Soil Enzyme Activity and Soil Nutrients Jointly Influence Post-Fire Habitat Models in Mixed-Conifer Forests of Yosemite National Park, USA
Abstract
1. Introduction
2. Materials and Methods
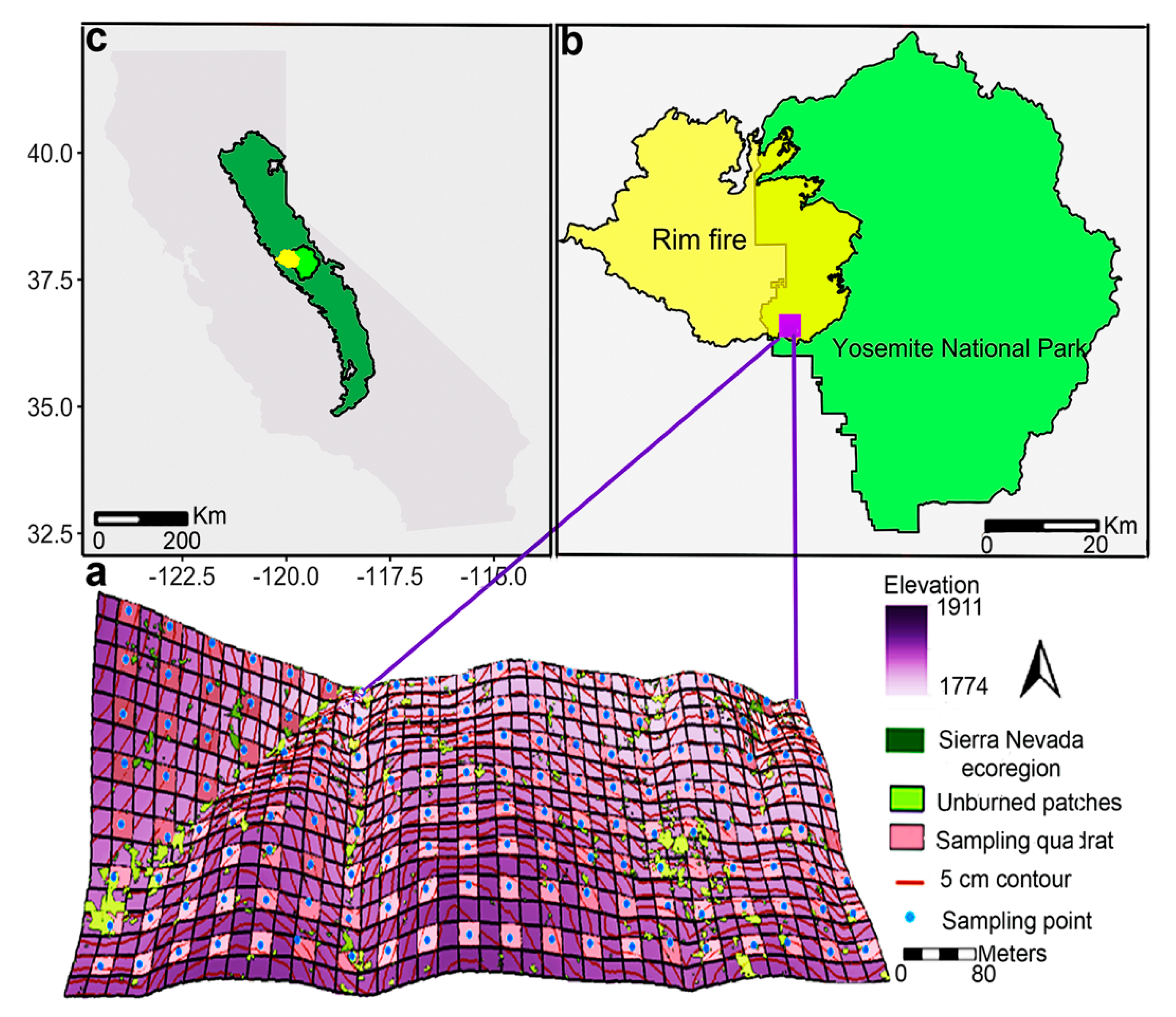
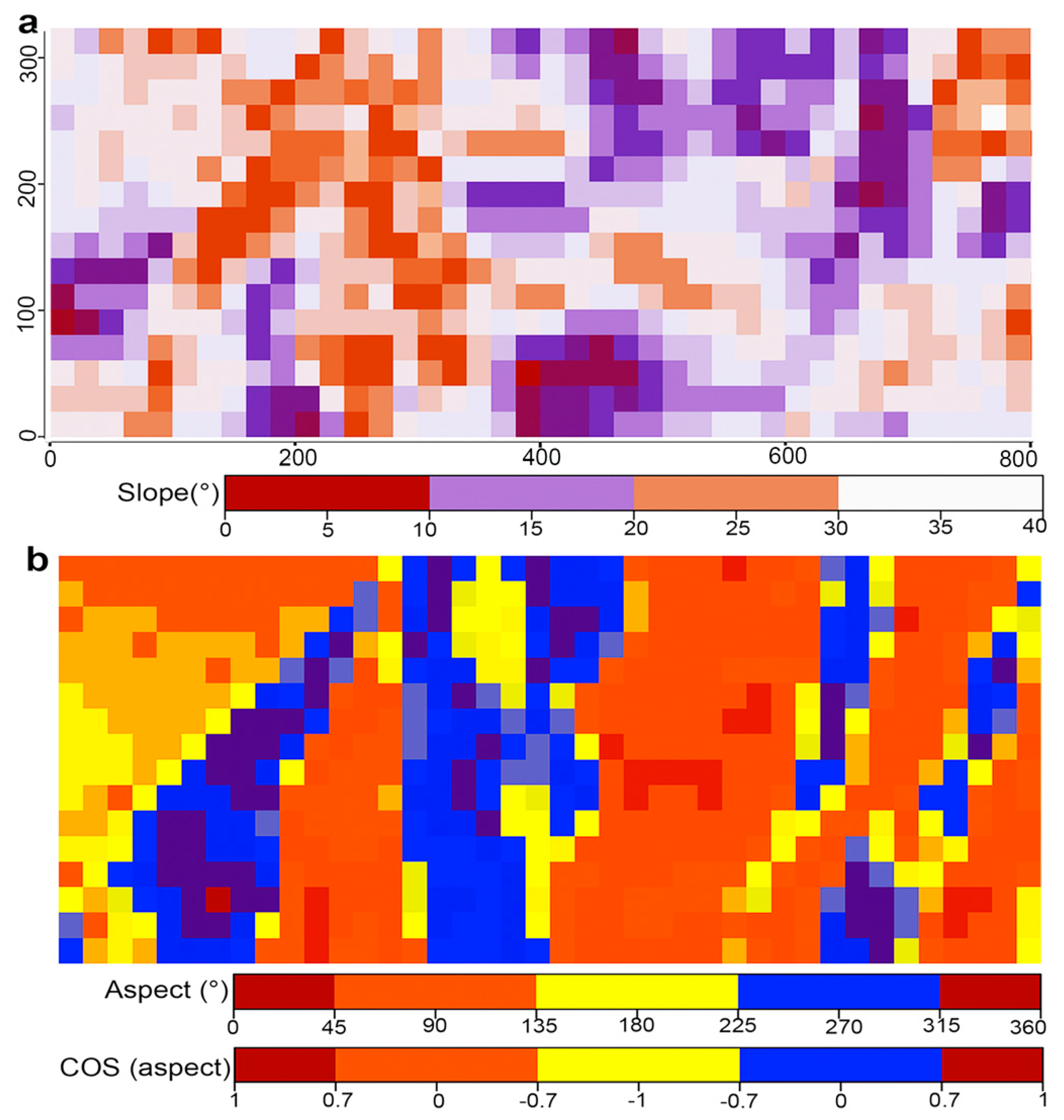
2.1. Study Area
2.2. Habitat Definition
2.3. Principal Coordinates of Neighbor Matrices
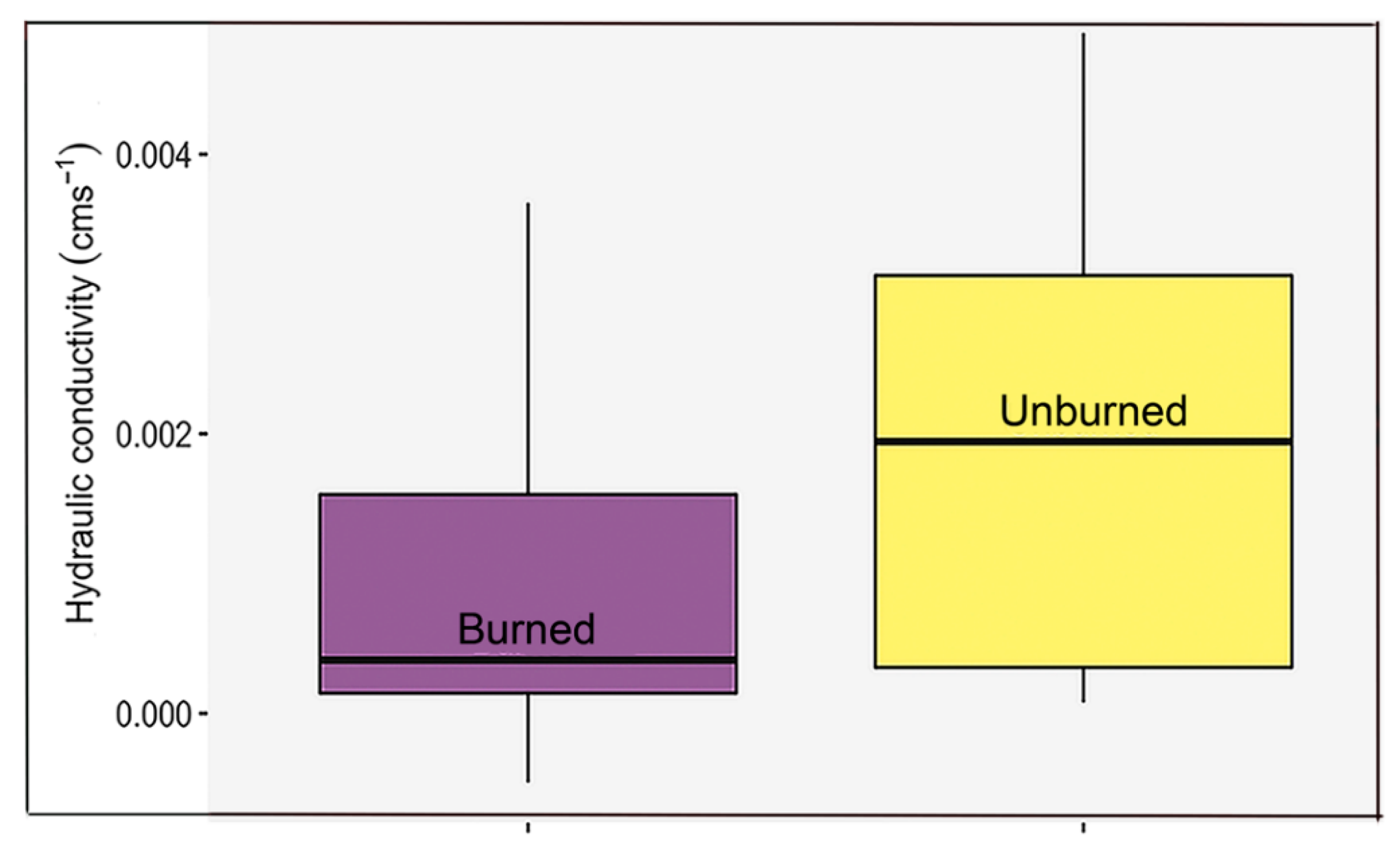
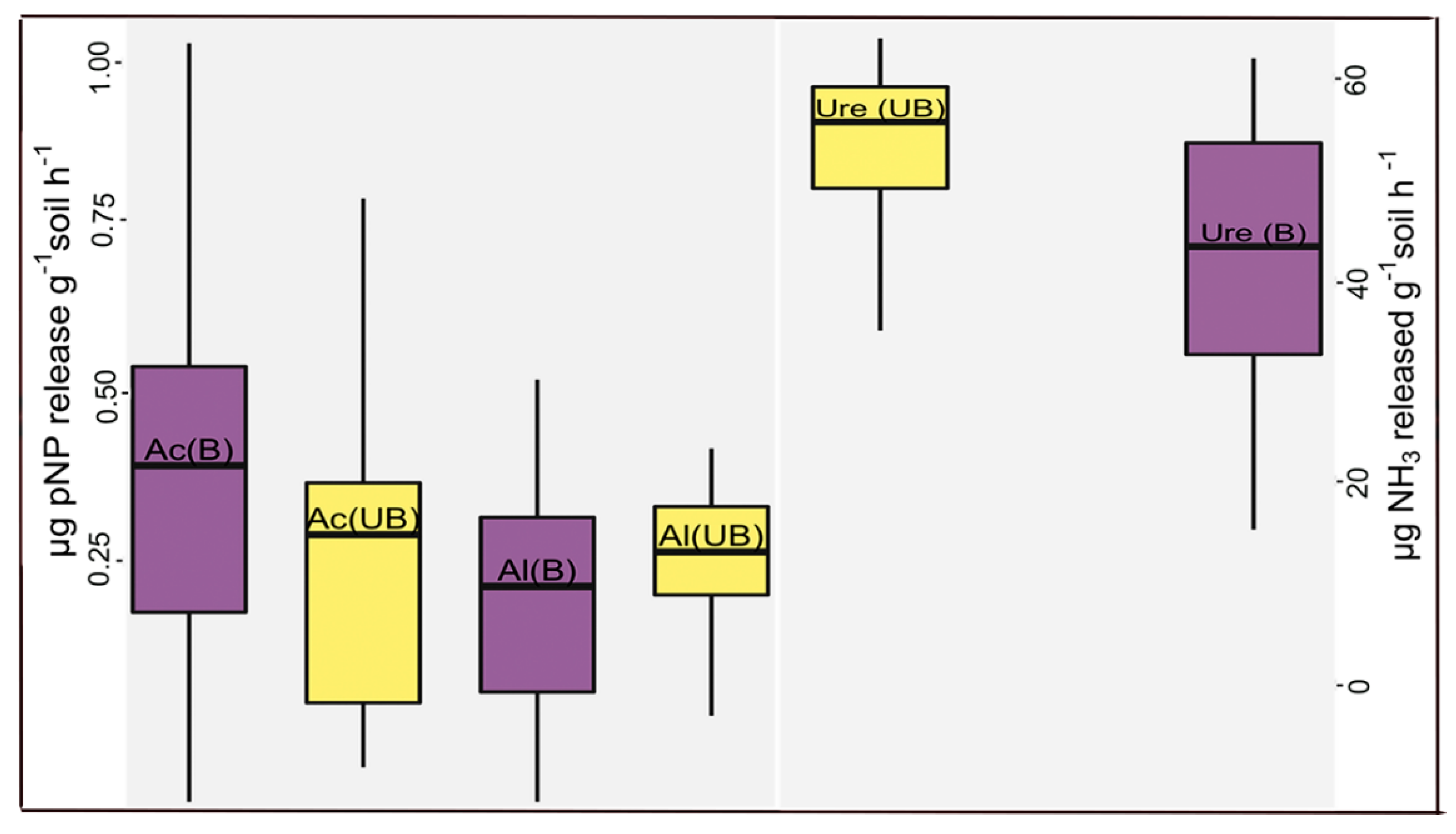
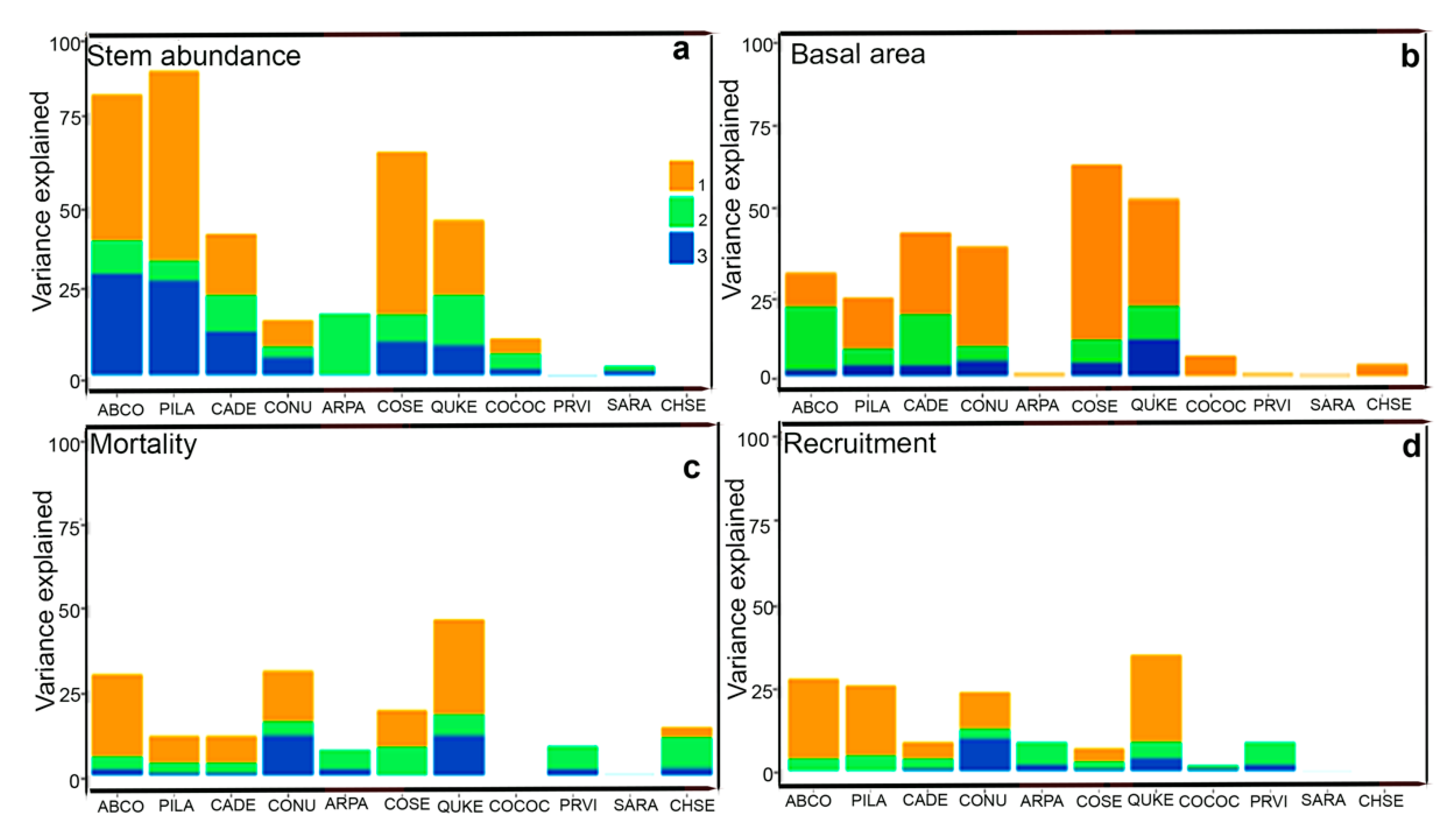
3. Results
4. Discussion
4.1. Associations of Different Species with Habitat Types
4.2. Niche vs. Dispersal Limitation Drive Variations in Species Abundance and Basal Area Increment
4.3. The Contribution of Environmental and Spatial Variables in Forming Mortality Abundances Across Species
4.4. The Contribution of Environmental and Spatial Variables in Forming Ingrowth Numbers Across Species
4.5. Edaphic Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Potts, M.D.; Davies, S.J.; Bossert, W.H.; Tan, S.; Supardi, M.N. Habitat heterogeneity and niche structure of trees in two tropical rain forests. Oecologia 2004, 139, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Keddy, P.A. Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef]
- Zhang, Z.-h.; Hu, G.; Ni, J. Effects of topographical and edaphic factors on the distribution of plant communities in two subtropical karst forests, southwestern China. J. Mt. Sci. 2013, 10, 95–104. [Google Scholar] [CrossRef]
