Kinetic Analysis of Thermal Decomposition of Polyvinyl Chloride at Various Oxygen Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. TG-FTIR Tests
2.3. Kinetics Analysis
2.3.1. Model-Free Method
2.3.2. Model-Fitting Method
3. Results and Discussion
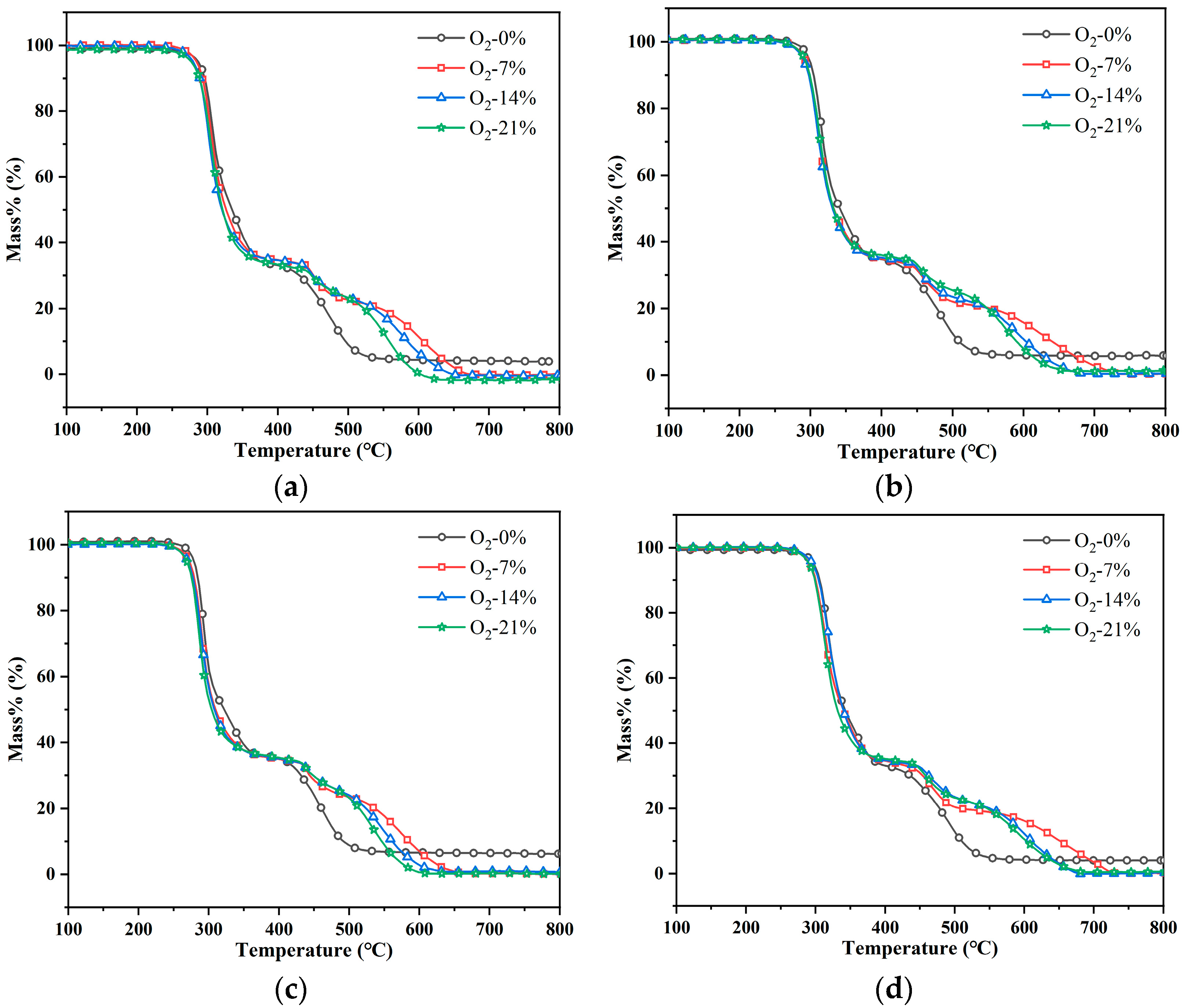
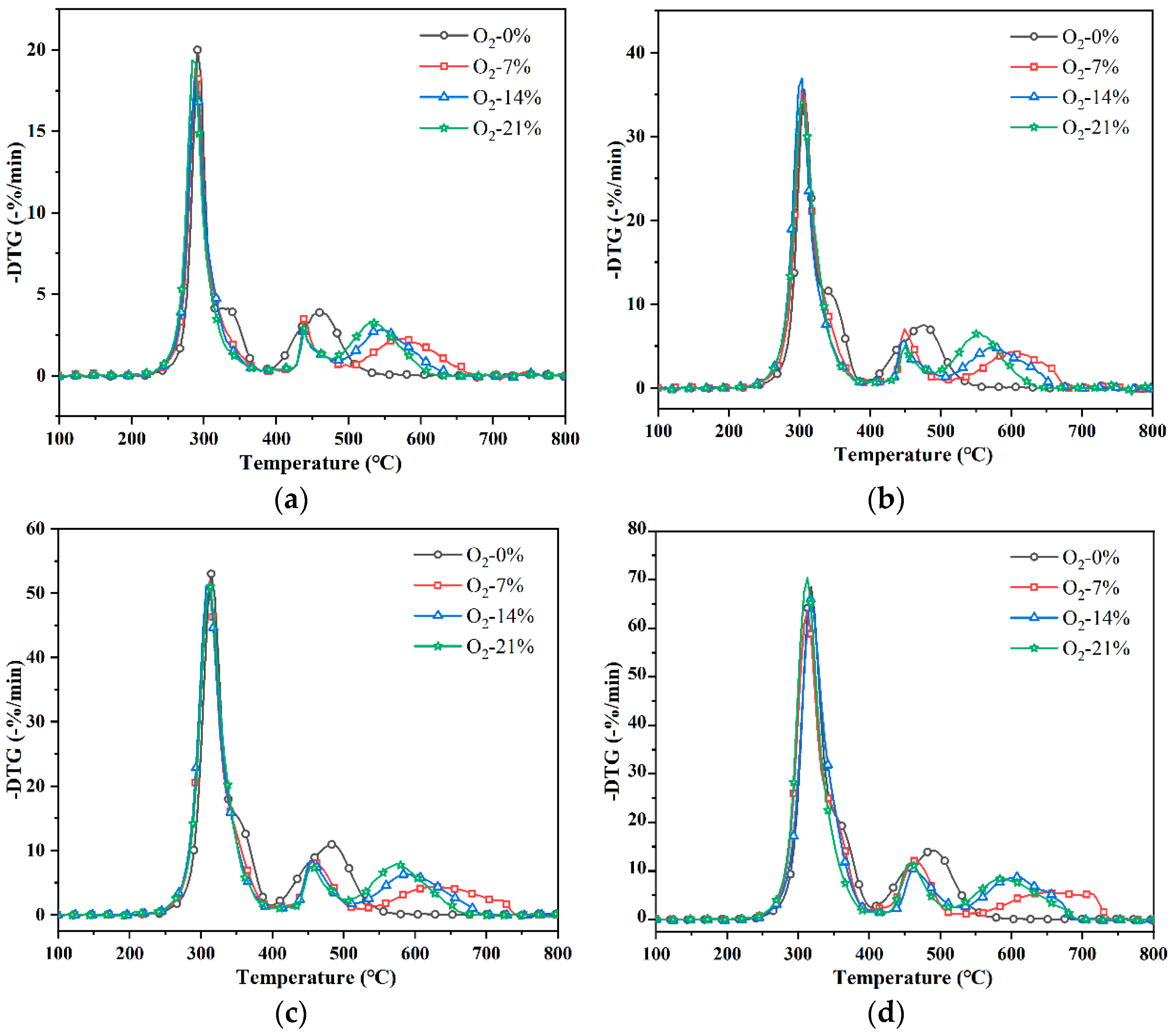
3.1. Thermogravimetric Analysis
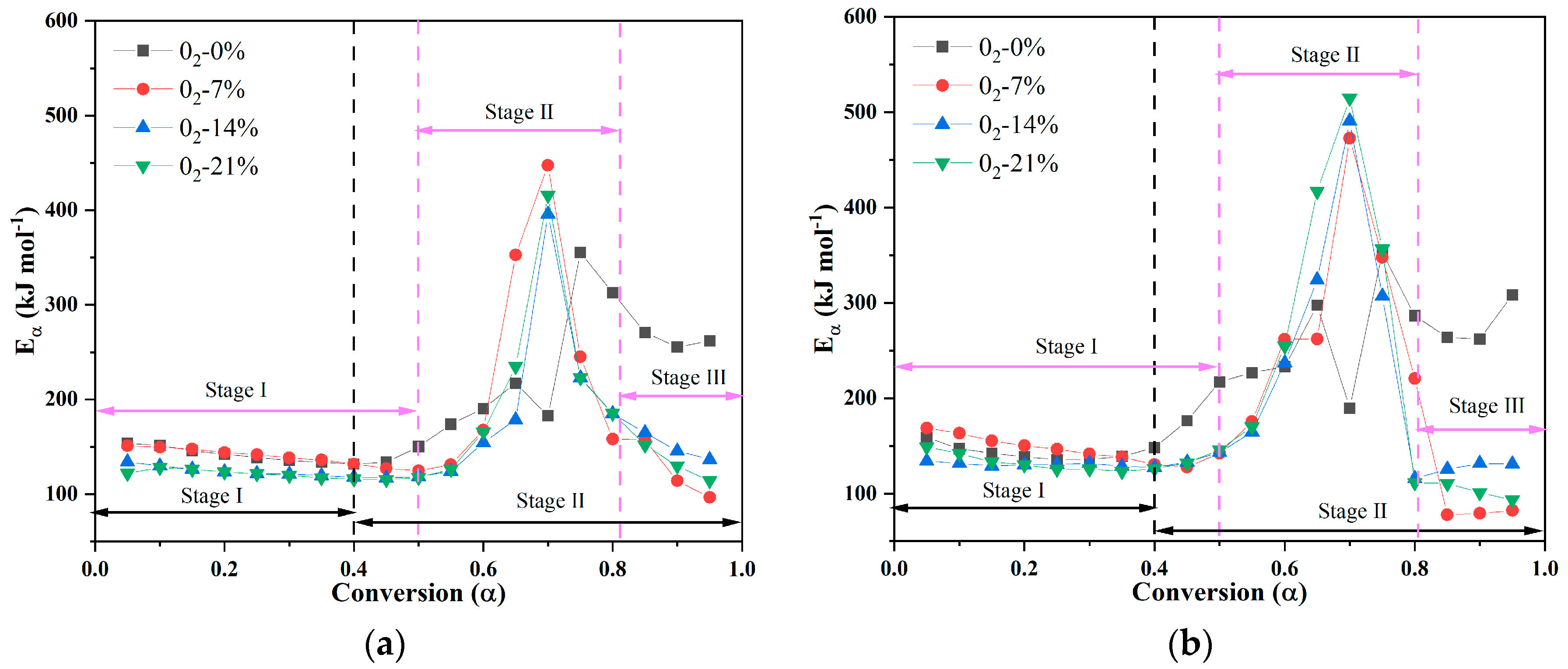
3.2. Activation Energy Analysis
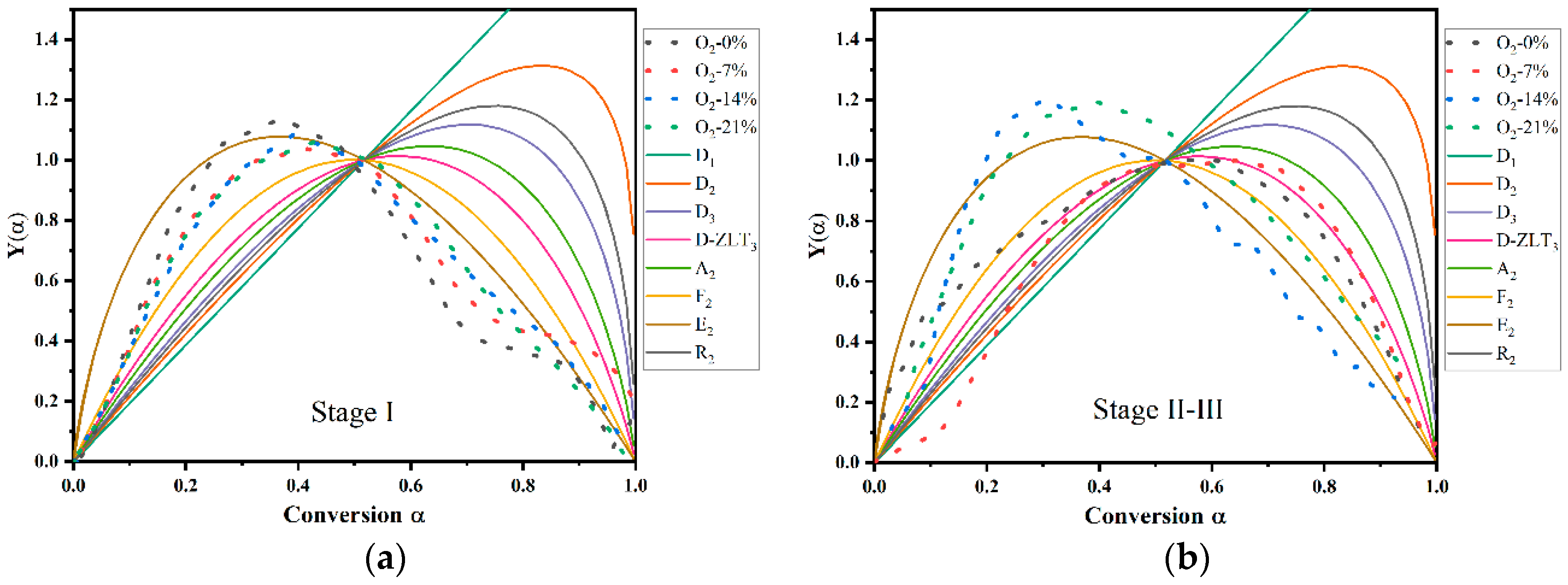
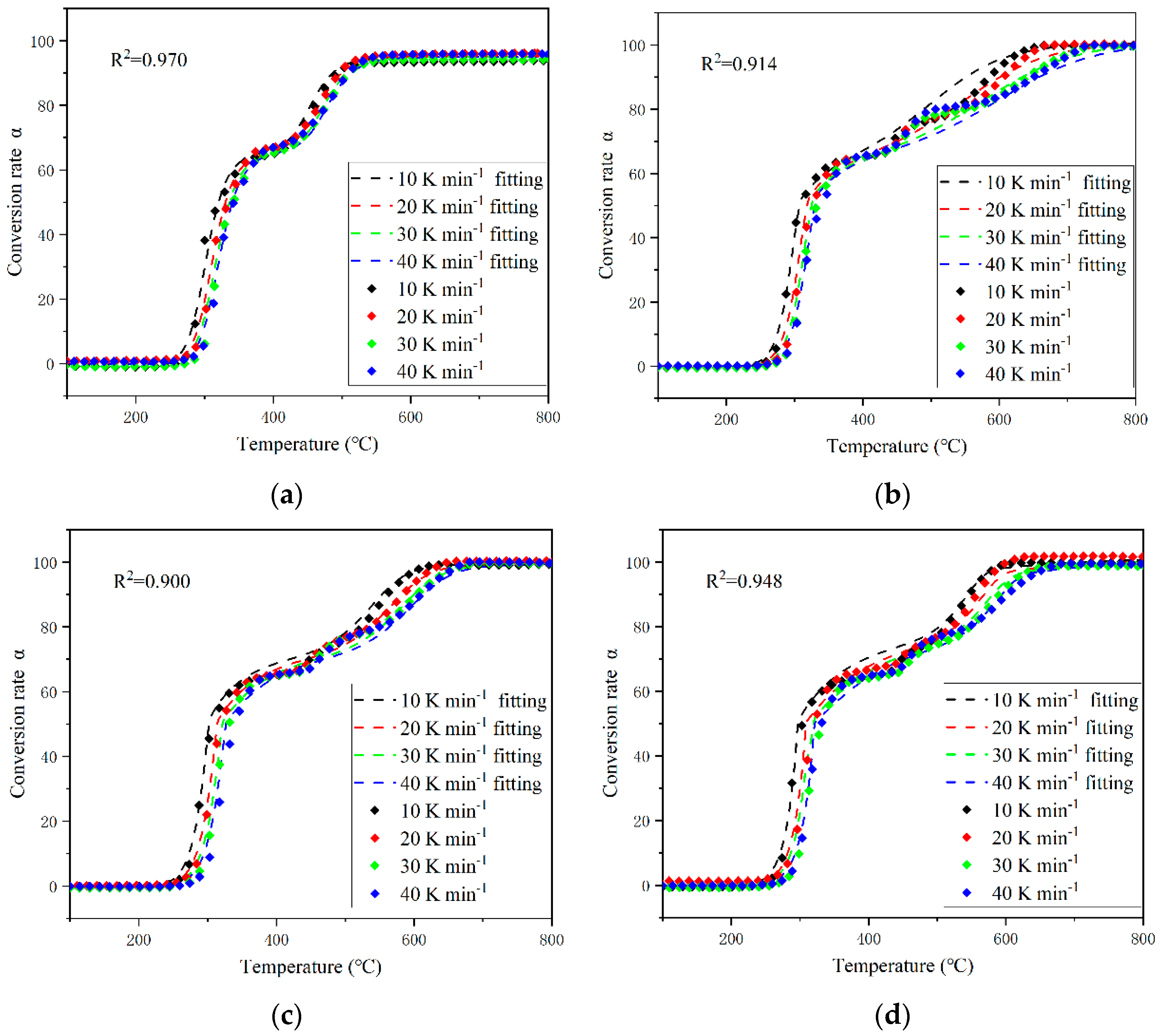
3.3. Mechanism Function Analysis
4. Conclusions
- (1)
