Inter-Month Nutrients Dynamic and Plant Growth in Calamagrostis angustifolia Community and Soil after Different Burning Seasons
Abstract
1. Introduction
2. Experimental Materials and Methods
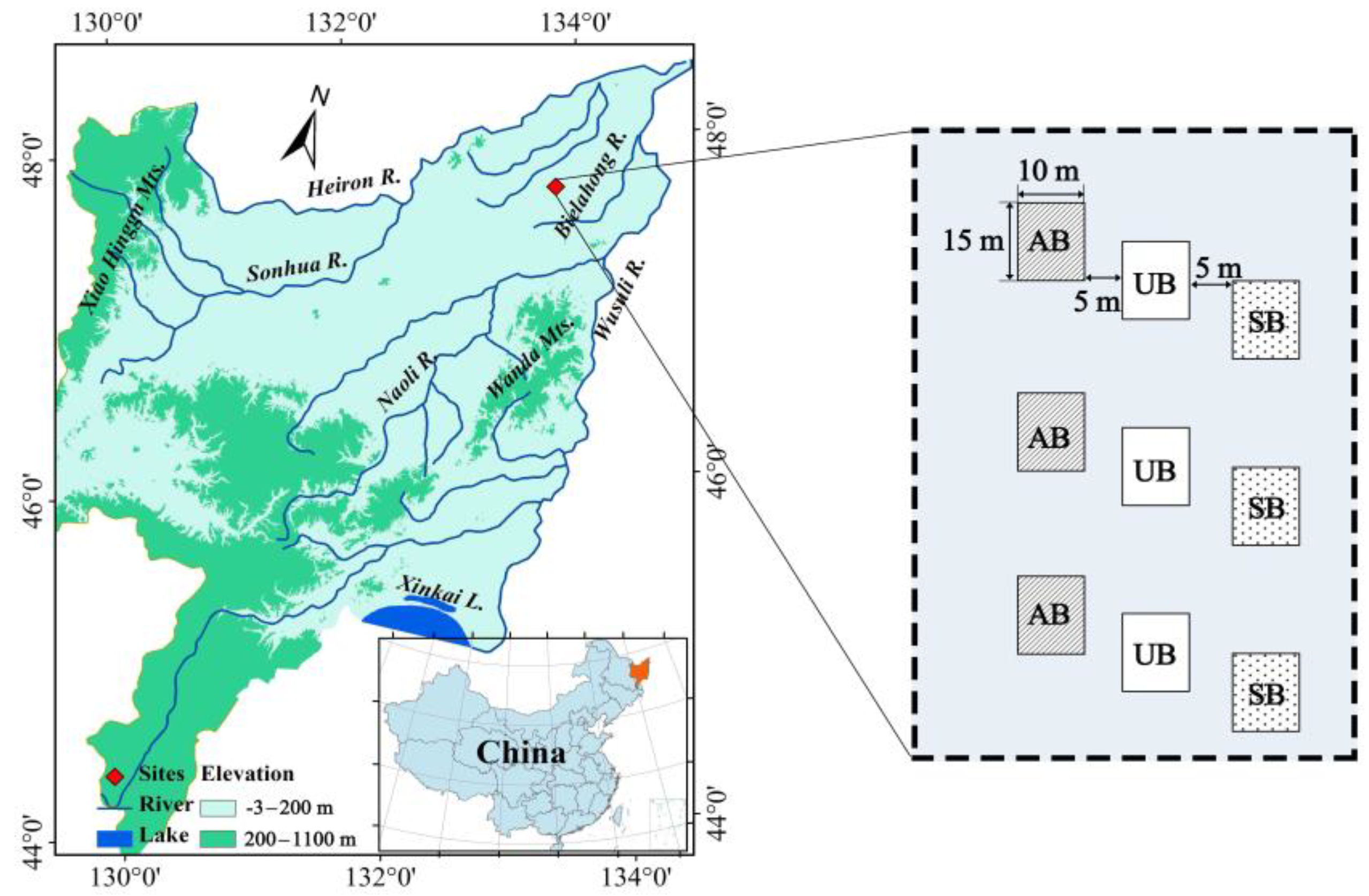
2.1. Study Site
2.2. Experimental Design and Treatments
2.3. Statistical Analysis
3. Results
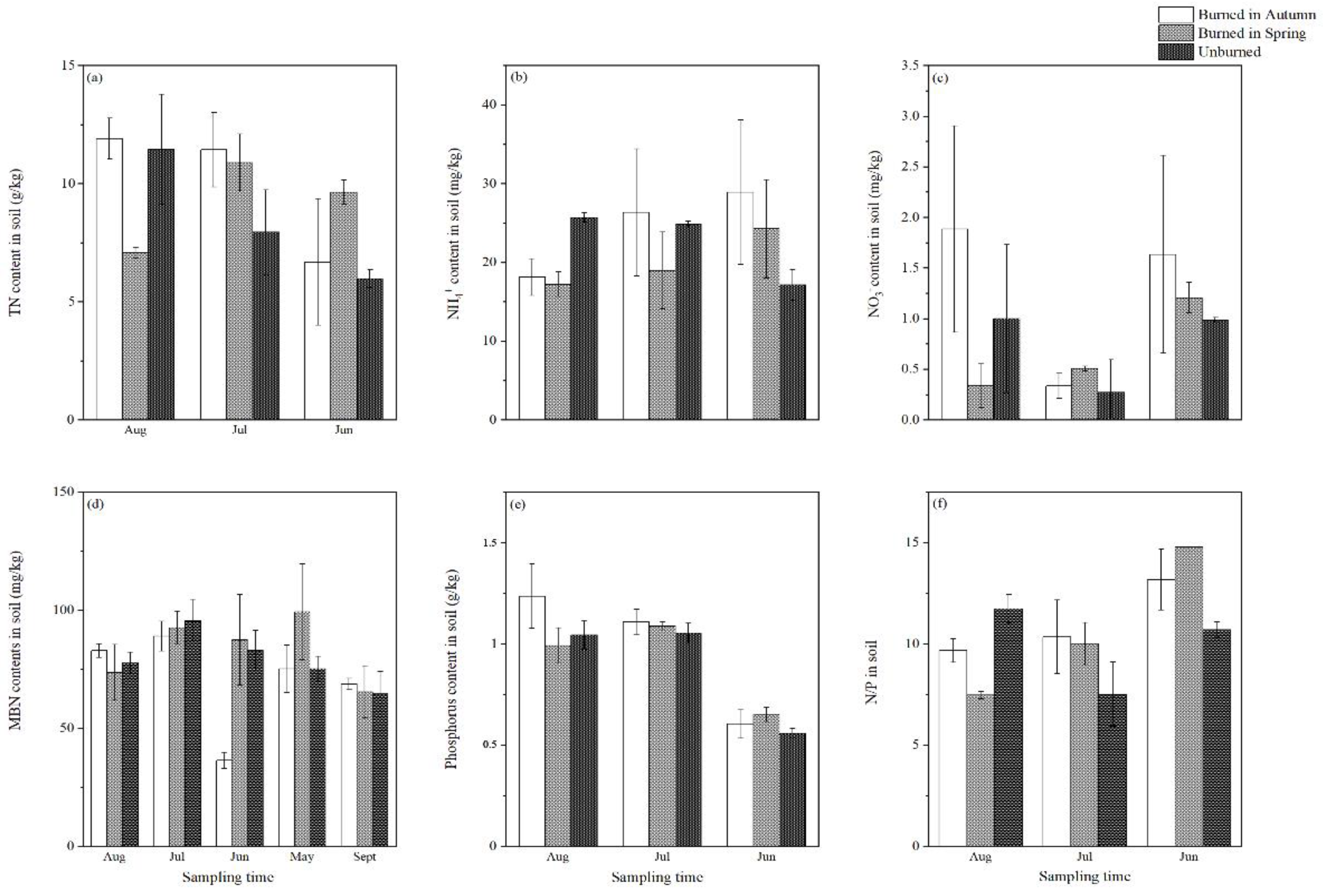
3.1. Effect of Burning on Soil Nitrogen and Phosphorus Contents
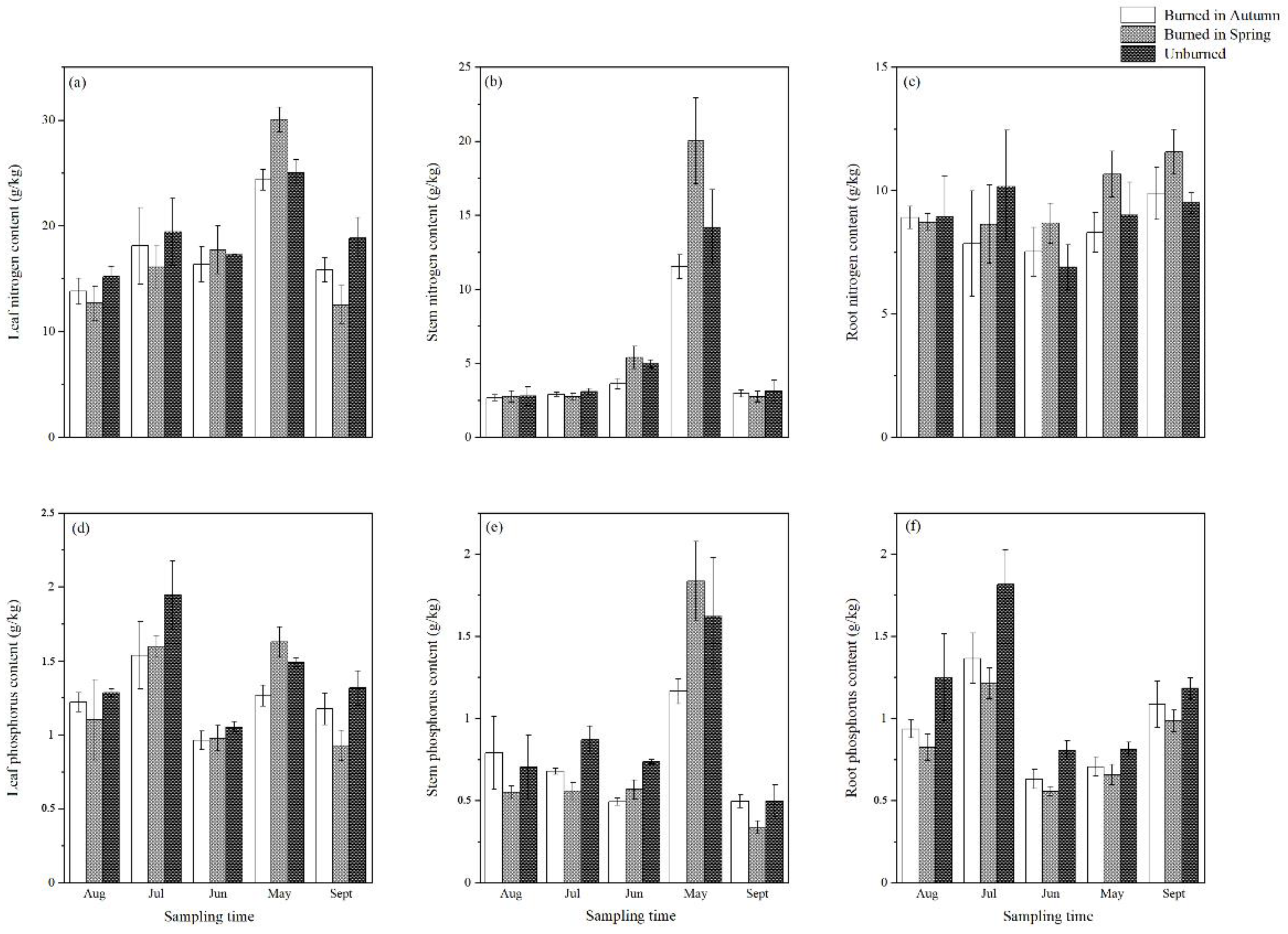
3.2. Effect of Burning on Plant Nitrogen and Phosphorus Contents
3.3. Effect of Burning on Plant Growth and Carbon Content
4. Discussion
4.1. The Effect of Burning on Soil Nitrogen and Phosphorus Contents
4.2. The Effect of Burning on Plant Nitrogen and Phosphorus Contents
4.3. Differences in the Effects of Burning on Plant Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majumdar, A.; Shrivastava, A.; Sarkar, S.R.; Sathyavelu, S.; Barla, A.; Bose, S. Hydrology, sedimentation and mineralisation: A wetland ecology perspective. Clim. Chang. Environ. Sustain. 2020, 8, 134–151. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, Ü.; Zhang, L.; Anderson, C.J.; Jørgensen, S.E.; Brix, H. Wetlands, carbon, and climate change. Landsc. Ecol. 2013, 28, 583–597. [Google Scholar] [CrossRef]
- Erwin, K.L. Wetlands and global climate change: The role of wetland restoration in a changing world. Wetl. Ecol. Manag. 2008, 17, 71–84. [Google Scholar] [CrossRef]
- Hale, K. Long-Term Carbon Storage in a Semi-Natural British Woodland; University of Liverpool: Liverpool, UK, 2015. [Google Scholar]
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global patterns in belowground communities. Ecol. Lett. 2009, 12, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Agren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin III, F.S. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 1999, 30, 1–67. [Google Scholar]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Stevens, C.J.; Lind, E.M.; Hautier, Y.; Harpole, W.S.; Borer, E.T.; Hobbie, S.; Seabloom, E.W.; Ladwig, L.; Bakker, J.D.; Chu, C.; et al. Anthropogenic nitrogen deposition predicts local grassland primary production worldwide. Ecology 2015, 96, 1459–1465. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Dennis, P.G.; Fedoseyenko, D.; Hajirezaei, M.R.; Borriss, R.; von Wirén, N. Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J. Plant Nutr. Soil Sci. 2011, 174, 3–11. [Google Scholar] [CrossRef]
- Widdig, M.; Heintz-Buschart, A.; Schleuss, P.-M.; Guhr, A.; Borer, E.T.; Seabloom, E.W.; Spohn, M. Effects of nitrogen and phosphorus addition on microbial community composition and element cycling in a grassland soil. Ecol. Appl. 2020, 151, 108041. [Google Scholar] [CrossRef]
- Reay, D.S.; Dentener, F.; Smith, P.; Grace, J.; Feely, R.A. Global nitrogen deposition and carbon sinks. Nat. Geosci. 2008, 1, 430–437. [Google Scholar] [CrossRef]
- Boerner, R.E. Fire and nutrient cycling in temperate ecosystems. Bioscience 1982, 32, 187–192. [Google Scholar]
- Butler, O.M.; Elser, J.J.; Lewis, T.; Mackey, B.; Chen, C. The phosphorus-rich signature of fire in the soil-plant system: A global meta-analysis. Ecol. Lett. 2018, 21, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Hardesty, J.; Myers, R.; Fulks, W. Fire, ecosystems, and people: A preliminary assessment of fire as a global conservation issue. In The George Wright Forum; George Wright Society: Hancock, MI, USA, 2005; pp. 78–87. [Google Scholar]
- Li, B.; Liu, G.; Li, W.; Liu, X. Effects of different wildfire intensities on soil organic carbon and soil nutrients in Pinus tabulaeformis forests in Pingquan County, Hebei Province. Ecol. Sci. 2018, 37, 35–44. [Google Scholar]
- Vitousek, P.; Howarth, R. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Wan, S.; Hui, D.; Luo, Y. Fire effects on nitrogen pools and dynamics in terrestrial ecosystems: A meta-analysis. Ecol. Appl. 2001, 11, 1349–1365. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Dangi, S.R.; Vermeire, L.T. The effect of fire intensity, nutrients, soil microbes, and spatial distance on grassland productivity. Plant Soil 2016, 409, 203–216. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Hatten, J.A.; Zabowski, D. Fire severity effects on soil organic matter from a ponderosa pine forest: A laboratory study. Int. J. Wildland Fire 2010, 19, 613–623. [Google Scholar] [CrossRef]
- Hume, A.; Chen, H.Y.H.; Taylor, A.R.; Kayahara, G.J.; Man, R. Soil C:N:P dynamics during secondary succession following fire in the boreal forest of central Canada. For. Ecol. Manag. 2016, 369, 1–9. [Google Scholar] [CrossRef]
- Dijkstra, F.; Adams, M. Fire eases imbalances of nitrogen and phosphorus in woody plants. Ecosystems 2015, 18, 769–779. [Google Scholar] [CrossRef]
- Hamman, S.T.; Burke, I.C.; Knapp, E.E. Soil nutrients and microbial activity after early and late season prescribed burns in a Sierra Nevada mixed conifer forest. For. Ecol. Manag. 2008, 256, 367–374. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Kolden, C.A.; Abatzoglou, J.T.; Johnston, F.H.; van der Werf, G.R.; Flannigan, M. Vegetation fires in the Anthropocene. Nat. Rev. Earth Environ. 2020, 1, 500–515. [Google Scholar] [CrossRef]
- Archibold, O.; Ripley, E.; Delanoy, L. Effects of season of burning on the microenvironment of fescue prairie in central Saskatchewan. Can. Field Nat. 2003, 117, 257–266. [Google Scholar] [CrossRef]
- Gao, C.; Wang, G.; Santin, C.; Doerr, S.H.; Cong, J.; Zhao, H. Response of Calamagrostis angustifolia to burn frequency and seasonality in the Sanjiang Plain wetlands (Northeast China). J. Environ. Manag. 2021, 300, 113759. [Google Scholar] [CrossRef]
- Zhao, H.; Tong, D.Q.; Lin, Q.; Lu, X.; Wang, G. Effect of fires on soil organic carbon pool and mineralization in a Northeastern China wetland. Geoderma 2012, 189–190, 532–539. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y. Remote sensing of methane (CH4) emission flux in the Sanjiang plain in northeastern China. In Proceedings of the 36th COSPAR Scientific Assembly, Beijing, China, 16–23 July 2006; p. 2592. [Google Scholar]
