Effects of Pre-Fire Vegetation on the Post-Fire Plant Community Response to Wildfire along a Successional Gradient in Western Juniper Woodlands
Abstract
1. Introduction
1.1. Extent of Sagebrush Steppe and Juniper Woodlands in the Great Basin
1.2. Woodland Development Phases, Mature Juniper and Juniper Expansion
1.3. Fire Regimes and Changes in Fuel Components
2. Methods
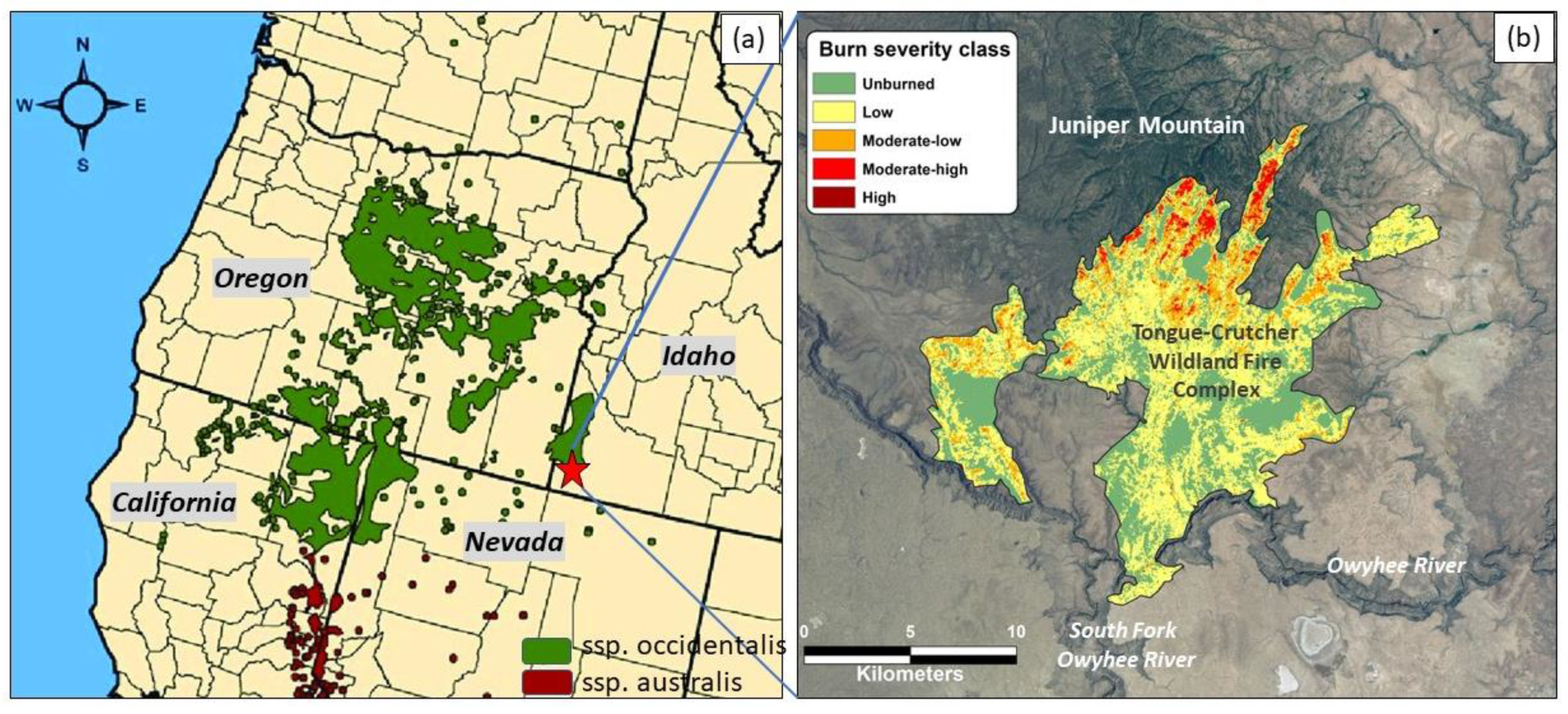
2.1. Site Description
2.2. Field Sampling
2.3. Statistical Analysis Methods
2.3.1. Species Richness and Diversity
2.3.2. Plant Community Turnover and Composition
2.3.3. Functional Groups and Dominant Species
3. Results
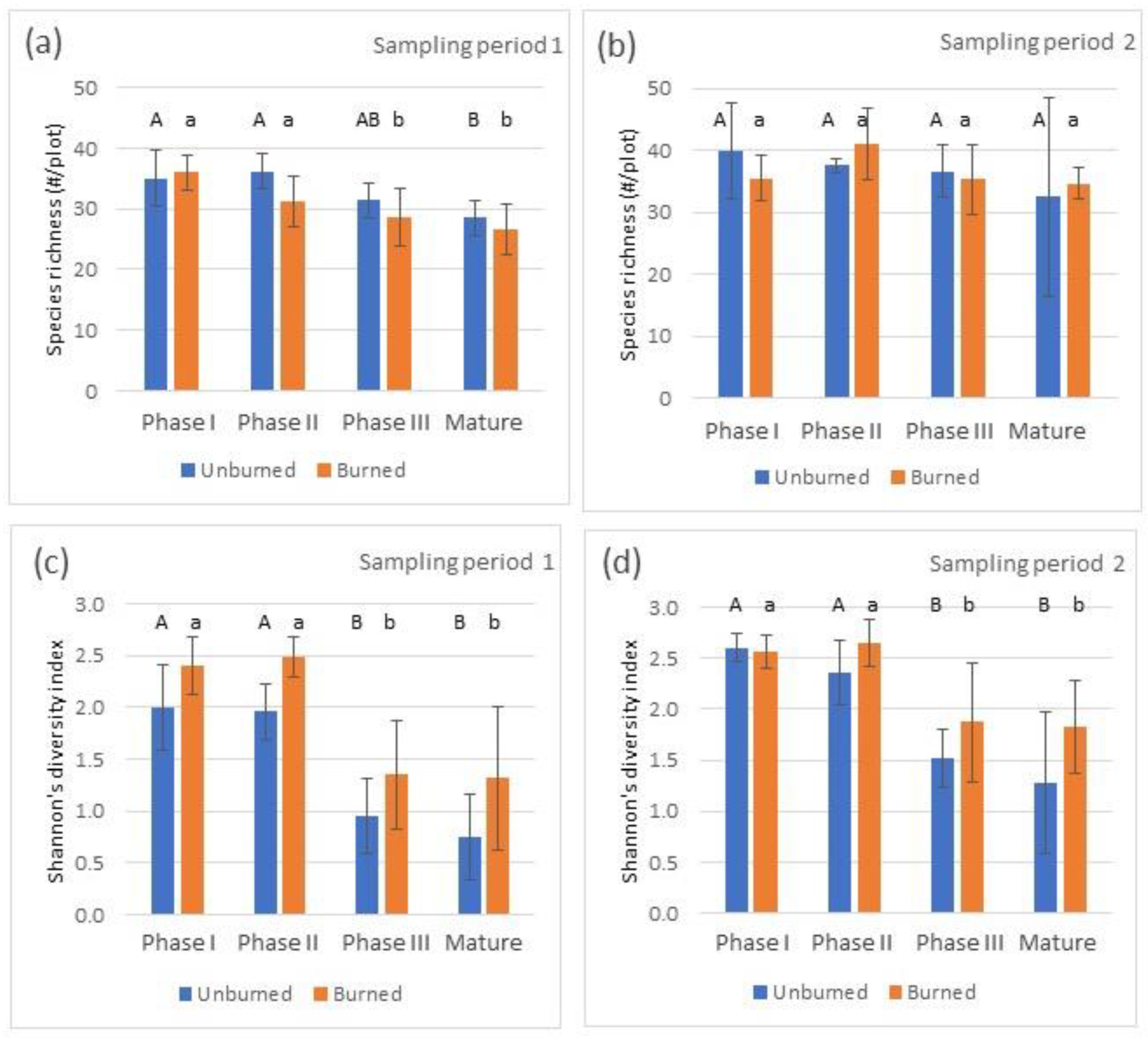
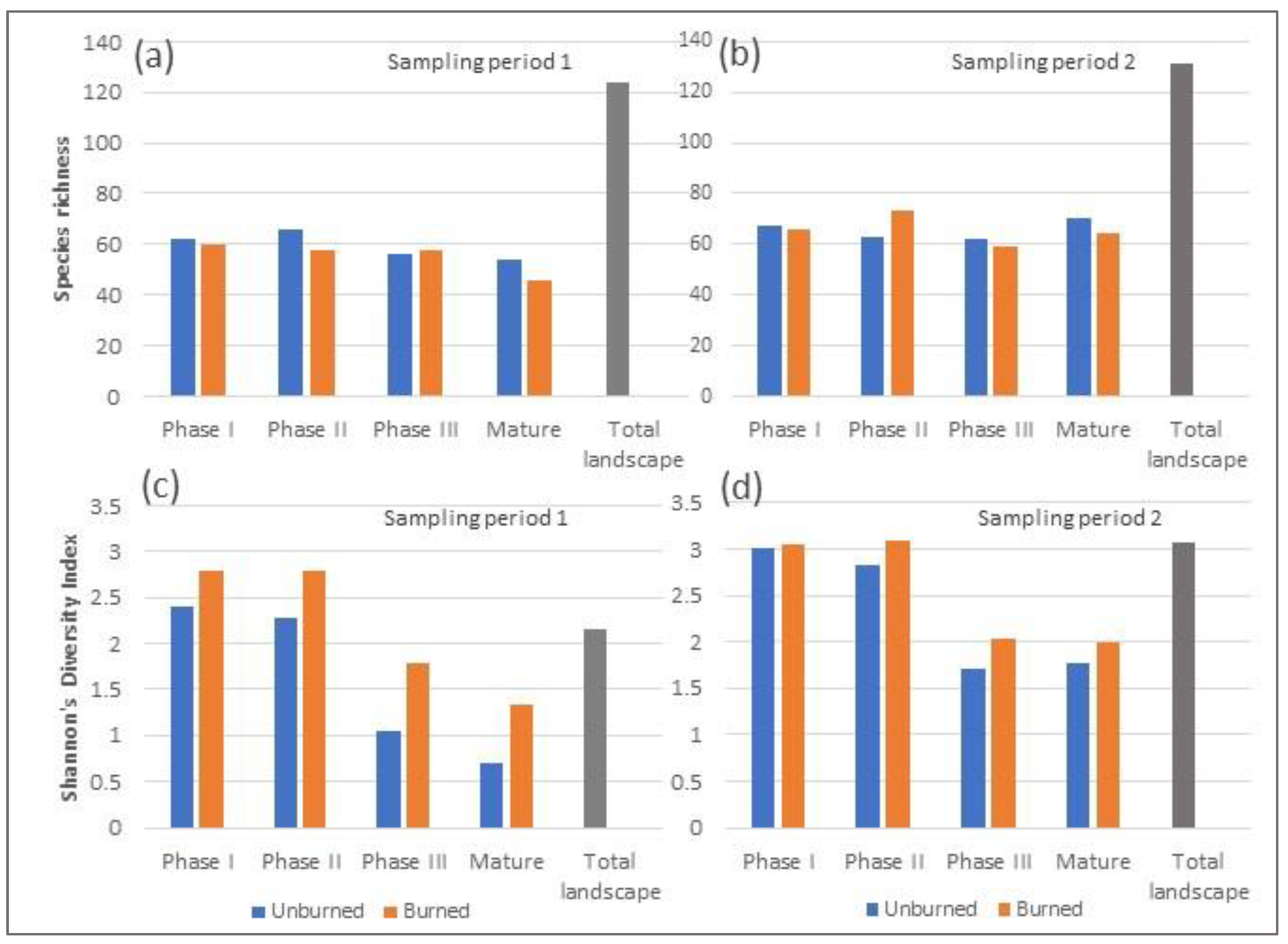
3.1. Richness and Diversity
3.2. Plant Community Turnover and Composition
3.3. Functional Groups
3.4. Changes in Cover of Dominant Species
3.5. Species with High Frequency but Low Cover
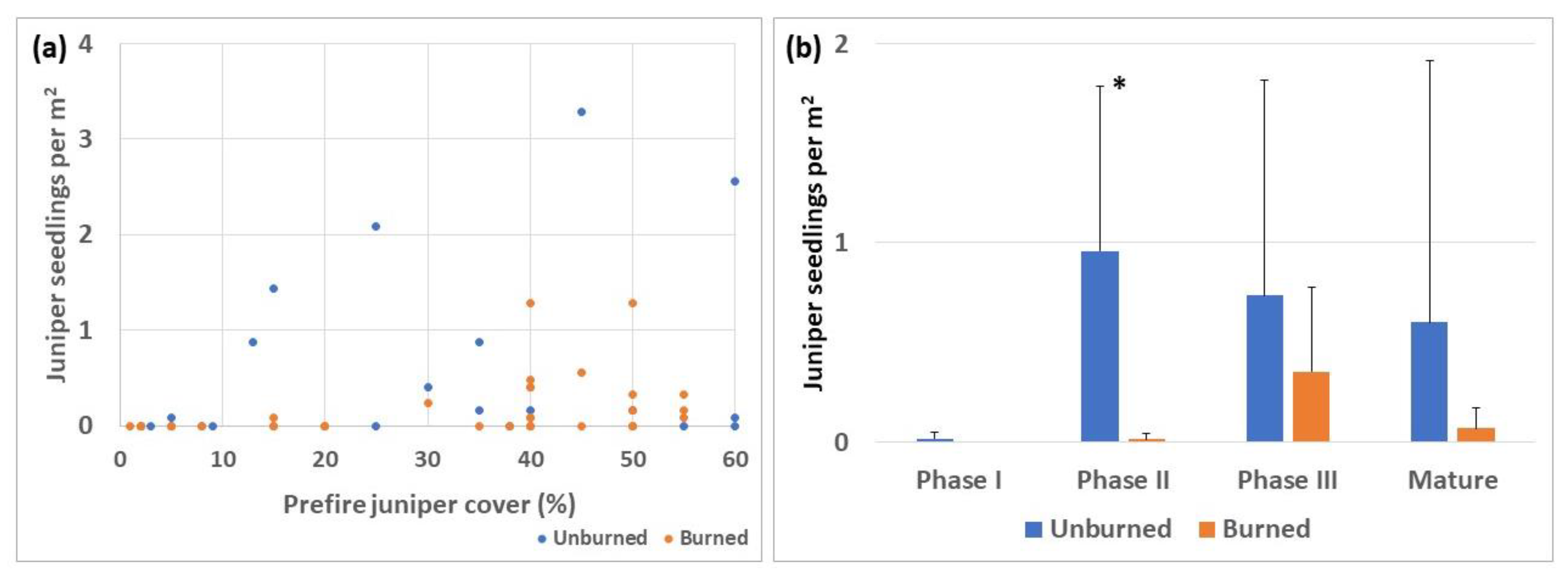
3.6. Juniper Seedlings
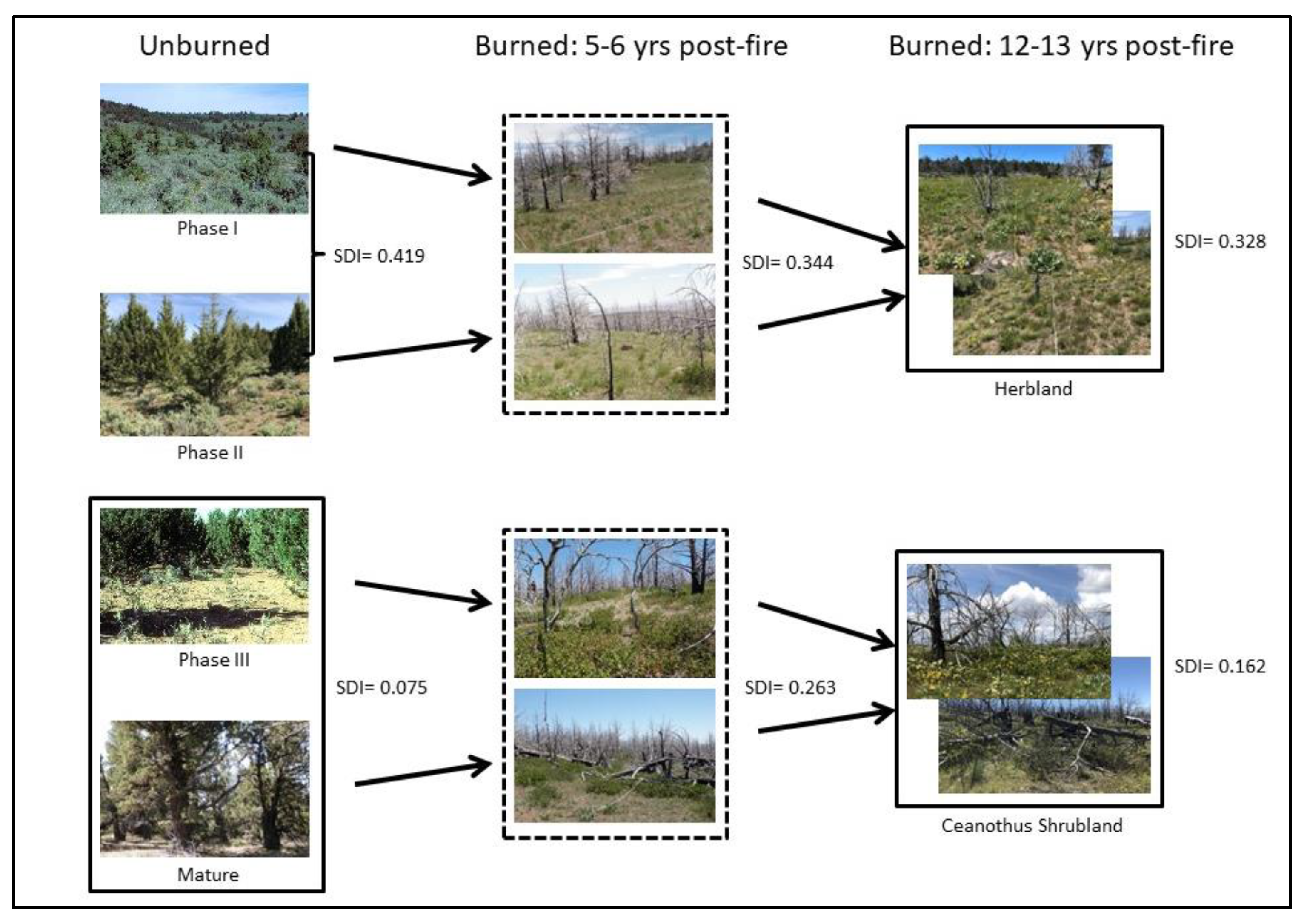
3.7. Successional Pathways
4. Discussion
4.1. Richness and Diversity
4.2. Plant Community Turnover and Composition
4.3. Functional Groups and Common Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, R.F.; Tausch, R.J. The role of fire in juniper and pinyon woodlands: A descriptive analysis. In Invasive Species Workshop: The Role of Fire in the Control and Spread of Invasive Species, Proceedings of the Fire Conference 2000: The First National Congress on Fire Ecology, Prevention, and Management, San Diego, CA, USA, 27 November–1 December 2000; Galley, K.E.M., Wilson, T.P., Eds.; Tall Timbers Research Station, Miscellaneous Publication: Tallahassee, FL, USA, 2001; Volume 11, pp. 15–30. [Google Scholar]
- Miller, R.F.; Svejcar, T.J.; Rose, J.A. Impacts of western juniper on plant community composition and structure. J. Range Manag. 2000, 53, 574–585. [Google Scholar] [CrossRef]