- Different oxygen concentrations and heating rates in a fire affected the pyrolysis process of PVC materials. The increase in heating rate caused the TG curve to shift to the high-temperature section and led to a higher DTG peak. Oxygen concentration mainly affected the second weight loss peak of the PVC pyrolysis process. When oxygen was involved in the pyrolysis reaction, the second weight loss peak changed to two weight loss peaks. The prolongation of pyrolysis and the increase in oxygen concentration led to the gradual acceleration of the oxidation reaction of PVC.
- (2)
- The activation energies of PVC materials in oxygenated and non-oxygenated atmospheres increased with an increase in the conversion rate in the first stage, and the activation energies were in the range of 130–175 kJ mol−1. The activation energies in the oxygenated atmosphere showed a wave-type trend in the first stage, and the activation energies were in the range of 230–320 kJ mol−1; the activation energies in the second stage increased significantly, and then decreased rapidly, in the range of 130–510 kJ mol−1. In the third stage, the activation energies rose sharply before falling quickly, ranging from 75 to 510 kJ mol−1. The activation energies of PVC in the oxygen-containing atmosphere did not change significantly as a result of the increase in oxygen concentration, and the overall trends of the activation energies were similar.
- (3)
- The effect of oxygen concentration on the mechanism function of PVC pyrolysis was mainly a change in the reaction mechanism of the second pyrolysis stage from D-ZLT3 to E at higher concentrations of oxygen. But there was no significant effect on the reaction mechanism in the first pyrolysis stage.
