Study on Spontaneous Combustion Characteristics and Early Warning of Coal in a Deep Mine
Abstract
:1. Introduction
2. Experimental Equipment and Processes
2.1. Experimental Coal Samples
2.2. Experimental Setup and Methods
2.3. Experimental Conditions
2.4. Gas Growth Rate Analysis Methods
3. Results and Discussion
3.1. Analysis of Changes in Single Gas Indicators
3.1.1. CO2 Concentration
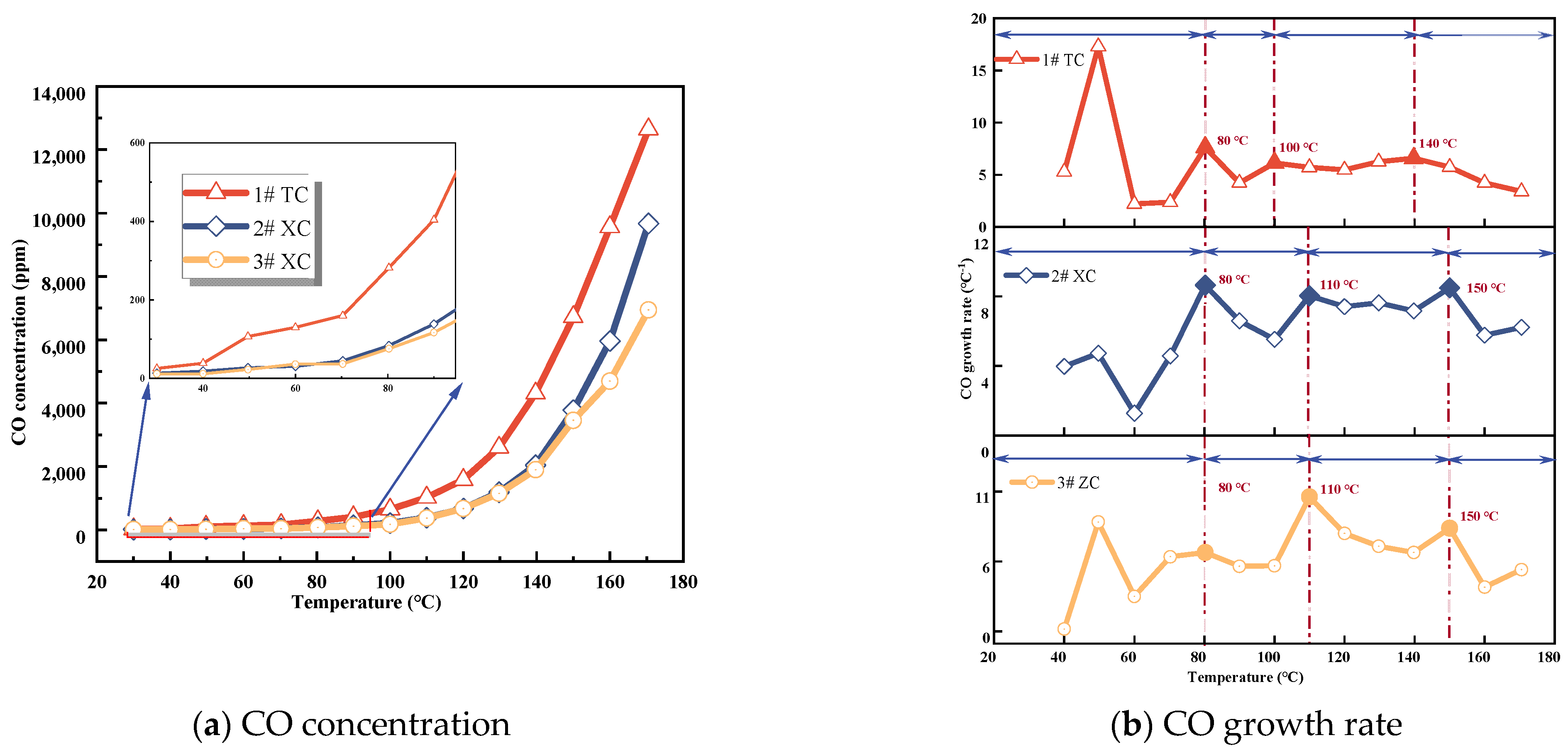
3.1.2. CO Concentration