- Weisberg, P.J.; Lingua, E.; Pillai, R.B. Spatial patterns of pinyon–juniper woodland expansion in central Nevada. Rangel. Ecol. Manag. 2007, 60, 115–124. [Google Scholar] [CrossRef]
- Miller, R.F.; Bates, J.D.; Svejcar, T.J.; Pierson, F.B.; Eddleman, L.E. Biology, Ecology, and Management of Western Juniper; Oregon State University, Agricultural Experiment Station: Corvallis, OR, USA, 2005; Volume 152, 82p. [Google Scholar]
- Jacobs, B.F. Spatial patterns and ecological drivers of historic piñon-juniper woodland expansion in the American southwest. Ecography 2011, 34, 1085–1095. [Google Scholar] [CrossRef]
- Flake, S.W.; Weisberg, P.J. Fine-scale stand structure mediates drought-induced tree mortality in pinyon–juniper woodlands. Ecol. Appl. 2019, 29, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.D.; Davies, K.W.; Bournoville, J.; Boyd, C.; O’Connor, R.; Svejcar, T.J. Herbaceous biomass response to prescribed fire in juniper-encroached sagebrush steppe. Rangel. Ecol. Manag. 2019, 72, 28–35. [Google Scholar] [CrossRef]
- Miller, R.F.; Tausch, R.J.; Waichler, W. Oldgrowth juniper and piñon woodlands. In Ecology and Management of Piñon-Juniper Communities within the Interior West; Monsen, S.B., Stevens, R., Tausch, R.J., Miller, R.F., Eds.; U.S. Depart. Agric. Forest Ser.: Provo, UT, USA, 1999; pp. 375–384. [Google Scholar]
- Keane, R.E.; Agee, J.K.; Fule, P.; Keeley, J.E.; Key, C.; Kitchen, S.G.; Miller, R.; Schulte, L.A. Ecological effects of large fires on US landscapes: Benefit or catastrophe? Int. J. Wildland Fire 2008, 17, 696–712. [Google Scholar] [CrossRef]