- (4)
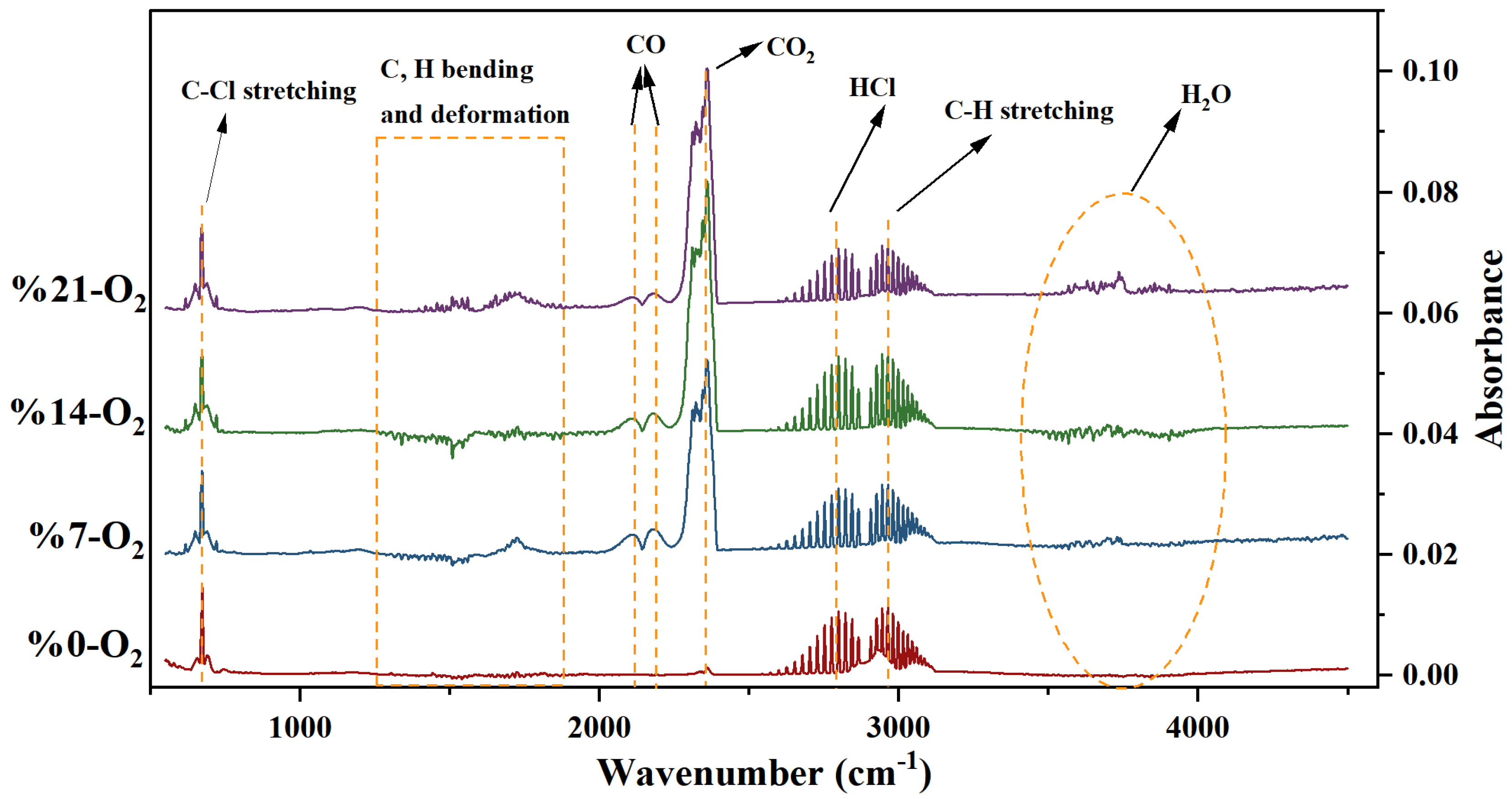
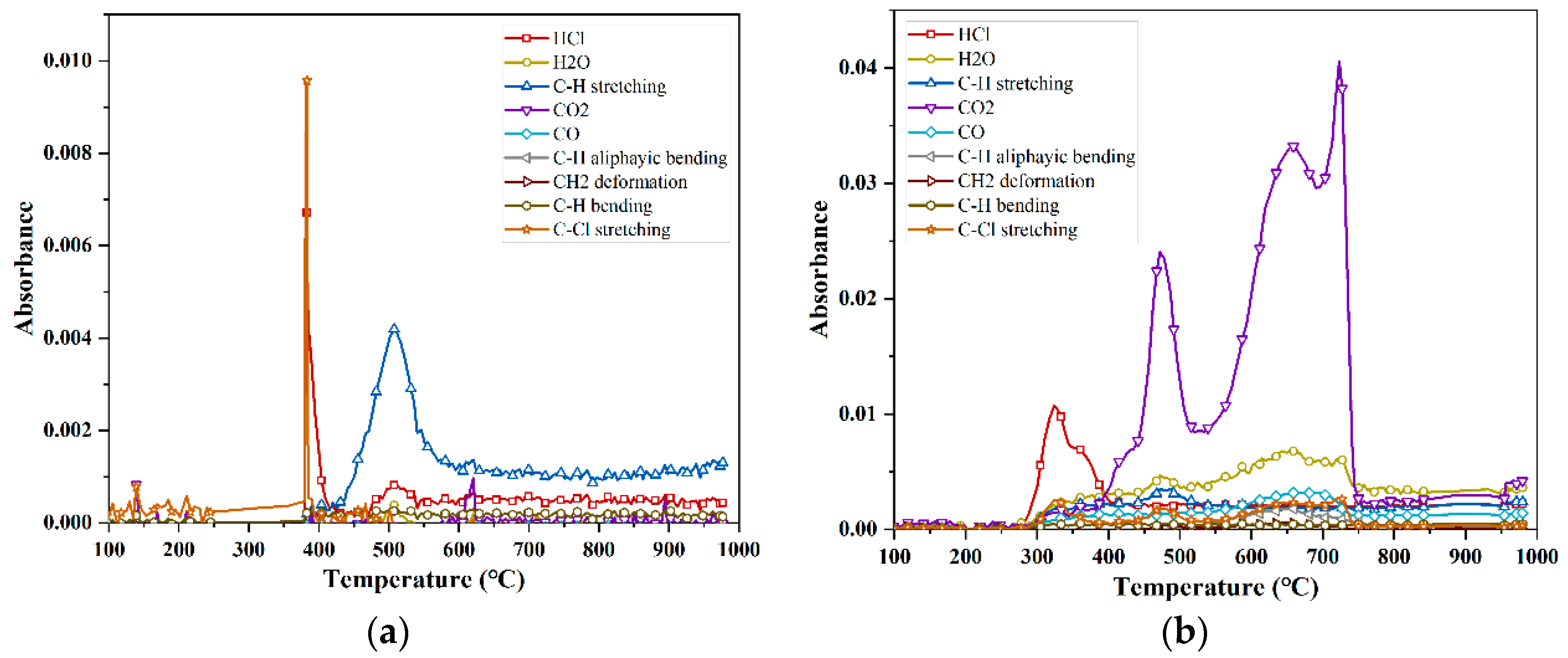
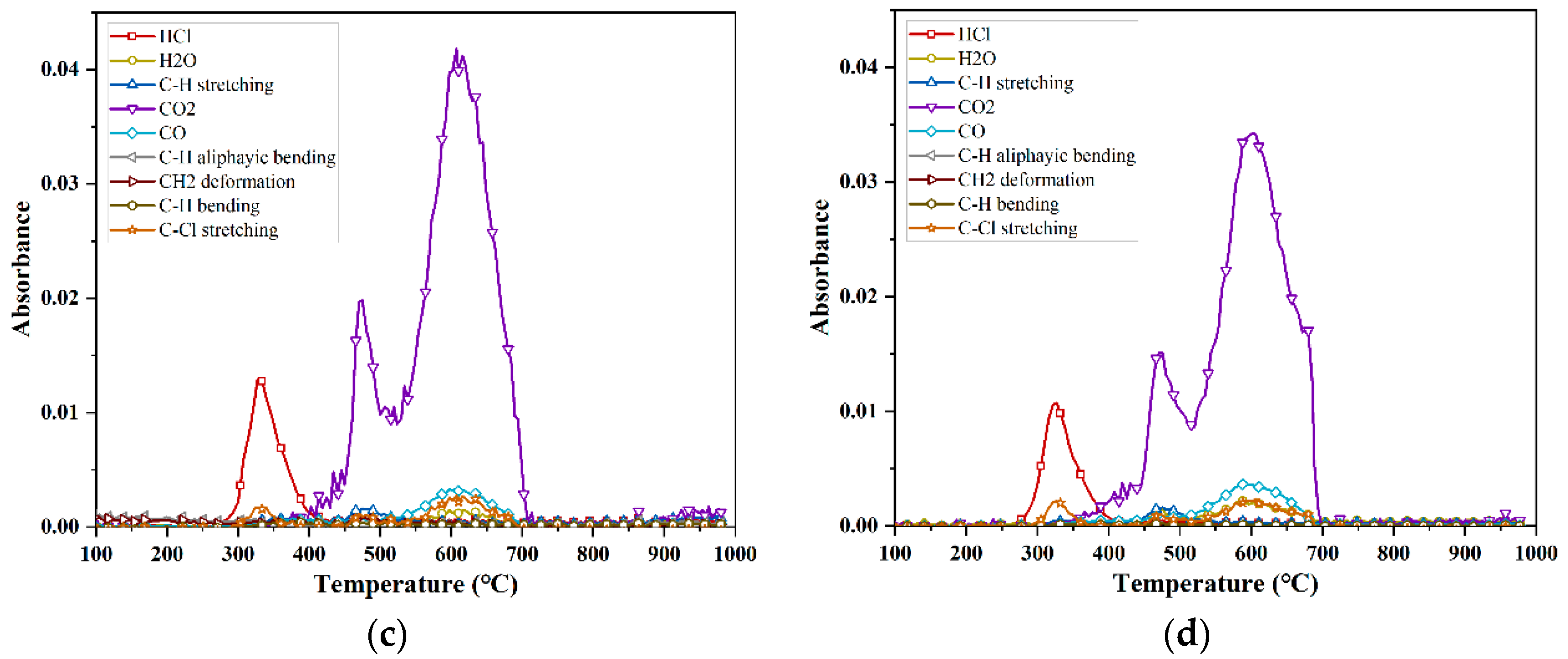
- The pyrolysis of PVC in a non-oxygenated atmosphere was investigated using infrared spectroscopy (FTIR), and the eight major released components, in descending order according to amount released, were C-H stretching > HCl > C-Cl stretching > H2O > CO2 > C-H bending > C-H aliphatic bending > CH2. For PVC at a 7% oxygen concentration, the nine major released components, in descending order according to amount released, were CO2 > HCl > H2O > CO > C-H stretching > C-Cl stretching > C-H aliphatic bending > C-H bending > CH2. For the PVC reaction at 14% and 21% oxygen concentrations, the five major released components, in descending order according to amount released, were: CO2 > HCl > CO > C-Cl stretching > H2O.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal Degradation of Pvc: A Review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Babrauskas, V. Mechanisms and Modes for Ignition of Low-Voltage, Pvc-Insulated Electrotechnical Products. Fire Mater. 2006, 30, 151–174. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Wang, X.; He, J.; Wang, J. Pyrolysis and Combustion of Polyvinyl Chloride (Pvc) Sheath for New and Aged Cables Via Thermogravimetric Analysis-Fourier Transform Infrared (Tg-Ftir) and Calorimeter. Materials 2018, 11, 1997. [Google Scholar] [CrossRef] [PubMed]
- Beneš, M.; Milanov, N.; Matuschek, G.; Kettrup, A.; Plaček, V. Thermal Degradation of Pvc Cable Insulation Studied by Simultaneous TG-FTIR and TG-EGA methods. J. Therm. Anal. Calorim. 2004, 78, 621–630. [Google Scholar] [CrossRef]
- Bhargava, A.; van Hees, P.; Andersson, B. Pyrolysis Modeling of Pvc and Pmma Using a Distributed Reactivity Model. Polym. Degrad. Stab. 2016, 129, 199–211. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Zhang, J.; Yang, S.; Zhang, Z.; Zhao, W. Thermal Degradation of Flame-Retarded High-Voltage Cable Sheath and Insulation Via Tg-Ftir. J. Anal. Appl. Pyrolysis 2018, 134, 167–175. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, T.; Ning, X.; Liu, Y.; Wang, J. Thermal Degradation Kinetics Study of Polyvinyl Chloride (Pvc) Sheath for New and Aged Cables. Waste Manag. 2019, 99, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.X.; Shen, D.K.; Li, Y.X.; Yu, C.J.; Luo, Z.Y.; Cen, K.F. Kinetic Study on Pyrolysis and Combustion of Wood under Different Oxygen Concentrations by Using Tg-Ftir Analysis. J. Anal. Appl. Pyrolysis 2006, 77, 22–27. [Google Scholar] [CrossRef]
- Ma, W.; Rajput, G.; Pan, M.; Lin, F.; Zhong, L.; Chen, G. Pyrolysis of Typical Msw Components by Py-Gc/Ms and Tg-Ftir. Fuel 2019, 251, 693–708. [Google Scholar] [CrossRef]
- Sun, C.; Li, C.; Tan, H.; Zhang, Y. Synergistic Effects of Wood Fiber and Polylactic Acid During Co-Pyrolysis Using Tg-Ftir-Ms and Py-Gc/Ms. Energy Convers. Manag. 2019, 202, 112212. [Google Scholar] [CrossRef]
- Volli, V.; Gollakota, A.R.K.; Shu, C.M. Comparative Studies on Thermochemical Behavior and Kinetics of Lignocellulosic Biomass Residues Using Tg-Ftir and Py-Gc/Ms. Sci. Total Environ. 2021, 792, 148392. [Google Scholar] [CrossRef] [PubMed]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. Ictac Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim. Acta 1999, 341, 53–68. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of Thermal Degradation of Char-Forming Plastics from Thermogravimetry. Application to a Phenolic Plastic. J. Polym. Sci. Part C Polym. Symp. 1954, 6, 183–195. [Google Scholar] [CrossRef]
- Málek, J. The kinetic analysis of non-isothermal data. Thermochim. Acta 1992, 200, 257–269. [Google Scholar] [CrossRef]
- Bai, F.; Guo, W.; Lü, X.; Liu, Y.; Guo, M.; Li, Q.; Sun, Y. Kinetic Study on the Pyrolysis Behavior of Huadian Oil Shale Via Non-Isothermal Thermogravimetric Data. Fuel 2015, 146, 111–118. [Google Scholar] [CrossRef]
- Wu, J.; Chen, T.; Luo, X.; Han, D.; Wang, Z.; Wu, J. Tg/Ftir Analysis on Co-Pyrolysis Behavior of Pe, Pvc and Ps. Waste Manag. 2014, 34, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.M.; Jiang, X.G.; Yan, J.H.; Chi, Y.; Cen, K.F. Tg-Ftir Analysis of Pvc Thermal Degradation and Hcl Removal. J. Anal. Appl. Pyrolysis 2008, 82, 1–9. [Google Scholar] [CrossRef]