- Valencia, R.; Foster, R.B.; Villa, G.; Condit, R.; Svenning, J.C.; Hernández, C.; Romoleroux, K.; Losos, E.; Magård, E.; Balslev, H. Tree species distributions and local habitat variation in the Amazon: Large forest plot in eastern Ecuador. J. Ecol. 2004, 92, 214–229. [Google Scholar] [CrossRef]
- Kanagaraj, R.; Wiegand, T.; Comita, L.S.; Huth, A. Tropical tree species assemblages in topographical habitats change in time and with life stage. J. Ecol. 2011, 99, 1441–1452. [Google Scholar] [CrossRef]
- Griffiths, R.; Madritch, M.; Swanson, A. The effects of topography on forest soil characteristics in the Oregon Cascade Mountains (USA): Implications for the effects of climate change on soil properties. For. Ecol. Manag. 2009, 257, 1–7. [Google Scholar] [CrossRef]
- Seibert, J.; Stendahl, J.; Sørensen, R. Topographical influences on soil properties in boreal forests. Geoderma 2007, 141, 139–148. [Google Scholar] [CrossRef]
- Aandahl, A.R. The characterization of slope positions and their influence on the total nitrogen content of a few virgin soils of western Iowa. Soil Sci. Soc. Am. J. 1949, 13, 449–454. [Google Scholar] [CrossRef]
- Fu, B.; Liu, S.; Ma, K.; Zhu, Y. Relationships between soil characteristics, topography and plant diversity in a heterogeneous deciduous broad-leaved forest near Beijing, China. Plant Soil 2004, 261, 47–54. [Google Scholar] [CrossRef]