- Miller, R.F.; Chambers, J.C.; Pyke, D.A.; Pierson, F.B.; Williams, C.J. A Review of Fire Effects on Vegetation and Soils in the Great Basin Region: Response and Ecological Site Characteristics; U.S. Dept. Agric., Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2013; Gen. Tech. Rep. RMRS-GTR-308; 126p. [Google Scholar]
- Miller, R.F.; Heyerdahl, E.K. Fine-scale variation of historical fire regimes in sagebrush-steppe and juniper woodland: An example from California, USA. Int. J. Wildland Fire 2008, 17, 245–254. [Google Scholar] [CrossRef]
- Burkhardt, W.J.; Tisdale, E.W. Causes of juniper invasion in southwestern Idaho. Ecology 1976, 57, 472–484. [Google Scholar] [CrossRef]
- Bukowski, B.E.; Baker, W.L. Historical fire regimes, reconstructed from land-survey data, led to complexity and fluctuation in sagebrush landscapes. Ecol. Appl. 2013, 23, 546–564. [Google Scholar] [CrossRef]
- Baker, W.L.; Shinneman, D.J. Fire and restoration of pinyon-juniper woodlands in the western United States: A review. For. Ecol. Manag. 2004, 189, 1–21. [Google Scholar] [CrossRef]
- Hester, D.A. The Piñon–Juniper Fuel Type Can Really Burn; U.S. Depart. Agric. Forest Ser. Fire Control Notes: Washington, DC, USA, 1952; Volume 13, pp. 26–29. [Google Scholar]
- Yanish, C.R. Western Juniper Succession: Changing Fuels and Fire Behavior. Master’s Thesis, University of Idaho, Moscow, ID, USA, 2002; 85p. [Google Scholar]
- Bernau, C.R.; Strand, E.K.; Bunting, S.C. Fuel bed response to vegetation treatments in juniper-invaded sagebrush steppe. Fire Ecol. 2018, 14, 1–13. [Google Scholar] [CrossRef]
- Weiner, N.I.; Strand, E.K.; Bunting, S.C.; Smith, A.M.S. Duff distribution influences fire severity in post-fire vegetation recovery in sagebrush steppe. Ecosystems 2016, 19, 1196–1209. [Google Scholar] [CrossRef]
- Sikkink, P.G.; Lutes, D.C.; Keane, R.E. Field Guide for Identifying Fuel Loading Models; U.S. Depart. Agric., Forest Ser., Rocky Mountain Research Station: Fort Collins, CO, USA, General Technical Report RMRS-GTR-225.; 2009; 33p. [Google Scholar]