- Aboulkas, A.; El harfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Koga, N.; Moukhina, E.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. Ictac Kinetics Committee Recommendations for Analysis of Multi-Step Kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]
- Çepelioğullar, Ö.; Pütün, A.E. Thermal and Kinetic Behaviors of Biomass and Plastic Wastes in Co-Pyrolysis. Energy Convers. Manag. 2013, 75, 263–270. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Perejón, A.; Criado, J.M.; Diánez, M.J.; Pérez-Maqueda, L.A. Kinetic model for thermal dehydrochlorination of poly (vinyl chloride). Polymer 2010, 51, 3998–4007. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Y.; Wang, L. Thermal decomposition of rape straw: Pyrolysis modeling and kinetic study via particle swarm optimization. Energy Convers. Manag. 2017, 146, 124–133. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Du, L.; Duan, Y. Tg–Ftir and Py–Gc/Ms Analysis on Pyrolysis and Combustion of Pine Sawdust. J. Anal. Appl. Pyrolysis 2013, 100, 26–32. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, N.; Lin, J.; Lu, S.; Liew, K.M. On the Pyrolysis Characteristic Parameters of Four Flame-Retardant Classes of Pvc Sheathless Cable Insulation Materials. J. Anal. Appl. Pyrolysis 2023, 170, 105901. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, H.; Tong, L. Experimental study on the fire protection properties of PVC sheath for old and new cables. J. Hazard. Mater. 2010, 179, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Pant, D. Polyvinyl Chloride Degradation by Hybrid (Chemical and Biological) Modification. Polym. Degrad. Stab. 2016, 123, 80–87. [Google Scholar] [CrossRef]


| Model-Free Method | Expressions |
|---|---|
| Kissinger–Akahira–Sunose (KAS) | |
| Friedman (FR) |
| Number | Model | Differential Form | Integral Form |
|---|---|---|---|
| Diffusion model | |||
| 1 | 1D diffusion D1 | α2 | |
| 2 | 2D diffusion–Valensi D-V2 | ||
| 3 | 3D diffusion–Jande r D-J3 | ||
| 4 | 3D Zhuravlev–Leskin–Tempelman D-ZLT3 | ||
| Sigmoidal rate equations | |||
| 5 | Avarami–Erofeev A2 | ||
| Reaction order models | |||
