Abstract
The combination of drought and fire can cause drastic changes in forest composition and structure. Given the predictions of more frequent and severe droughts and forecasted increases in fire size and intensity in the western United States, we assessed the impact of drought and different fire intensities on Pinus ponderosa saplings. In a controlled combustion laboratory, we exposed saplings to surface fires at two different fire intensity levels (quantified via fire radiative energy; units: MJ m−2). The recovery (photosynthesis and bud development) and mortality of saplings were monitored during the first month, and at 200- and 370-days post-fire. All the saplings subjected to high intensity surface fires (1.4 MJ m−2), regardless of the pre-fire water status, died. Seventy percent of pre-fire well-watered saplings recovered after exposure to low intensity surface fire (0.7 MJ m−2). All of the pre-fire drought-stressed saplings died, even at the lower fire intensity. Regardless of the fire intensity and water status, photosynthesis was significantly reduced in all saplings exposed to fire. At 370 days post-fire, burned well-watered saplings that recovered had similar photosynthesis rates as unburned plants. In addition, all plants that recovered or attempted to recover produced new foliage within 35 days following the fire treatments. Our results demonstrate that the pre-fire water status of saplings is an important driver of Pinus ponderosa sapling recovery and mortality after fire.
1. Introduction
Since the start of the 21st Century, studies have documented widespread forest die-off caused by heat stress and droughts [1,2,3]. Although high temperatures associated with severe droughts are of considerable concern in relation to tree mortality, wildfires are also predicted to increase in frequency, size, and intensity in response to anthropogenic climate change [4,5,6]. This combination of prolonged drought events and wildfires can cause drastic changes in forest cover [7,8]. To forecast tree mortality caused by drought or fire, forest managers rely on tree mortality models, such as components within LANDIS-II [9] and First Order Fire Effects Model (FOFEM) [10]. However, the majority of these models are empirical, and have been developed from only a limited number of species and tree ages [11,12], are often logistic regressions that produce only a binary live/dead prediction [13], and lack the mechanistic underpinnings required to make them robust to changing climate conditions or transferable across geographical regions [14,15]. Although some Earth system models, such as the community land model, have built-in mechanistic relations of drought induced tree mortality, fire-induced mortality is still modelled via the non-mechanistic SPITFIRE model, using bark thickness derived from diameter measurements and fire duration [16,17,18]. Some Earth systems models, such as BioEarth [19], have ecohydrological components, including Regional Hydro-Ecological Simulation System (RHESSys) [20,21], which can allow a mechanistic understanding of fire-effects to be directly incorporated into the modeling frameworks.
An alternative to improving fire severity and plant mortality predictions is the use of controlled combustion experiments to create or refine process-based models that include the physiological conditions of plants pre-fire, such as water stress, and the physiological responses after the fire [14,22]. Recent syntheses have highlighted that such ‘pyroecophysiology’ approaches have considerable potential to improve predictions of post-fire tree recovery or mortality [15,23]. Although several studies have assessed how discrete fire intensity levels impact the probability of mortality and post-fire growth over a range of spatiotemporal scales [14,24,25,26,27,28,29,30], limited studies have evaluated the interaction between drought and subsequent fire intensity on tree physiology and mortality [27].
In general, trees under drought stress are believed to exhibit increased vulnerability to fire-induced mortality [24,31]. During a water shortage, trees can close their stomata for extended durations to lower the risk of cavitation in xylem tissues [32,33], thereby reducing CO2 uptake and assimilation [34,35]. Trees that close their stomata for long periods of time can deplete their non-structural carbohydrate (NSC) reserves, which are critical for metabolic maintenance and other functions [33,36,37]. When trees are exposed to fire, these NSC reserves are used to recover from heat-induced injuries in the foliage, bole and roots [38]. Drought followed by fire is therefore hypothesized to compound the depletion of NSC reserves, leading to a higher probability of tree mortality [27].
Independent of drought stress, many potential mechanisms of fire-induced tree mortality exist [31]. One proposed mechanism is the failure of water transport due to xylem embolism [39] and deformation of xylem conduits [40,41,42]. Fire is also thought to lead to tree mortality through damage to the meristematic tissues and tree crown [27], where severe crown damage can significantly reduce photosynthesis [14,24] forcing the plants to rely on their reserves (i.e., NSC and other nutrients) to recover. Severe crown damage can also cause death of buds, preventing the plant from regrowing foliage [38], particularly in non-resprouting species. During the fire, heat can be transferred through the bark leading some studies to propose that mortality could arise through damage to the phloem and vascular cambium [43,44]. If the phloem is damaged, the movement of sugars would be impaired and, if severe enough, could eventually result in tree mortality [42].
Although numerous studies have focused on how fires kill trees [15,23,31], the compounded effects of drought stress and fire on mortality and recovery of surviving trees are not well understood [7,27,45]. Studies in temperate [46] and tropical forests [47,48] have observed that pre-fire drought stress significantly amplifies tree mortality in subsequent fires. Complex interactions have also been observed between drought and fire in some species. Prior studies [27] showed that Larix occidentalis saplings subjected to severe drought stress that caused the foliage to senesce prior to exposure to low-intensity surface fires exhibited increased bud counts and lower mortality rates 1-year post fire, compared to moderately drought-stressed saplings. Other studies have observed no mortality in drought-stressed resprouting species when subjected to surface fires [49,50]. Complex fire-drought interactions are also apparent when fire follows droughts in variable density forests. For example, one study [51] showed that mature trees of Pinus lambertiana and Pinus ponderosa exhibited significantly higher rates of mortality in a subsequent severe drought, compared to their fire-affected counterparts that benefited from less competition for resources.
In this study, we build off [27] and assess the influence of pre-fire water status on post-fire tree mortality and the effects of different fire intensity levels on Pinus ponderosa sapling physiology by testing the following hypotheses: (1) Pinus ponderosa saplings subjected to pre-fire drought stress are more likely to die than well-watered saplings; and (2) Pinus ponderosa saplings subjected to pre-fire drought stress are less likely to develop new foliage than well-watered saplings post-fire. We further seek to assess how the interaction of fire and drought on Pinus ponderosa saplings compares to that observed in a similar study [27] on Larix occidentalis saplings. Finally, we compare and seek to confirm the fire intensity levels required to cause mortality in similarly sized Pinus ponderosa saplings that were reported in an earlier study [29]. Following past studies [24,29], Pinus ponderosa saplings were used given under projected fire regimes, fires will likely occur more frequently leading to an increased incident of fire effects on younger cohorts. This knowledge will also be of value to help land managers seeking to predict how many of such younger trees, whether planted or grown through natural regeneration, will need to be replanted following landscape fires [29]. In addition, conducting experiments on young trees enables a worst-case scenario to be assessed given they are less likely to have developed fire-resistant morphological traits observed in mature trees [52].
2. Materials and Methods
2.1. Pinus Ponderosa Saplings and Study Treatments
A total of 60 Pinus ponderosa saplings were purchased from Franklin H. Pitkin Forest Nursery at the University of Idaho, Moscow, ID and potted in 3.7 L pots with 50% Sungro@ Professional Growing mix media and 50% perlite. We follow the nomenclature in a recent study [53] and use the term ‘saplings’, as the Pinus ponderosa used in this study were not in the emergent or established stage and were not producing seeds. The saplings were grown in a climate-controlled greenhouse at University of Idaho for 6 months. The temperature average in the greenhouse ranged between 15 to 25 ℃, with a photoperiod of ~16 hours. During this period, all saplings were watered to field capacity every other day and fertilized once a month with a 200 ppm solution of N,P,K (20:20:20) Technigro@ fertilizer. At the time of the fire treatments root collar diameter and mean height (±SE) were 1.11 ± 0.01 cm and 0.44 ± 0.06 m, respectively.
In fall 2017, saplings were randomly placed in two water status treatments: well-watered (n = 30) and drought-stressed (n = 30). Saplings in the well-watered treatment were watered every other day to field capacity, to avoid water stress. For drought-stressed saplings, we targeted the drought treatment, so that saplings would reach a predawn leaf water potential (Ψp) equal or more negative than −1.6 MPa just prior to the fire experiment. This Ψp coincides with previous minimum water potentials measured on Pinus ponderosa saplings during an abnormally dry summer in Idaho [54], and also corresponds to the xylem tension resulting in the onset of embolism in Pinus ponderosa saplings [30]. Water was withheld from all saplings in the drought-stress treatment until the desired Ψp was reached. All water potential measurements were conducted using a pressure chamber (PMS Instruments Co., Albany, OR, USA).