- Sherene, T. Role of soil enzymes in nutrient transformation: A review. Bio Bull. 2017, 3, 109–131. [Google Scholar]
- Burns, R. Extracellular enzyme-substrate interactions in soil. In Microbes in their Natural Environment; Slater, J.H., Wittenbury, R., Wimpenny, J.W.T., Eds.; Cambridge University Press: London, UK, 1983; pp. 249–298. [Google Scholar]
- Sinsabaugh, R.L.; Antibus, R.K.; Linkins, A.E. An enzymic approach to the analysis of microbial activity during plant litter decomposition. Agric. Ecosyst. Environ. 1991, 34, 43–54. [Google Scholar] [CrossRef]
- Bielińska, E.J.; Kołodziej, B.; Sugier, D. Relationship between organic carbon content and the activity of selected enzymes in urban soils under different anthropogenic influence. J. Geochem. Explor. 2013, 129, 52–56. [Google Scholar] [CrossRef]
- Siles, J.A.; Cajthaml, T.; Minerbi, S.; Margesin, R. Effect of altitude and season on microbial activity, abundance and community structure in Alpine forest soils. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef]
- Boerner, R.E.; Decker, K.L.; Sutherland, E.K. Prescribed burning effects on soil enzyme activity in a southern Ohio hardwood forest: A landscape-scale analysis. Soil Biol. Biochem. 2000, 32, 899–908. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ceccanti, B.; Conti, C.; Bianchi, D. Hydrolases extracted from soil: Their properties and activities. Soil Biol. Biochem. 1982, 14, 257–263. [Google Scholar] [CrossRef]