- Roth, A.D.; Bunting, S.C.; Strand, E.K. Relationships between landscape patterns and fire occurrence within a successional gradient in sagebrush steppe-juniper woodland. Int. J. Wildland Fire 2011, 20, 69–77. [Google Scholar] [CrossRef]
- Key, C.H.; Benson, N.C. Landscape assessment: Ground measure of severity, the Composite Burn Index, and remote sensing of severity, the Normalized Burn Ratio. In FIREMON: Fire Effects Monitoring and Inventory System. USDA Forest Service General Technical Report RMRS-GTR-164-CD; Lutes, D.C., Keane, R.E., Caratti, J.F., Key, C.H., Benson, N.C., Sutherland, S., Gangi, L.J., Eds.; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2006; pp. LA 1–LA 51. [Google Scholar]
- Strand, E.K.; Bunting, S.C.; Keefe, R. Influence of wildland fire along a successional gradient in sagebrush steppe and western juniper woodlands. Rangel. Ecol. Manag. 2013, 66, 667–679. [Google Scholar] [CrossRef]
- Holling, C.S. Resilience and Stability of Ecological Systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- Pimm, S.L. The complexity and stability of ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Donohue, I.; Petchey, O.L.; Montoya, J.M.; Jackson, A.L.; McNally, L.; Viana, M.; Healy, K.; Lurgi, M.; O’Connor, N.E.; Emmerson, M.C. On the dimensionality of ecological stability. Ecol. Lett. 2013, 6, 421–429. [Google Scholar] [CrossRef]
- Bunting, S.C.; Robberecht, R.; Defosse, G.E. Length and timing of grazing on postburn productivity of two bunchgrasses in an Idaho experimental range. Int. J. Wildland Fire 1998, 8, 15–20. [Google Scholar] [CrossRef]
- Davies, K.W.; Bates, J.D. Re-introducing fire in sagebrush steppe experiencing decreased fire frequency: Does burning promote spatial and temporal heterogeneity? Int. J. Wildland Fire 2020, 29, 686–695. [Google Scholar] [CrossRef]