Following the drought treatments, saplings in each water status treatment were randomly divided into three fire treatments: (1) unburned, (2) subjected to surface fires with fire radiative energy (FRE) of 0.7 MJ m−2, and (3) subjected to surface fires with FRE of 1.4 MJ m−2. FRE has been used in past studies assessing the impacts of fire intensity levels on plant physiology responses [24,25,26,27,28,29,30]. The use of FRE, as opposed to the maximum fire radiative power [26], enables a readily repeatable stratification of the experiment into discrete fire intensity levels. FRE is also widely used in Earth system science, as it is directly proportion to the total quantity of biomass consumed [55,56]. Although maximum and integrated temperatures with time were not measured in this study given they are not valid descriptors of heat [14,57], maximum bark surface temperatures between 750 and 1050 K, for 0.4 MJ m−2 and 1.2 MJ m−2 FRE fires, have been observed in companion sapling experiments to occur up to durations of a few seconds [24].
Each water status × fire treatment had 10 replicates. The number of Pinus ponderosa saplings used in each treatment and the fire intensity levels were selected following preliminary data from Pinus ponderosa saplings burned at 0.7 m−2 (n = 5) and 1.0 MJ m−2 (n = 5) [29]. Saplings were subjected to the surface fire treatments under controlled conditions at the Idaho Fire Initiative for Research and Education (IFIRE), which is a climatically controlled indoor combustion laboratory [56,58,59]. Following established methods to minimize pseudoreplication [24,25,26,27,28,29,30], saplings were individually placed in a custom-cut concrete board with each sapling’s soil leveled with the board. Following [31], we created a relation between dry (0% moisture content) pre-fire fuel load of Pinus ponderosa needles and FRE as measured via dual-band thermometry [13]. Specifically, a linear regression between FRE and fuel consumption of Pinus ponderosa needles (kg m−2) was determined using 10 burned pure fuel beds at ~0% moisture content, where FRE (MJ) = 5.74 × fuel consumed (kg), where mean consumption completeness was ~80%. Based on this information, to generate FRE levels of ~0.7 and ~1.4 MJ m−2, 152g and 304 g of oven dried (<1% fuel moisture content) Pinus ponderosa needles were used as the fuelbed [30]. Flame height was similar to that reported in [29], where at 0.7 MJ m−2 it 0.7 ranged between 0.025 and 0.12 m and at 1.4 MJ m−2 it ranged between 0.06 and 0.16m. Dried needles were evenly distributed in a 1 m2 circular area around the saplings and the fuel gently tamped down with a flat metal board to ensure similar bulk densities between fire treatment replicates. Each fuel bed was ignited on one side using ~2 g of ethanol [29]. After the burns, all saplings were returned to the greenhouse, and watered to field capacity every other day until the end of the experiment.
2.2. Physiological Measurements
On the day prior to sapling exposure to fire, Ψp and photosynthesis (A) were measured in 10 randomly chosen well-watered and 8 randomly chosen drought-stressed saplings. After the fire treatments, A was measured at 1, 14, 27, and 370 days post-fire on 5 saplings in each water status/fire treatment (Table 1). A was measured between 8:00 to 11:00 in the morning using a LICOR 6400XT portable photosynthesis system (LI-COR, Lincoln, NE) with a photosynthetic photon flux density of 1400 µmol m−2 s−1, CO2 concentration of 400 ppm, temperature of ~25 ℃, and relative humidity of ~37%.

Table 1.
List of parameters measured.
2.3. Bud Development and Sapling Mortality
New foliage was evaluated at 27, 35, 68, 125, 141, and 200 days post-fire (Table 1). At each evaluation day we counted the number of the buds that flushed. Here, we define bud flush as the emergence and subsequent growth of new leaves. The number of buds per sapling (bud density) and status (live, dying, and dead) were recorded. ‘Live’ buds were defined as those having green bud tissue and needles, ‘dying’ when some of the new needles started turning brown, and ‘dead’ when all needles that had emerged from the bud were brown. Sapling mortality was assessed at 200 days post-fire (Table 1), and was defined as the death of all foliage, vascular cambium, and phloem and the inability to regenerate new shoots. To confirm the death of vascular cambium and phloem, all saplings with dead foliage and no development of new shoots were harvested, and if the tissue between the bark and xylem (i.e., phloem and cambium cells) did not have a green coloration, then the cambium and phloem were considered dead.
2.4. Statistical Analysis
Before saplings were exposed to the fire treatments, we tested for significant differences in Ψp and A between well-watered and drought-stressed treatments using Student’s t-test. To test the fire effects on Ψp and A in the following days post-fire, we used a mixed effects model with saplings as random effects. Tukey’s HSD post-hoc test was used to assess the differences in gas exchange and Ψp between treatments. We also assessed the differences in bud density between the treatments for each post-fire sampling date using ANOVA, and, if significant (α = 0.05), a Tukey’s HSD post-hoc test. R version 3.6.0 (R Core Team) and the packages ‘lme4’ [60] and ‘emmeans’ [61] were used to conduct the data analysis.
3. Results
3.1. Sapling Physiology
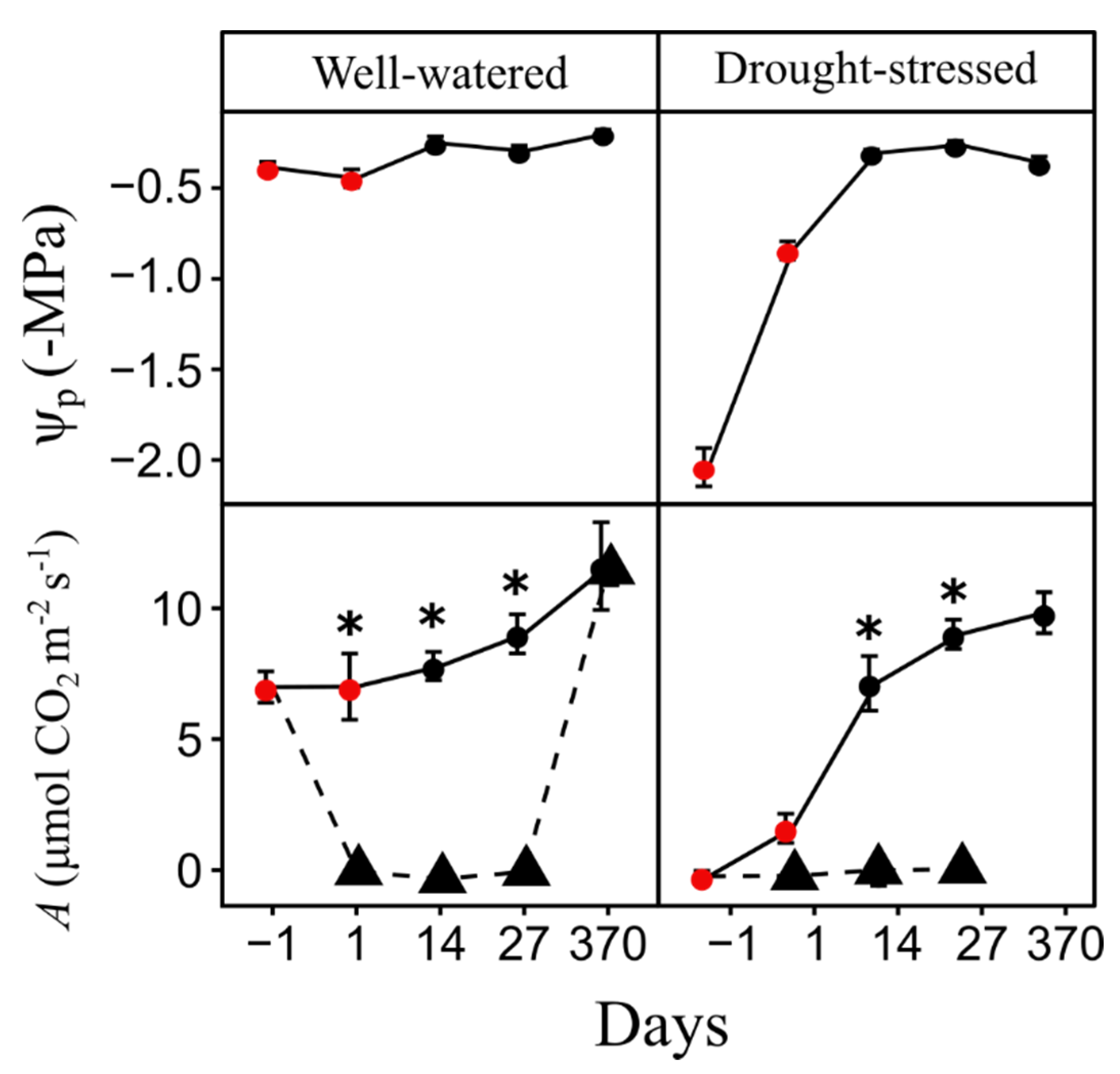
The day before saplings were exposed to the fire, Ψp in drought-stressed saplings was more negative (−2.04 ± 0.03 MPa) than well-watered saplings (−0.39 ± 0.1 MPa; P < 0.0001). A in well-watered saplings was six times greater than drought-stressed saplings (P < 0.0001, Figure 1) at the day before the fire treatments. Ψp was measured post-fire in saplings exposed to 0.7 MJ m−2, however, we recognize that fire-damaged foliage may have altered leaf balance pressures due to potential osmotic effects (i.e., cell death). Thus, we only report Ψp for unburned well-watered and drought-stressed saplings when regular watering resumed. Drought-stressed, unburned sapling Ψp was lower (−0.84 ± 0.05 MPa) than well-watered saplings (−0.45 ± 0.05 MPa) one-day post-fire (P < 0.0001), but not significantly different at 14 (P = 0.553), 27- (P = 0.709) and 370 (P = 0.07) days post-fire. A was significantly reduced (P < 0.0001) in well-watered saplings burned at 0.7 MJ m−2 one day post-fire, with values approaching zero, compared to unburned well-watered saplings and remained near zero at 27 days post-fire (Figure 1). However, at 370 days post-fire, saplings burned at 0.7 MJ m−2 had similar A to unburned saplings (Figure 1). A comparison between drought-stressed unburned and burned saplings in the 0.7 MJ m−2 fire treatment showed that A was similar one-day after the fire but differed at 14 (P < 0.0001) and 27 (P < 0.0001) days post-fire (Figure 1). All saplings exposed to the 1.4 MJ m−2 fire treatment had significant foliage loss, due to partial crown combustion, and subsequently died, thus, measurements of post-fire Ψp and A were not possible.