- Lutz, J.A.; Matchett, J.R.; Tarnay, L.W.; Smith, D.F.; Becker, K.M.; Furniss, T.J.; Brooks, M.L. Fire and the distribution and uncertainty of carbon sequestered as aboveground tree biomass in Yosemite and Sequoia & Kings Canyon National Parks. Land 2017, 6, 10. [Google Scholar] [CrossRef]
- Meddens, A.J.; Kolden, C.A.; Lutz, J.A.; Smith, A.M.; Cansler, C.A.; Abatzoglou, J.T.; Meigs, G.W.; Downing, W.M.; Krawchuk, M.A. Fire refugia: What are they, and why do they matter for global change? BioScience 2018, 68, 944–954. [Google Scholar] [CrossRef]
- Page, N.V.; Shanker, K. Environment and dispersal influence changes in species composition at different scales in woody plants of the Western Ghats, India. J. Veg. Sci. 2018, 29, 74–83. [Google Scholar] [CrossRef]
- Beckage, B.; Clark, J.S. Seedling survival and growth of three forest tree species: The role of spatial heterogeneity. Ecology 2003, 84, 1849–1861. [Google Scholar] [CrossRef]
- Neumann, M.; Mues, V.; Moreno, A.; Hasenauer, H.; Seidl, R. Climate variability drives recent tree mortality in Europe. Glob. Chang. Biol. 2017, 23, 4788–4797. [Google Scholar] [CrossRef]
- Furniss, T.J.; Larson, A.J.; Kane, V.R.; Lutz, J.A. Multi-scale assessment of post-fire tree mortality models. Int. J. Wildland Fire 2019, 28, 46–61. [Google Scholar] [CrossRef]
- Furniss, T.J.; Kane, V.R.; Larson, A.J.; Lutz, J.A. Detecting tree mortality with Landsat-derived spectral indices: Improving ecological accuracy by examining uncertainty. Remote Sens. Environ. 2020, 237, 111497. [Google Scholar] [CrossRef]