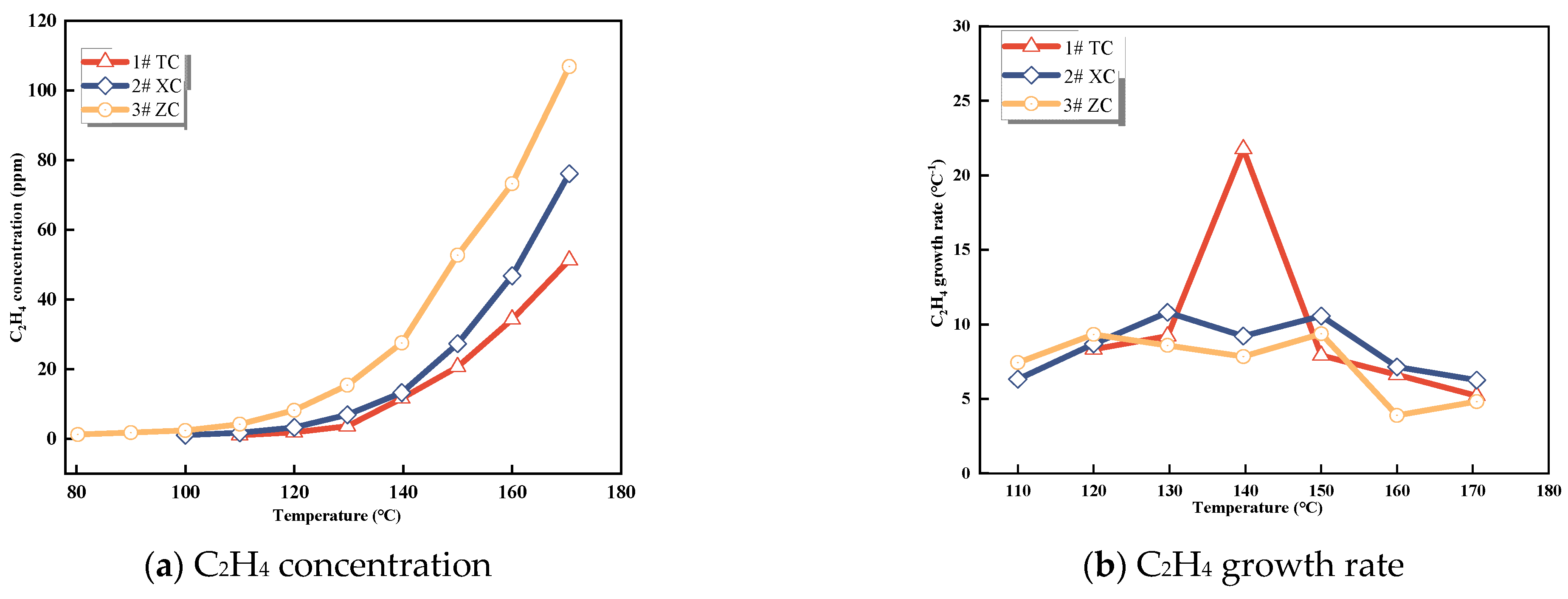
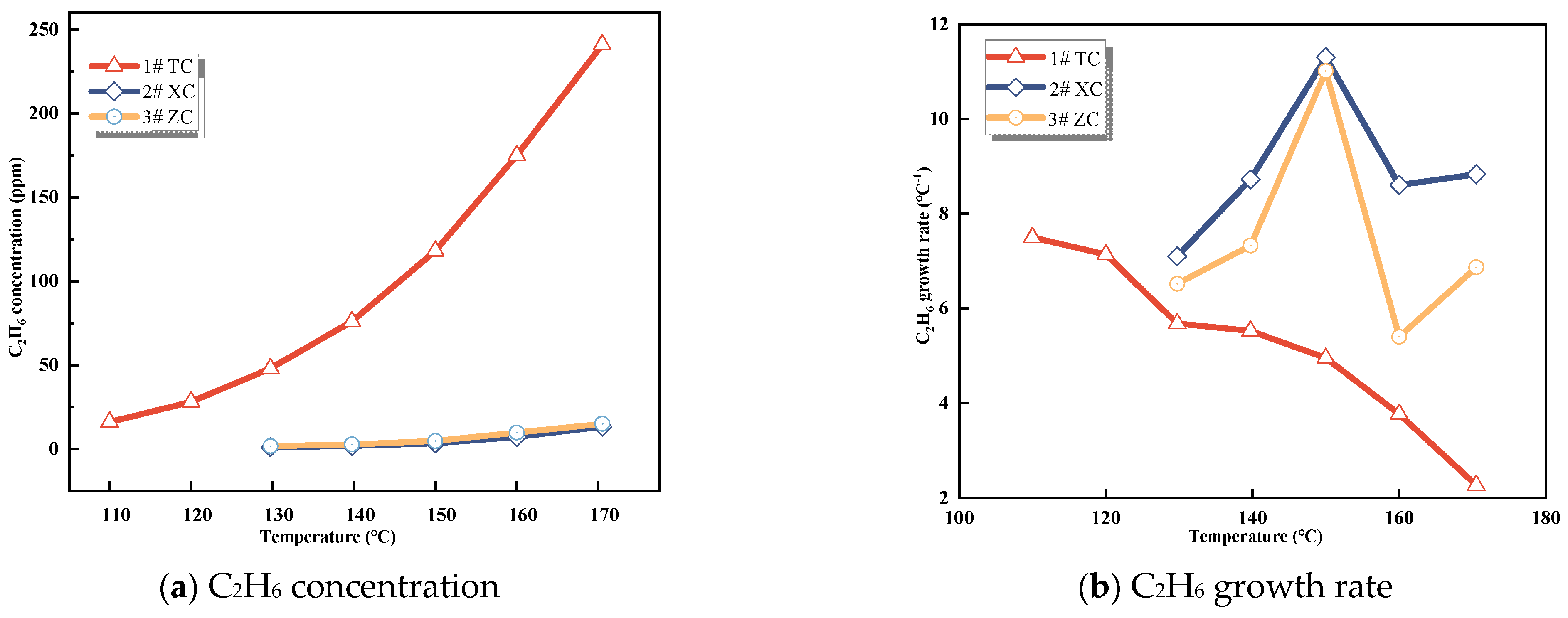
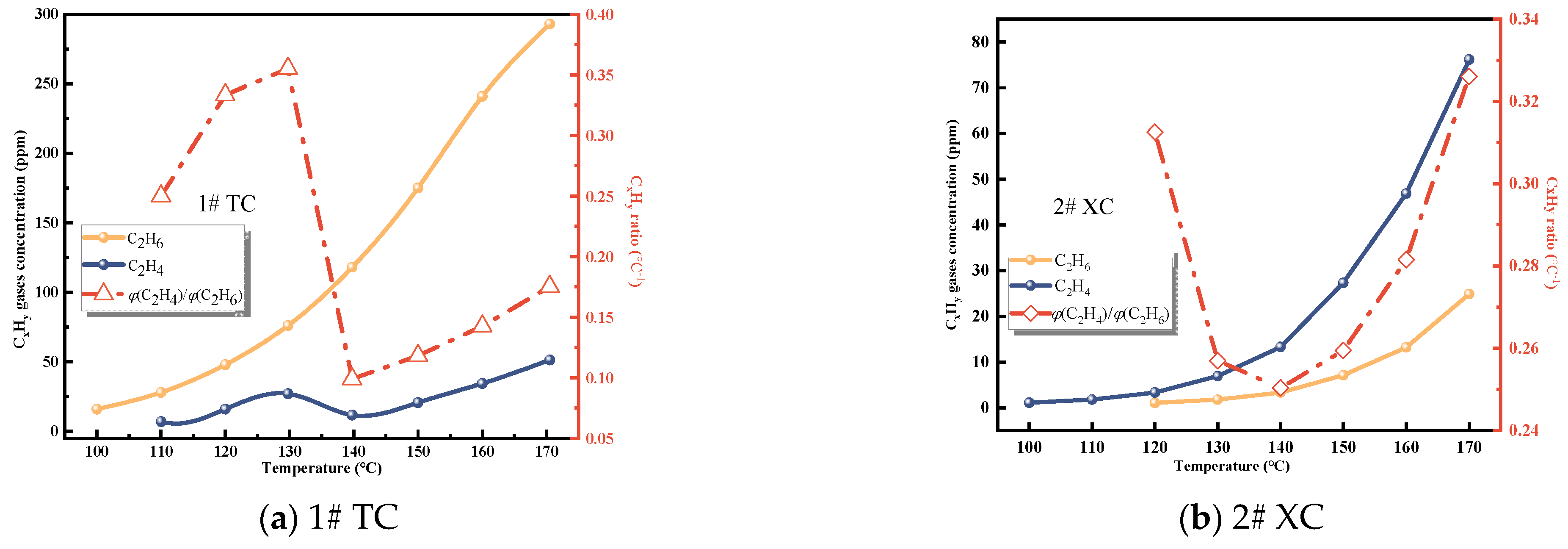
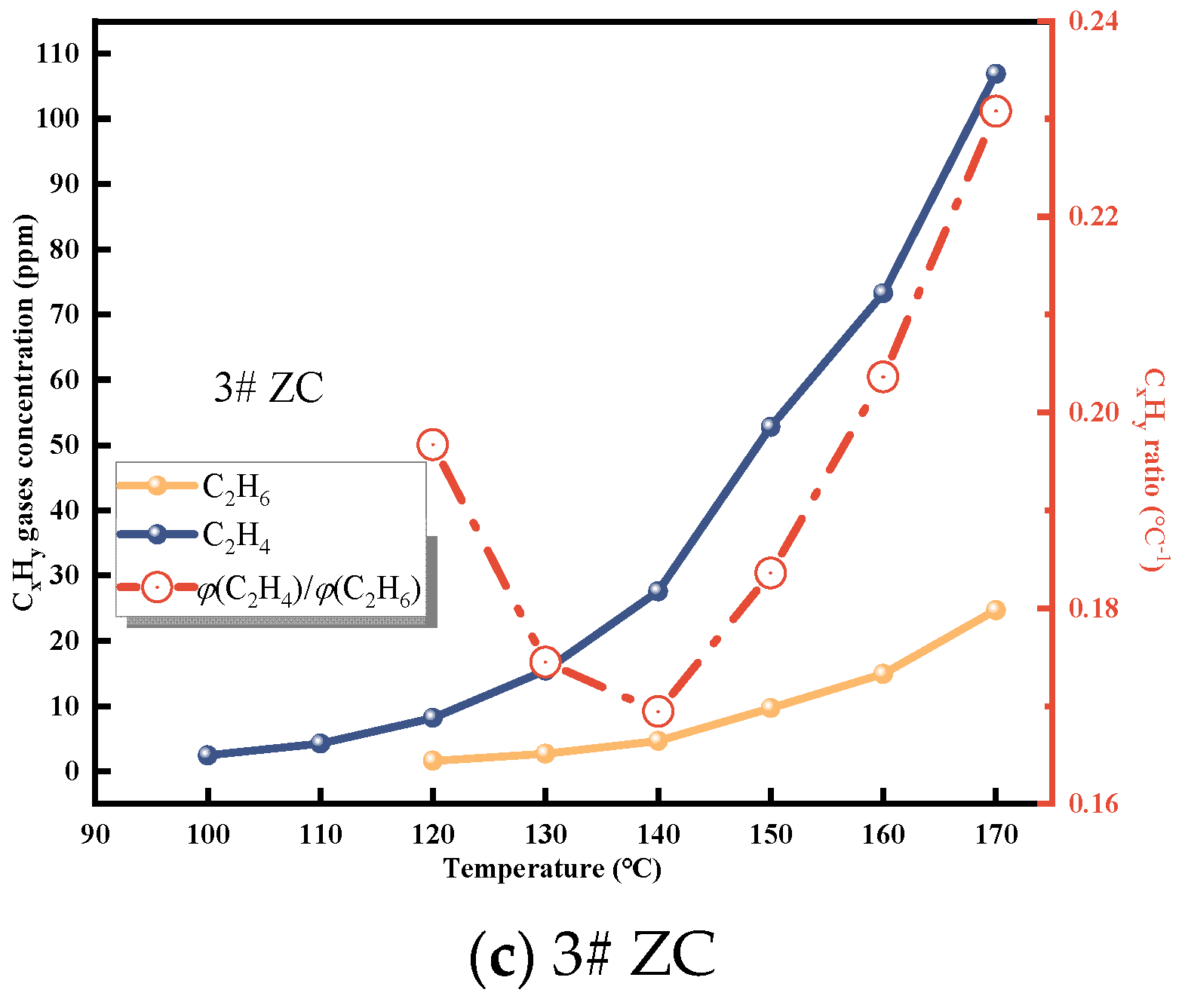
3.1.3. CxHy Gases
3.2. Analysis of Changes in Composite Indicators
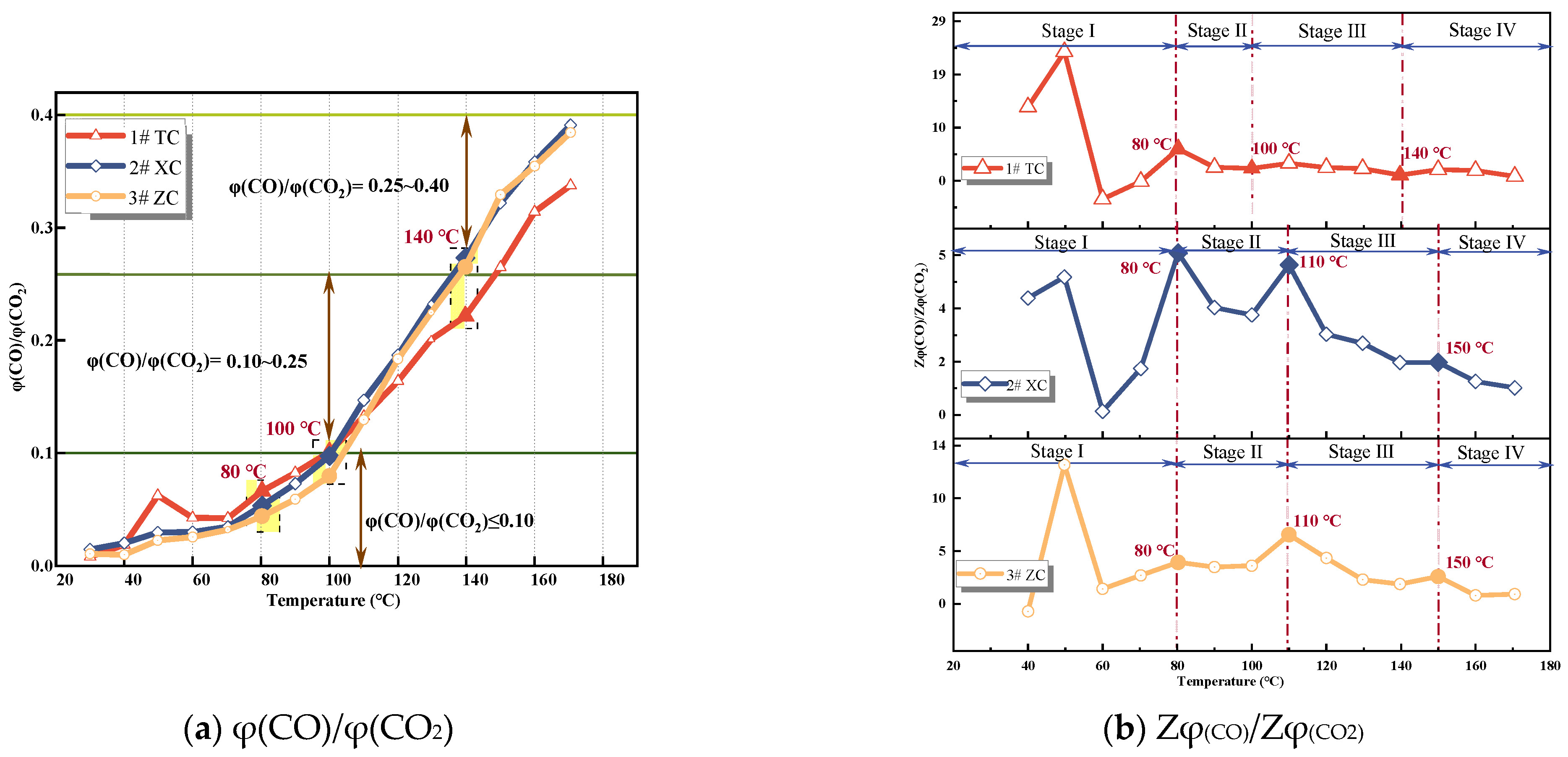
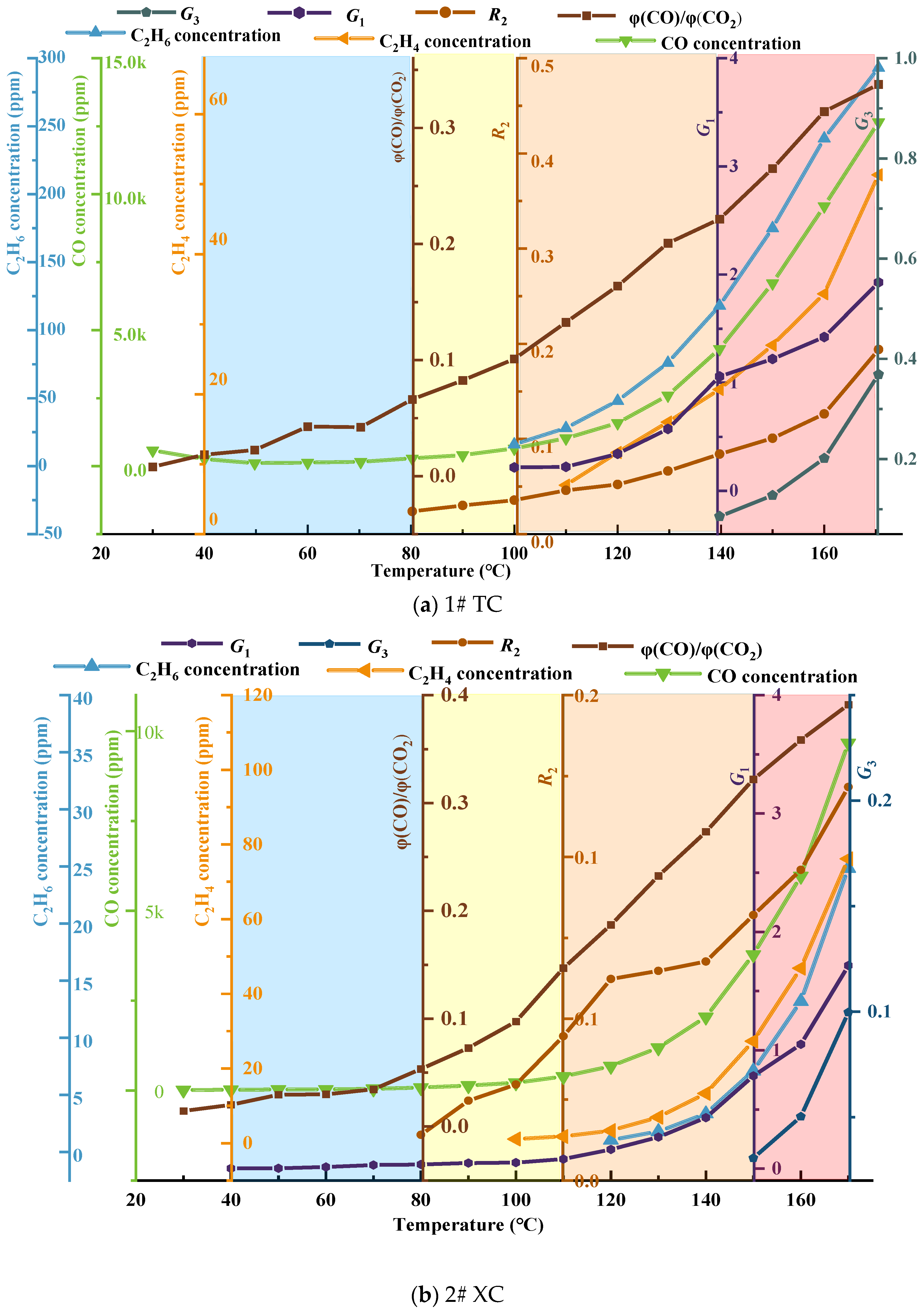
3.2.1. φ(CO)/φ(CO2) and Alkane Ratio
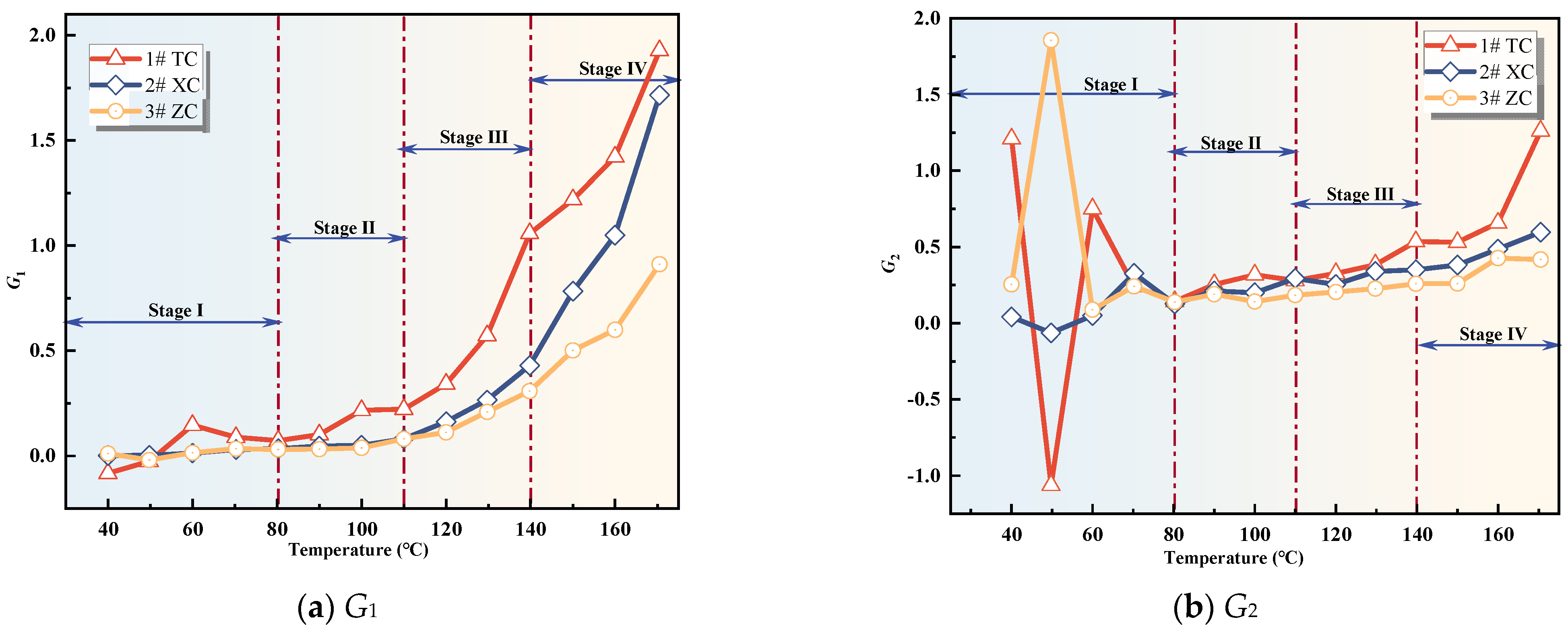
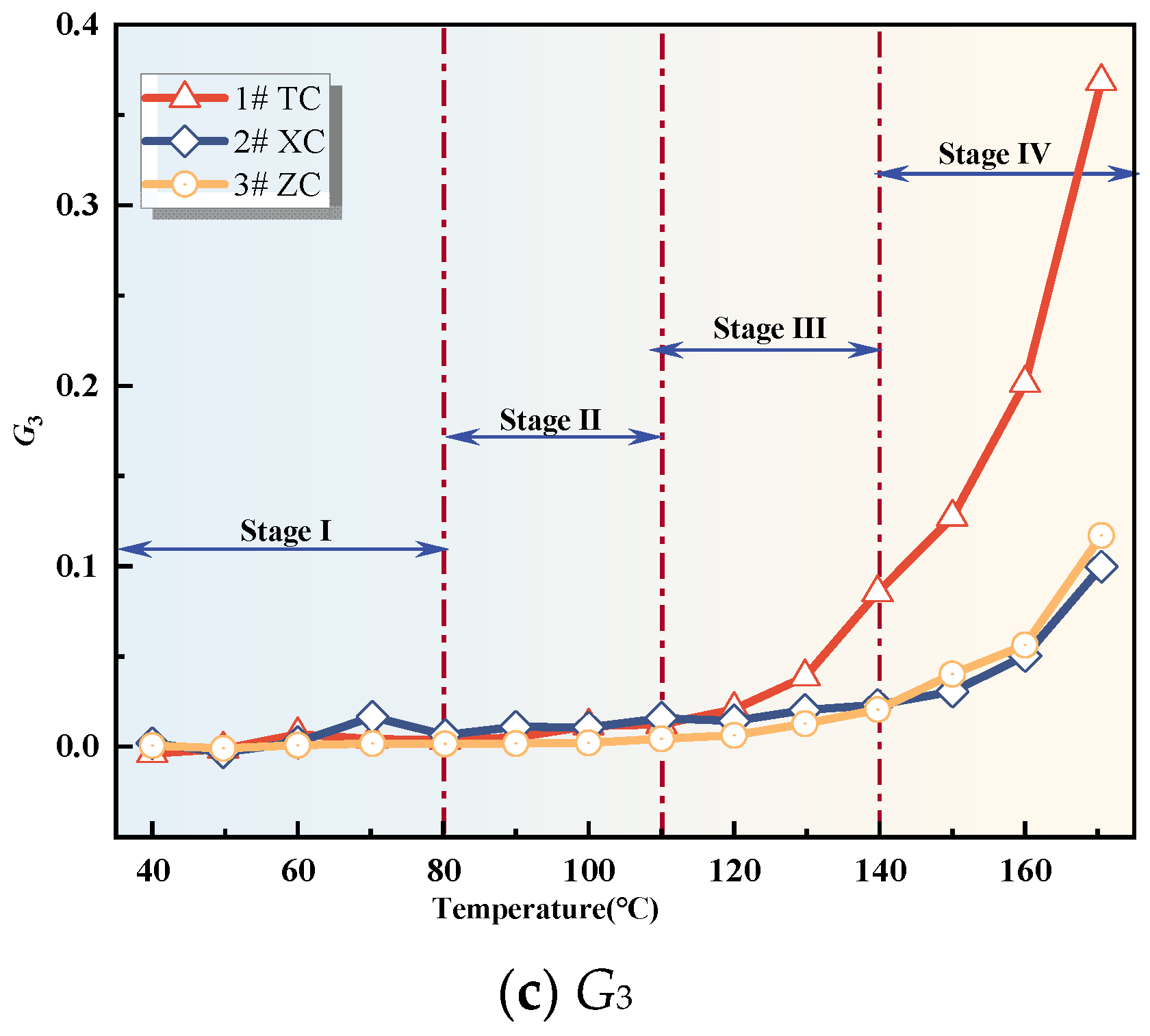