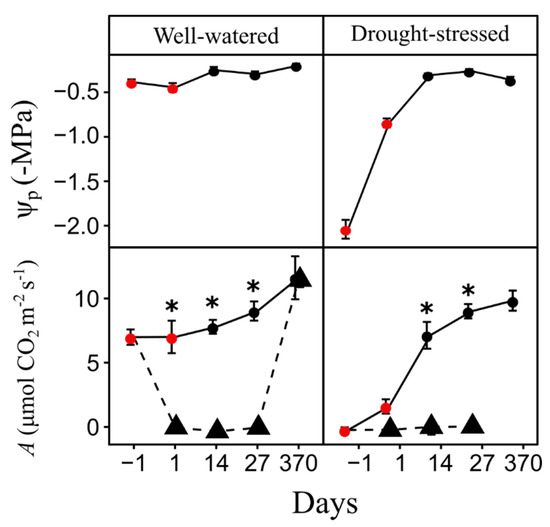
Figure 1.
Mean predawn leaf water potential (Ψp) and photosynthesis (A) in unburned (circle) and burned (triangle) Pinus ponderosa saplings. Ψp values are only reported for the unburned treatment and A data shows results for control and 0.7 MJ m−2 treatments. Asterisks denote days where Ψp and A significantly differed (*P < 0.05) between unburned and burned saplings in each water status treatment. Red circles denote days where Ψp and A significantly differed (P < 0.05) between well-watered and drought-stressed saplings in each fire treatment. Bars are SE.
3.2. Bud Development and Sapling Mortality
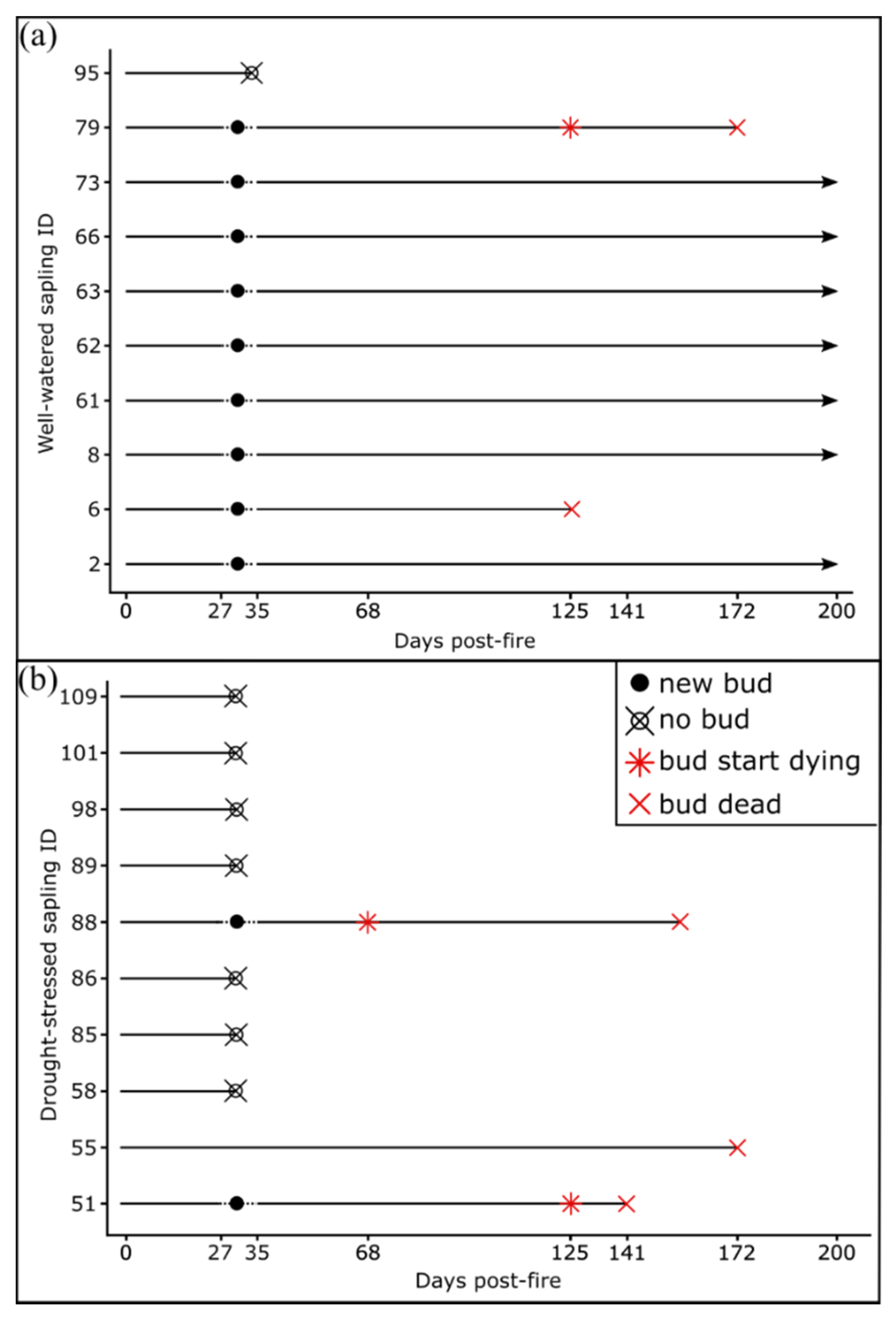
Newly flushing buds were observed in saplings burned at 0.7 MJ m−2 between 27- and 35- days post-fire (Figure 2). No saplings exposed to 1.4 MJ m−2 surface fires flushed any buds within 35 days. Of the 10 well-watered saplings exposed to 0.7 MJ m−2 surface fires, nine of them had flushed buds (Figure 2). However, for sapling 6 (Figure 2a) newly developed leaves were dead by 125 days post-fire, while the new leaves of sapling 79 (Figure 2a and Figure 3) started dying at 125 days, and were dead by 172 days post-fire. Two drought-stressed saplings burned at 0.7 MJ m−2 flushed buds within 35 days post-fire (Figure 2b). However, the new leaves started dying at 68 and 125 days post-fire, and by 141 days post-fire, all new leaves for both saplings were dead (Figure 2b).
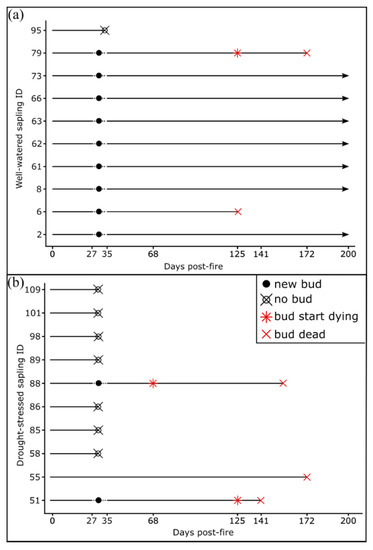
Figure 2.