- Lutz, J.A.; Larson, A.J.; Swanson, M.E.; Freund, J.A. Ecological importance of large-diameter trees in a temperate mixed-conifer forest. PLoS ONE 2012, 7, e36131. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.A. The evolution of long-term data for forestry: Large temperate research plots in an era of global change. Northwest Sci. 2015, 89, 255–269. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Davies, S.J.; Bennett, A.C.; Gonzalez-Akre, E.B.; Muller-Landau, H.C.; Joseph Wright, S.; Abu Salim, K.; Almeyda Zambrano, A.M.; Alonso, A.; Baltzer, J.L.; et al. CTFS-Forest GEO: A worldwide network monitoring forests in an era of global change. Glob. Chang. Biol. 2015, 21, 528–549. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.A.; van Wagtendonk, J.W.; Franklin, J.F. Climatic water deficit, tree species ranges, and climate change in Yosemite National Park. J. Biogeogr. 2010, 37, 936–950. [Google Scholar] [CrossRef]
- Keeler-Wolf, T.; Moore, P.; Reyes, E.; Menke, J.; Johnson, D.; Karavidas, D. Yosemite National Park vegetation classification and mapping project report. In Natural Resource Technical Report NPS/YOSE/NRTR—2012/598; National Park Service: Fort Collins, CO, USA, 2012. [Google Scholar]
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Web Soil Survey. Available online: http://websoilsurvey.sc.egov.usda.gov/ (accessed on 8 May 2018).