- Zhao, H.; Bao, K.; Tong, D.Q.; Lin, Q.; Lu, X.; Wang, G. Ecophysiological influences of prescribed burning on wetland plants: A case study in Sanjiang Plain wetlands, northeast China. Fresen. Environ. Bull. 2011, 20, 2932–2938. [Google Scholar]
- Wang, G.P.; Liu, J.S.; Wang, J.D.; Yu, J.B. Soil phosphorus forms and their variations in depressional and riparian freshwater wetlands (Sanjiang Plain, Northeast China). Geoderma 2006, 132, 59–74. [Google Scholar] [CrossRef]
- Boby, L.A.; Schuur, E.A.; Mack, M.C.; Verbyla, D.; Johnstone, J.F. Quantifying fire severity, carbon, and nitrogen emissions in Alaska’s boreal forest. Ecol. Appl. 2010, 20, 1633–1647. [Google Scholar] [CrossRef]
- Rietl, A.J.; Jackson, C.R. Effects of the ecological restoration practices of prescribed burning and mechanical thinning on soil microbial enzyme activities and leaf litter decomposition. Soil Biol. Biochem. 2012, 50, 47–57. [Google Scholar] [CrossRef]
- Silva, J.; Raventos, J.; Caswell, H. Growth, survivorship and the effects of fire exclusion in two savanna grasses. Acta Oecol. 1990, 11, 783–800. [Google Scholar]
- Huang, W.; Liu, J.; Wang, Y.P.; Zhou, G.; Han, T.; Li, Y. Increasing phosphorus limitation along three successional forests in southern China. Plant Soil 2013, 364, 181–191. [Google Scholar] [CrossRef]
- Pereira-Silva, E.F.L.; Casals, P.; Sodek, L.; Delitti, W.B.C.; Vallejo, V.R. Post-fire nitrogen uptake and allocation by two resprouting herbaceous species with contrasting belowground traits. Environ. Exp. Bot. 2019, 159, 157–167. [Google Scholar] [CrossRef]
- Coutinho, L.M. As queimadas e seu papel ecológico. Bras. Florest. 1980, 10, 7–23. [Google Scholar]
- Pivello, V.R. An Expert System for the Use of Prescribed Fires in the Management of Brazilian Savannas; Department of Environmental Technology, Imperial College: London, UK, 1992. [Google Scholar]