Bud emergence, development and mortality of (a) well-watered and (b) drought-stressed Pinus ponderosa saplings burned at 0.7 MJ m−2. All saplings burned at 1.4 MJ m−2 did not have ‘new buds’ by day 35 post-fire. ‘New buds’ indicates saplings with at least one flushed bud between day 27 and 35, ‘bud start dying’ indicates buds where new leaves started turning brown and ‘bud dead’ indicates buds where all the new leaves died. All saplings were watered one day before day 0. The drought-stressed sapling ID 55 burned at 0.7 MJ m−2 did not flush any new buds but retained some green foliage post-fire.

Figure 3.
Bud and foliage mortality between 125 and 172 days post-fire for two example Pinus ponderosa saplings burned at 0.7 MJ m−2. Bud mortality was observed in a well-watered sapling (sapling ID 79) and crown mortality in a drought-stressed sapling (sapling ID 55). Numbers above each panel represent days post-fire.
Although the percentage of green crown remaining after the fire was not assessed in this study, we observed that the saplings which retained a green crown after the fire did not flush new buds, while the saplings that had the majority of their crown damaged flushed new buds (Figure 3, sapling 55).
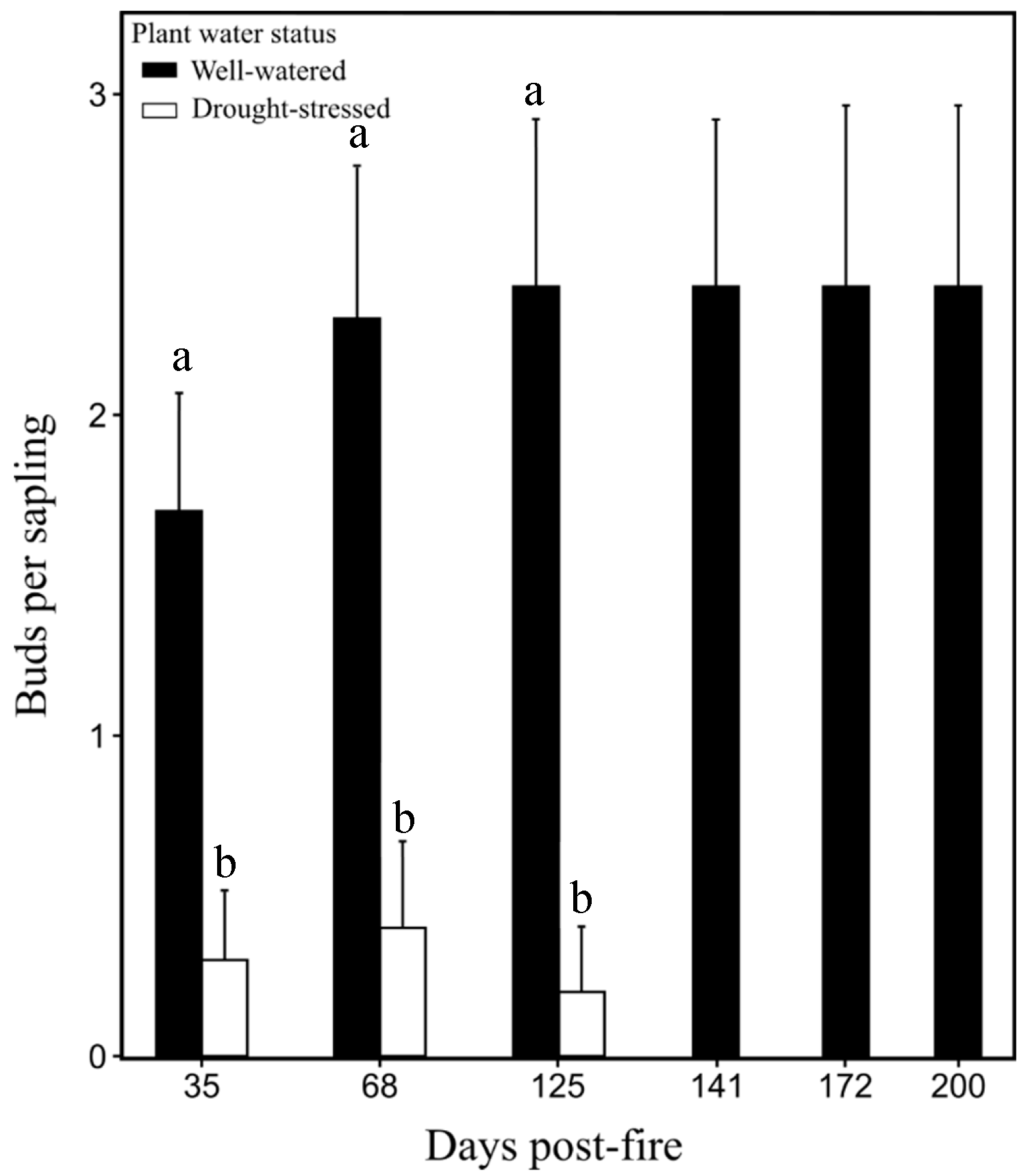
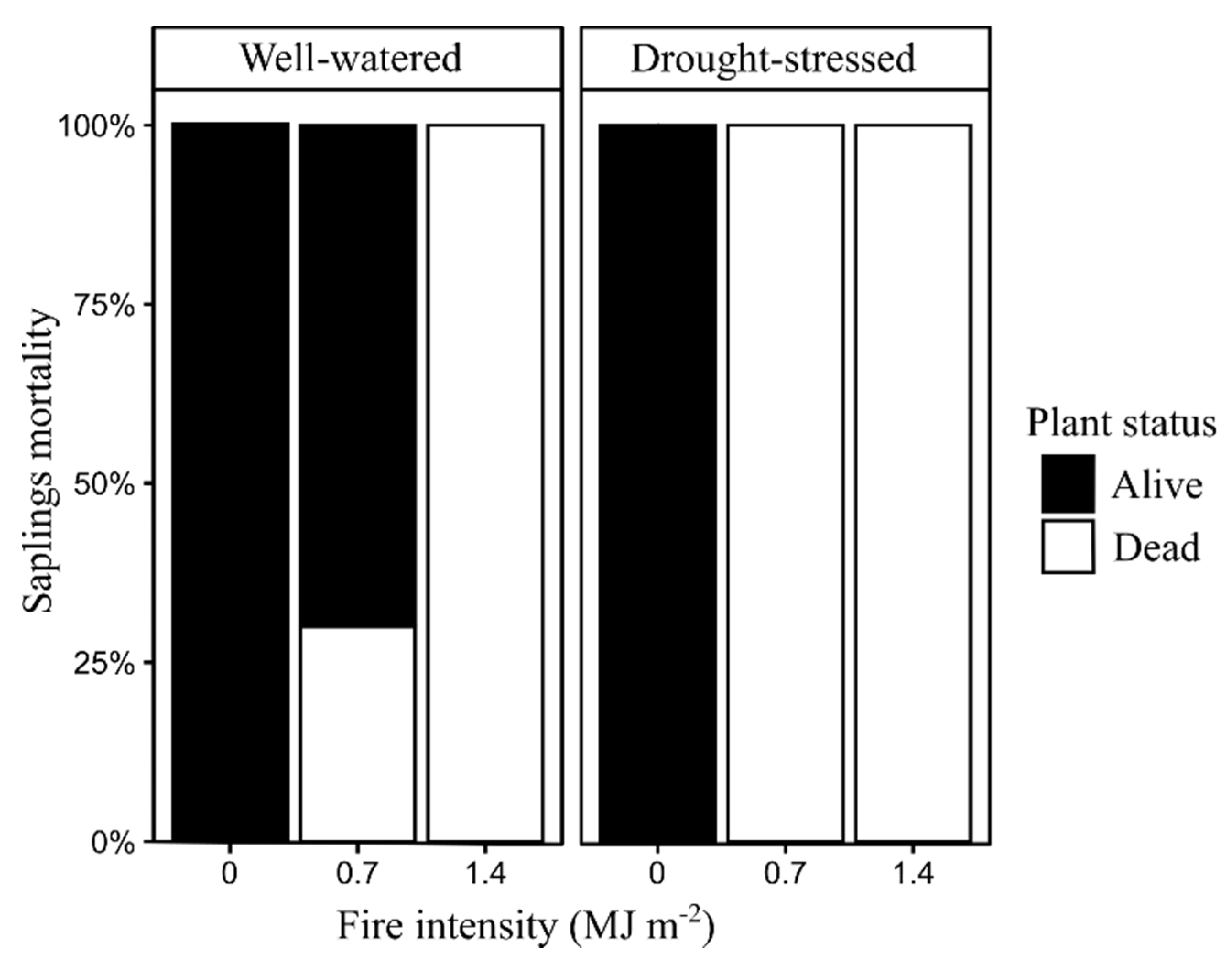
Bud density for the well-watered saplings burned at 0.7 MJ m−2 was significantly greater (P < 0.05) than in drought-stressed saplings burned at 0.7 MJ m−2 at 35, 68, and 125 days post-fire (Figure 4). All unburned saplings were alive at 200 days post-fire (Figure 5). Well-watered saplings burned at 0.7 MJ m−2 had 30% mortality, while saplings burned at 1.4 MJ m−2 had 100% mortality. All burned saplings in the drought-stressed treatment were dead at 200 days post-fire (Figure 5).