- Barth, M.A.; Larson, A.J.; Lutz, J.A. A forest reconstruction model to assess changes to Sierra Nevada mixed-conifer forest during the fire suppression era. For. Ecol. Manag. 2015, 354, 104–118. [Google Scholar] [CrossRef]
- Scholl, A.E.; Taylor, A.H. Fire regimes, forest change, and self-organization in an old-growth mixed-conifer forest, Yosemite National Park, USA. Ecol. Appl. 2010, 20, 362–380. [Google Scholar] [CrossRef]
- Stavros, E.N.; Tane, Z.; Kane, V.R.; Veraverbeke, S.; McGaughey, R.J.; Lutz, J.A.; Ramirez, C.; Schimel, D. Unprecedented remote sensing data over King and Rim megafires in the Sierra Nevada Mountains of California. Ecology 2016, 97, 3244. [Google Scholar] [CrossRef]
- Kane, V.R.; Cansler, C.A.; Povak, N.A.; Kane, J.T.; McGaughey, R.J.; Lutz, J.A.; Churchill, D.J.; North, M.P. Mixed severity fire effects within the Rim fire: Relative importance of local climate, fire weather, topography, and forest structure. For. Ecol. Manag. 2015, 358, 62–79. [Google Scholar] [CrossRef]
- Blomdahl, E.M.; Kolden, C.A.; Meddens, A.J.; Lutz, J.A. The importance of small fire refugia in the central Sierra Nevada, California, USA. For. Ecol. Manag. 2019, 432, 1041–1052. [Google Scholar] [CrossRef]
- Cansler, C.A.; Swanson, M.E.; Furniss, T.J.; Larson, A.J.; Lutz, J.A. Fuel dynamics after reintroduced fire in an old-growth Sierra Nevada mixed-conifer forest. Fire Ecol. 2019, 15, 16. [Google Scholar] [CrossRef]
- Larson, A.J.; Cansler, C.A.; Cowdery, S.G.; Hiebert, S.; Furniss, T.J.; Swanson, M.E.; Lutz, J.A. Post-fire morel (Morchella) mushroom abundance, spatial structure, and harvest sustainability. For. Ecol. Manag. 2016, 377, 16–25. [Google Scholar] [CrossRef]
- van Wagtendonk, J.W.; Lutz, J.A. Fire regime attributes of wildland fires in Yosemite National Park, USA. Fire Ecol. 2007, 3, 34–52. [Google Scholar] [CrossRef]
- Lutz, J.; Larson, A.; Swanson, M. Advancing fire science with large forest plots and a long-term multidisciplinary approach. Fire 2018, 1, 5. [Google Scholar] [CrossRef]
- Furniss, T.J.; Larson, A.J.; Lutz, J.A. Reconciling niches and neutrality in a subalpine temperate forest. Ecosphere 2017, 8, e01847. [Google Scholar] [CrossRef]
- Zhang, R. Determination of soil sorptivity and hydraulic conductivity from the disk infiltrometer. Soil Sci. Soc. Am. J. 1997, 61, 1024–1030. [Google Scholar] [CrossRef]
- Carsel, R.F.; Parrish, R.S. Developing joint probability distributions of soil water retention characteristics. Water Resour. Res. 1988, 24, 755–769. [Google Scholar] [CrossRef]
- Jönsson, U.; Rosengren, U.; Nihlgård, B.; Thelin, G. A comparative study of two methods for determination of pH, exchangeable base cations, and aluminum. Commun. Soil Sci. Plant Anal. 2002, 33, 3809–3824. [Google Scholar] [CrossRef]
- Dick, R.P. Methods of Soil Enzymology; Soil Science Society of America: Madison, WI, USA, 2020; pp. 154–196. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Tabatabai, M.; Bremner, J. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Package ‘Factoextra’. Extract and Visualize the Results of Multivariate Data Analyses. 2017, 76. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 23 September 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.4.3; R Core Team, R fundation for statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Pitman, N.C.; Terborgh, J.; Silman, M.R.; Nuñez, V.P. Tree species distributions in an upper Amazonian forest. Ecology 1999, 80, 2651–2661. [Google Scholar] [CrossRef]
- Harms, K.E.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol. Model. 2002, 153, 51–68. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘Vegan’. Community Ecology Package, Version 2013, 2. Available online: http://CRAN.R-project.org/package=vegan (accessed on 23 September 2020).