| 6 | Second-order chemical reaction F2 | ||
| Exponent power models | |||
| 7 | Second-order E2 | − | |
| Geometrical contraction models | |||
| 8 | Contracting area R2 | ||
| Atmosphere | Heat Rate /°C min−1 | Tset/°C | Tfinal/°C | DTGpeak1 /% min−1 | DTGpeak2 /% min−1 | DTGpeak3 /% min−1 |
|---|---|---|---|---|---|---|
| O2-0% | 10 | 275 | 506 | 20.3 | 3.9 | / |
| 20 | 286 | 523 | 35.7 | 7.5 | / | |
| 30 | 289 | 535 | 53.0 | 10.9 | / | |
| 40 | 292 | 544 | 68.7 | 14.2 | / | |
| O2-7% | 10 | 270 | 715 | 19.2 | 3.7 | 2.3 |
| 20 | 282 | 795 | 36.0 | 7.0 | 4.3 | |
| 30 | 285 | 814 | 52.1 | 8.3 | 4.4 | |
| 40 | 285 | 826 | 63.8 | 12.1 | 5.6 | |
| O2-14% | 10 | 268 | 642 | 18.4 | 2.8 | 2.9 |
| 20 | 279 | 661 | 37.5 | 5.8 | 4.8 | |
| 30 | 283 | 689 | 52.1 | 8.5 | 6.3 | |
| 40 | 289 | 692 | 66.1 | 10.3 | 8.8 | |
| O2-21% | 10 | 270 | 610 | 20.0 | 2.9 | 3.27 |
| 20 | 280 | 636 | 35.0 | 5.0 | 6.6 | |
| 30 | 285 | 663 | 51.4 | 7.5 | 7.9 | |
| 40 | 285 | 740 | 70.5 | 11.7 | 8.4 |
| Description of Vibrations | Wave Numbers (cm−1) |
|---|---|
| HCl | 3150–2500 |
| H2O | 4000–3500, 1800–1300 |
| C-H stretching | 2934 |
| CO2 | 2400–2260 |
| CO | 2173, 2120 |
| aliphatic bending of C-H | 1458 |
| deformation of CH2 | 1338 |
| C-H bending | 1240 |
| stretching of C-Cl | 660, 852 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Wang, Y.; Man, P. Kinetic Analysis of Thermal Decomposition of Polyvinyl Chloride at Various Oxygen Concentrations. Fire 2023, 6, 404. https://doi.org/10.3390/fire6100404
Yang S, Wang Y, Man P. Kinetic Analysis of Thermal Decomposition of Polyvinyl Chloride at Various Oxygen Concentrations. Fire. 2023; 6(10):404. https://doi.org/10.3390/fire6100404
Chicago/Turabian StyleYang, Shuo, Yong Wang, and Pengrui Man. 2023. "Kinetic Analysis of Thermal Decomposition of Polyvinyl Chloride at Various Oxygen Concentrations" Fire 6, no. 10: 404. https://doi.org/10.3390/fire6100404
APA StyleYang, S., Wang, Y., & Man, P. (2023). Kinetic Analysis of Thermal Decomposition of Polyvinyl Chloride at Various Oxygen Concentrations. Fire, 6(10), 404. https://doi.org/10.3390/fire6100404