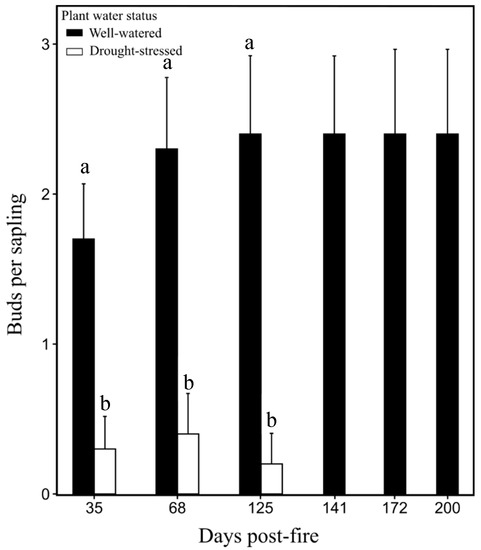
Figure 4.
Average bud count per sapling (±SE) in well-watered and drought-stressed Pinus ponderosa saplings burned at 0.7 MJ m−2 from day 35 to 200 days post-fire. Mean values sharing the same letter are not significantly different (P < 0.05).
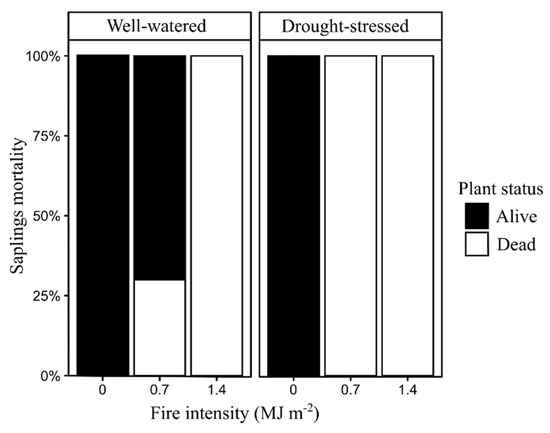
Figure 5.
Pinus ponderosa sapling mortality at 200 days after exposure to surface fires with different fire intensity (n = 10).
4. Discussion and Conclusions
Here, we tested two hypotheses: (1) Pinus ponderosa saplings subjected to pre-fire drought stress are more likely to die than well-watered saplings; and (2) Pinus ponderosa saplings subjected to pre-fire drought stress are less likely to develop new foliage than well-watered saplings post-fire. This study did not focus on the specific mechanisms of fire-induced mortality. We also sought to confirm the fire intensity levels required to cause mortality in similarly sized Pinus ponderosa saplings that were reported in an earlier study [29], and assess how interaction of fire and drought on Pinus ponderosa saplings compares to that observed in a study focused on Larix occidentalis [27]. In this study and [27] similar reductions in photosynthesis one day after the saplings were exposed to fire were observed. In contrast to Larix occidentalis saplings where photosynthesis recovered within 30 days in all drought treatments, regardless of the fire intensity and pre-fire water status, photosynthesis was reduced in all burned saplings for at least 27 days post-fire in this study. Our bud density and mortality results also contrast with the results in study [27]. We found that drought-stressed saplings had greater mortality than well-watered saplings burned at the same fire intensity and bud density was higher in burned well-watered saplings (Figure 4 and Figure 5). This, however, was in contrast to the study by [27] in Larix occidentalis where saplings under severe drought stress had similar mortality of saplings under low water stress. Thus, these contrasting results highlight that species and evolutionary fire adaptation differences may be important factors.
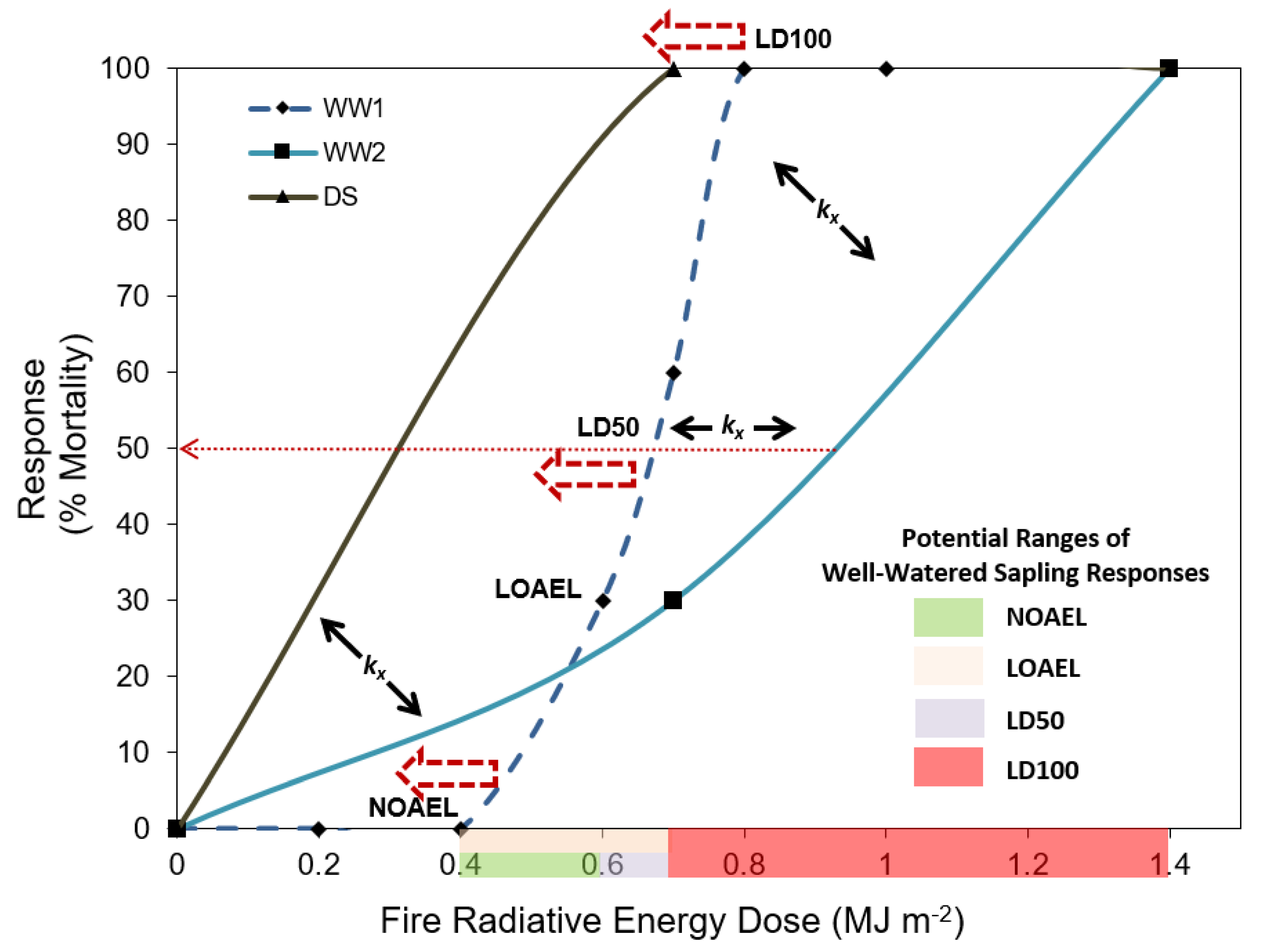
A comparison of this study with prior experiments that have assessed Pinus ponderosa sapling response to fire [29,30] is shown in Figure 6. Given that no data points exist in well-watered saplings in this study (WW2) between 0.7 MJ m−2 and 1.4 MJ m−2, the intercept with the LD 100 line (i.e., dosage that produces 100% mortality) could occur anywhere over this range. As such, the WW2 curve is not in disagreement with the well-watered plants curve in the study [29] (WW1), and highlights the need for more studies to confirm where these various thresholds occur for this and other species. The current study confirms the hypothesis proposed by [24] that, for individual species, drought stress would result in increased negative effects at lower FRE dosage levels. This is demonstrated by the ‘leftwards’ shift of the NOAEL (no-observed-adverse-effect level), the LOAEL (lowest-observed-adverse-effect level), and the LD50 (the ‘dose’ that causes 50% mortality) when comparing well-watered to drought-stressed saplings in this species. Shifts to the right have been observed in prior studies comparing laboratory to field-based modeling, which may be expected when considering plants that are more relatively more resistant to fire [24]. Species mortality curves like these are contained within the BioEarth Earth system model [19] through the RHESSys-WMFire component, where the ‘left’ and right’ shifts are described by a series of scaling, slope, and centerpoint (LD50) coefficients denoted by kx [21]. Therefore, results such as those presented herein can be directly incorporated within such Earth system models to enable improved assessments of fire-induced species mortality. Future studies are needed to confirm where these various thresholds are and how they may change due to plant stressors and across different species. Differences can also be accounted for by age of plants at time of burn, environmental variations between the two studies, among other factors.