- Pivello, V.R.; Oliveras, I.; Miranda, H.S.; Haridasan, M.; Sato, M.N.; Meirelles, S.T. Effect of fires on soil nutrient availability in an open savanna in Central Brazil. Plant Soil 2010, 337, 111–123. [Google Scholar] [CrossRef]
- Anderson, R.C.; Menges, E.S. Effects of fire on sandhill herbs: Nutrients, mycorrhizae, and biomass allocation. Am. J. Bot. 1997, 84, 938–948. [Google Scholar] [CrossRef]
- Fortunel, C.; Fine, P.V.A.; Baraloto, C.; Dalling, J. Leaf, stem and root tissue strategies across 758 Neotropical tree species. Funct. Ecol. 2012, 26, 1153–1161. [Google Scholar] [CrossRef]
- Minden, V.; Kleyer, M. Internal and external regulation of plant organ stoichiometry. Plant Biol. 2014, 16, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.-L.; Reich, P.B. Dark respiration rate increases with plant size in saplings of three temperate tree species despite decreasing tissue nitrogen and nonstructural carbohydrates. Tree Physiol. 2006, 26, 915–923. [Google Scholar] [CrossRef][Green Version]
- Reich, P.B.; Tjoelker, M.G.; Machado, J.L.; Oleksyn, J. Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 2006, 439, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Elser, J.J.; He, N.; Wu, H.; Chen, Q.; Zhang, G.; Han, X. Stoichiometric homeostasis of vascular plants in the Inner Mongolia grassland. Oecologia 2011, 166, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huanosto Magana, R.; Adamowicz, S.; Pages, L. Diel changes in nitrogen and carbon resource status and use for growth in young plants of tomato (Solanum lycopersicum). Ann. Bot. 2009, 103, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, Z.; Luo, Y.; Liu, Y. Allocation strategies of carbon, nitrogen and phosphorus following a gradient of wildfire severities. J. Plant. Ecol. 2022, 15, 347–358. [Google Scholar] [CrossRef]
- Knapp, A.K. Post-burn differences in solar radiation, leaf temperature and water stress influencing production in a lowland tallgrass prairie. Am. J. Bot. 1984, 71, 220–227. [Google Scholar] [CrossRef]