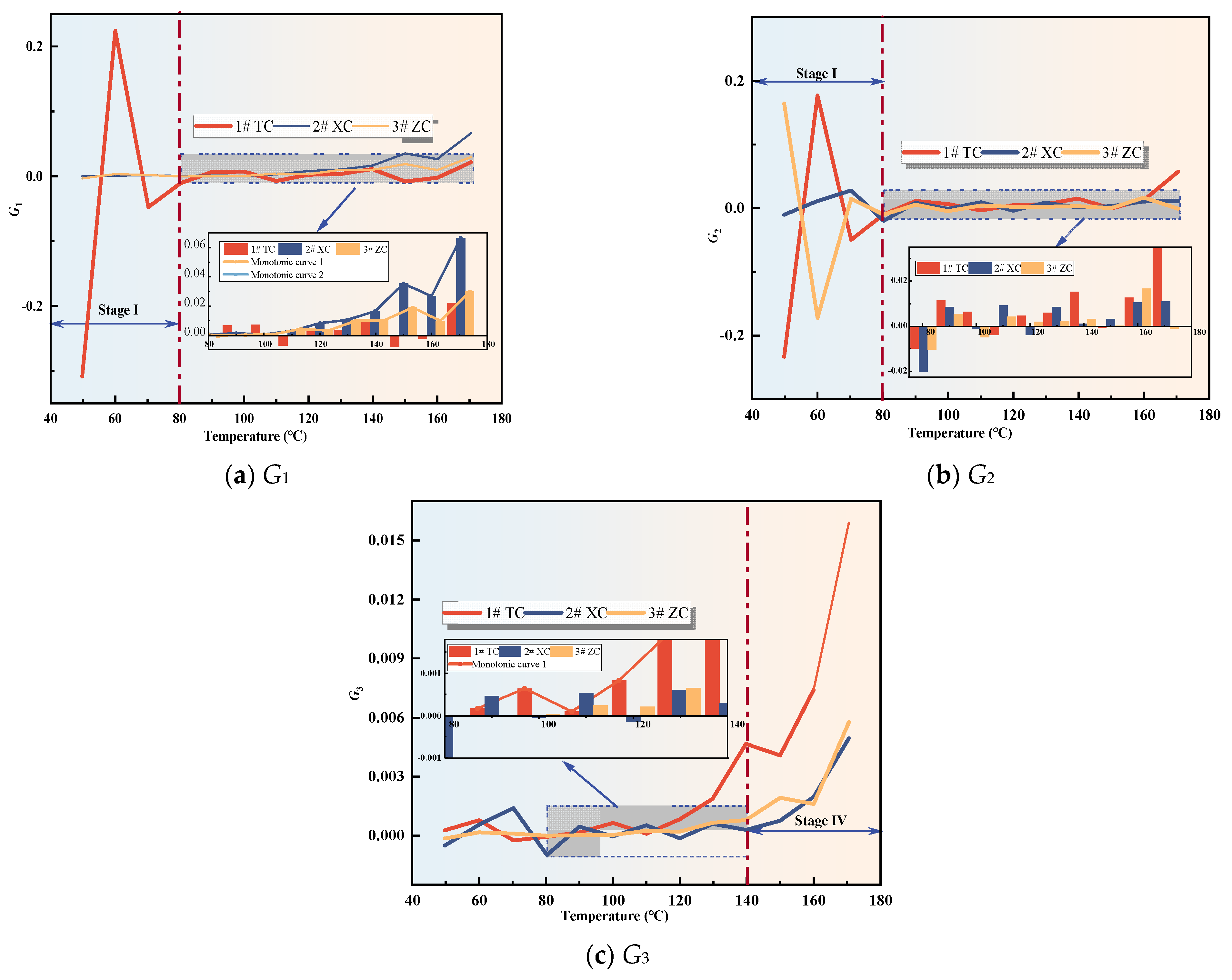
3.2.2. Analysis of Fire Hazard Index Variation
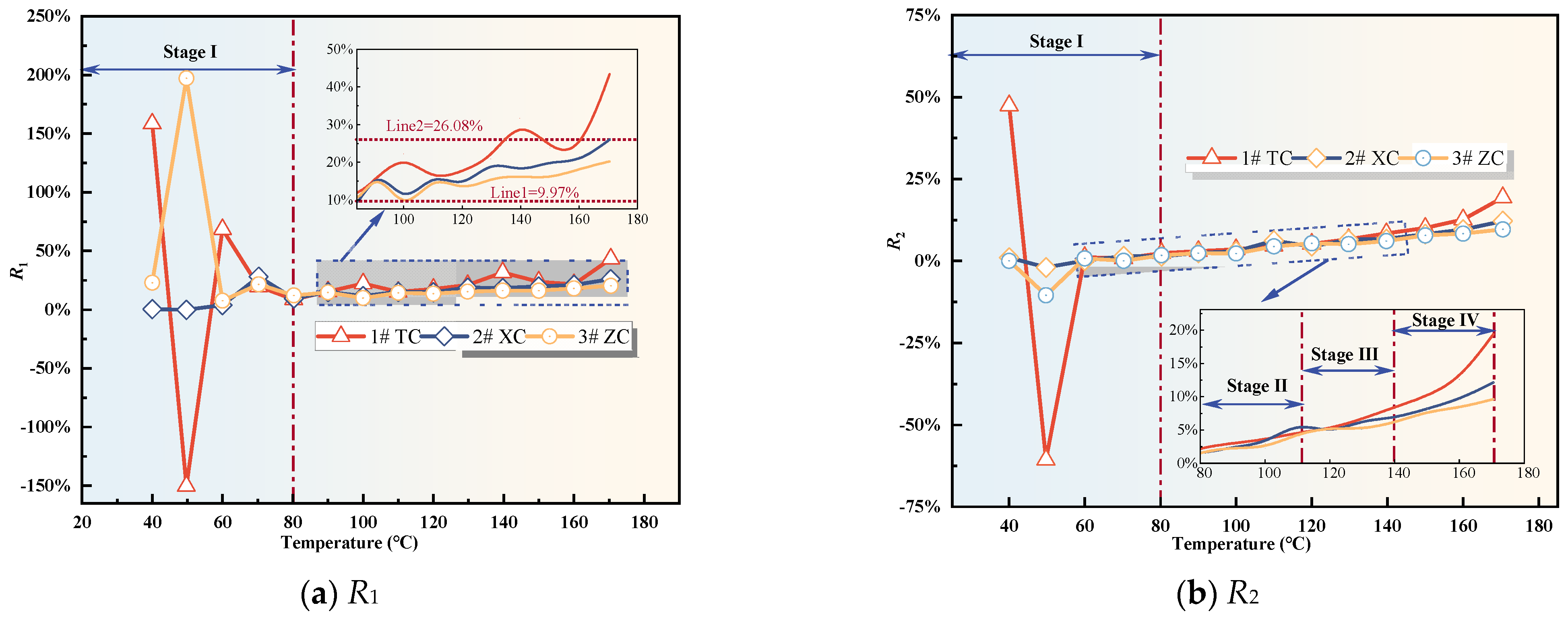
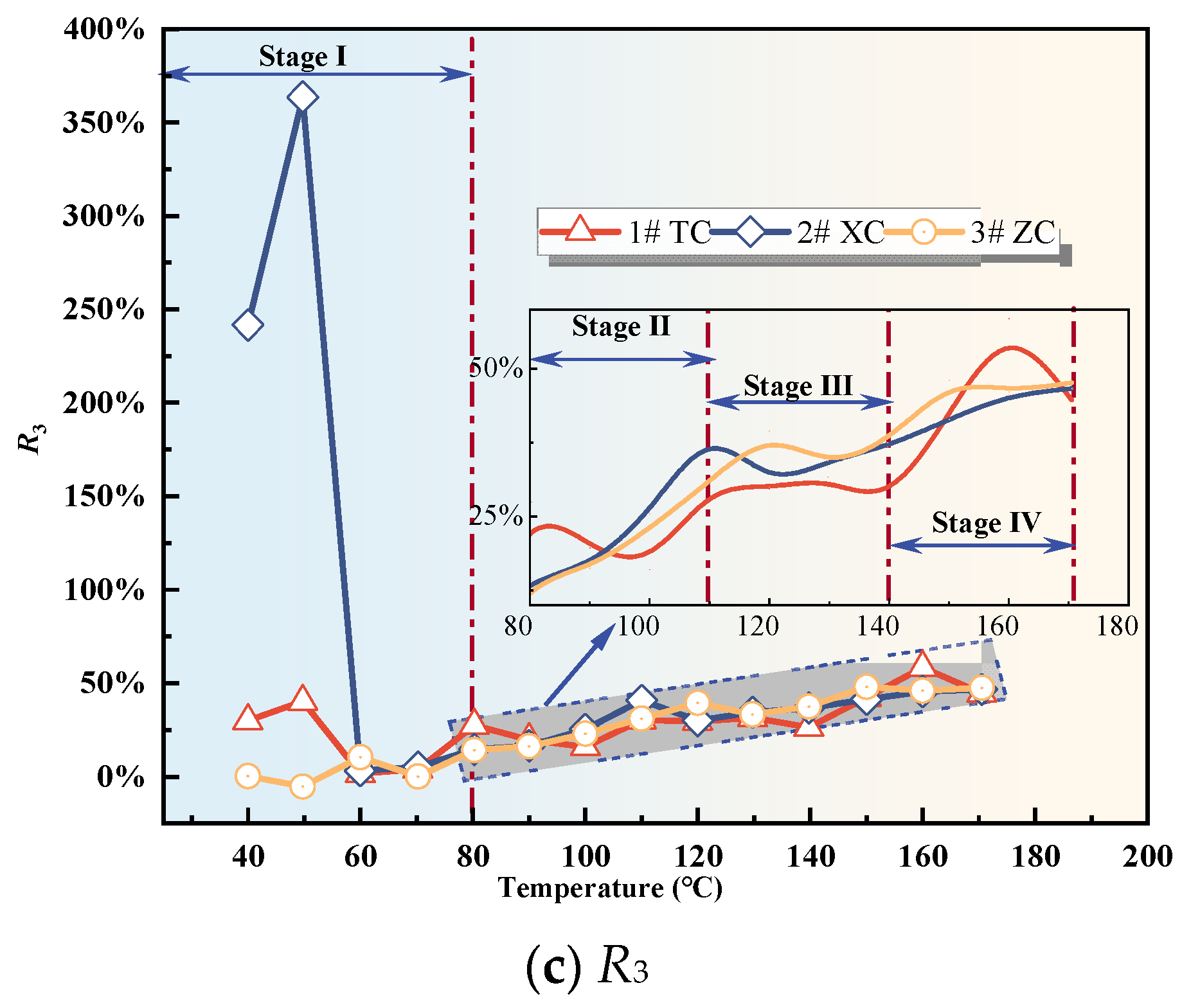
3.2.3. Analysis of Changes in Composite Gas Indicators
3.3. Classification and Warning Indicator System for CSC Hazard
4. Conclusions
- (1)
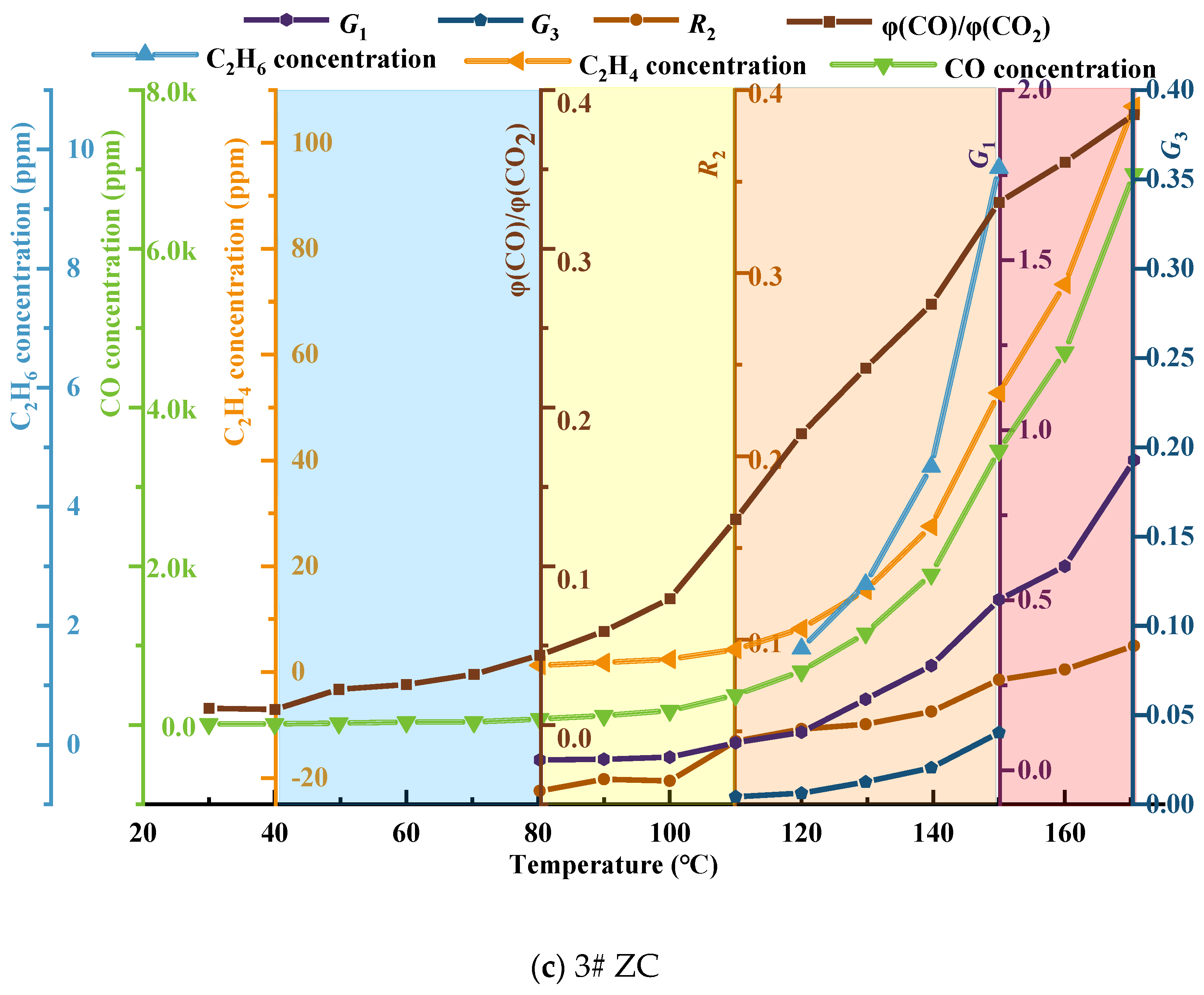
- The growth rate of CO and C2H4 gas concentration was calculated according to the growth rate analysis method and verified by analyzing the growth rate of φ(CO)/φ(CO2); it was determined that the critical temperature of the No. 3 coal seam in the Juye coalfield was near 80 °C, the dry cracking temperature was in the range of 100−110 °C, and the fissure temperature was about 130−150 °C.
- (2)
- The variation of single and composite indicator gases during the coal oxidation process was analyzed, and predictive indicators were selected for different oxidation stages. RCO, R2, G1, and G3 were identified as predictive indicators for Tang Kou coal, New Julong coal, and Zhao Lou coal in the initial oxidation and accelerated oxidation stages. R2, G1, and G3 were selected as predictive indicators in the intense oxidation and oxidative decomposition stages.
- (3)
- Based on the characteristic temperatures of coal and in combination with the carbon oxide ratio, a four-level warning system consisting of blue, yellow, orange, and red levels was established for the No. 3 coal seam in the Juye coalfield. The composite indicator gases G1 and G2, as well as the second fire hazard coefficient R2, were annotated in their respective warning level regions. Along with the concentration curves of CO and C2H6, these indicators were used to construct the threshold curves for the classification and warning of the self-ignition hazard in the No. 3 coal seam of the Juye coalfield.