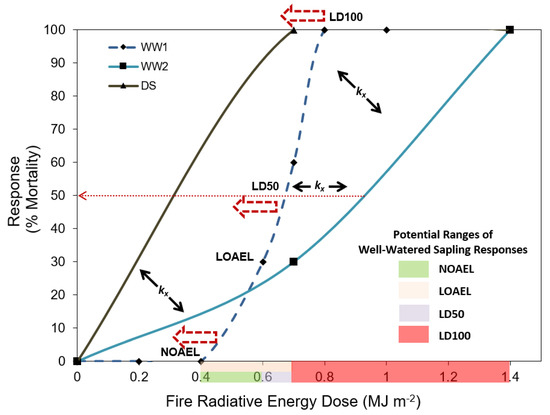
Figure 6.
Comparison of Pinus ponderosa sapling mortality after exposure to surface fires with different fire intensities across several studies. WW1 represents data from well-watered saplings reported in [29]. WW2 and DS represent the well-watered and drought-stressed saplings in the current study. Adapted from [24]. LDY denotes the lethal dose to cause Y% mortality, NOEAL represents the no observed adverse effect level, and LOAEL represents the lowest observed adverse effect level. The kx refer to center point, slope, and scale coefficients used within the Regional Hydro-Ecological Simulation System model (RHESSys) [21].
The shaded areas on the Figure 6 x-axis represent the current unknowns of where the various thresholds occur for well-watered Pinus ponderosa saplings of this age and size. Specifically, the green shaded area represents the range at which the NOAEL may occur, given that [29] observed 100% survival at 0.4 MJ m−2, and all doses greater than that value have shown some degree of mortality. Given the evidence to date, we contend that the 100% survival threshold is likely between 0.4 and 0.6 MJ m−2 for well-watered Pinus ponderosa saplings of this age and size. As hypothesized in [24], we would expect a rightwards and downshift for older plants, given that they may have developed more fire resistant features, such as thicker bark or vertical avoidance of key components from the fire [52]. Similarly, the LOAEL for well-watered Pinus ponderosa saplings of this size and age is likely between 0.6 and 0.7 MJ m−2, as indicated by the blue shaded area. The lethal threshold for any Pinus ponderosa sapling in this size and age group regardless of the degree of water stress is likely between 0.7 and 1.4 MJ m−2 indicated by the red shaded area. Therefore, this study reconfirmed the prior study of [29] that 1.4 MJ m−2 will result in 100% mortality in Pinus ponderosa saplings of this age and size.
The observed increase in well-watered sapling mortality with greater fire intensity supports previous studies conducted with other conifer saplings [24,25,26,27,28,29,30]. To our knowledge, no studies exist that have evaluated sapling mortality under pre-fire water stress exposed to different fire intensities. Limited studies have observed different mortality patterns in drought-stressed trees subjected to surface fires with a single fire intensity [27,49]. For example, a study conducted with the deciduous conifer Larix occidentalis saplings showed that saplings under moderate drought stress (Ψp = −1.5 MPa) were more susceptible to mortality (83% mortality) than saplings not under water stress (14% mortality) when subjected to surface fires of the same fire intensity (0.4 MJ m−2) [27]. However, severely drought-stressed Larix (Ψp = −2.3 MPa) only had 14% mortality, potentially due to nutrient and NSC translocation during drought-induced foliar senescence before the fires [27]. In contrast, [49] and [50] observed that drought-stressed Quercus spp. seedlings exhibited no mortality, despite 100% foliage loss due to surface fires. The high survival rate was likely owing to Quercus’ ability to resprout new stems and foliage from fire-protected buds at the root collar and NSC reserves in the roots.
Our results suggest that the pre-fire water status can influence the recovery and mortality of ponderosa pine saplings after the fire. For instance, the water stressed saplings exposed to the low fire intensity (0.7 MJ m−2) had 100% mortality, while well-watered saplings burned at the same intensity suffered only 30% mortality. It is possible that the low moisture content in the foliage due to water stress contributed to more consumption of the crown and increased bud damage. For instance, in high-elevation conifers, the high moisture content of the crown foliage just after bud flush contributes to the lower probability of crown fire in these forests [62]. Additionally, the low moisture content in the bark might increase the magnitude of phloem and cambium heating and, thus, tissue death. However, more applied research is needed to identify these mechanisms in drought-stressed plants. Our data also suggest that there is a fire intensity threshold, above which the pre-fire water stress no longer influences sapling mortality. As observed in this study, all saplings exposed to 1.4 MJ m−2, regardless of the pre-fire water stress, died. A prior study [29] found that, in well-watered ponderosa pine saplings, there was 100% mortality in saplings subjected to a fire intensity of 0.8 MJ m−2. Thus, it is likely that when Pinus ponderosa saplings, of similar size to this study, are subjected to fire doses above 0.8 MJ m−2, they will experience 100% mortality regardless of their pre-fire water status. As fire-induced tree mortality is size-dependent (smaller trees are more likely to be killed than larger trees) [63], more research is needed to assess how larger drought-stressed trees are impacted by varying fire intensities.
We observed that, regardless of the pre-fire water status and fire intensity, all of the saplings that were dead by the end of this experiment did not flush buds between 27 and 35 days post-fire. Bud death is one of the potential fire injuries in these saplings that could have led to sapling mortality. When 100% of the buds are either killed by the convective heat or conductive heat (through direct contact with the flames) during the fire, it can cause immediate mortality, particularly in non-resprouting species such as ponderosa pine [15]. However, we also observed that some saplings flushed new buds but then eventually died (Figure 2). Thus, our data also leaves open the possibility that cambium death and/or depletion of NSC could be the cause of mortality. The saplings that flushed new buds and eventually died had most of their crown damaged (Figure 4) and significantly reduced photosynthesis (Figure 1). Large reductions in photosynthesis can force the sapling to depend on their NSC reserves to rebuild the foliage and repair the damage caused in other organs, such as the stem and roots. Although our data suggests that cambium necrosis and/or depletion of NSC could be the potential causes of some mortality, more studies are needed to evaluate these mechanisms, particularly NSC depletion. This research need is particularly relevant, given the predicted increases in drought events and the potential increase in fire activity in the coming decades [64,65]. A logical extension of this research and the previous study on Larix occidentalis [27] is to explore what happens to saplings that continue to be exposed to drought conditions following fire treatments, as well as to assess other environmental interactions, such as disease or frost shock, followed by fire treatments.
Here, we demonstrated that saplings under drought stress are more vulnerable than well-watered saplings to mortality when exposed to non-lethal fire intensity for well-watered plants. Although this experiment was conducted in a controlled environment, we expect saplings under drought stress in the field will have higher mortality when exposed to fire. The results from this study serve as a first step toward that effort and give us important insights into how saplings under drought stress might respond to fire in the future.