- Rice, E.L.; Parenti, R.L. Causes of decreases in productivity in undisturbed tall grass prairie. Am. J. Bot. 1978, 65, 1091–1097. [Google Scholar] [CrossRef]
- Gonzalez, S.L.; Ghermandi, L.; Peláez, D.V. Growth and reproductive post-fire responses of two shrubs in semiarid Patagonian grasslands. Int. J. Wildland Fire 2015, 24, 809–818. [Google Scholar] [CrossRef]
- Saltonstall, K.; Bonnett, G.D. Fire promotes growth and reproduction of Saccharum spontaneum (L.) in Panama. Biol. Invasions 2012, 14, 2479–2488. [Google Scholar] [CrossRef]
- Czimczik, C.I.; Schmidt, M.W.I.; Schulze, E.D. Effects of increasing fire frequency on black carbon and organic matter in Podzols of Siberian Scots pine forests. Eur. J. Soil Sci. 2005, 56, 417–428. [Google Scholar] [CrossRef]
- Oluwole, F.A.; Sambo, J.M.; Sikhalazo, D. Long-term effects of different burning frequencies on the dry savannah grassland in South Africa. Afr. J. Agric. Res. 2008, 3, 147–153. [Google Scholar]

| Autumn Burned * Unburned | Spring Burned * Autumn Burned | Spring Burned * Unburned | ||||||
|---|---|---|---|---|---|---|---|---|
| Chisq | p | z Value | p | z Value | p | z Value | p | |
| Total nitrogen | 3.08 | 0.21 | −1.64 | 0.23 | −1.32 | 0.38 | 0.30 | 0.95 |
| NH4+-N | 2.82 | 0.24 | −0.75 | 0.73 | −1.68 | 0.21 | −0.91 | 0.64 |
| NO3−-N | 6.31 | <0.05 | −2.35 | <0.05 | −1.90 | 0.14 | 0.44 | 0.90 |
| MBN | 6.00 | <0.05 | 1.52 | 0.28 | 2.41 | <0.05 | 0.78 | 0.72 |
| Phosphorus | 6.86 | <0.05 | −2.48 | <0.05 | −1.92 | 0.13 | 0.55 | 0.85 |
| N:P | 3.48 | 0.18 | −1.87 | 0.15 | −0.86 | 0.67 | 0.99 | 0.59 |
| Autumn Burned * Unburned | Spring Burned * Autumn Burned | Spring Burned * Unburned | ||||||
|---|---|---|---|---|---|---|---|---|
| Chisq | p | z Value | p | z Value | p | z Value | p | |
| Stem nitrogen | 12.16 | <0.01 | 1.51 | 0.29 | 3.49 | <0.01 | 1.72 | 0.20 |
| Leaf nitrogen | 4.16 | 0.13 | 1.86 | 0.15 | 0.11 | 0.99 | −1.76 | 0.19 |
| Root nitrogen | 10.29 | <0.01 | 0.89 | 0.65 | 3.14 | <0.01 | 2.02 | 0.11 |