- (4)
- Based on the refined theory of self-ignition stages in coal, as well as regulations regarding coal self-ignition monitoring, indicative gases, critical values, fire initiation precursors, and fire management, the self-ignition hazard classification and warning system, along with the threshold values, were reconstructed for the No. 3 coal seam in the Juye coalfield. The system was based on the classification criteria and threshold curves for self-ignition risk levels. It established a six-level warning system, including the initial warning level, blue, yellow, orange, red, and black levels, and their respective indicator thresholds, to predict the occurrence of CSC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ni, G.H.; Xie, H.C.; Li, Z.; Zhuansun, L.X.; Niu, Y.Y. Improving the permeabilityof coal seam with pulsating hydraulic fracturing technique: A case study in changping coal mine. Process Saf. Environ. 2018, 117, 565–572. [Google Scholar]
- Chen, X.J.; Li, L.Y.; Wang, L.; Qi, L.L. The current situation and prevention and control countermeasures for typical dynamic disasters in kilometer-deep mines in China. Saf. Sci. 2019, 114, 229–236. [Google Scholar] [CrossRef]
- Li, X.B.; Gong, F.Q.; Tao, M.; Dong, L.J.; Du, K.; Ma, C.D.; Zhou, Z.L.; Yin, T.B. Failure mechanism and coupled static-dynamic loading theory in deep hard rock mining: A review. J. Rock Mech. Geotech. Eng. 2017, 9, 767–782. [Google Scholar] [CrossRef]
- Belle, B.; Biffi, M. Cooling pathways for deep Australian longwall coal mines of the future. Int. J. Min. Sci. Technol. 2018, 28, 865–875. [Google Scholar] [CrossRef]
- Bukowski, P. Water hazard assessment in active shafts in upper silesian coal basin mines. Mine Water Environ. 2011, 30, 302–311. [Google Scholar] [CrossRef]
- Ranjith, P.G.; Zhao, J.; Ju, M.H.; De Silva, R.V.; Rathnaweera, T.D.; Bandara, A.K. Opportunities and challenges in deep mining: A brief review. Engineering 2017, 3, 546–551. [Google Scholar] [CrossRef]
- Pan, R.K.; Ma, Z.H.; Yu, M.G.; Chao, J.K.; Li, C.; Wang, J. Study on the mechanism of coal oxidation under stress disturbance. Fuel 2020, 275, 117901. [Google Scholar] [CrossRef]
- Wang, K.; Li, K.N.; Du, F. Study on Prediction of Coal-Gas Compound Dynamic Disaster Based on GRA-PCA-BP Model. Geofluids 2021, 2021, 3508806. [Google Scholar] [CrossRef]
- Chao, J.K.; Chu, T.X.; Yu, M.G.; Han, X.F.; Hu, D.M.; Liu, W.; Yang, X.L. An experimental study on the oxidation kinetics characterization of broken coal under stress loading. Fuel 2021, 287, 119515. [Google Scholar] [CrossRef]
- Niu, H.Y.; Sun, Q.Q.; Bu, Y.C.; Chen, H.Y.; Yang, Y.X.; Li, S.P.; Sun, S.W.; Mao, Z.H.; Tao, M. Study of the microstructure and oxidation characteristics of residual coal in deep mines. J. Clean. Prod. 2022, 373, 133923. [Google Scholar] [CrossRef]
- Jia, H.; Yang, Y.; Ren, W.; Kang, Z.; Shi, J. Experimental study on the characteristics of the spontaneous combustion of coal at high ground temperatures. Combust. Sci. Technol. 2022, 194, 2880–2893. [Google Scholar] [CrossRef]
- Odintsev, V.N.; Miletenko, N.A. Water inrush in mines as a consequence of spontaneous hydrofracture. J. Min. Sci. 2015, 51, 423–434. [Google Scholar] [CrossRef]
- Wang, K.; Hu, L.H.; Deng, J.; Zhang, Y.N. Multiscale thermal behavioral characterization of spontaneous combustion of pre-oxidized coal with different air exposure time. Energy 2023, 262, 125397. [Google Scholar] [CrossRef]
- Adamus, A.; Šancer, J.; Guřanová, P.; Zubíček, V. An investigation of the factors associated with interpretation of mine atmosphere for spontaneous combustion in coal mines. Fuel Process. Technol. 2011, 92, 663–670. [Google Scholar] [CrossRef]
- Wojtacha-Rychter, K.; Smoliński, A. A study of dynamic adsorption of propylene and ethylene emitted from the process of coal self-heating. Sci. Rep. 2019, 9, 18277. [Google Scholar] [CrossRef]
- Baris, K.; Kizgut, S.; Didari, V. Low-temperature oxidation of some Turkish coals. Fuel 2012, 93, 423–432. [Google Scholar] [CrossRef]
- Onifade, M.; Genc, B. Spontaneous combustion of coals and coal-shales. Int. J. Min. Sci. Technol. 2018, 28, 933–940. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R.V.K.; Singh, M.P.; Chandra, H.; Shukla, N.K. Mine fire gas indices and their application to Indian underground coal mine fires. Int. J. Coal Geol. 2007, 69, 192–204. [Google Scholar] [CrossRef]
- Baris, K.; Aydin, H.; Didari, V. Statistical modeling of the effect of rank, temperature, and particle size on low-temperature oxidation of Turkish coals. Combust. Sci. Technol. 2010, 183, 105–121. [Google Scholar] [CrossRef]
- Kong, B.; Niu, S.Y.; Cao, H.M.; Lu, W.; Wen, J.M.; Yin, J.L.; Zhang, W.R.; Zhang, X.L. Study on the application of coal spontaneous combustion positive pressure beam tube classification monitoring and early warning. Environ. Sci. Pollut. Res. 2023, 30, 75735–75751. [Google Scholar] [CrossRef]
- Wang, C.P.; Deng, Y.; Xiao, Y.; Deng, J.; Shu, C.M.; Jiang, Z.G. Gas-heat characteristics and oxidation kinetics of coal spontaneous combustion in heating and decaying processes. Energy 2022, 250, 123810. [Google Scholar] [CrossRef]
- Gbadamosi, A.R.; Onifade, M.; Genc, B.; Rupprecht, S. Analysis of spontaneous combustion liability indices and coal recording standards/basis. Int. J. Min. Sci. Technol. 2020, 30, 723–736. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Zhang, Y.N.; Deng, J. An approach for evaluation of grading forecasting index of coal spontaneous combustion by temperature-programmed analysis. Environ. Sci. Pollut. Res. 2023, 30, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Misra, S. A review of experimental research on Enhanced Coal Bed Methane (ECBM) recovery via CO2 sequestration. Earth-Sci. Rev. 2018, 179, 392–410. [Google Scholar] [CrossRef]
- Lu, H.; Li, J.L.; Lu, W.; Xu, Z.; Li, J.H.; He, Q.L. Variation laws of CO2/CO and influence of key active groups on it during low-temperature oxidation of coal. Fuel 2023, 339, 127415. [Google Scholar] [CrossRef]
- Wieckowski, M.; Howaniec, N.; Smolinski, A. Natural desorption of carbon monoxide during the crushing of coal simulating natural rock mass pressure. Sci. Total Environ. 2020, 736, 139639. [Google Scholar] [CrossRef]
- Zhou, B.Z.; Yang, S.Q.; Jiang, X.Y.; Cai, J.W.; Xu, Q.; Song, W.X.; Zhou, Q.C. The reaction of free radicals and functional groups during coal oxidation at low temperature under different oxygen concentrations. Process Saf. Environ. Prot. 2021, 150, 148–156. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Sun, L.L.; Liu, Z.Y.; Wang, G.; Ma, J.Y. Effects of air volume and pre-oxidation on re-ignition characteristics of bituminous coal. Energy 2023, 265, 126124. [Google Scholar] [CrossRef]