Author Contributions
Conceptualization, R.P.-F, D.M.J, A.M.S.S.; Formal analysis, R.P.-F.; Methodology, R.P.-F., D.M.J., A.M.S.S; Writing—original draft, R.P.-F., D.M.J, A.M.S.S.; Writing—review & editing, R.P.-F., D.M.J., A.M.S., H.D.A., C.A.K., A.S.N., A.M.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported with partial funding from the National Science Foundation award DMS-1520873 and IOS-1852976, the USDA McIntire-Stennis award IDAZ-MS0117, and the Joint Fire Sciences Program award 19-1-01-51. Smith was also partially supported though the NASA Carbon Monitoring System Program Award NNH15AZ06I. Adams was supported by the USDA National Institute of Food and Agriculture, McIntire Stennis project WNP00009.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Breshears, D.D.; Cobb, N.S.; Rich, P.M.; Price, K.P.; Allen, C.D.; Balice, R.G.; Romme, W.H.; Kastens, J.H.; Floyd, M.L.; Belnap, J.; et al. Regional vegetation die-off in response to global-change-type drought. Proc. Natl. Acad. Sci. USA 2005, 102, 15144–15148. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2009, 259, 660–684. [Google Scholar] [CrossRef]
- Adams, H.D.; Luce, C.H.; Breshears, D.D.; Weiler, M.; Hale, C.H.; Allen, C.D.; Smith, A.M.S.; Huxman, T.E. Ecohydrological consequences of drought- and infestation-triggered tree die-off. Ecohydrology 2012, 5, 145–159. [Google Scholar] [CrossRef]
- Barbero, R.; Abatzoglou, J.T.; Larkin, N.K.; Kolden, C.A.; Stocks, B. Climate change presents increased potential for very large fires in the contiguous United States. Int. J. Wildland Fire 2015, 24, 892–899. [Google Scholar] [CrossRef]
- Abatzoglou, J.T.; Williams, A.P. Impact of anthropogenic climate change on wildfire across western US forests. Proc. Natl. Acad. Sci. USA 2016, 113, 11770–11775. [Google Scholar] [CrossRef] [PubMed]
- Bowman, D.M.J.S.; Williamson, G.; Kolden, C.A.; Abatzoglou, J.T.; Cochrane, M.A.; Smith, A.M.S. Human exposure and sensitivity to globally extreme wildfire events. Nat. Ecol. Evol. 2017, 1, 58. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging mega disturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef]
- Davis, K.T.; Dobrowski, S.Z.; Higuera, P.E.; Holden, Z.A.; Veblen, T.T.; Rother, M.T.; Parks, S.A.; Sala, A.; Maneta, M. Wildfires and climate change push low-elevation forests across a critical climate threshold for tree regeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 6193–6198. [Google Scholar] [CrossRef]
- Gustafson, E.J.; Sturtevant, B.R. Modeling forest mortality caused by drought stress: Implications for climate change. Ecosystems 2013, 16, 60–74. [Google Scholar] [CrossRef]
- Reinhardt, E.D.; Keane, R.E.; Brown, J.K. First Order Fire Effects Model: FOFEM 4.0 User’s Guide INT-GTR-344 US Department of Agriculture; Forest Service, Intermountain Research Station: Ogden, UT, USA, 1997. [Google Scholar]
- Rebain, S.A. The Fire and Fuels Extension to the Forest Vegetation Simulator: Updated Model Documentation; Internal Rep. U.S. Department of Agriculture 2015, Forest Service, Forest Management Service Center: Fort Collins, CO, USA, 2015; p. 403.
- Lutes, D. FOFEM 6.5 First Order Fire Effects Model User Guide, Fire and Aviation Management, Rocky Mountain Research Station Fire Modelling Institute; United States Department of Agriculture: Fort Collins, CO, USA, 2018; p. 86.
- Woolley, T.; Shaw, D.C.; Ganio, L.M.; Fitzgerald, S. A review of logistic regression models used to predict post-fire tree mortality of western North American conifers. Int. J. Wildland Fire 2012, 21, 1–35. [Google Scholar] [CrossRef]
- Smith, A.M.S.; Sparks, A.M.; Kolden, C.A.; Abatzoglou, J.T.; Talhelm, A.F.; Johnson, D.M.; Boschetti, L.; Lutz, J.A.; Apostol, K.G.; Yedinak, K.M.; et al. Towards a new paradigm in fire severity research using dose-response experiments. Int. J. Wildland Fire 2016, 25, 158–166. [Google Scholar] [CrossRef]
- Hood, S.M.; Varner, J.M.; van Mantgem, P.; Cansler, C.A. Fire and tree death: Understanding and improving modeling of fire-induced tree mortality. Environ. Res. Lett. 2018, 13, 113004. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Spessa, A.; Kaplan, J.O. A model for global biomass burning in preindustrial time: LPJ-LMfire (v1. 0). Geosci. Model Dev. 2013, 6, 643–685. [Google Scholar] [CrossRef]
- Thonicke, K.; Spessa, A.; Prentice, I.C.; Harrison, S.P.; Dong, L.; Carmona-Moreno, C. The influence of vegetation, fire spread and fire behaviour on biomass burning and trace gas emissions: Results from a process-based model. Biogeosciences 2010, 7, 1991–2011. [Google Scholar] [CrossRef]
- Lawrence, D.M.; Fisher, R.A.; Koven, C.D.; Oleson, K.W.; Swenson, S.C.; Bonan, G.; Collier, N.; Ghimire, B.; van Kampenhout, L.; Kennedy, D.; et al. The Community Land Model Version 5: Description of New Features, Benchmarking, and Impact of Forcing Uncertainty. J. Adv. Modeling Earth Syst. 2019, 11, 4245–4287. [Google Scholar] [CrossRef]
- Adam, J.C.; Stephens, J.C.; Chung, S.H.; Brady, M.P.; Evans, R.D.; Kruger, C.E.; Lamb, B.K.; Liu, M.; Stökle, C.O.; Vaughan, J.K.; et al. BioEarth: Envisioning and developing a new regional earth system model to inform natural and agricultural resource management. Clim. Chang. 2015, 129, 555–571. [Google Scholar] [CrossRef][Green Version]
- Zierl, B.; Bugmann, H.; Tague, C.L. Water and carbon fluxes of European ecosystems: An evaluation of the ecohydrological model RHESSys. Hydrol. Process. 2007, 21, 3328–3339. [Google Scholar] [CrossRef]
- Bart, R.R.; Kennedy, M.C.; Tague, C.L.; McKenzie, D. Integrating fire effects on vegetation carbon cycling within an ecohydrologic model. Ecol. Model. 2000, 416, 108880. [Google Scholar] [CrossRef]
- Jolly, W.M.; Johnson, D.M. Pyro-Ecophysiology: Shifting the Paradigm of Live Wildland Fuel Research. Fire 2018, 1, 8. [Google Scholar] [CrossRef]
- O’Brien, J.J.; Hiers, J.K.; Varner, J.M.; Hoffman, C.M.; Dickinson, M.B.; Michaletz, S.T.; Loudermilk, E.L.; Butler, B.W. Advances in Mechanistic Approaches to Quantifying Biophysical Fire Effects. Curr. For. Rep. 2018, 4, 161–177. [Google Scholar] [CrossRef]
- Smith, A.M.S.; Talhelm, A.F.; Johnson, D.M.; Sparks, A.M.; Yedinak, K.M.; Apostol, K.G.; Tinkham, W.T.; Kolden, C.A.; Abatzoglou, J.T.; Lutz, J.A.; et al. Effects of fire radiative energy density intensity on Pinus contorta and Larix occidentalis seedling physiology and mortality. Int. J. Wildland Fire 2017, 26, 82–94. [Google Scholar] [CrossRef]
- Sparks, A.M.; Kolden, C.A.; Talhelm, A.F.; Smith, A.M.S.; Apostol, K.G.; Johnson, D.M.; Boschetti, L. Spectral indices accurately quantify changes in seedling physiology following fire: Towards mechanistic assessments of post-fire carbon cycling. Remote Sens. 2016, 8, 572. [Google Scholar] [CrossRef]
- Sparks, A.M.; Smith, A.M.S.; Talhelm, A.F.; Kolden, C.A.; Yedinak, K.M.; Johnson, D.M. Impacts of fire radiative flux on mature Pinus ponderosa growth and vulnerability to secondary mortality agents. Int. J. Wildland Fire 2017, 26, 95–106. [Google Scholar] [CrossRef]
- Sparks, A.M.; Talhelm, A.F.; Partelli-Feltrin, R.; Smith, A.M.S.; Johnson, D.M.; Kolden, C.A.; Boschetti, L. An experimental assessment of the impact of drought and fire on western larch mortality and recovery. Int. J. Wildland Fire 2018, 27, 490–497. [Google Scholar] [CrossRef]
- Sparks, A.M.; Kolden, C.A.; Smith, A.M.S.; Boschetti, L.; Johnson, D.M.; Cochrane, M.A. Fire intensity impacts on post-fire response of temperate coniferous forest net primary productivity. Biogeosciences 2018, 15, 1173–1183. [Google Scholar] [CrossRef]
- Steady, W.D.; Partelli-Feltrin, R.; Johnson, D.M.; Sparks, A.M.; Kolden, C.A.; Talhelm, A.F.; Lutz, J.A.; Boschelli, L.; Hudak, A.T.; Nelson, A.S.; et al. The survival of Pinus ponderosa saplings subjected to increasing levels of fire behavior and impacts on post-fire growth. Fire 2019, 2, 23. [Google Scholar] [CrossRef]