- Moffet, C.A.; Taylor, B.J.; Booth, D.T. Postfire shrub cover dynamics: A 70-year fire chronosequence in mountain big sagebrush communities. J. Arid Environ. 2015, 114, 116–123. [Google Scholar] [CrossRef]
- Martin, R.E. Fire manipulation and effects in western juniper (Juniperus occidentalis Hook). In Western Juniper Ecology and Management Workshop; Martin, R.E., Dealy, J.E., Caraher, D.L., Eds.; Bend, OR, USA. General Technical Report PNW-74; U.S. Depart. Agric., Forest Ser., Pacific Northwest Forest and Range Exper. Sta.: Portland, OR, USA, 1978; pp. 121–136. [Google Scholar]
- Pierson, F.B.; Williams, C.J.; Hardegree, S.P.; Clark, P.E.; Kormos, P.R.; Al-Hamdan, O.Z. Hydrologic and erosion responses of sagebrush steppe following juniper encroachment, wildfire, and tree cutting. Rangel. Ecol. Manag. 2013, 66, 274–289. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Cleveland, OH, USA, 2002; Version 7.02. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef]
- Turner, M.G.; Romme, W.H.; Gardner, R.H. Prefire heterogeneity, fire severity, and early postfire plant reestablishment in subalpine forests of Yellowstone National Park, Wyoming. Int. J. Wildland Fire 1999, 9, 21–36. [Google Scholar] [CrossRef]
- Strand, E.K.; Satterberg, K.L.; Hudak, A.T.; Byrne, J.; Khalyani, A.H.; Smith, A.M.S. Does burn severity affect plant community diversity and composition in mixed conifer forests of the United States Intermountain West one decade post fire? Fire Ecol. 2019, 15, 1–22. [Google Scholar] [CrossRef]
- PRISM Climate Group, Oregon State University. Annual Average Mean Temperature by Month, 1991–2020 Normals. Available online: https://prism.oregonstate.edu/downloads/ (accessed on 3 January 2022).
