fuel fractions below the value corresponding to the curve minimum. If the fuel mass fraction is beyond this minimum, any increase in the mass of the pellet batches will lead to an increase in ignition energy or ignition delay.
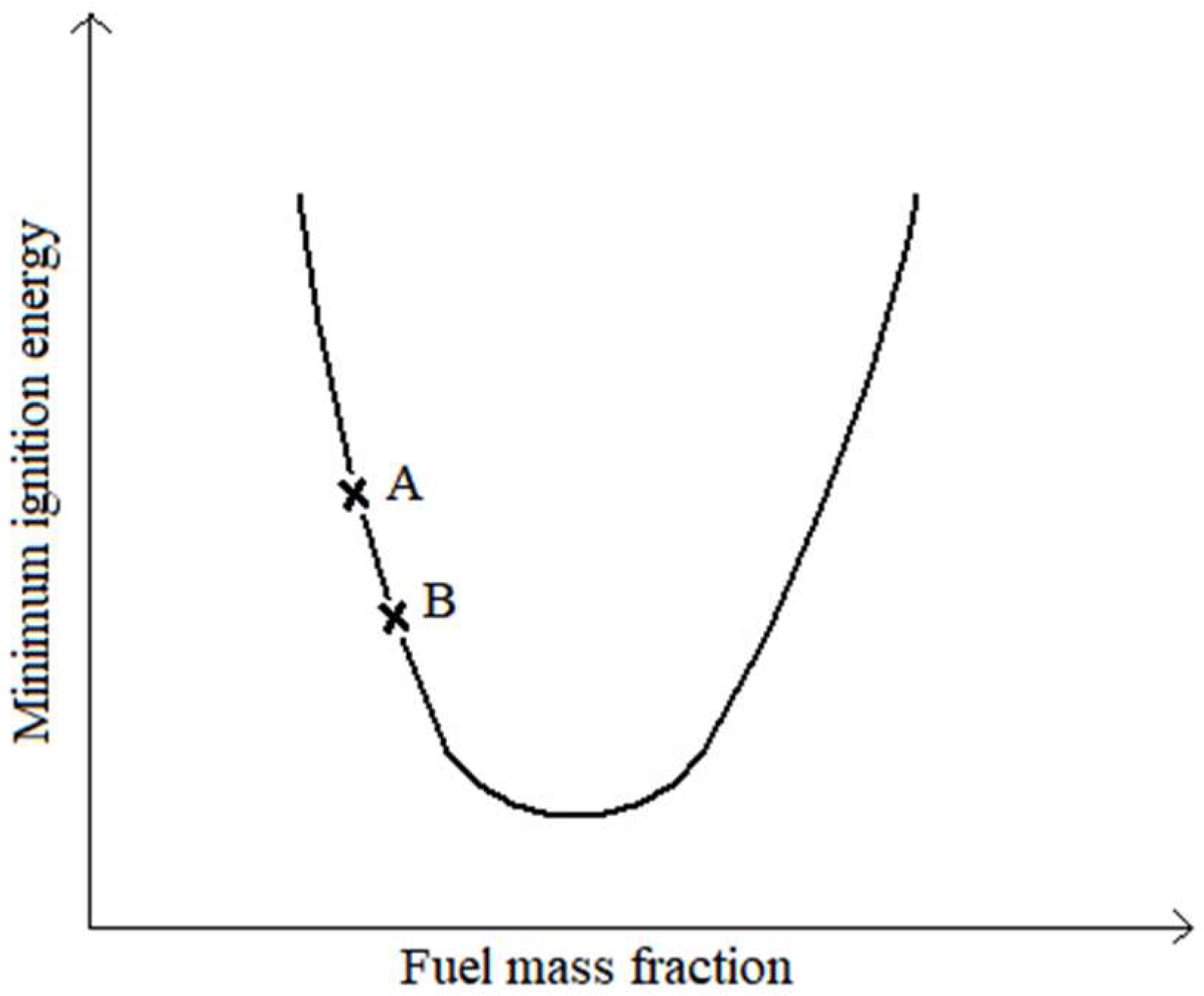
In general terms, the evolution of ignition time, when going from a 6 g to an 8 g batch, can be easily understood through the scheme in
Figure 10, where two generic points, A and B, have been marked.
In this figure, the abscissa axis is parameterized with the fuel mass fraction in the reactant mixture, air and volatiles are released from the biomass and the ordinate axis is parameterized either by the minimum ignition energy or by the ignition time. Either of these two parameters can be used on the abscissa axis, and the U-shaped curve is equally valid for both cases [
27]. Taking into consideration that the air mass flow rate used in all the tests was the same, it can be said that for a 6 g batch the fuel equivalence ratio of the mixture is such that the ignition condition will be represented by point A. When changing to a batch of 8 g, it can be seen that the fuel equivalence ratio of the mixture will increase, because if the mass of the batch is greater, the mass of volatiles released will also be greater for this case, and therefore it can be said that, in general, the representative point of this reactant mixture will be point B, i.e., its ignition time will be shorter.
It was previously mentioned that these tests were the result of a preliminary study of the problem of the ignition of volatiles released from wood pellets, and as such no other batch sizes were used. It should also be noted that if the weight of the pellet batch to be introduced into the moving bed was greatly increased, the thermal conditions inside the furnace would change greatly, and it would be difficult to guarantee the constancy of the furnace temperature in all the tests. This problem of finding the ignition times or limits for volatiles released from wood pellet loads will require further analysis and the use of a different type of test reactor, perhaps one more similar to reactors or pumps for explosivity tests [
29,
30,
31].
5. Conclusions
In this study, the ignition time of volatiles released from six types of wood pellets made from Pinus pinaster, Acacia dealbata, Cytisus scoparius, Cistus ladanifer, Paulownia cotevisa and Eucalyptus globulus were determined under five different furnace temperatures, between 359 and 443 °C. For the studied pellets, there are significant differences in the ignition time, particularly at the lowest temperature, 359 °C.
The influence of the initial mass of the pellet batches and their average length on the ignition time was analyzed.
The increase in furnace temperature led, as expected, to a decrease in the pellets’ ignition time; its variation with furnace temperature proved to be linear.
The first tests were performed with batches of 6 g, with particles of average length between 14 and 15 mm, at five different furnace temperatures. The Pinus pinaster pellets were those that presented a higher ignition time, and this difference was sharper at the lowest temperatures. In the tests performed at 359 °C, the average ignition time of Pinus pinaster pellets was 170 s; Cytisus scoparius was the species that, at this temperature, presented the shortest ignition time, at 136 s.
The second group of tests was performed only with Paulownia cotevisa and Cistus ladanifer pellets. The obtained results indicated that for the same pellets batch mass, a smaller particle size led to a shorter ignition time. For the same particle size, an increase in the mass of the batches, from 6 to 8 g, led to a lower ignition time. As the tests were performed with the same air mass flow rate, the increase in the mass of the batches resulted in an increase in the fuel equivalence ratio of the mixture; the mass of volatiles released was obviously higher; the verified conditions allowed a lower minimum ignition energy and consequently a shorter ignition time.