- Partelli-Feltrin, R.; Smith, A.M.S.; Adams, H.D.; Kolden, C.A.; Johnson, D.M. Short- and long-term effects of fire on stem hydraulics in Pinus ponderosa saplings. Plant. Cell Environ. 2020. [Google Scholar] [CrossRef]
- Bär, A.; Michaeltz, S.T.; Mayr, S. Fire effects on tree physiology. New Phytol. 2019, 223, 1728–1741. [Google Scholar] [CrossRef]
- Cochard, H.; Coll, L.; Roux, X.L.; Améglio, T. Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant. Physiol. 2002, 128, 282–290. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Beerling, D.J.; Breshears, D.D.; Fisher, R.A.; Raffa, K.F.; Stitt, M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 2011, 26, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Grieu, P.; Guehl, J.M.; Aussenac, G. The effects of soil and atmospheric drought on photosynthesis and stomatal control of gas exchange in three coniferous species. Physiol. Plant. 1988, 73, 97–104. [Google Scholar] [CrossRef]
- Rouhi, V.; Samson, R.; Lemeur, R.; Damme, P.V. Photosynthetic gas exchange characteristics in three different almond species during drought stress and subsequent recovery. Environ. Exp. Bot. 2007, 59, 117–129. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhaüsser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hartmann, H.; Adams, H.D.; Zhang, H.; Jin, C.; Zhao, C.; Guan, D.; Wang, A.; Yuan, F.; Wu, J. The sweet side of global change-dynamic responses of non-structural carbohydrates to drought, elevated CO2 and nitrogen fertilization in tree species. Tree Physiol. 2018, 38, 1–18. [Google Scholar] [CrossRef]
- Michaletz, S.T.; Johnson, E.A. How forest fires kill trees: A review of the fundamental biophysical processes. Scand. J. For. Res. 2007, 22, 500–515. [Google Scholar] [CrossRef]
- Kavanagh, K.L.; Dickinson, M.B.; Bova, A.S. A way forward for fire-caused tree mortality prediction: Modeling a physiological consequence of fire. Fire Ecol. 2010, 6, 80–94. [Google Scholar] [CrossRef]
- Michaletz, S.T.; Johnson, E.A.; Tyree, M.T. Moving beyond the cambium necrosis hypothesis of post-fire tree mortality: Cavitation and deformation of xylem in forest fires. New Phytol. 2012, 194, 254–263. [Google Scholar] [CrossRef]
- West, A.G.; Nel, J.A.; Bond, W.J.; Midgley, J.J. Experimental evidence for heat plume-induced cavitation and xylem deformation as a mechanism of rapid post-fire tree mortality. New Phytol. 2016, 211, 828–838. [Google Scholar] [CrossRef]
- Bär, A.; Nardini, A.; Mayr, S. Post-fire effects in xylem hydraulics of Picea abies, Pinus sylvestris and Fagus sylvatica. New Phytol. 2018, 217, 1484–1493. [Google Scholar] [CrossRef]
- Ryan, K.C.; Frandsen, W.H. Basal injury from smoldering fires in mature Pinus ponderosa Laws. Int. J. Wildland Fire 1991, 2, 107–118. [Google Scholar] [CrossRef]
- Dickinson, M.B.; Johnsonm, E.A. Temperature-dependent rate models of vascular cambium cell mortality. Can. J. For. Res. 2004, 34, 546–559. [Google Scholar] [CrossRef]
- Kleinman, J.S.; Goode, J.D.; Fries, A.C.; Hart, J.L. Ecological consequences of compound disturbances in forest ecosystems: A systematic review. Ecosphere 2019, 10, e02962. [Google Scholar] [CrossRef]
- van Mantgem, P.J.; Nesmith, J.C.B.; Keifer, M.; Knapp, E.E.; Flint, A.; Flint, L. Climatic stress increases forest fire severity across the western United States. Ecol. Lett. 2013, 16, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwstadt, M.G.L.; Sheil, D. Drought, fire and tree survival in a Borneo rain forest, East Kalimantan, Indonesia. J. Ecol. 2005, 93, 191–201. [Google Scholar] [CrossRef]
- Brando, P.M.; Balch, J.K.; Nepstad, D.C.; Morton, D.C.; Putz, F.E.; Coe, M.T.; Silverio, D.; Macedo, M.N.; Davidson, E.A.; Nobrega, C.C.; et al. Abrupt increases in Amazonian tree mortality due to drought–fire interactions. Proc. Natl. Acad. Sci. USA 2014, 111, 6347–6352. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, A.; Montagnoli, A.; Scippa, G.S.; Chiatante, D. Fine root growth of Quercus pubescens seedlings after drought stress and fire disturbance. Environ. Exp. Bot. 2011, 74, 272–279. [Google Scholar] [CrossRef]
- Chiatante, D.; Tognetti, R.; Scippa, G.S.; Congiu, T.; Baesso, B.; Terzaghi, M.; Montagnoli, A. Interspecific variation in functional traits of oak seedlings (Quercus ilex, Quercus trojana, Quercus virgiliana) grown under artificial drought and fire conditions. J. Plant. Res. 2015, 128, 595–611. [Google Scholar] [CrossRef]
- van Mantgem, P.J.; Caprio, A.C.; Stephenson, N.L.; Das, A.J. Does prescribed fire promote resistance to drought in low-elevation forests of the Sierra Nevada, California, USA? Fire Ecol. 2016, 12, 13–25. [Google Scholar] [CrossRef]
- Smith, A.M.S.; Kolden, C.A.; Bowman, D.M.J.S. Biomimicry can help humans to sustainably coexist with fire. Nat. Ecol. Evol. 2018, 2, 1827–1829. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Germino, M.J.; Johnson, D.M.; Reinhardt, K.; Smith, W.K.; Resler, L.M.; Bader, M.Y.; Sala, A.; Kueppers, L.M.; Broll, G.; et al. Seedling survival at timberline is critical to conifer mountain forest elevation and extent. Front. For. Glob. Chang. 2019, 2, 9. [Google Scholar] [CrossRef]
- Baker, K.V.; Tai, X.; Miller, M.L.; Johnson, D.M. Six co-occurring conifer species in northern Idaho exhibit a continuum of hydraulic strategies during an extreme drought year. AoB Plants 2019, 11, plz056. [Google Scholar] [CrossRef] [PubMed]
- Wooster, M.J.; Roberts, G.; Smith, A.M.S.; Johnson, J.; Freeborn, P.; Amici, S.; Hudak, A.T. Thermal Remote Sensing of Active Vegetation Fires and Biomass Burning Events, chapter 18. In Thermal Infrared Remote Sensing; Kuenzer, C., Dech, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-94-007-6638-9. [Google Scholar]
- Smith, A.M.S.; Tinkham, W.T.; Roy, D.P.; Boschetti, L.; Kumar, S.; Sparks, A.M.; Kremens, R.L.; Falkowski, M.J. Quantification of fuel moisture effects on biomass consumed derived from fire radiative energy retrievals. Geophys. Res. Lett. 2013, 40, 6298–6302. [Google Scholar] [CrossRef]
- Kremens, R.; Smith, A.M.S.; Dickinson, M. Fire Metrology: Current and future directions in physics-based measurements. Fire Ecol. 2010, 6, 13–35. [Google Scholar] [CrossRef]
- Tinkham, W.T.; Smith, A.M.S.; Higuera, P.E.; Hatten, J.A.; Brewer, N.B.; Doerr, S.H. Replacing time with space: Using laboratory fires to explore the effects of repeated burning on black carbon degradation. Int. J. Wildland Fire 2016, 25, 242–248. [Google Scholar] [CrossRef]
- Brewer, N.W.; Smith, A.M.S.; Hatten, J.A.; Higuera, P.E.; Hudak, A.T.; Ottmar, R.D.; Tinkham, W.T. Fuel Moisture Influences on Fire-altered Carbon in Masticated Fuels: An Experimental Study. J. Geophys. Res. 2013, 118, 30–40. [Google Scholar] [CrossRef]
- Bates, D.; Mäechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.4.3.01. 2019. Available online: https://www.r-project.org/ (accessed on 1 August 2020).
- Agee, J.K.; Wright, C.S.; Williamson, N.; Huff, M.H. Foliar moisture content of Pacific Northwest vegetation and its relation to wildland fire behavior. For. Ecol. Manag. 2002, 167, 57–66. [Google Scholar] [CrossRef]
- McDowell, N.G.; Michaletz, S.T.; Bennett, K.E.; Solander, K.C.; Xu, C.; Maxwell, R.M.; Allen, C.D.; Middleton, R.S. Predicting chronic climate- driven disturbances and their mitigation. Trends Ecol. Evol. 2018, 33, 15–27. [Google Scholar] [CrossRef]
- Flannigan, M.; Stocks, B.J.; Wotton, B.M. Climate change and forest fires. Sci. Total Environ. 2000, 262, 221–229. [Google Scholar] [CrossRef]
- Flannigan, M.; Cantin, A.S.; De Groot, W.J.; Wotton, M.; Newbery, A.; Gowman, L.M. Global wildland fire season severity in the 21st century. For. Ecol. Manag. 2013, 294, 54–61. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).