- Harkness, A. Soil Survey of Owyhee County Area, Idaho; U.S. Depart. Agric., Nat. Res. Conser. Ser.: Washington, DC, USA, 1998; 809p. [Google Scholar]
- USDA—NRCS. Owyhee County Digital Soil Survey, SSURGO, Natural Resources Conservation Service, U.S. Department of Agriculture. Online via WebSoilSurvey. 1998. Available online: https://websoilsurvey.sc.egov.usda.gov/App/WebSoilSurvey.aspx (accessed on 5 February 2007).
- Chambers, J.C.; Bradley, B.A.; Brown, C.S.; D’Antonio, C.; Germino, M.J.; Grace, J.B.; Hardegree, S.P.; Miller, R.F.; Pyke, D.A. Resilience to stress and disturbance, and resistance to Bromus tectorum L. invasion in cold desert shrublands of western North America. Ecosystems 2014, 17, 360–375. [Google Scholar]
- SYSTAT 2009, Statistical Software, version 13; Systat Software Inc.: San Jose, CA, USA, 2009.
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data; MjM Software Design: Cleveland, OH, USA, 2011; Version 7.02. [Google Scholar]
- Anderson, M.D. Ceanothus velutinus. In Fire Effects Information System. U.S. Depart. Agric. Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). 2001. Available online: https://www.fs.usda.gov/database/feis/plants/shrub/ceavel/all.html (accessed on 10 September 2022).
- Parker, K.G. Some Important Utah Range Plants. Extension Service Bulletin EC-383; Utah State University: Logan, UT, USA, 1975; 174p. [Google Scholar]
- Pavlacky, D.C., Jr.; Anderson, S.H. Comparative habitat use in a juniper woodland bird community. West. N. Am. Nat. 2004, 64, 376–384. [Google Scholar]
- Holmes, A.L.; Maestas, J.D.; Naugled, D.E. Bird Responses to Removal of Western Juniper in Sagebrush-Steppe. Rangel. Ecol. Manag. 2017, 70, 87–94. [Google Scholar] [CrossRef]
- Forbis, T.A. Germination phenology of some Great Basin native annual forb species. Plant Species Biol. 2010, 25, 221–230. [Google Scholar] [CrossRef]
- Ledger, E.A.; Goergen, E.M.; Forbis de Queiroz, T. Can native annual forbs reduce Bromus tectorum biomass and indirectly facilitate establishment of a native perennial grass? J. Arid. Environ. 2014, 102, 9–16. [Google Scholar] [CrossRef]
- Bunting, S.C.; Kingery, J.L.; Strand, E.K. Effects of succession on species richness of the western juniper woodland/sagebrush steppe mosaic. In Ecology and Management of Pinyon–Juniper Communities in the Interior West; Monsen, S.B., Stevens, R., Eds.; U.S. Depart. Agric., Forest Ser., Rocky Mountain Research Station: Ogden, UT, USA, 1999; pp. 76–81, Proceedings RMRS-P-9. [Google Scholar]
- Conrad, C.E.; Poulton, C.E. Effect of a wildfire on Idaho fescue and bluebunch wheatgrass. J. Range Manag. 1966, 19, 138–141. [Google Scholar] [CrossRef]
- Pechanec, J.F.; Stewart, G.; Blaisdell, J.P. Sagebrush Burning Good and Bad; Farmers’ Bull. No. 1948; U.S. Depart. Agric.: Washington, DC, USA, 1954; p. 34. [Google Scholar]
- Ziegenhagen, L.L.; Miller, R.F. Postfire recovery of two shrubs in the interiors of large burns in the Intermountain West, USA. West. N. Nat. 2009, 69, 195–205. [Google Scholar] [CrossRef]
- Harvey, S.J. Life History and Reproductive Strategies in Artemisia. Master’s Thesis, Montana State University, Bozeman, MT, USA, 1981; 132p. [Google Scholar]
- Young, J.A.; Evans, R.A. Dispersal and germination of big sagebrush (Artemisia tridentata) seeds. Weed Sci. 1989, 37, 201–206. [Google Scholar] [CrossRef]
- Innes, R.J.; Zouhar, K. Fire Regimes of Mountain Big Sagebrush Communities. In Fire Effects Information System, U.S. Depart. Agric., Forest Ser., Rocky Mountain Research Station, Fire Sciences Laboratory. 2018. Available online: https://www.fs.usda.gov/database/feis/fire_regimes/mountain_big_sagebrush/all.html (accessed on 10 September 2022).