- Borcard, D.; Legendre, P.; Avois-Jacquet, C.; Tuomisto, H. Dissecting the spatial structure of ecological data at multiple scales. Ecology 2004, 85, 1826–1832. [Google Scholar] [CrossRef]
- Blanchet, F.G.; Legendre, P.; Borcard, D. Forward selection of explanatory variables. Ecology 2008, 89, 2623–2632. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Y.; Zhao, X.; Gadow, K. Species-habitat associations in a northern temperate forest in China. Silva Fenn 2012, 46, 501–519. [Google Scholar] [CrossRef]
- Kutiel, P.; Lavee, H. Effect of slope aspect on soil and vegetation properties along an aridity transect. Isr. J. Plant Sci. 1999, 47, 169–178. [Google Scholar] [CrossRef]
- Punchi-Manage, R.; Getzin, S.; Wiegand, T.; Kanagaraj, R.; Savitri Gunatilleke, C.; Nimal Gunatilleke, I.; Wiegand, K.; Huth, A. Effects of topography on structuring local species assemblages in a Sri Lankan mixed dipterocarp forest. J. Ecol. 2013, 101, 149–160. [Google Scholar] [CrossRef]
- Méndez-Toribio, M.; Ibarra-Manríquez, G.; Navarrete-Segueda, A.; Paz, H. Topographic position, but not slope aspect, drives the dominance of functional strategies of tropical dry forest trees. Environ. Res. Lett. 2017, 12, 085002. [Google Scholar] [CrossRef]
- Laacke, R. Chapter Fir. In Silvics of North America; Burns, R., Honkala, B., Eds.; United States Department of Agriculture, Forest Service: Washington, DC, USA, 1990; Volume 1, pp. 36–46. [Google Scholar]
- Neba, G.A.; Newbery, D.M.; Chuyong, G.B. Limitation of seedling growth by potassium and magnesium supply for two ectomycorrhizal tree species of a Central African rain forest and its implication for their recruitment. Ecol. Evol. 2016, 6, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Aydin, I.; Uzun, F. Nitrogen and phosphorus fertilization of rangelands affects yield, forage quality and the botanical composition. Eur. J. Agron. 2005, 23, 8–14. [Google Scholar] [CrossRef]
- Baribault, T.W.; Kobe, R.K.; Finley, A.O. Tropical tree growth is correlated with soil phosphorus, potassium, and calcium, though not for legumes. Ecol. Monogr. 2012, 82, 189–203. [Google Scholar] [CrossRef]
- Gagnon, J. Effect of magnesium and potassium fertilization on a 20-year-old red pine plantation. For. Chron. 1965, 41, 290–294. [Google Scholar] [CrossRef]
- Baldeck, C.A.; Harms, K.E.; Yavitt, J.B.; John, R.; Turner, B.L.; Valencia, R.; Navarrete, H.; Davies, S.J.; Chuyong, G.B.; Kenfack, D. Soil resources and topography shape local tree community structure in tropical forests. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122532. [Google Scholar] [CrossRef]
- Legendre, P.; Mi, X.; Ren, H.; Ma, K.; Yu, M.; Sun, I.F.; He., F. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 2009, 90, 663–674. [Google Scholar] [CrossRef]
- Gilbert, B.; Lechowicz, M.J. Neutrality, niches, and dispersal in a temperate forest understory. Proc. Natl. Acad. Sci. USA 2004, 101, 7651–7656. [Google Scholar] [CrossRef]
- Girdler, E.B.; Barrie, B.T.C. The scale-dependent importance of habitat factors and dispersal limitation in structuring Great Lakes shoreline plant communities. Plant Ecol. 2008, 198, 211–223. [Google Scholar] [CrossRef]
- Lin, G.; Stralberg, D.; Gong, G.; Huang, Z.; Ye, W.; Wu, L. Separating the effects of environment and space on tree species distribution: From population to community. PLoS ONE 2013, 8, e56171. [Google Scholar] [CrossRef]
- Yuan, Z.; Gazol, A.; Wang, X.; Lin, F.; Ye, J.; Bai, X.; Li, B.; Hao, Z. Scale specific determinants of tree diversity in an old growth temperate forest in China. Basic Appl. Ecol. 2011, 12, 488–495. [Google Scholar] [CrossRef]
- Shipley, B.; Paine, C.T.; Baraloto, C. Quantifying the importance of local niche-based and stochastic processes to tropical tree community assembly. Ecology 2012, 93, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, B.B.; Scheuner, W.H. Chapter Sugar Pine. In Silvics of North America; Burns, R., Honkala, B., Eds.; United States Department of Agriculture, Forest Service: Washington, DC, USA, 1990; Volume 1, pp. 370–379. [Google Scholar]
- Ma, L.; Lian, J.; Lin, G.; Cao, H.; Huang, Z.; Guan, D. Forest dynamics and its driving forces of sub-tropical forest in South China. Sci. Rep. 2016, 6, 22561. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.J.; Lutz, J.A.; Donato, D.C.; Freund, J.A.; Swanson, M.E.; HilleRisLambers, J.; Sprugel, D.G.; Franklin, J.F. Spatial aspects of tree mortality strongly differ between young and old-growth forests. Ecology 2015, 96, 2855–2861. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J. Tree mortality and growth in 11 sympatric Macaranga species in Borneo. Ecology 2001, 82, 920–932. [Google Scholar] [CrossRef]
- Bazzaz, F. The physiological ecology of plant succession. Annu. Rev. Ecol. Syst. 1979, 10, 351–371. [Google Scholar] [CrossRef]
- Eriksson, O. Seedling recruitment in deciduous forest herbs: The effects of litter, soil chemistry and seed bank. Flora 1995, 190, 65–70. [Google Scholar] [CrossRef]