| Stem phosphorus | 6.04 | <0.05 | 2.43 | <0.05 | 0.82 | 0.69 | −1.67 | 0.22 |
| Leaf phosphorus | 15.63 | <0.01 | 0.91 | 0.64 | 0.97 | 0.60 | −0.01 | 1.00 |
| Root phosphorus | 66.77 | <0.01 | 5.65 | <0.01 | −2.61 | <0.05 | −8.07 | <0.01 |
| Autumn Burned * Unburned | Spring Burned * Autumn Burned | Spring Burned * Unburned | ||||||
|---|---|---|---|---|---|---|---|---|
| Chisq | p | z Value | p | z Value | p | z Value | p | |
| Stem density | 24.83 | <0.01 | −3.09 | <0.01 | 2.04 | 0.10 | 4.97 | <0.01 |
| Aboveground biomass | 59.99 | <0.01 | −7.69 | <0.01 | −4.39 | <0.01 | 3.62 | <0.01 |
| Belowground biomass | 13.41 | <0.01 | −2.04 | 0.10 | 1.75 | 0.19 | 3.66 | <0.01 |
| Stem carbon | 9.18 | <0.05 | −2.82 | <0.05 | −0.32 | 0.94 | 2.52 | <0.05 |
| Leaf carbon | 1.21 | 0.55 | 3.62 | <0.01 | 0.28 | 0.96 | −3.37 | <0.01 |
| Root carbon | 7.81 | <0.05 | −2.65 | <0.05 | −0.44 | 0.90 | 2.24 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Zhao, H.; Wang, G.; Cong, J.; Han, D.; Sun, L.; Gao, C. Inter-Month Nutrients Dynamic and Plant Growth in Calamagrostis angustifolia Community and Soil after Different Burning Seasons. Fire 2023, 6, 405. https://doi.org/10.3390/fire6100405
Xu Z, Zhao H, Wang G, Cong J, Han D, Sun L, Gao C. Inter-Month Nutrients Dynamic and Plant Growth in Calamagrostis angustifolia Community and Soil after Different Burning Seasons. Fire. 2023; 6(10):405. https://doi.org/10.3390/fire6100405
Chicago/Turabian StyleXu, Ziyang, Hongmei Zhao, Guoping Wang, Jinxin Cong, Dongxue Han, Long Sun, and Chuanyu Gao. 2023. "Inter-Month Nutrients Dynamic and Plant Growth in Calamagrostis angustifolia Community and Soil after Different Burning Seasons" Fire 6, no. 10: 405. https://doi.org/10.3390/fire6100405
APA StyleXu, Z., Zhao, H., Wang, G., Cong, J., Han, D., Sun, L., & Gao, C. (2023). Inter-Month Nutrients Dynamic and Plant Growth in Calamagrostis angustifolia Community and Soil after Different Burning Seasons. Fire, 6(10), 405. https://doi.org/10.3390/fire6100405