- Stickney, P.F. Initial stages of a natural forest succession following wildfire in the Northern Rocky Mountains, a case study. In Proceedings of the Symposium and Workshop on Wilderness Fire, Missoula, MT, USA, 15–18 November 1983; Lotan, J.E., Kilgore, B.M., Fischer, W.C., Mutch, R.W., Eds.; U.S. Depart. Agric., Forest Ser. Intermountain Research Station: Ogden, UT, USA, 1985; pp. 383–384, General Technical Report INT-181. [Google Scholar]
- Johnson, C.G., Jr. Vegetation Response after Wildfires in National Forests of Northeastern Oregon; U.S. Depart. Agric., Forest Ser., Pacific Northwest Region: Portland, OR, USA, 1998; 128p. [Google Scholar]
- Conard, S.G.; Jaramillo, A.E.; Cromack, K., Jr.; Rose, S. Compilers. In The Role of the Genus Ceanothus In Western Forest Ecosystems; U.S. Depart. Agric., Forest Service, Pacific Northwest Forest and Range Exper. Sta.: Portland, OR, USA, 1985; 72p, Gen. Tech. Rep. PNW-182. [Google Scholar]
- Gratkowski, H. Heat as a Factor in Germination of Seeds of Ceanothus Velutinus var. Laevigatus T. & G. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 1962; 122p. [Google Scholar]
- Wozniak, S.S.; Strand, E.K. Fuels Guide for Sagebrush and Pinyon-Juniper Treatments: 10 Years Post-Treatment; U.S. Dep. Int., Bureau of Land Manage.: Boise, ID, USA, 2019; Technical Note 451. [Google Scholar]
- Chambers, J.C.; Miller, R.F.; Board, D.I.; Pyke, D.A.; Roundy, B.A.; Grace, J.B.; Schupp, E.W.; Tausch, R.J. Resilience and Resistance of Sagebrush Ecosystems: Implications for State and Transition Models and Management Treatments. Rangel. Ecol. Manag. 2014, 67, 440–454. [Google Scholar] [CrossRef]



| Variable | Richness | Shannon’s Diversity Index | ||||||
|---|---|---|---|---|---|---|---|---|
| df | MS | F-Ratio | p | df | MS | F-Ratio | p | |
| TIME | 1 | 611.02 | 24.34 | <0.001 | 1 | 4.73 | 31.06 | <0.001 |
| TIME*BURN | 1 | 26.27 | 1.05 | 0.311 | 1 | 0.23 | 1.50 | 0.226 |
| TIME*PHASE | 3 | 20.07 | 0.80 | 0.500 | 3 | 0.10 | 0.64 | 0.593 |
| TIME*BURN*PHASE | 3 | 48.18 | 1.92 | 0.139 | 3 | 0.06 | 0.38 | 0.766 |
| BURN | 1 | 30.46 | 0.80 | 0.375 | 1 | 3.66 | 14.22 | <0.001 |
| PHASE | 3 | 217.36 | 5.72 | 0.002 | 3 | 8.82 | 34.21 | <0.001 |
| BURN*PHASE | 3 | 6.61 | 0.17 | 0.914 | 3 | 0.15 | 0.57 | 0.635 |
| Sample Year 1 | Unburned Plots | Burned Plots | ||||
|---|---|---|---|---|---|---|
| T | A | p | T | A | p | |
| Overall | −6.186 | 0.305 | <0.001 | −8.063 | 0.162 | <0.001 |
| PI vs. PII | −1.522 | 0.060 | 0.079 | −1.778 | 0.039 | 0.054 |
| PI vs. PIII | −5.532 | 0.383 | 0.001 | −6.360 | 0.124 | <0.001 |
| PI vs. Mature | −5.958 | 0.360 | 0.001 | −7.696 | 0.240 | <0.001 |
| PII vs. PIII | −4.439 | 0.246 | 0.003 | −5.048 | 0.107 | 0.001 |
| PII vs. Mature | −4.207 | 0.215 | 0.004 | −6.186 | 0.207 | 0.001 |
| PIII vs. Mature | 0.399 | −0.020 | 0.550 | −1.222 | 0.024 | 0.112 |
| Sample Year 2 | T | A | p | T | A | p |
| Overall | −7.448 | 0.294 | <0.001 | −7.570 | 0.144 | <0.001 |
| PI vs. PII | −3.900 | 0.144 | 0.005 | −0.591 | 0.011 | 0.240 |
| PI vs. PIII | −5.591 | 0.387 | 0.002 | −8.418 | 0.158 | <0.001 |
| PI vs. Mature | −6.187 | 0.358 | 0.001 | −7.837 | 0.233 | <0.001 |
| PII vs. PIII | −4.551 | 0.167 | 0.003 | −4.297 | 0.077 | 0.004 |
| PII vs. Mature | −4.872 | 0.162 | 0.002 | −5.267 | 0.137 | 0.001 |
| PIII vs. Mature | −0.468 | 0.012 | 0.306 | 0.311 | −0.005 | 0.515 |
| Period | Phase | Burn | N | dNBR 1-yr | Bromus tectorum | Collomia linearis | Collinsia parviflora | Crepis acuminata | Cryptantha spp. | Epilobium brachty-carpum | Lactuca serriola | Phacelia heterophylla | Phlox longifolia | Stellaria spp. | Tragopogon dubius | Viola purpurea |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 5 | −36 (11) | 0.0 | 76.0 (15.5) | 49.2 (9.7) | 22.8 (17.5) | 8.4 (18.8) | 5.6 (11.4) | 0.0 | 0.0 | 46.4 25.5) | 47.6 (24.6) | 1.6 (2.6) | 10.8 (11.4) |