- Dalling, J.W.; Hubbell, S.P. Seed size, growth rate and gap microsite conditions as determinants of recruitment success for pioneer species. J. Ecol. 2002, 90, 557–568. [Google Scholar] [CrossRef]
- Vera, M. Effects of altitude and seed size on germination and seedling survival of heathland plants in north Spain. Plant Ecol. 1997, 133, 101–106. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Gawroński, S. Influence of litter and weather on seedling recruitment in a mixed oak–pine woodland. Ann. Bot. 2002, 90, 245–251. [Google Scholar] [CrossRef]
- Baraloto, C.; Forget, P.M. Seed size, seedling morphology, and response to deep shade and damage in neotropical rain forest trees. Am. J. Bot. 2007, 94, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Holdridge, L.R. Determination of world plant formations from simple climatic data. Science 1947, 105, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Naples, B.K.; Fisk, M.C. Belowground insights into nutrient limitation in northern hardwood forests. Biogeochemistry 2010, 97, 109–121. [Google Scholar] [CrossRef]
- Fay, P.A.; Prober, S.M.; Harpole, W.S.; Knops, J.M.; Bakker, J.D.; Borer, E.T.; Lind, E.M.; MacDougall, A.S.; Seabloom, E.W.; Wragg, P.D. Grassland productivity limited by multiple nutrients. Nat. Plants 2015, 1, 1–5. [Google Scholar] [CrossRef]
- John, R.; Dalling, J.W.; Harms, K.E.; Yavitt, J.B.; Stallard, R.F.; Mirabello, M.; Hubbell, S.P.; Valencia, R.; Navarrete, H.; Vallejo, M. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl. Acad. Sci. USA 2007, 104, 864–869. [Google Scholar] [CrossRef]
- Gleason, S.M.; Read, J.; Ares, A.; Metcalfe, D.J. Species–soil associations, disturbance, and nutrient cycling in an Australian tropical rainforest. Oecologia 2010, 162, 1047–1058. [Google Scholar] [CrossRef]
- Hernández, T.; Garcia, C.; Reinhardt, I. Short-term effect of wildfire on the chemical, biochemical and microbiological properties of Mediterranean pine forest soils. Biol. Fertil. Soils 1997, 25, 109–116. [Google Scholar] [CrossRef]
- Xue, L.; Li, Q.; Chen, H. Effects of a wildfire on selected physical, chemical and biochemical soil properties in a Pinus massoniana forest in South China. Forests 2014, 5, 2947–2966. [Google Scholar] [CrossRef]


| 2014 | 2019 | 2014–2019 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Stems ≥ 1 cm DBH | Stems ≥ 60 cm DBH | BA ≥ 1 cm DBH | BA ≥ 60 cm DBH | Stems ≥ 1 cm DBH | Stems ≥ 60 cm DBH | BA ≥ 1 cm DBH | BA ≥ 60 cm BDH | BAI ≥ 1 cm DBH | BAI ≥ 60 cm DBH |
| Abies concolor | 2815 | 403 | 15.25 | 8.56 | 2815 | 420 | 15.89 | 8.92 | 0.64 | 0.36 |
| Pinus lambertiana | 855 | 398 | 15.29 | 13.77 | 855 | 409 | 15.67 | 14.17 | 0.38 | 0.4 |
| Cornus nuttallii | 439 | 0.06 | 439 | 0.07 | 0.01 | |||||
| Calocedrus decurrens | 440 | 85 | 3.41 | 2.52 | 440 | 89 | 3.50 | 2.59 | 0.09 | 0.07 |
| Quercus kelloggii | 278 | 1 | 0.48 | 0.01 | 278 | 1 | 0.51 | 0.01 | 0.03 | t |
| Arctostaphylos patula | 82 | t | t | |||||||
| Cornus sericea | 11 | t | 11 | t | t | |||||
| Corylus cornuta var. californica | 275 | t | t | |||||||
| Prunus virginiana | 2 | t | 2 | t | t | |||||
| Sambucus racemosa | 35 | t | t | |||||||
| Chrysolepis sempervirens | 36 | t | t | |||||||
| Topography | Edaphic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Density (stems ha−1) | Stems ≥ 1 cm dbh | Abundance | BAI | Mortality | Recruit. | Abundance | BAI | Mortality | Recruit. |
| Abies concolor | 111.8 | 2862 | LSN+ | LSN- | h3+ | |||||
| Quercus kelloggii | 50.1 | 1282 | h3- | h7+/h5- | h6+ | |||||
| Pinus lambertiana | 33.5 | 857 | LSN+/LSS- | h3+/h7- | ||||||
| Cornus nuttallii | 32 | 817 | LSN- | |||||||
| Calocedrus decurrens | 17.6 | 450 | LSN- | h7+/h5- | ||||||
| Corylus cornuta var. californica | 10.7 | 275 | h6+/h2- | |||||||
| Cornus sericea | 9.8 | 252 | HSS/HSN- | h1+ | ||||||
| Arctostaphylos patula | 34.5 | 82 | ||||||||
| Chrysolepis sempervirens | 1.4 | 36 | ||||||||
| Sambucus racemosa | 1.4 | 35 | ||||||||
| Prunus virginiana | 1 | 25 | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamjidi, J.; Lutz, J.A. Soil Enzyme Activity and Soil Nutrients Jointly Influence Post-Fire Habitat Models in Mixed-Conifer Forests of Yosemite National Park, USA. Fire 2020, 3, 54. https://doi.org/10.3390/fire3040054
Tamjidi J, Lutz JA. Soil Enzyme Activity and Soil Nutrients Jointly Influence Post-Fire Habitat Models in Mixed-Conifer Forests of Yosemite National Park, USA. Fire. 2020; 3(4):54. https://doi.org/10.3390/fire3040054
Chicago/Turabian StyleTamjidi, Jelveh, and James A. Lutz. 2020. "Soil Enzyme Activity and Soil Nutrients Jointly Influence Post-Fire Habitat Models in Mixed-Conifer Forests of Yosemite National Park, USA" Fire 3, no. 4: 54. https://doi.org/10.3390/fire3040054
APA StyleTamjidi, J., & Lutz, J. A. (2020). Soil Enzyme Activity and Soil Nutrients Jointly Influence Post-Fire Habitat Models in Mixed-Conifer Forests of Yosemite National Park, USA. Fire, 3(4), 54. https://doi.org/10.3390/fire3040054