- Wojtacha-Rychter, K.; Smoliński, A. Coal oxidation with air stream of varying oxygen content and flow rate-Fire gas emission profile. Fire Saf. J. 2020, 116, 103182. [Google Scholar] [CrossRef]
- Liang, Y.T.; Zhang, J.; Wang, L.C.; Luo, H.Z.; Ren, T. Forecasting spontaneous combustion of coal in underground coal mines by index gases: A review. J. Loss Prev. Process Ind. 2019, 57, 208–222. [Google Scholar] [CrossRef]
- Guo, Q.; Ren, W.X.; Lu, W. A Method for Predicting Coal Temperature Using CO with GA-SVR Model for Early Warning of the Spontaneous Combustion of Coal. Combust. Sci. Technol. 2020, 194, 523–538. [Google Scholar] [CrossRef]
- Wang, C.P.; Zhao, X.Y.; Bai, Z.J.; Deng, J.; Shu, C.M.; Zhang, M. Comprehensive index evaluation of the spontaneous combustion capability of different ranks of coal. Fuel 2021, 291, 120087. [Google Scholar] [CrossRef]
- Rua, M.O.B.; Aragon, A.J.D.; Baena, P.B. A study of fire propagation in coal seam with numerical simulation of heat transfer and chemical reaction rate in mining field. Int. J. Min. Sci. Technol. 2019, 29, 873–879. [Google Scholar]
- Zhang, D.; Cen, X.; Wang, W.F.; Deng, J.; Wen, H.; Xiao, Y.; Shu, C.M. The graded warning method of coal spontaneous combustion in Tangjiahui Mine. Fuel 2021, 288, 119635. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Zhang, Y.L.; Song, J.J.; Guo, T.; Deng, J.; Shu, C.M. Oxygen distribution and gaseous products change of coal fire based upon the semi-enclosed experimental system. Energy 2023, 263, 125721. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Ming, H.Q.; Song, J.J.; Lu, S.P.; Xiao, Y.Y.; Zhang, Y.L.; Shu, C.M. Preoptimal analysis of phase characteristic indicators in the entire process of coal spontaneous combustion. J. Loss Prev. Process Ind. 2023, 84, 105131. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, S.G.; Wei, L.; Ying, J.Z.; Cao, Y.J.Z.; Li, J.L. Coal spontaneous combustion characteristics based on constant temperature difference guidance method. Process Saf. Environ. 2019, 131, 223–234. [Google Scholar]

| Coal Samples | Mad (%) | Aad (%) | Vad (%) | FCad (%) |
|---|---|---|---|---|
| 1# TC | 2.16 | 17.02 | 34.20 | 46.62 |
| 2# XC | 1.22 | 24.23 | 34.54 | 40.01 |
| 3# ZC | 1.09 | 8.40 | 34.88 | 55.63 |
| Coal Samples | Oxidation Stage (°C) | T1 (°C) | Accelerated Oxidation Stage (°C) | T2 (°C) | Intense Oxidation Stage (°C) | T3 (°C) | Oxidation Decomposition Stage (°C) |
|---|---|---|---|---|---|---|---|
| 1# TC | 40−80 | 80 | 80−100 | 100 | 100−140 | 140 | 140−170 |
| 2# XC | 40−80 | 80 | 80−110 | 110 | 110−150 | 150 | 150−170 |
| 3# ZC | 40−80 | 80 | 80−110 | 110 | 110−150 | 150 | 150−170 |
| Coal Samples | Characteristic Temperature (°C) | Coal Seam Temperature Range (°C) | Preferred Metrics |
|---|---|---|---|
| 1# TC | 40 | (40−80] | Rco |
| 2# XC | 40 | (40−80] | Rco |
| 3# ZC | 40 | (40−80] | Rco |
| 1# TC | 80 | (80−100] | R2, G1, G3 |
| 2# XC | 80 | (80−110] | R2, G1 |
| 3# ZC | 80 | (80−110] | R2, G1 |
| 1# TC | 100 | (100−140] | R2, G1, G3 |
| 2# XC | 110 | (110−150] | R2, G1 |
| 3# ZC | 110 | (110−150] | R2, G2 |
| 1# TC | 140 | (140−170] | R2, G1, G3 |
| 2# XC | 150 | (150−170] | R2, G1, G3 |
| 3# ZC | 150 | (150−170] | R2, G1, G3 |
| Early Warning Level | Coal Samples | Early Warning Temperature Range (°C) | RCO ((φ(CO)/φ(CO2)) |
|---|---|---|---|
| Blue alert | 1# TC | (40−80] | RΙ ≤ 0.066 |
| 2# XC | (40−80] | RΙ ≤ 0.053 | |
| 3# ZC | (40−80] | RΙ ≤ 0.044 | |
| Yellow alert | 1# TC | (80−100] | 0.066 < RⅡ ≤ 0.101 |
| 2# XC | (80−110] | 0.053 < RⅡ ≤ 0.147 | |
| 3# ZC | (80−110] | 0.044 < RⅡ ≤ 0.130 | |
| Orange alert | 1# TC | (110−140] | 0.101 < RⅢ ≤ 0.221 |
| 2# XC | (110−150] | 0.147 < RⅢ ≤ 0.322 | |
| 3# ZC | (110−150] | 0.130 < RⅢ ≤ 0.329 | |
| Red alert | 1# TC | (140−170] | RⅣ > 0.221 |
| 2# XC | (150−170] | RⅣ > 0.322 | |
| 3# ZC | (150−170] | RⅣ > 0.329 |
| Coal Samples | Characteristic Temperature (°C) | Coal Seam Temperature Range (°C) | Preferred Metrics | Judgment Threshold |
|---|---|---|---|---|
| 1# TC | 40 | (40−80] | Rco | I1 = {RΙ ≤ 0.066} |
| 80 | (80−100] | R2, G1, G3 | I2 = I1 ∩ {0.024 < R2 < 0.036} | |
| 100 | (100−140] | R2, G1, G3, C2H4 | I3 = I2 ∩ {0.036 < R2 < 0.084} ∩ {0.217 < G1 < 1.058} ∩ {0.011 < G3 < 0.081} ∩ {φ(C2H4) > 0} | |
| 140 | (140−170] | R2, G1, G3, C2H4 | I4 = I3 ∩ {0.084 < R2 < 0.194} ∩ {0.085 < G3 < 0.369} ∩{ 11.675 < φ(C2H4) < 51.34} | |
| >170 | φ(C2H4)/φ(C2H6) rapid growth | I5 = I4 ∩ {φ(C2H2) > 0} | ||
| 2# XC | 40 | (40−80] | Rco | I1 = {RΙ ≤ 0.053} |
| 80 | (80−110] | R2, G1 | I2 = I1 ∩ {0.014 < R2 < 0.062} | |
| 110 | (110−150] | R2, G1, C2H6 | I3 = I2 ∩ {0.0062 < R2 < 0.082} ∩ {0.080 < G1 < 0.783} ∩ {φ(C2H6) > 0} | |
| 150 | (150−170] | R2, G1, G3, C2H4 | I4 = I3 ∩ {0.082 < R2 < 0.122} ∩ {0.783 < G1 < 1.716} ∩ {0.036 < G3 < 0.100} ∩ {φ(C2H6) > 0} ∩ {1.777 < φ(C2H4) < 24.84} | |
| >170 | φ(C2H4)/φ(C2H6) rapid growth | I5 = I4 ∩ {φ(C2H2) > 0} | ||
| 3# ZC | 40 | (40−80] | Rco | I1 = {RΙ ≤ 0.044} |
| 80 | (80−110] | R2, G1 | I2 = I1 ∩ {0.017 < R2 < 0.045} ∩ {0.031 < G1 < 0.081} | |
| 110 | (110−150] | R2, G1, G3, C2H6 | I3 = I2 ∩ {0.045 < R2 < 0.078} ∩ {0.081 < G2 < 0.502} ∩ {φ(C2H4) > 0} | |
| 150 | (150−170] | R2, G1, C2H4 | I4 = I3 ∩ {0.078 < R2 < 0.097} ∩ {0.502 < G1 < 0.912} ∩ {φ(C2H4) > 0} ∩ {0.184 < φ(C2H4)/φ(C2H6) < 0.231} | |
| >170 | φ(C2H4)/φ(C2H6) rapid growth | I5 = I4 ∩ {φ(C2H2) > 0} |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Du, Y.; Deng, Y.; Zhang, Y.; Deng, J.; Zhao, X.; Duan, X. Study on Spontaneous Combustion Characteristics and Early Warning of Coal in a Deep Mine. Fire 2023, 6, 396. https://doi.org/10.3390/fire6100396
Wang C, Du Y, Deng Y, Zhang Y, Deng J, Zhao X, Duan X. Study on Spontaneous Combustion Characteristics and Early Warning of Coal in a Deep Mine. Fire. 2023; 6(10):396. https://doi.org/10.3390/fire6100396
Chicago/Turabian StyleWang, Caiping, Yuxin Du, Yin Deng, Yu Zhang, Jun Deng, Xiaoyong Zhao, and Xiadan Duan. 2023. "Study on Spontaneous Combustion Characteristics and Early Warning of Coal in a Deep Mine" Fire 6, no. 10: 396. https://doi.org/10.3390/fire6100396
APA StyleWang, C., Du, Y., Deng, Y., Zhang, Y., Deng, J., Zhao, X., & Duan, X. (2023). Study on Spontaneous Combustion Characteristics and Early Warning of Coal in a Deep Mine. Fire, 6(10), 396. https://doi.org/10.3390/fire6100396