| 1 | 1 | 1 | 7 | 47 (93) | 11.4 (18.8) | 60.9 (10.0) | 35.1 (22.4) | 51.1 (20.3) | 24.3 (16.1) | 33.1 (36.0) | 12.6 (14.6) | 0.0 | 53.7 (14.9) | 65.1 (21.9) | 4.6 (4.6) | 7.1 (8.8) |
| 1 | 2 | 0 | 5 | −23 (35) | 0.0 | 53.6 (49.9) | 48 (48.4) | 36.8 (33.4) | 3.6 (3.1) | 8.0 (9.6) | 0.0 | 0.0 | 44.8 (42.6) | 60.0 (62.0) | 0.0 | 15.2 (12.6) |
| 1 | 2 | 1 | 5 | 139 (123) | 4.7 (8.2) | 51.0 (25.3) | 37.7 (19.4) | 46.0 (10.9) | 53.3 (16.1) | 29.3 (22.1) | 19.3 (18.3) | 8.7 (17.4) | 32.7 (20.7) | 73.7 (14.6) | 8.3 (6.3) | 3.0 (2.1) |
| 1 | 3 | 0 | 5 | −20 (12) | 0.0 | 27.2 (22.3) | 52.4 (32.1) | 5.2 (6.4) | 5.6 (6.7) | 0.4 (0.9) | 0.0 | 0.8 (1.8) | 10.4 (10.9) | 49.6 (13.0) | 0.0 | 32.8 (10.6) |
| 1 | 3 | 1 | 15 | 284 (16) | 21.7 (25.3) | 19.9 (16.0) | 3.0 (23.3) | 10.0 (13.7) | 50.0 (28.5) | 27.6 (25.7) | 36.9 (29.8) | 18.7 (18.3) | 6.3 (8.0) | 61.5 (15.9) | 7.2 (9.9) | 8.5 (7.5) |
| 1 | 4 | 0 | 5 | −12 (17) | 11.7 (15.1) | 38.7 (14.8) | 76.0 (11.8) | 8.7 (12.2) | 5.3 (4.3) | 4.3 (5.0) | 0.3 (0.8) | 1.3 (1.6) | 10.7 (9.4) | 50.3 (32.3) | 0.0 | 40.0 (15.4) |
| 1 | 4 | 1 | 8 | 396 (74) | 23.5 (20.5) | 19.8 (16.5) | 23.5 (19.9) | 25.5 (17.3) | 77.3 (14.8) | 67.5 (20.2) | 47.8 (29.7) | 30.8 (22.9) | 14.5 (11.1) | 49.8 (26.8) | 5.0 (4.4) | 5.8 (5.7) |
| 2 | 1 | 0 | 7 | −36 (11) | 2.8 (5.2) | 57.2 (19.6) | 9.2 (3.0) | 24.8 (15.4) | 12.8 (13.2) | 12.0 (10.8) | 0.4 (0.9) | 0.0 | 57.2 (23.8) | 23.2 (14.5) | 0.4 (0.9) | 12.8 (10.0) |
| 2 | 1 | 1 | 7 | 47 (93) | 10.3 (14.3) | 63.7 (15.3) | 14.0 (13.0) | 47.1 (14.3) | 23.1 (17.2) | 54.3 (29.4) | 2.6 (4.3) | 0.3 (0.8) | 54.0 (15.6) | 10.3 (10.5) | 17.7 (19.2) | 12.0 (13.1) |
| 2 | 2 | 0 | 5 | −23 (35) | 1.2 (1.1) | 37.2 (27.5) | 23.6 (15.1) | 18.0 (14.8) | 17.6 22.7) | 9.6 (10.4) | 0.0 | 1.2 (1.1) | 33.2 (23.9) | 47.2 (20.5) | 0.4 (0.9) | 28.4 (16.9) |
| 2 | 2 | 1 | 5 | 139 (123) | 11.3 (18.4) | 59.3 (16.5) | 9.0 (10.5) | 58.0 (11.0) | 31.0 (20.1) | 43.3 (14.1) | 7.7 (9.4) | 3.7 (9.0) | 40.7 (12.6) | 11.3 (9.3) | 22.7 (10.4) | 7.3 (6.2) |
| 2 | 3 | 0 | 5 | −20 (12) | 4.8 (7.8) | 34.8 (31.3) | 56.0 (15.0) | 2.0 (2.8) | 11.6 (9.0) | 17.2 (15.0) | 1.2 (1.8) | 6.8 (8.3) | 11.2 (11.2) | 47.6 (28.8) | 0.0 | 53.2 (6.6) |
| 2 | 3 | 1 | 14 | 284 (165) | 22.3 (18.8) | 52.6 (17.3) | 9.3 (10.2) | 24.9 (17.8) | 28.1 (15.0) | 36.0 (26.0) | 10.7 (8.4) | 21.6 (19.8) | 11.9 (13.3) | 17.9 (12.7) | 32.9 (19.5) | 8.6 (6.3) |
| 2 | 4 | 0 | 5 | −12 (17) | 19.2 (20.5) | 35.6 (19.1) | 31.2 (17.9) | 11.2 (9.2) | 12.0 (9.9) | 11.6 (6.2) | 1.2 (1.1) | 4.4 (5.4) | 19.6 (15.6) | 57.2 (22.5) | 0.4 (0.9) | 43.6 (16.8) |
| 2 | 4 | 1 | 8 | 396 (74) | 39.0 (21.3) | 49.5 (21.7) | 6.0 (4.4) | 27.5 (17.1) | 32.8 (12.7) | 37.8 (17.0) | 13.8 (9.0) | 38.5 (27.9) | 13.5 (12.1) | 18.3 (15.6) | 42.0 (27.6) | 7.5 (10.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strand, E.K.; Bunting, S.C. Effects of Pre-Fire Vegetation on the Post-Fire Plant Community Response to Wildfire along a Successional Gradient in Western Juniper Woodlands. Fire 2023, 6, 141. https://doi.org/10.3390/fire6040141
Strand EK, Bunting SC. Effects of Pre-Fire Vegetation on the Post-Fire Plant Community Response to Wildfire along a Successional Gradient in Western Juniper Woodlands. Fire. 2023; 6(4):141. https://doi.org/10.3390/fire6040141
Chicago/Turabian StyleStrand, Eva K., and Stephen C. Bunting. 2023. "Effects of Pre-Fire Vegetation on the Post-Fire Plant Community Response to Wildfire along a Successional Gradient in Western Juniper Woodlands" Fire 6, no. 4: 141. https://doi.org/10.3390/fire6040141
APA StyleStrand, E. K., & Bunting, S. C. (2023). Effects of Pre-Fire Vegetation on the Post-Fire Plant Community Response to Wildfire along a Successional Gradient in Western Juniper Woodlands. Fire, 6(4), 141. https://doi.org/10.3390/fire6040141






